Abstract
In this study, the fabrication of Ti-12%Zr-4%Ta-2%Sn alloy, Ti-12%Zr-4%Ta-4%Sn alloy, and Ti-12%Zr-4%Ta-6%Sn alloy using powder metallurgy fabrication technique has been carried out. The influence of Sn addition on the corrosion of these alloys after 30 min and 3 days in 3.5% NaCl solution using various techniques has been reported. The Nyquist spectra revealed that boosting Sn content from 2 to 4% and further to 6% increases the corrosion resistance of the alloy through increasing the diameter of the obtained semicircle. Bode spectra also elucidated that the increased percentage of Sn increases the values of the impedance of the interface |Z| and the maximum degree of the phase angle (Φ). It was indicated from the cyclic polarization curves that the increased Sn content increases the passivation of the alloy through decreasing its rate of corrosion and increasing its corrosion resistance. The measured current over time at −0.10 V showed that the alloy with low Sn content, 2%, records the highest currents, which pronouncedly decreases when Sn content increases to 4% and further to 6%. Prolonging the time of exposure from 30 min to 3 days greatly enhances the passivation of the TiZrTaSn alloys due to the formation of mixed oxides of TiO2, ZrO2, TaO2, and SnO2. The results of these electrochemical measurements were confirmed by the surface investigations carried out by scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS). The results collectively proved that the uniform corrosion remarkably decreases with the increase in the Sn% and that the pitting corrosion is not likely to take place.
1 Introduction
Nowadays, the need for substituting the fractured or broken or flawed bones of the human body such as hips, fibula, tibia, humerus, tarsals, the knee hinge joint, etc., has significantly increased the necessity for developing biomedical implants. There are at least a million people who need to replace their hips. Based on the high need for replacing some of the human body bone parts, it is important to select high-quality materials for such applications. These materials must have excellent corrosion resistance with strong mechanical and tribological behavior, and biocompatibility [1,2,3]. One of the most employed materials in the biomedicine applications is the Ti-based alloys, which have also been employed long time in the aerospace and automotive industry [4,5,6,7]. This is because these alloys have an outstanding biocompatibility, great mechanical strength, good tribological behavior, and extraordinary corrosion resistance [8,9,10,11]. The continuous developments of Ti-based alloys have been reported to overcome some deficiencies in the current used alloys. Several studies have reported about the use of Ti-6Al-4V [10,11,12,13,14]. The presence of Al and V as main alloying elements could cause undesirable influence [10,11,12,13,14]. The dissolution of Al and V occur through their oxidation into Al3+ and V5+, which may cause an interaction with the bloodstream, thereby developing the risk of Alzheimer’s disease to occur [15,16]. There have been numerous trails to implant new alloying elements to replace Al and V [16,17]. Other researchers have reported the alloying Ti with different percentages of Zr, Nb, Sn, Ta, etc., to manufacture diverse alloys to be employed in different biomedical applications [13,18,19,20,21,22,23,24,25,26,27].
The influence of alloying Zr with Ti has been investigated and was found to improve the mechanical strength and remarkably increase the passivation to corrosion and for these reasons, these alloys can be of good use as biomedical implants [2,3,4,5,24,25,26,27,28,29,30,31,32,33,34]. The influence of Ta within Ti has been reported for its favorable usage in surgical sutures, prosthetic joints, and cranial plates, because of which Ta was found not to irritate the body [17,19,27,35,36,37]. Alloying Sn with Ti alloys improves its mechanical properties through improving its ductility and also increases its corrosion resistance [3,4,24,37,38,39]. Ijaz et al. [24] studied the mechanical characters and corrosion of Ti-Zr-Mo-Sn alloy and found that this alloy shows a large superelastic recovery strain and Young’s modulus and found also that this alloy has a thick protective film of the natively formed TiO2, ZrO2, MoO2, and SnO2 that passivate its surface. Torres-Sánchez et al. [38] have reported that the presence of 5 at.% Sn in different alloys of Ti-Nb increases the passivation of these alloys through forming a protective oxide film. This positive effect of Sn addition on improving the mechanical and corrosion behavior of Ti-base alloys was further confirmed by other research works [39,40,41].
The objective of the current research is to fabricate new alloys of Ti12Zr4Ta that contains 2% Sn, 4% Sn, and 6% Sn and characterize their corrosion in 3.5% NaCl solution. The fabrication process included the use of high energy ball mill for mixing and grinding, as well as, the use of high frequency heat induction furnace for sintering. The corrosion behavior was measured using methods including electrochemical and spectroscopic ones. The corrosion experiments were carried out after 30 min and 3 days immersion using polarization, impedance, and chronoamperometry experiments. The spectroscopic investigations were performed by employing SEM and EDS surface analysis. It is expected that these alloys show excellent corrosion resistance as compared to the other fabricated alloys, which enables our newly fabricated alloys to be employed in the biomedical applications.
2 Materials and methods
Ti, Zr, Ta, and Sn powders with > 99% purity have been utilized as received. 1 L of the 3.5% sodium chloride (NaCl, 99.9% purity, delivered by Merck) solution was prepared by weighing 35 gm NaCl and dissolving it in distilled water to have 1 L in a measuring flask. The Ti-base alloys, namely, Ti12%Zr4%Ta2%Sn, Ti12%Zr4%Ta4%Sn, and Ti12%Zr4%Ta6%Sn, were manufactured from their powders using the same procedures reported in a previous study [3]. In brief, the three alloys were manufactured by placing the desired mixture of powders of Ti, Zr, Ta, and Sn in a steel jar. A number of steel balls were also added to the powder mixture (the ball to powder ratio was 9:1). The jar was perfectly closed and placed in the high energy vibratory ball mill. The powders were ball milled at a speed of 2,000 rpm for 30 min to ensure its homogenous distribution. The mixture was sintered inside a cylindrical graphite die. The die with powders were placed inside a high frequency induction heat furnace sintered at 1,200°C for 5 min under 40 MPa pressure. The sintered samples were left to be cooled down before taking it out of the die. The fabricated alloys were mounted in an inert epoxy resin and polished with different emery papers up to 800 grits. A traditional three-electrode cell that can be filled with 60 mL test solution was employed. In this cell, the manufactured alloys were the working electrode, an Ag/AgCl was the reference electrode, and a platinum sheet was the counter electrode.
For all corrosion tests, an Autolab Model PGSTAT302N was used. The polarization data were gathered with a starting potential of −970 mV that is scanned to +170 mV and again in reverse way at 0.1667 mV/s scan rate. The chronoamperometric current measured at −100 mV was carried out for 30 min. The electrochemical impedance spectroscopy for both Nyquist and Bode spectra were collected from the corrosion potential after 30 min and 3 days immersion at a frequency range between 100 kHz and 100 mHz. The impedance data were obtained using the Powersine software at a rate of 10 points for every decade variation in the scanned frequency. To ensure the reproducibility of the experiments, each run was repeated three times by the use of a new surface of the examined alloys and in a new portion of the electrolyte. The surfaces of the alloys were investigated after performing the corrosion tests by the use of a scanning electron microscopy JEOL model (SEM, Tokyo, Japan) model JSM-7400F and an attached unit of energy dispersive spectroscopy (EDS).
3 Results and discussion
3.1 Electrochemical impedance spectroscopy (EIS)
This technique has been frequently employed in the investigating materials corrosion because it provides necessary information about the reactions at the material and solution interface [3,26,27,42,43,44,45,46,47]. Figure 1 displays the Nyquist plots of (1) TiZrTa-2%Sn alloy, (2) TiZrTa-4%Sn alloy, and (3) TiZrTa-6%Sn alloy, which were immersed in the solutions for (a) 30 min and (b) 3 days, respectively. These collected data were best-fitted to the equivalent circuit, which is exhibited in Figure 2. It is worth mentioning that the used circuit has been successfully employed in many investigations [3,26,27,48,49,50,51]. The parameters of Figure 2 are: R S is the solution resistance, Q 1 and Q 2 are the constant phase elements, and R P1 and R P2 are the polarization resistances and their values are exhibited in Table 1. In addition, the values of the open-circuit potential (OCP), which were shown on the Autolab potentiostat for each alloy after immersion in the chloride solution for 30 min and 3 days are also listed in Table 1.

Nyquist plots for (1) TiZrTa-2%Sn alloy, (2) TiZrTa-4%Sn alloy and (3) TiZrTa-6%Sn alloy after (a) 30 min and (b) 3 days exposure to the NaCl solution.

Circuit employed to fit EIS data.
Data obtained from fitting the EIS results
| Alloy/time | OCP (mV) | R S (Ω cm2) | Q 1 | R P1 (Ω cm2) | Q 2 | R P2 (Ω cm2) | Chi squared | ||
|---|---|---|---|---|---|---|---|---|---|
| Y Q1 (F/cm2) | n | Y Q2 (F/cm2) | n | ||||||
| TiZrTa-2%Sn/30 min | −625 | 46.4 | 0.000452 | 1.00 | 1,517 | 0.001825 | 0.82 | 5,262 | 6.8 × 10−3 |
| TiZrTa-4%Sn/30 min | −602 | 52.3 | 0.000434 | 1.00 | 1,780 | 0.000973 | 0.80 | 5,696 | 5.9 × 10−3 |
| TiZrTa-6%Sn/30 min | −587 | 58.8 | 0.000355 | 1.00 | 1,920 | 0.000331 | 0.80 | 7,167 | 5.3 × 10−3 |
| TiZrTa-2%Sn/3 days | −420 | 51.1 | 0.000153 | 0.90 | 1,966 | 0.000972 | 0.79 | 5,687 | 6.3 × 10−3 |
| TiZrTa-4%Sn/3 days | −398 | 55.8 | 0.000136 | 0.88 | 3,098 | 0.0002126 | 0.84 | 9,150 | 5.4 × 10−3 |
| TiZrTa-6%Sn/3 days | −384 | 60.5 | 0.000121 | 1.00 | 4,362 | 0.000073 | 0.83 | 18,698 | 5.2 × 10−3 |
The Nyquist plots of Figure 1 exhibit one semicircle for all alloys, whose diameter increased with the increase in the content of Sn from 2 to 6%. The increase in the diameter indicates the increase in the passivity of the alloy’s surface. Table 1 along with the plots of Figure 1 confirmed that the resistances, R S, R P1, and R P2 values, boost with duplicate and triplicate of Sn concentration in the alloy. The value of “n” component that accompanied Y Q1 is exactly “1” for all alloys. This means that Q 1 represents an ideal double layer capacitor (C dl), the value of Y Q1 decreases with the increase in the content of Sn, which proves that the corrosion resistance increases. As well as, the values of “n” component that accompanied Y Q2 is close to unity and indicates that Q 2 also represents another C dl that has some pores. The value of Y Q2 also decreases with the increase in the percentage of Sn. Therefore, the presence of Q 1 and Q 2 supports the assumption that rising Sn percentage supports the alloy’s passivation in chloride solution.
The Nyquist plots of Figure 1 indicated that the semicircles obtained after 3 days have wider diameter than those obtained after only 30 min exposure, which reveals that the alloys become more passivated after the longer immersion time of 3 days. Table 1 also supported these plots, as all resistances recorded higher values. In addition, both Q 1 and Q 2 represent double layer capacitors because the values the component “n” accompanying them are slightly lower than “1.” Y Q1 and Y Q2 pronouncedly decrease in values with the increase in the Sn content. The Nyquist plots and Table 1 together supported the fact of duplicating or even triplicating Sn to 4 and 6% mitigates the corrosion of the Ti alloy and extending the immersion to 3 days greatly enhanced.
Figure 3 exhibits the values of |Z| (Bode impedance of the interface) plotted against the change in frequency of (1) TiZrTa-2%Sn alloy, (2) TiZrTa-4%Sn alloy, and (3) TiZrTa-6%Sn alloy for (a) 30 min and (b) 3 days in NaCl solutions. These kinds of plots have been reported [3,26,27] to provide important information on the behavior of corrosion and even the protection from corrosion for most materials in their surrounding environments. The values of |Z| depicted in the figure are the lowest at higher values of frequency and exponentially increase with the scanning frequency toward the lower values. It is reported that recording higher values of |Z| when the values of frequency are lower indicates more passivation of the surface [3,26,27]. Therefore, increasing Sn% enhances passivation to some extent for the alloys under investigation when the immersion time was only 30 min, while it greatly increases the passivation of the surface after 3 days. This is because of the great difference seen in Figure 3(b), particularly at 6% Sn. This can be explained by the increase in the thickness of the formed oxides, which are TiO2, ZrO2, TaO2, and SnO2. This is not the case after only 30 min immersion, where the formed oxides do not have enough time to be thick. This also interprets the reason why prolonging the exposure time to the chloride solution before measurements allow the surface to show more passivation vs corrosion.

Bode |Z| plots of (1) TiZrTa-2%Sn alloy, (2) TiZrTa-4%Sn alloy, and (3) TiZrTa-6%Sn alloy for (a) 30 min and (b) 3 days in NaCl solutions.
The Bode Φ plots of (1) TiZrTa-2%Sn alloy, (2) TiZrTa-4%Sn alloy, and (3) TiZrTa-6%Sn alloy that were exposed for (a) 30 min and (b) 3 days in NaCl solutions are exhibited in Figure 5. The higher maximum value of Φ confirms the better resistance to corrosion reactions [26]. Highest maximum Φ value is seen in Figure 4(a) for TiZrTa6%Sn alloy followed by TiZrTa4%Sn and the least was recorded for TiZrTa2%Sn alloy. When the time was increased to 3 days, Figure 4(b), the maximum Φ value was seen by far for TiZrTa6%Sn alloy, while the other two alloys with lower Sn% was much lower. This indicates that TiZrTa6%Sn alloy has the highest protection against corrosion and also extending the time of immersion boosts the passivation for the three alloys. EIS plots and Table 1 collectively reveal that increasing the Sn contents from 2 to 4% and 6% improve the corrosion resistance and this effect increases with extending the time of immersion in Cl solution from 30 min to 3 days.

Bode Φ plots of (1) TiZrTa-2%Sn alloy, (2) TiZrTa-4%Sn alloy, and (3) TiZrTa-6%Sn alloy immersed for (a) 30 min and (b) 3 days in NaCl solutions.
3.2 Polarization and chronoamperometric data
The cyclic potentiodynamic polarization (CPP) measurements collected for the three TiZrTaSn alloys in 3.5% NaCl solution for 30 min is shown in Figure 5. Another set of polarization measurement was also performed after 3 days as demonstrated by the curves of Figure 6. The CPP investigating method has been frequently employed in understanding the corrosion and its mitigation of materials in aggressive media [3,26,27,47]. These curves were employed to collect the values of the cathodic Tafel slope, βc, and anodic Tafel slope, βa, corrosion potential, E Corr, and corrosion current, j Corr, corrosion rate, R Corr, and polarization resistance, R P. The values of these parameters are listed in Table 2 and are as reported previously [3,26,27,52,53,54].

CPP curves of TiZrTaSn alloys that was immersed in NaCl solution for 30 min.

CPP curves of TiZrTaSn alloys that were immersed in NaCl solution for 3 days.
Data obtained from the CPP measurements
| Alloy/time | βc (mV/dec) | E Corr (mV) | βa (mV/dec) | j Corr (μA/cm2) | R P (kΩ cm2) | R Corr (mm/year) |
|---|---|---|---|---|---|---|
| TiZrTa-2%Sn/30 min | 90 | −643 | 100 | 2.0 | 10.297 | 0.0204 |
| TiZrTa-4%Sn/30 min | 98 | −638 | 104 | 1.6 | 13.711 | 0.0163 |
| TiZrTa-6%Sn/30 min | 105 | −635 | 110 | 0.5 | 46.714 | 0.0051 |
| TiZrTa-2%Sn/3 days | 120 | −625 | 103 | 1.5 | 16.066 | 0.0153 |
| TiZrTa-4%Sn/3 days | 132 | −500 | 108 | 1.2 | 21.522 | 0.0122 |
| TiZrTa-6%Sn/3 days | 135 | −580 | 114 | 0.2 | 134.36 | 0.0020 |
Scanning the potential from −0.97 V led to the decrease in the current in the cathodic branch for all alloys. This is because of the occurrence of the oxygen reduction reaction that consumes oxygen to produce hydroxide ions on the surface of the alloys [26,27]. The cathodic side continues till the current values reach the values of j Corr after which the anodic reaction begins. Further scanning the potential led to further current increases mostly because of a formed oxide film dissolution from the alloys surface. Reversing the scan in the potential backward direction leads to increasing the obtained currents, which means the pitting corrosion takes place for all alloys at these conditions. The recorded currents for TiZrTa-2%Sn (Figure 5, curve 1) were the highest in both cathodic and anodic branches. Increasing the content of Sn to 4% (Figure 5, curve 2) provides pronounced current decreases and the highest decreases were recorded when the Sn content was increased to 6% as seen from Figure 5, curve 3.
The curves of Figure 6 also show a similar behavior to those of Figure 5 but at lower values of currents for all alloys. This means that the immersion for 3 days enhances the passivation of all alloys, which is definitely due to the thickening of the formed oxides of TiO2, ZrO2, TaO2, and SnO2. The impact of increasing Sn% from 2 to 6 is also seen to remarkably reduce the currents, particularly on the anodic side, which is also the case seen in Figure 5 and is probably resulted from the increase in the thickness of the formed SnO2 amongst the other formed oxides. The beneficial influence of increasing Sn% is also confirmed by the value of j Corr that is listed in Table 2, which decreases with the increase in the Sn%. Table 2 also presented that the value of R Corr also decreases and the value of R P increases with the increase in Sn%. The lowest j Corr and R Corr values, as well as, the highest R P values are for TiZrTa-6%Sn when prolonging the time of exposure to 3 days.
To understand whether a pitting attack at this condition takes place, an active anodic value of potential was selected from the CPP plots, where the dissolution reactions occur. The current vs time for (1) TiZrTa-2%Sn alloy, (2) TiZrTa-4%Sn alloy, and (3) TiZrTa-6%Sn alloy at −0.1 V and after 30 min of the immersion in NaCl solutions is exhibited in Figure 7. Same measurements were again performed for the same alloys after being exposed for 3 days and the change in their currents with time is demonstrated in Figure 8. The curves of Figures 7 and 8 show that the initial current recorded low values and rapidly increased in the first few seconds. This behavior results from the dissolution of a formed oxide film during immersing the alloys before measurement, this dissolution is because of the aggressiveness of the chloride anions and the active potential value.

Current vs time curves at −0.1 V for (1) TiZrTa-2%Sn alloy, (2) TiZrTa-4%Sn alloy, and (3) TiZrTa-6%Sn alloy after 30 min exposure to the NaCl solutions.

Current vs time for (1) TiZrTa-2%Sn alloy, (2) TiZrTa-4%Sn alloy, and (3) TiZrTa-6%Sn alloy at −0.1 V after 3 days exposure to the NaCl solutions.
The recorded currents for TiZrTa-2%Sn alloy (curve 1) is seen to be the highest recording, almost 45 µA/cm2. The current then decreases up to circa 30 µA/cm2 in the first 600 s as a result of oxide film formation. This film starts to partially dissolve causing the increase in the current again for few hundreds of seconds to finally stabilize with a slight decrease till the end of the experiment. Increasing the content of Sn to 4% (Figure 7, curve 2) causes a large decrease in the absolute currents, while the increase in Sn to 6% (Figure 7, curve 3) provided the lowest absolute currents. This supports the assumption that increasing Sn% enhances the resistance to corrosion for the alloys even at −0.1 V.
Extending the time of immersion to 3 days as shown in Figure 8 provided much lower current values when compared to those shown after only 30 min (Figure 7). The currents in Figure 8 increase abruptly in the first 60 s before being stabilized with time with little decrease in its value up to end of the run. The current first increased by the partial dissolution of the formed oxide film. The highest current was almost 7.5 µA/cm2 that was recorded for TiZrTa-2%Sn alloy, which means that immersing this alloy for 3 days decreases its current from 40 µA/cm2 to only 7.5 µA/cm2 and proves the ability of prolonging the immersion time to 3 days on decreasing the dissolution of the alloy in the chloride solution. Currents obtained from TiZrTa-4%Sn alloy were lower, where its highest current value was about 4.5 µA/cm2. Further increasing the content of Sn to 6%, TiZrTa-6%Sn alloy, decreased the obtained current to about 3.5 µA/cm2 at its maximum and this value decreases to its minimum at the end of the application of the potential. The reduction in the values of current with Sn% confirms the Sn ability on passivating the corrosion of the alloys. Also, increasing the time from 30 min to 3 days immersion in Cl solutions provides more passivation results from the thickening of the formed layer of mixed oxides, TiO2 + ZrO2 + TaO2 + SnO2.
3.3 Surface investigation
To understand the influence of Sn on the corrosion passivation of different alloys in the chloride solution, SEM and EDS analyses were performed before and after corrosion. Figure 9 shows (a) SEM image and (b) EDS profile taken from the polished surface of TiZrTa-2%Sn alloy before corrosion. It is seen that the surface is free of uniform or pitting corrosion. The EDS profile shown in Figure 9(b) depicts that the TiZrTaSn alloy has almost the same percentages of its starting elements. Where the EDS indicated that the surface has 82.41% Ti, 11.80% Zr, 3.83% Ta, and 1.96% Sn. The presence of these alloying elements at the present percentages proves the correct composition (82%Ti-12%Zr-4%Ta-2%Sn) of the alloy.

(a) SEM image and (b) EDS profile taken from a part of the surface of 82%Ti-12%Zr-4%Ta-2%Sn alloy before corrosion.
Another example, the SEM and EDS analyses taken for the alloy with composition 78%Ti-12%Zr-4%Ta-6%Sn are shown in Figure 10(a) and (b), respectively. It is seen that the surface of the alloy does not suffer uniform or pitting attacks. The EDS spectrum taken for the surface shown in the SEM image depicts that the alloy has 77.96% Ti, 11.89% Zr, 3.97% Ta, and 6.18% Sn. This also confirms the third alloy elemental composition, which is 78%Ti-12%Zr-4%Ta-6%Sn) of the alloy.

(a) SEM image and (b) EDS profile taken from a part of the surface of 78%Ti-12%Zr-4%Ta-6%Sn alloy before corrosion.
The surface of TiZrTa-2%Sn alloy was imaged by SEM after performing the current vs time experiment seen by curve 1 in Figure 8 and the image is presented in Figure 11(a). The EDS profile taken for that surface is exhibited also in Figure 11(b). The image shows a regular face except in few areas where there is a possibility of the presence of a pit. This is due to the aggressive action of the Cl− plus the application of −0.1 V for about 1,800 s. The EDS profile shows the presence of all alloying elements along with the detection of oxygen. The equivalent weight percent of each detected element is as follows, 59.43 Ti(K), 25.88 O(K), 10.32 Zr(L), 1.42 Sn(L), and 2.95 Ta(M). All these values for the main alloying elements were lower than its presence in the alloy due to the presence of O. The detection of O at high wt% implies that it is incorporated with other alloying elements to form their oxides, namely, TiO2, ZrO2, TaO2, and SnO2 [39,40]. The detection of few pits is due to the formed oxide layer, which may have some flaws that allow the chloride ions to penetrate it causing the occurrence of pitting attack.
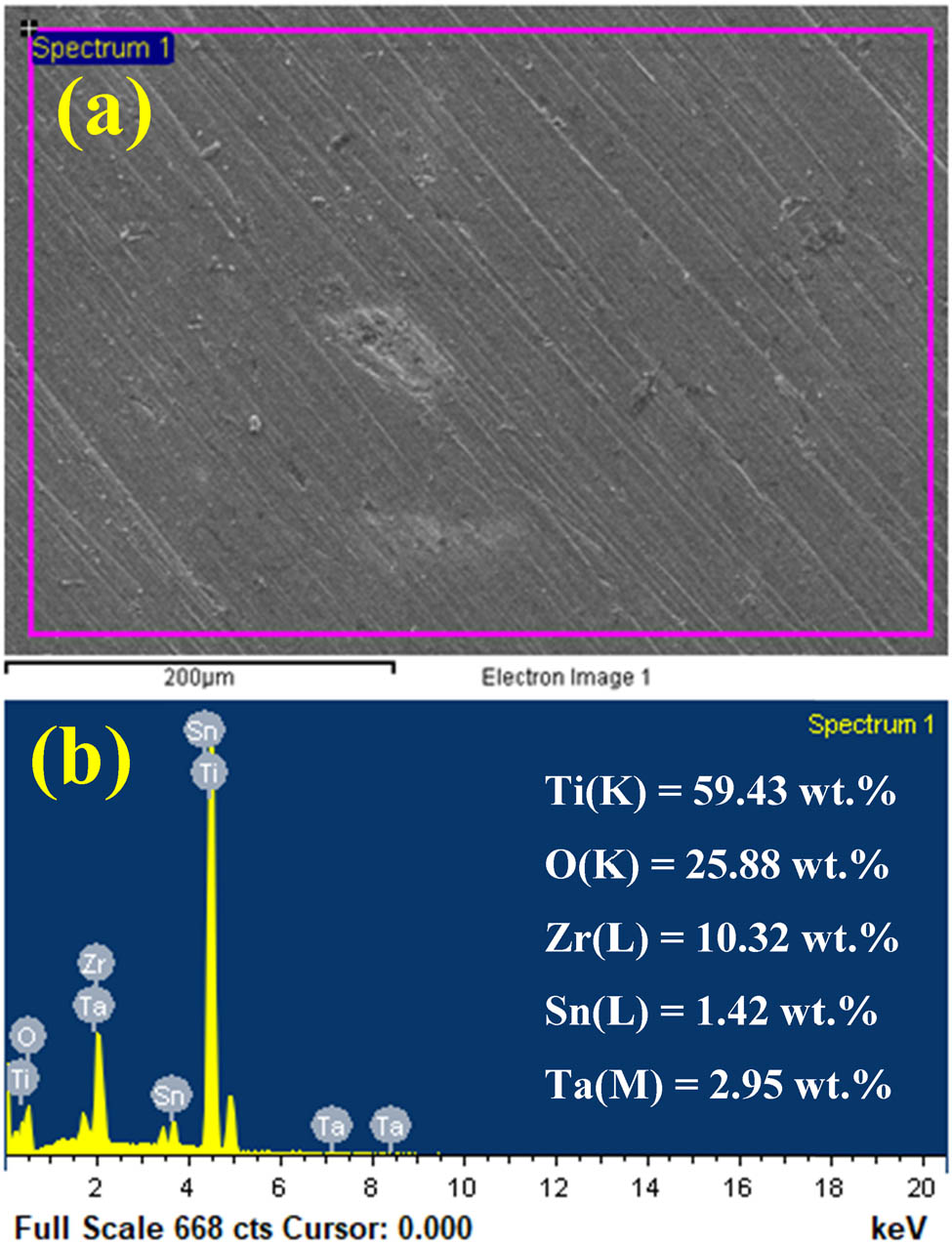
(a) SEM image and (b) EDS profile taken from a part of the surface of TiZrTa-2%Sn alloy after performing the test shown in Figure 8 (curve 1).
The effect of extending the time of immersion to 3 days on the morphology of TiZrTa-6%Sn alloy’s surface and the detected wt% of elements on its surface is also investigated. The SEM image and the corresponding EDS profile for TiZrTa-6%Sn alloy are exhibited in Figure 12(a) and (b). The SEM image, Figure 12(a), displays a homogenous surface that is clear from having any pits. The EDS profile, Figure 12(b) presents the detection of all alloying elements in addition to oxygen. The wt% for all detected elements are as follows: 54.89 Ti(K), 27.64 O(K), 9.82 Zr(L), 4.20 Sn(L), and 3.45 Ta(M). All these values for the main alloying elements were lower than its presence in the alloy due to the presence of O, where the detection of O at high wt% says that it is incorporated with other alloying elements to form their oxides, namely, TiO2, ZrO2, TaO2, and SnO2 [39,40]. The surface investigation thus confirmed the chronoamperometric experiments that the high content of Sn, 6%, provides better passivation than the alloys that have 4 and 2% Sn.

(a) SEM image and (b) EDS profile taken from a part of the surface of TiZrTa-6%Sn alloy after performing the test shown in Figure 8 (curve 3).
4 Conclusion
Three new TiZrTaSn alloys were fabricated by mixing the powders of high purity Ti, Zr, Ta, and Sn. Those alloys are Ti12%Zr4%Ta2%Sn, Ti12%Zr4%Ta4%Sn, and Ti12%Zr4%Ta6%Sn. The behavior of corrosion for these alloys after exposure in NaCl (3.5%) solution for 30 min and 3 days was reported using EIS, CPP, and chronoamperometric current over the running time at an active anodic potential of –0.1 V. The surface of the alloys after being tested for corrosion possibility was examined by SEM and EDS investigations. Nyquist and Bode spectra revealed that increasing Sn from 2 to 6% highly boosts the resistances (surface and polarization) and thus, increases the passivation of alloys. CPP experiments elucidated that increasing Sn% reduces the cathodic and anodic currents along with j Corr values, which reflects on the direct decrease of R Corr values. The chronoamperometric experiments exhibited that the increase in Sn% minimizes the absolute currents over time and eliminates the occurrence of pitting corrosion. The surface investigations using SEM and EDS showed that the surface of the alloys does not suffer a sever corrosion and the appearance of a considerable wt% of oxygen along with Ti, Zr, Ta, and Sn elements, which indicates that the surface develops a mixture of oxide layers, i.e., TiO2 + ZrO2 + TaO2 + SnO2. Measurements collectively substantiated that these new alloys have a great corrosion resistance that enables them to be used in the field of biomedical applications.
Acknowledgement
Researchers Supporting Project Number (RSP2024R33), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: This work was financially supported by the Researchers Supporting Project Number (RSP2024R33), King Saud University, Riyadh, Saudi Arabia.
-
Author contributions: The author confirms the sole responsibility for the conception of the study, presented results, and manuscript preparation.
-
Conflict of interest: The author states no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available within the manuscript.
References
[1] Yu Z. Titanium alloys for biomedical development and applications. In Design, microstrcature, properties, and application. Amsterdam, The Netherlands: Elsevier Inc; 2022. 10.1016/C2020-0-00127-1. ISBN: 978-0-12-823927-8.Search in Google Scholar
[2] Luo Y, Yang L, Tian M. Application of biomedical-grade titanium alloys in trabecular bone and artificial joints. In: Davim JP, editor. Biomaterials and medical tribology, research and development. A volume in Woodhead Publishing series in biomaterials. The Netherlands: Elsevier Inc.; 2013. p. 181–216. 10.1533/9780857092205.181. ISBN: 978-0-85709-017-1.Search in Google Scholar
[3] Sherif ESM, Bahri YA, Alharbi HF, Ijaz MF. Corrosion passivation in simulated body fluid of Ti-Zr-Ta-xSn alloys as biomedical materials. Materials. 2023;16:4603. 10.3390/ma16134603.Search in Google Scholar PubMed PubMed Central
[4] Ijaz MF, Alharbi HF, Bahri YA, Sherif ESM. Alloy design and fabrication of duplex titanium-based alloys by spark plasma sintering for biomedical implant applications. Materials. 2022;15:8562.10.3390/ma15238562Search in Google Scholar PubMed PubMed Central
[5] Abdulmenova EV, Buyakova SP, Kulkov SN. Electrochemical hydrogenation of Ti–Ni powder mechanochemically alloyed with titanium. Intermetallics. 2022;151:107739.10.1016/j.intermet.2022.107739Search in Google Scholar
[6] Davim JP. Materials and surface engineering, research and development. A volume in Woodhead Publishing reviews: Mechanical engineering series. 1st edn. The Netherlands: Elsevier Inc., eBook; 2012 Feb. ISBN: 9780857096036.Search in Google Scholar
[7] Chicardi E, García-Garrido C, Sayagués M, Torres Y, Amigó C, Aguilar V. Development of a novel FCC structure for an amorphous-nanocrystalline Ti-33Nb-4Mn (at.%) ternary alloy. Mater Charact. 2018;135:46–56.10.1016/j.matchar.2017.11.021Search in Google Scholar
[8] Héraud L, Castany P, Ijaz MF, Gordin DM, Gloriant T. Large-strain functional fatigue properties of superelastic metastable β titanium and NiTi alloys: A comparative study. J Alloy Compd. 2023;953:170170.10.1016/j.jallcom.2023.170170Search in Google Scholar
[9] Davim JP. Mechanical behavior of biomaterials. A volume in Woodhead Publishing series in biomaterials. 1st edn. The Netherlands: Elsevier Inc., eBook; 2019 June. ISBN: 9780081021750.Search in Google Scholar
[10] Smith SM, Gilbert JL. Interfacial compliance, energy dissipation, frequency effects, and long-term fretting corrosion performance of Ti-6Al-4V/CoCrMo interfaces. J Biomed Mater Res A. 2022;110:409–23.10.1002/jbm.a.37299Search in Google Scholar PubMed
[11] Sitek R, Kaminski J, Borysiuk J, Matysiak H, Kubiak K, Kurzydlowski KJ. Microstructure and properties of titanium aluminides on Ti6Al4V titanium alloy produced by chemical vapor deposition method. Intermetallics. 2013;36:36–44.10.1016/j.intermet.2012.12.017Search in Google Scholar
[12] Krawiec H, Vignal V, Loch J, Erazmus-Vignal P. Influence of plastic deformation on the microstructure and corrosion behaviour of Ti–10Mo–4Zr and Ti–6Al–4V alloys in the Ringer’s solution at 37°C. Corros Sci. 2015;96:160–70.10.1016/j.corsci.2015.04.006Search in Google Scholar
[13] Chong Y, Bhattacharjee T, Tsuji N. Bi-lamellar microstructure in Ti–6Al–4V: Microstructure evolution and mechanical properties. Mater Sci Eng A. 2019;762:138077.10.1016/j.msea.2019.138077Search in Google Scholar
[14] Kurtz MA, Wessinger AC, Mace A, Moreno-Reyes A, Gilbert JL. Additively manufactured Ti-29Nb-21Zr shows improved oxide polarization resistance versus Ti-6Al-4V in inflammatory simulating solution. J Biomed Mater Res. 2023;111:1538–53.10.1002/jbm.a.37552Search in Google Scholar PubMed
[15] Kandimalla R, Vallamkondu J, Corigat EB, Gill KD. Understanding aspects of aluminum exposure in Alzheimer’s disease development. Brain Pathol. 2016;26:139–54.10.1111/bpa.12333Search in Google Scholar PubMed PubMed Central
[16] Kawahara M, Kato-Negishi M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimer’s Dis. 2011;2011:276393.10.4061/2011/276393Search in Google Scholar PubMed PubMed Central
[17] Lin J, Ozan S, Li Y, Ping D, Tong X, Li G, et al. Novel Ti-Ta-Hf-Zr alloys with promising mechanical properties for prospective stent applications. Sci Rep. 2016;6:1–11.10.1038/srep37901Search in Google Scholar PubMed PubMed Central
[18] Tahara M, Kim HY, Hosoda H, Miyazaki S. Cyclic deformation behavior of a Ti-26 at.% Nb alloy. Acta Mater. 2009;57:2461–9.10.1016/j.actamat.2009.01.037Search in Google Scholar
[19] Stenlund P, Omar O, Brohede U, Norgren S, Norlindh B, Johansson A, et al. Bone response to a novel Ti-Ta-Nb-Zr alloy. Acta Biomater. 2015;20:165–75.10.1016/j.actbio.2015.03.038Search in Google Scholar PubMed
[20] Abdel-Hady M, Fuwa H, Hinoshita K, Kimura H, Shinzato Y, Morinaga M. Phase stability change with Zr content in β-type Ti–Nb alloys. Scr Mater. 2007;57(11):1000–3.10.1016/j.scriptamat.2007.08.003Search in Google Scholar
[21] Fojt J, Joska L, Malek J. Corrosion behaviour of porous Ti–39Nb alloy for biomedical applications. Corros Sci. 2013;71:78–83.10.1016/j.corsci.2013.03.007Search in Google Scholar
[22] Kim J, Park HW. Influence of a large pulsed electron beam (LPEB) on the corrosion resistance of Ti6Al7Nb alloys. Corros Sci. 2015;96:153–60.10.1016/j.corsci.2014.10.008Search in Google Scholar
[23] Mohan L, Anandan C, Rajendran N. Electrochemical behavior and effect of heat treatment on morphology, crystalline structure of self-organized TiO2 nanotube arrays on Ti–6Al–7Nb for biomedical applications. Mater Sci Eng C. 2015;50:394–401.10.1016/j.msec.2015.02.013Search in Google Scholar PubMed
[24] Ijaz MF, Vasilescu C, Drob SI, Osiceanu P, Marcu M, Kim HY, et al. Electrochemical characterization of the superelastic (Ti-Zr)-Mo-Sn biomedical alloy displaying a large recovery strain. Mater Corros. 2017;68:1220–7.10.1002/maco.201709484Search in Google Scholar
[25] Grandin HM, Berner S, Dard M. A review of titanium zirconium (TiZr) alloys for use in endosseous dental implants. Materials. 2012;5:1348–60.10.3390/ma5081348Search in Google Scholar
[26] Alharbi HF, Bahri YA, Sherif ESM. Influence of zirconium on the corrosion passivation of titanium in simulated body fluid. Crystals. 2021;11:1391. 10.3390/cryst11111391.Search in Google Scholar
[27] Sherif ESM, Bahri YA, Alharbi HF, Ijaz MF, Alnaser IA. Influence of tantalum addition on the corrosion passivation of titanium-zirconium alloy in simulated body fluid. Materials. 2022;15:8812. 10.3390/ma15248812.Search in Google Scholar PubMed PubMed Central
[28] Hu M, Wang L, Li G, Huang Q, Liu Y, He J, et al. Investigations on microstructure and properties of Ti-Nb-Zr medium-entropy alloys for metallic biomaterials. Intermetallics. 2022;145:107568.10.1016/j.intermet.2022.107568Search in Google Scholar
[29] Correa DRN, Kuroda PAB, Lourenço ML, Buzalaf MAR, Grandini CR. Adjustment of the microstructure and selected mechanical properties of biomedical Ti-15Zr-Mo alloys through oxygen doping. J Alloy Compd. 2019;775:158–67.10.1016/j.jallcom.2018.10.105Search in Google Scholar
[30] Sanchez AG, Schreiner W, Duffó G, Ceré S. Surface characterization of anodized zirconium for biomedical applications. Appl Surf Sci. 2011;257:6397–405.10.1016/j.apsusc.2011.02.005Search in Google Scholar
[31] Yang S, Zhang DC, Wei M, Su HX, Wu W, Lin JG. Effects of the Zr and Mo contents on the electrochemical corrosion behavior of Ti–22Nb alloy. Mater Corros. 2013;64:402–7. 10.1002/maco.201106478.Search in Google Scholar
[32] Chelariu R, Mareci D, Munteanu C. Preliminary electrochemical testing of some Zr–Ti alloys in 0.9% NaCl solution. Mater Corros. 2013;64:585–91. 10.1002/maco.201206717.Search in Google Scholar
[33] Trincă LC, Mareci D, Cimpoeşu N, Călin M, Stan T. Influence of hydrogen peroxide on the corrosion of thermally oxidized ZrTi alloys in phosphate-buffered saline solution. Mater Corros. 2016;67:1088–95. 10.1002/maco.201508785.Search in Google Scholar
[34] Wang BL, Zheng YF, Zhao LC. Electrochemical corrosion behavior of biomedical Ti–22Nb and Ti–22Nb–6Zr alloys in saline medium. Mater Corros. 2009;60:788–94. 10.1002/maco.200805173.Search in Google Scholar
[35] Jeong S. Basic knowledge about metal stent development. Clin Endosc. 2016;49:108–12.10.5946/ce.2016.029Search in Google Scholar PubMed PubMed Central
[36] Festas AJ, Ramos A, Davim JP. Medical devices biomaterials–A review. Proc Inst Mech Eng Part L J Mater Des Appl. 2020;234:218–28. 10.1177/1464420719882458.Search in Google Scholar
[37] Keshtta A, Gepreel MAH. Effect of Sn-addition on the properties of the biomedical Ti17Nb-6Ta alloy. IOP Conf Ser Mater Sci Eng. 2019;553:012032.10.1088/1757-899X/553/1/012032Search in Google Scholar
[38] Torres-Sánchez C, Wang J, Norrito M, Zani L, Conway PP. Addition of Sn to TiNb alloys to improve mechanical performance and surface properties conducive to enhanced cell activity. Mater Sci Eng C. 2020;115:110839.10.1016/j.msec.2020.110839Search in Google Scholar PubMed
[39] Zhang C, Zhang S, Pan Y, Xu W, Singh HP, Liu B, et al. Effect of Sn addition on the mechanical properties and high-temperature oxidation resistance of intermetallic TiAl alloys by first principles study and experimental investigation. J Mater Res Technol. 2022;21:3666–77.10.1016/j.jmrt.2022.11.007Search in Google Scholar
[40] Drob SI, Ijaz MF, Vasilescu C, Osiceanu P, Gordin DM, Cimpean A, et al. Surface characterization, corrosion resistance and in vitro biocompatibility of a new Ti-Hf-Mo-Sn alloy. Materials. 2016;9:818. 10.3390/ma9100818.Search in Google Scholar PubMed PubMed Central
[41] Dal Bó MR, Salvador AF, CMello MG, Lima DD, Faria GA, Ramirez AJ, et al. The effect of Zr and Sn additions on the microstructure of Ti-Nb-Fe gum metals with high elastic admissible strain. Mater Des. 2018;160:1186–95.10.1016/j.matdes.2018.10.040Search in Google Scholar
[42] Sherif EM, Park SM. Effects of 1,5-naphthalenediol on aluminum corrosion as a corrosion inhibitor in 0.50 M NaCl. J Electrochem Soc. 2005;152:B205–11. 10.1149/1.1914752.Search in Google Scholar
[43] Gopi D, Sherif ESM, Manivannan V, Rajeswari D, Surendiran M, Kavitha L. Corrosion and corrosion inhibition of mild steel in groundwater at different temperatures by newly synthesized benzotriazole and phosphono derivatives. Ind Eng Chem Res. 2014;53:4286–94.10.1021/ie4039357Search in Google Scholar
[44] Sherif EM, Park SM. Effects of 1,4-naphthoquinone on aluminum corrosion in 0.50 M sodium chloride solutions. Electrochim Acta. 2006;51:1313–21. 10.1016/j.electacta.2005.06.018.Search in Google Scholar
[45] Afzali P, Ghomashchi R, Oskouei RH. On the corrosion Behaviour of low modulus titanium alloys for medical implant applications: A review. Metals. 2019;9:878.10.3390/met9080878Search in Google Scholar
[46] Sherif ESM, Potgieter JH, Comins JD, Cornish L, Olubambi PA, Machio CN. The beneficial effect of ruthenium additions on the passivation of duplex stainless-steel corrosion in sodium chloride solutions. Corros Sci. 2009;51:1364–71.10.1016/j.corsci.2009.03.022Search in Google Scholar
[47] Latief FH, Sherif ESM, Almajid AA, Junaedi H. Fabrication of exfoliated graphite nanoplatelets-reinforced aluminum composites and evaluating their mechanical properties and corrosion behavior. J Anal Appl Pyrol. 2011;92:485–92.10.1016/j.jaap.2011.09.003Search in Google Scholar
[48] Alam MA, Samad UA, Sherif ESM, Seikh AH, Al-Zahrani SM, Alharthi NH, et al. Synergistic effect of Ag and ZnO nanoparticles on polyaniline incorporated epoxy/2pack coatings for splash zone applications. J Coat Technol Res. 2019;16:835–45.10.1007/s11998-018-00156-4Search in Google Scholar
[49] Alam MA, Sherif ESM, Al-Zahrani SM. Mechanical properties and corrosion behavior of different coatings fabricated by diglycidyl ether of bisphenol-a epoxy resin and Aradur®-3282 curing agent. Int J Electrochem Sci. 2013;8:8388–400.10.1016/S1452-3981(23)12896-3Search in Google Scholar
[50] Alam MA, Samad UA, Seikh A, Mohammed JA, Al-Zahrani SM, Sherif ESM. Development and characterization of PA 450 and PA 3282 epoxy coatings as anti-corrosion materials for offshore applications. Materials. 2022;15:2562. 10.3390/ma15072562.Search in Google Scholar PubMed PubMed Central
[51] Samad UA, Alam MA, Seikh AH, Mohammed JA, Al-Zahrani SM, Sherif ESM. Corrosion resistance performance of epoxy coatings incorporated with unmilled micro aluminium pigments. Crystals. 2023;13:558. 10.3390/cryst13040558.Search in Google Scholar
[52] Alshammri GA, Fathy N, Al-Shomar SM, Alshammari AH, Sherif ESM, Ramadan M. Effect of Al2O3 and NiO nanoparticle additions on the structure and corrosion behavior of Sn—4% Zn alloy coating carbon steel. Sustainability. 2023;15:2511. 10.3390/su15032511.Search in Google Scholar
[53] Sivasankaran S, Sherif ESM, Ammar HR, Alaboodi AS, Mekky ABH. Influence of oxide dispersions (Al2O3, TiO2, and Y2O3) in CrFeCuMnNi high-entropy alloy on microstructural changes and corrosion resistance. Crystals. 2023;13:605. 10.3390/cryst13040605.Search in Google Scholar
[54] Ammar HR, Sherif E-SM, Sivasankaran S, Almufadi FA, Mekky A-BH. Developing improved corrosion-resistant AA5083—BN/WC composites for tribological applications. Materials. 2023;16:1663. 10.3390/ma16041663.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Research on damage evolution mechanisms under compressive and tensile tests of plain weave SiCf/SiC composites using in situ X-ray CT
- Structural optimization of trays in bolt support systems
- Continuum percolation of the realistic nonuniform ITZs in 3D polyphase concrete systems involving the aggregate shape and size differentiation
- Multiscale water diffusivity prediction of plain woven composites considering void defects
- The application of epoxy resin polymers by laser induction technologies
- Analysis of water absorption on the efficiency of bonded composite repair of aluminum alloy panels
- Experimental research on bonding mechanical performance of the interface between cementitious layers
- A study on the effect of microspheres on the freeze–thaw resistance of EPS concrete
- Influence of Ti2SnC content on arc erosion resistance in Ag–Ti2SnC composites
- Cement-based composites with ZIF-8@TiO2-coated activated carbon fiber for efficient removal of formaldehyde
- Microstructure and chloride transport of aeolian sand concrete under long-term natural immersion
- Simulation study on basic road performance and modification mechanism of red mud modified asphalt mixture
- Extraction and characterization of nano-silica particles to enhance mechanical properties of general-purpose unsaturated polyester resin
- Roles of corn starch and gellan gum in changing of unconfined compressive strength of Shanghai alluvial clay
- A review on innovative approaches to expansive soil stabilization: Focussing on EPS beads, sand, and jute
- Experimental investigation of the performances of thick CFRP, GFRP, and KFRP composite plates under ballistic impact
- Preparation and characterization of titanium gypsum artificial aggregate
- Characteristics of bulletproof plate made from silkworm cocoon waste: Hybrid silkworm cocoon waste-reinforced epoxy/UHMWPE composite
- Experimental research on influence of curing environment on mechanical properties of coal gangue cementation
- Multi-objective optimization of machining variables for wire-EDM of LM6/fly ash composite materials using grey relational analysis
- Synthesis and characterization of Ag@Ni co-axial nanocables and their fluorescent and catalytic properties
- Beneficial effect of 4% Ta addition on the corrosion mitigation of Ti–12% Zr alloy after different immersion times in 3.5% NaCl solutions
- Study on electrical conductive mechanism of mayenite derivative C12A7:C
- Fast prediction of concrete equivalent modulus based on the random aggregate model and image quadtree SBFEM
- Research on uniaxial compression performance and constitutive relationship of RBP-UHPC after high temperature
- Experimental analysis of frost resistance and failure models in engineered cementitious composites with the integration of Yellow River sand
- Influence of tin additions on the corrosion passivation of TiZrTa alloy in sodium chloride solutions
- Microstructure and finite element analysis of Mo2C-diamond/Cu composites by spark plasma sintering
- Low-velocity impact response optimization of the foam-cored sandwich panels with CFRP skins for electric aircraft fuselage skin application
- Research on the carbonation resistance and improvement technology of fully recycled aggregate concrete
- Study on the basic properties of iron tailings powder-desulfurization ash mine filling cementitious material
- Preparation and mechanical properties of the 2.5D carbon glass hybrid woven composite materials
- Improvement on interfacial properties of CuW and CuCr bimetallic materials with high-entropy alloy interlayers via infiltration method
- Investigation properties of ultra-high performance concrete incorporating pond ash
- Effects of binder paste-to-aggregate ratio and polypropylene fiber content on the performance of high-flowability steel fiber-reinforced concrete for slab/deck overlays
- Interfacial bonding characteristics of multi-walled carbon nanotube/ultralight foamed concrete
- Classification of damping properties of fabric-reinforced flat beam-like specimens by a degree of ondulation implying a mesomechanic kinematic
- Influence of mica paper surface modification on the water resistance of mica paper/organic silicone resin composites
- Impact of cooling methods on the corrosion behavior of AA6063 aluminum alloy in a chloride solution
- Wear mechanism analysis of internal chip removal drill for CFRP drilling
- Investigation on acoustic properties of metal hollow sphere A356 aluminum matrix composites
- Uniaxial compression stress–strain relationship of fully aeolian sand concrete at low temperatures
- Experimental study on the influence of aggregate morphology on concrete interfacial properties
- Intelligent sportswear design: Innovative applications based on conjugated nanomaterials
- Research on the equivalent stretching mechanical properties of Nomex honeycomb core considering the effect of resin coating
- Numerical analysis and experimental research on the vibration performance of concrete vibration table in PC components
- Assessment of mechanical and biological properties of Ti–31Nb–7.7Zr alloy for spinal surgery implant
- Theoretical research on load distribution of composite pre-tightened teeth connections embedded with soft layers
- Coupling design features of material surface treatment for ceramic products based on ResNet
- Optimizing superelastic shape-memory alloy fibers for enhancing the pullout performance in engineered cementitious composites
- Multi-scale finite element simulation of needle-punched quartz fiber reinforced composites
- Thermo-mechanical coupling behavior of needle-punched carbon/carbon composites
- Influence of composite material laying parameters on the load-carrying capacity of type IV hydrogen storage vessel
- Review Articles
- Effect of carbon nanotubes on mechanical properties of aluminum matrix composites: A review
- On in-house developed feedstock filament of polymer and polymeric composites and their recycling process – A comprehensive review
- Research progress on freeze–thaw constitutive model of concrete based on damage mechanics
- A bibliometric and content analysis of research trends in paver blocks: Mapping the scientific landscape
- Bibliometric analysis of stone column research trends: A Web of Science perspective
Articles in the same Issue
- Regular Articles
- Research on damage evolution mechanisms under compressive and tensile tests of plain weave SiCf/SiC composites using in situ X-ray CT
- Structural optimization of trays in bolt support systems
- Continuum percolation of the realistic nonuniform ITZs in 3D polyphase concrete systems involving the aggregate shape and size differentiation
- Multiscale water diffusivity prediction of plain woven composites considering void defects
- The application of epoxy resin polymers by laser induction technologies
- Analysis of water absorption on the efficiency of bonded composite repair of aluminum alloy panels
- Experimental research on bonding mechanical performance of the interface between cementitious layers
- A study on the effect of microspheres on the freeze–thaw resistance of EPS concrete
- Influence of Ti2SnC content on arc erosion resistance in Ag–Ti2SnC composites
- Cement-based composites with ZIF-8@TiO2-coated activated carbon fiber for efficient removal of formaldehyde
- Microstructure and chloride transport of aeolian sand concrete under long-term natural immersion
- Simulation study on basic road performance and modification mechanism of red mud modified asphalt mixture
- Extraction and characterization of nano-silica particles to enhance mechanical properties of general-purpose unsaturated polyester resin
- Roles of corn starch and gellan gum in changing of unconfined compressive strength of Shanghai alluvial clay
- A review on innovative approaches to expansive soil stabilization: Focussing on EPS beads, sand, and jute
- Experimental investigation of the performances of thick CFRP, GFRP, and KFRP composite plates under ballistic impact
- Preparation and characterization of titanium gypsum artificial aggregate
- Characteristics of bulletproof plate made from silkworm cocoon waste: Hybrid silkworm cocoon waste-reinforced epoxy/UHMWPE composite
- Experimental research on influence of curing environment on mechanical properties of coal gangue cementation
- Multi-objective optimization of machining variables for wire-EDM of LM6/fly ash composite materials using grey relational analysis
- Synthesis and characterization of Ag@Ni co-axial nanocables and their fluorescent and catalytic properties
- Beneficial effect of 4% Ta addition on the corrosion mitigation of Ti–12% Zr alloy after different immersion times in 3.5% NaCl solutions
- Study on electrical conductive mechanism of mayenite derivative C12A7:C
- Fast prediction of concrete equivalent modulus based on the random aggregate model and image quadtree SBFEM
- Research on uniaxial compression performance and constitutive relationship of RBP-UHPC after high temperature
- Experimental analysis of frost resistance and failure models in engineered cementitious composites with the integration of Yellow River sand
- Influence of tin additions on the corrosion passivation of TiZrTa alloy in sodium chloride solutions
- Microstructure and finite element analysis of Mo2C-diamond/Cu composites by spark plasma sintering
- Low-velocity impact response optimization of the foam-cored sandwich panels with CFRP skins for electric aircraft fuselage skin application
- Research on the carbonation resistance and improvement technology of fully recycled aggregate concrete
- Study on the basic properties of iron tailings powder-desulfurization ash mine filling cementitious material
- Preparation and mechanical properties of the 2.5D carbon glass hybrid woven composite materials
- Improvement on interfacial properties of CuW and CuCr bimetallic materials with high-entropy alloy interlayers via infiltration method
- Investigation properties of ultra-high performance concrete incorporating pond ash
- Effects of binder paste-to-aggregate ratio and polypropylene fiber content on the performance of high-flowability steel fiber-reinforced concrete for slab/deck overlays
- Interfacial bonding characteristics of multi-walled carbon nanotube/ultralight foamed concrete
- Classification of damping properties of fabric-reinforced flat beam-like specimens by a degree of ondulation implying a mesomechanic kinematic
- Influence of mica paper surface modification on the water resistance of mica paper/organic silicone resin composites
- Impact of cooling methods on the corrosion behavior of AA6063 aluminum alloy in a chloride solution
- Wear mechanism analysis of internal chip removal drill for CFRP drilling
- Investigation on acoustic properties of metal hollow sphere A356 aluminum matrix composites
- Uniaxial compression stress–strain relationship of fully aeolian sand concrete at low temperatures
- Experimental study on the influence of aggregate morphology on concrete interfacial properties
- Intelligent sportswear design: Innovative applications based on conjugated nanomaterials
- Research on the equivalent stretching mechanical properties of Nomex honeycomb core considering the effect of resin coating
- Numerical analysis and experimental research on the vibration performance of concrete vibration table in PC components
- Assessment of mechanical and biological properties of Ti–31Nb–7.7Zr alloy for spinal surgery implant
- Theoretical research on load distribution of composite pre-tightened teeth connections embedded with soft layers
- Coupling design features of material surface treatment for ceramic products based on ResNet
- Optimizing superelastic shape-memory alloy fibers for enhancing the pullout performance in engineered cementitious composites
- Multi-scale finite element simulation of needle-punched quartz fiber reinforced composites
- Thermo-mechanical coupling behavior of needle-punched carbon/carbon composites
- Influence of composite material laying parameters on the load-carrying capacity of type IV hydrogen storage vessel
- Review Articles
- Effect of carbon nanotubes on mechanical properties of aluminum matrix composites: A review
- On in-house developed feedstock filament of polymer and polymeric composites and their recycling process – A comprehensive review
- Research progress on freeze–thaw constitutive model of concrete based on damage mechanics
- A bibliometric and content analysis of research trends in paver blocks: Mapping the scientific landscape
- Bibliometric analysis of stone column research trends: A Web of Science perspective

