Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
Abstract
C37H56NO4.5, tetragonal, P41212/c (no. 92), a = 10.4368(10) Å, b = 10.4268(10) Å, c = 61.5166(11) Å, V = 6700.80(18) Å3, Z = 8, R gt (F) = 0.0557, wR ref (F2) = 0.1350, T = 100 K.
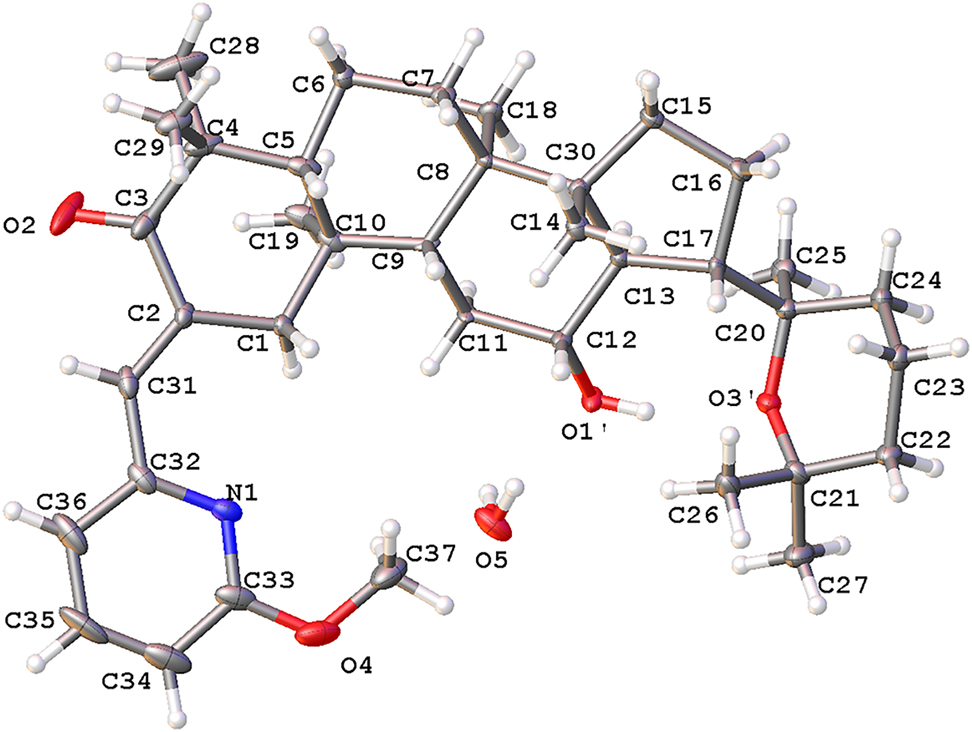
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.12 × 0.10 × 0.08 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.59 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 73.6°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 14308, 6554, 0.046 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5487 |
| N(param)refined: | 612 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1a | 0.0487 (4) | 0.2764 (5) | 0.52841 (7) | 0.0287 (11) |
| H1a | −0.019314 | 0.307386 | 0.524310 | 0.043* |
| O1b | 0.0368 (4) | 0.1529 (5) | 0.52199 (7) | 0.0314 (12) |
| H1′b | −0.033400 | 0.174839 | 0.517318 | 0.047* |
| O2 | 0.6118 (3) | −0.0279 (4) | 0.61718 (5) | 0.0736 (12) |
| O3a | −0.1901 (5) | 0.3522 (5) | 0.52263 (9) | 0.0250 (11) |
| O3′b | −0.2106 (9) | 0.1794 (8) | 0.51019 (11) | 0.030 (2) |
| O4 | 0.3865 (3) | 0.5363 (3) | 0.55606 (5) | 0.0557 (8) |
| N1 | 0.4692 (3) | 0.3783 (3) | 0.57838 (4) | 0.0297 (6) |
| C1 | 0.3573 (3) | 0.1239 (3) | 0.58524 (4) | 0.0214 (6) |
| H1A | 0.381442 | 0.153712 | 0.570877 | 0.026* |
| H1B | 0.299619 | 0.186981 | 0.591428 | 0.026* |
| C2 | 0.4756 (3) | 0.1174 (3) | 0.59910 (4) | 0.0235 (6) |
| C3 | 0.5028 (3) | −0.0033 (4) | 0.61148 (5) | 0.0381 (9) |
| C4 | 0.3924 (4) | −0.0907 (3) | 0.61793 (5) | 0.0377 (9) |
| C5 | 0.2665 (3) | −0.0598 (3) | 0.60592 (5) | 0.0313 (8) |
| H5Ab | 0.221451 | 0.004934 | 0.614558 | 0.038* |
| H5Ba | 0.229698 | 0.011163 | 0.614269 | 0.038* |
| C6b | 0.1938 (11) | −0.1821 (12) | 0.60828 (18) | 0.026 (2) |
| H6Ab | 0.193562 | −0.210965 | 0.623269 | 0.032* |
| H6Bb | 0.230150 | −0.248853 | 0.599198 | 0.032* |
| C6′a | 0.1512 (9) | −0.1606 (11) | 0.60396 (19) | 0.026 (2) |
| H6′Aa | 0.177938 | −0.228886 | 0.594282 | 0.031* |
| H6′Ba | 0.137180 | −0.198347 | 0.618173 | 0.031* |
| C7b | 0.0590 (11) | −0.1472 (10) | 0.60080 (14) | 0.027 (2) |
| H7Ab | 0.024788 | −0.081482 | 0.610319 | 0.032* |
| H7Bb | 0.004327 | −0.222053 | 0.602043 | 0.032* |
| C7′a | 0.0238 (10) | −0.1084 (9) | 0.59579 (14) | 0.027 (2) |
| H7′Aa | −0.007572 | −0.046295 | 0.606238 | 0.033* |
| H7′Ba | −0.037344 | −0.178341 | 0.595331 | 0.033* |
| C8b | 0.0552 (8) | −0.0980 (8) | 0.57699 (12) | 0.0184 (15) |
| C8′a | 0.0273 (8) | −0.0442 (8) | 0.57315 (12) | 0.0216 (16) |
| C9b | 0.1552 (13) | 0.0108 (9) | 0.5750 (2) | 0.017 (2) |
| H9b | 0.119666 | 0.080640 | 0.583752 | 0.021* |
| C9′a | 0.1427 (12) | 0.0529 (9) | 0.5729 (2) | 0.018 (2) |
| H9′a | 0.117468 | 0.118354 | 0.583498 | 0.021* |
| C10 | 0.2842 (3) | −0.0035 (3) | 0.58280 (5) | 0.0266 (7) |
| C11b | 0.1552 (8) | 0.0644 (8) | 0.55145 (13) | 0.0214 (17) |
| H11Ab | 0.217508 | 0.133201 | 0.550453 | 0.026* |
| H11Bb | 0.181509 | −0.002792 | 0.541521 | 0.026* |
| C11′a | 0.1489 (7) | 0.1265 (8) | 0.55135 (14) | 0.0222 (16) |
| H11Ca | 0.223226 | 0.182361 | 0.551366 | 0.027* |
| H11Da | 0.158198 | 0.066365 | 0.539428 | 0.027* |
| C12b | 0.0246 (6) | 0.1149 (7) | 0.54451 (10) | 0.0231 (13) |
| H12b | 0.003447 | 0.190426 | 0.553267 | 0.028* |
| C12′a | 0.0284 (6) | 0.2060 (7) | 0.54803 (9) | 0.0241 (13) |
| H12′a | 0.018435 | 0.265792 | 0.560192 | 0.029* |
| C13b | −0.0788 (6) | 0.0146 (6) | 0.54766 (9) | 0.0201 (12) |
| H13b | −0.057066 | −0.057228 | 0.538074 | 0.024* |
| C13′a | −0.0853 (5) | 0.1160 (6) | 0.54738 (8) | 0.0207 (13) |
| H13′a | −0.069965 | 0.055564 | 0.535475 | 0.025* |
| C14b | −0.0779 (7) | −0.0392 (8) | 0.57149 (10) | 0.0208 (14) |
| C14′a | −0.0973 (6) | 0.0349 (8) | 0.56874 (9) | 0.0206 (13) |
| C15b | −0.1914 (6) | −0.1319 (6) | 0.57022 (10) | 0.0248 (14) |
| H15Ab | −0.226368 | −0.147749 | 0.584574 | 0.030* |
| H15Bb | −0.165082 | −0.212906 | 0.563911 | 0.030* |
| C15′a | −0.2222 (7) | −0.0394 (7) | 0.56373 (11) | 0.0285 (14) |
| H15Ca | −0.203838 | −0.115446 | 0.555215 | 0.034* |
| H15Da | −0.264253 | −0.065156 | 0.577098 | 0.034* |
| C16b | −0.2912 (7) | −0.0650 (6) | 0.55563 (10) | 0.0259 (14) |
| H16Ab | −0.360629 | −0.031153 | 0.564386 | 0.031* |
| H16Bb | −0.326506 | −0.125349 | 0.545242 | 0.031* |
| C16′a | −0.3077 (6) | 0.0537 (7) | 0.55078 (11) | 0.0302 (15) |
| H16Ca | −0.379259 | 0.081804 | 0.559638 | 0.036* |
| H16Da | −0.341211 | 0.011738 | 0.537911 | 0.036* |
| C17b | −0.2203 (6) | 0.0465 (6) | 0.54347 (10) | 0.0220 (13) |
| H17b | −0.239396 | 0.125914 | 0.551328 | 0.026* |
| C17′a | −0.2232 (6) | 0.1705 (6) | 0.54420 (9) | 0.0230 (13) |
| H17′a | −0.235938 | 0.236705 | 0.555283 | 0.028* |
| C18b | 0.0861 (7) | −0.2141 (7) | 0.56226 (10) | 0.0253 (14) |
| H18Ab | 0.169509 | −0.246741 | 0.565821 | 0.038* |
| H18Bb | 0.084820 | −0.187729 | 0.547307 | 0.038* |
| H18Cb | 0.023218 | −0.279912 | 0.564500 | 0.038* |
| C18′a | 0.0429 (7) | −0.1505 (7) | 0.55607 (11) | 0.0307 (15) |
| H18Da | 0.121650 | −0.195580 | 0.558595 | 0.046* |
| H18Ea | 0.044361 | −0.113128 | 0.541808 | 0.046* |
| H18Fa | −0.027686 | −0.209187 | 0.557119 | 0.046* |
| C19 | 0.3641 (4) | −0.0901 (3) | 0.56776 (6) | 0.0427 (10) |
| H19A | 0.358775 | −0.058838 | 0.553113 | 0.064* |
| H19B | 0.331433 | −0.176037 | 0.568363 | 0.064* |
| H19C | 0.451861 | −0.089516 | 0.572425 | 0.064* |
| C20b | −0.2670 (6) | 0.0649 (6) | 0.51962 (10) | 0.0255 (14) |
| C20′a | −0.2572 (6) | 0.2295 (7) | 0.52252 (10) | 0.0228 (13) |
| C21b | −0.2625 (7) | 0.3083 (7) | 0.51346 (12) | 0.0296 (15) |
| C21′a | −0.2228 (6) | 0.4608 (7) | 0.50916 (10) | 0.0287 (14) |
| C22b | −0.4087 (8) | 0.3092 (10) | 0.51373 (16) | 0.0303 (19) |
| H22Ab | −0.440266 | 0.301944 | 0.498957 | 0.036* |
| H22Bb | −0.438373 | 0.390191 | 0.519594 | 0.036* |
| C22′a | −0.3666 (6) | 0.4819 (7) | 0.50904 (10) | 0.0339 (16) |
| H22Ca | −0.388009 | 0.543840 | 0.497870 | 0.041* |
| H22Da | −0.392652 | 0.517134 | 0.522947 | 0.041* |
| C23b | −0.4632 (7) | 0.1997 (7) | 0.52735 (11) | 0.0295 (15) |
| H23Ab | −0.437503 | 0.209427 | 0.542415 | 0.035* |
| H23Bb | −0.556085 | 0.200592 | 0.526694 | 0.035* |
| C23′a | −0.4400 (7) | 0.3586 (9) | 0.50488 (12) | 0.0365 (17) |
| H23Ca | −0.531294 | 0.375435 | 0.505533 | 0.044* |
| H23Da | −0.419999 | 0.326880 | 0.490447 | 0.044* |
| C24b | −0.4132 (6) | 0.0763 (7) | 0.51848 (11) | 0.0313 (15) |
| H24Ab | −0.439896 | 0.068179 | 0.503440 | 0.038* |
| H24Bb | −0.451012 | 0.005960 | 0.526539 | 0.038* |
| C24′a | −0.4049 (7) | 0.2589 (9) | 0.52157 (13) | 0.0263 (16) |
| H24Ca | −0.450440 | 0.180224 | 0.518288 | 0.032* |
| H24Da | −0.432958 | 0.288003 | 0.535764 | 0.032* |
| C25b | −0.2218 (7) | −0.0410 (7) | 0.50468 (9) | 0.0311 (16) |
| H25Ab | −0.261083 | −0.031147 | 0.490675 | 0.047* |
| H25Bb | −0.245263 | −0.122554 | 0.510718 | 0.047* |
| H25Cb | −0.130333 | −0.036640 | 0.503184 | 0.047* |
| C25′a | −0.2177 (15) | 0.1499 (15) | 0.50306 (18) | 0.032 (3) |
| H25Da | −0.264797 | 0.177448 | 0.490488 | 0.049* |
| H25Ea | −0.235796 | 0.061227 | 0.505864 | 0.049* |
| H25Fa | −0.127648 | 0.160510 | 0.500475 | 0.049* |
| C26b | −0.2075 (8) | 0.3678 (8) | 0.53420 (16) | 0.0372 (18) |
| H26Ab | −0.244289 | 0.326551 | 0.546666 | 0.056* |
| H26Bb | −0.227363 | 0.457591 | 0.534545 | 0.056* |
| H26Cb | −0.116160 | 0.356621 | 0.534384 | 0.056* |
| C26′a | −0.1540 (7) | 0.5707 (7) | 0.51987 (12) | 0.0389 (17) |
| H26Da | −0.181017 | 0.577572 | 0.534744 | 0.058* |
| H26Ea | −0.173794 | 0.648842 | 0.512345 | 0.058* |
| H26Fa | −0.063253 | 0.555921 | 0.519358 | 0.058* |
| C27b | −0.2119 (8) | 0.3812 (8) | 0.49376 (14) | 0.0435 (19) |
| H27Ab | −0.119980 | 0.379206 | 0.493797 | 0.065* |
| H27Bb | −0.240727 | 0.468483 | 0.494380 | 0.065* |
| H27Cb | −0.243267 | 0.341792 | 0.480696 | 0.065* |
| C27′a | −0.1712 (7) | 0.4422 (9) | 0.48588 (11) | 0.0413 (18) |
| H27Da | −0.085139 | 0.409851 | 0.486514 | 0.062* |
| H27Ea | −0.171711 | 0.522868 | 0.478388 | 0.062* |
| H27Fa | −0.224527 | 0.382256 | 0.478253 | 0.062* |
| C28 | 0.4374 (6) | −0.2307 (4) | 0.61539 (7) | 0.0728 (17) |
| H28A | 0.439185 | −0.252966 | 0.600247 | 0.109* |
| H28B | 0.379208 | −0.286541 | 0.622906 | 0.109* |
| H28C | 0.521695 | −0.239815 | 0.621424 | 0.109* |
| C29 | 0.3740 (4) | −0.0638 (4) | 0.64256 (5) | 0.0405 (9) |
| H29A | 0.449087 | −0.090514 | 0.650345 | 0.061* |
| H29B | 0.300994 | −0.110608 | 0.647791 | 0.061* |
| H29C | 0.360414 | 0.026194 | 0.644774 | 0.061* |
| C30b | −0.1175 (10) | 0.0690 (8) | 0.58766 (19) | 0.027 (2) |
| H30Ab | −0.123582 | 0.034313 | 0.602081 | 0.040* |
| H30Bb | −0.199102 | 0.103454 | 0.583436 | 0.040* |
| H30Cb | −0.054314 | 0.135796 | 0.587423 | 0.040* |
| C30′a | −0.1313 (9) | 0.1246 (9) | 0.58818 (17) | 0.025 (2) |
| H30Da | −0.160802 | 0.074003 | 0.600214 | 0.038* |
| H30Ea | −0.197559 | 0.183146 | 0.583854 | 0.038* |
| H30Fa | −0.056578 | 0.171986 | 0.592441 | 0.038* |
| C31 | 0.5602 (3) | 0.2131 (4) | 0.60169 (5) | 0.0313 (8) |
| H31 | 0.627148 | 0.194916 | 0.611165 | 0.038* |
| C32 | 0.5645 (3) | 0.3409 (4) | 0.59210 (5) | 0.0326 (8) |
| C33 | 0.4794 (4) | 0.4935 (3) | 0.56961 (6) | 0.0413 (9) |
| C34 | 0.5805 (5) | 0.5780 (4) | 0.57309 (8) | 0.0587 (13) |
| H34 | 0.584061 | 0.656908 | 0.566091 | 0.070* |
| C35 | 0.6739 (5) | 0.5406 (4) | 0.58714 (8) | 0.0645 (15) |
| H35 | 0.741805 | 0.595335 | 0.590243 | 0.077* |
| C36 | 0.6681 (4) | 0.4205 (4) | 0.59686 (6) | 0.0515 (12) |
| H36 | 0.732004 | 0.393666 | 0.606355 | 0.062* |
| C37 | 0.2867 (4) | 0.4481 (4) | 0.55072 (7) | 0.0533 (11) |
| H37A | 0.248291 | 0.416382 | 0.563834 | 0.080* |
| H37B | 0.321910 | 0.377810 | 0.542606 | 0.080* |
| H37C | 0.222944 | 0.490767 | 0.542116 | 0.080* |
| O5 | 0.2546 (2) | 0.2546 (2) | 0.500000 | 0.0464 (10) |
| H5Cc | 0.278688 | 0.186144 | 0.493598 | 0.070* |
| H5Dc | 0.196893 | 0.229105 | 0.508802 | 0.070* |
-
aOccupancy: 0.507 (3), bOccupancy: 0.493 (3), cOccupancy: 0.5.
Source of material
(8R,10R,14R)-12-Hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H- cyclopenta[a]phenanthren-3-one (100 mg, 0.22 mmol) and 6-methoxy-2-pyridinecarboxaldehyde (30.0 mg, 0.22 mmol) were dissolved in 1.4 mL methanol. After adding 0.72 mL of 25% NaOH aqueous solution, the reaction system was opalescent. The reaction system was stirred at room temperature for 5 h. The response endpoint was detected by thin layer chromatography (TLC). When the reaction was stopped, a moderate amount of water was added to the container and the mixture was extracted with ethyl acetate (twice). The combined organic phase was washed with saturated sodium chloride solution, dried with anhydrous sodium sulfate, filtered, reduced pressure concentration, yellow solid is obtained. The crude product was purified by silica-gel thin layer chromatography (petroleum ether: ethyl acetate = 3/1, v/v). The single crystal of the target compound was obtained by recrystallization with ethyl acetate solution.
Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C–H) = 0.97 Å (methylene), d(C–H) = 0.93 Å (aromatic), d(C–H) = 0.96 Å (methyl) d(C–H) = 0.93 Å (alkenyl) and d(O–H) = 0.82 Å (–OH). The absolute configuration was derived from the synthesis and the configuration of the educts. Almost the whole organic molecule shows a disorder, which limits the significance of all bond lengths and angles. The figure shows only one of the two overlayed models.
Comment
Ginseng is a traditional Chinese medicinal plant, which has been widely used for the treatment of heart failure [4, 5] and various tissue damages under cellular and environmental stress. Ginseng is effective in improving blood circulation and brain function, enhancing immune function, preventing diabetes, as well as having anti-cancer, anti-inflammatory [6, 7] and antibacterial properties. Ginsenosides are the major active components of ginseng responsible for pharmacological actions. Ginsenoside are divided into two groups according to their glycosidic structures: dammarane and oleanane. There are two types of dammaranes: protopanaxadiol (PD) type [8, 9] and protopanaxatriol type sharing a tetrahydrofuran ring and a dammarane skeleton. Moreover, the conformation of the compound will have a certain influence on the biological activity [10]. Our group has done a lot of research work on panaxadiol derivatives [11] and panaxatriol derivatives [12], and obtained molecular data based on crystal structures [13, 14]. The title compound is a PD derivative. It is possible to modify the structure of ginsenoside which is an important saponin in ginsenoside.
Single-crystal structure analysis reveals that the title compound contains one drug molecule and one half of a water molecule in the asymmetric unit (cf. the Figure). Except for the substituents on C(2), the structure of the title compound is the same as that of panaxadiol. In the crystal structure, the pyridine ring has a planar conformation, and the other six membered rings except the ring with the keto group which has a chair conformation. The bond lengths and angles are all in the expected ranges.
Funding source: National Natural Science Foundation of China 10.13039/501100001809
Award Identifier / Grant number: 81473104
Award Identifier / Grant number: 81773563
Award Identifier / Grant number: 81573585
Funding source: Science Foundation
Award Identifier / Grant number: 13ZJZ06
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the National Natural Science Foundation of China (No. 81473104, 81773563). Meanwhile, this work was also supported by National Natural Science Foundation of China (Grant no. 81573585) and other Science Foundation (No. 13ZJZ06).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Streit, W. J., Xue, Q. S., Tischer, J., Bechmann, I. Microglial pathology. Acta Neuropathol. Commun. 2014, 2, 142; https://doi.org/10.1186/s40478-014-0142-6.Suche in Google Scholar PubMed PubMed Central
5. Wang, J. Z., Wang, H. Y., Mou, X. D., Luan, M. Z., Zhang, X. F., He, X. T., Zhao, F. L., Meng, Q. G. The advances on the protective effects of ginsenosides on myocardial ischemia and ischemia-reperfusion injury. Mini Rev. Med. Chem. 2020, 20, 1610–1618; https://doi.org/10.2174/1389557520666200619115444.Suche in Google Scholar PubMed
6. Zhang, J. Q., Zhang, Q., Xu, Y. R., Li, H. X., Zhao, F. L., Wang, C. M., Liu, Z., Liu, P., Liu, Y. N., Meng, Q. G., Zhao, F. Synthesis and in vitro anti-inflammatory activity of C20 epimeric ocotillol-type triterpenes and protopanaxadiol. Planta Med. 2019, 85, 292–301; https://doi.org/10.1055/a-0770-0994.Suche in Google Scholar PubMed
7. Zhang, S. N., Zhao, Y. Q. Crystallization separation of anti-tumor constituent 20(R) 25–OCH3–PPD. Chin. Tradit. Herb. Drugs 2014, 45, 770–773.Suche in Google Scholar
8. Liu, J., Xu, Y. R., Yang, J. J., Wang, W. Z., Zhang, J. Q., Zhang, R. M., Meng, Q. G. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J. Ginseng Res. 2017, 41, 373–378; https://doi.org/10.1016/j.jgr.2017.01.001.Suche in Google Scholar PubMed PubMed Central
9. Ma, Y., Wang, H. Y., Zhang, X. F., Zhao, F. L., Meng, Q. G. Crystal structure of (3S,8R,10R,12R,14R)-12- hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro- 2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren- 3-yl acetate C32H54O4. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 163–166; https://doi.org/10.1515/ncrs-2020-0311.Suche in Google Scholar
10. Yang, Q. W., Wang, N., Zhang, J., Chen, G., Xu, H., Meng, Q. G., Du, Y., Yang, X., Fan, H. Y. In vitro and in silico evaluation of stereoselective effect of ginsenosideisomers on platelet P2Y12 receptor. Phytomedicine 2019, 64, 152889; https://doi.org/10.1016/j.phymed.2019.152899.Suche in Google Scholar PubMed
11. Wang, J. Z., Weng, W. Z., Ma, Y., He, X. T., Meng, Q. G. Crystal structure of (1S,3aR,3bR,10aR, 10bR,12aR)-8-amino-3a,3b,6,6,10a-pentamethyl-1- ((S)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)- 2,3,3a,3b,4,5,5a,6,10,10a,10b,11,12,12a-tetradecahydro-1H- cyclopenta [7,8] phenanthro [2,3-d]thiazol-12-ol - a panaxadiol dervative, C31H50N2O2S. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 397–400; https://doi.org/10.1515/ncrs-2018-0238.Suche in Google Scholar
12. Sun, K. W., Wang, X. H., Ma, G. Q., Luo, Q., Wang, Y. H., Meng, Q. G. The crystal structure of (5R,8R,9R,10R, 12R,13R,14R)-12-hydroxy-4,4,8,10,14- pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H- pyran-2-yl) tetradecahydro-3H-cyclopenta[a] phenanthrene- 3,6(2H)-dione, C30H48O4. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 17–20; https://doi.org/10.1515/ncrs-2020-0459.Suche in Google Scholar
13. Zhao, R. L., Wang, H. Y., Luan, M. Z., Zheng, X., Zhao, F. L., Meng, Q. G. Crystal structure of (3H,5R,8R,9R,10R, 12R,13R,14R)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6- trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H- cyclopenta[a]phenanthrene-3,12-diol, C30H52O3. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1329–1331.10.1515/ncrs-2019-0533Suche in Google Scholar
14. Wang, C. M., Liu, J., Deng, J. Q., Wang, J. Z., Weng, W. Z., Chu, H. X., Meng, Q. G. Advances in the chemistry, pharmacological diversity, and metabolism of 20(R)-ginseng saponins. J. Ginseng Res. 2020, 44, 14–23; https://doi.org/10.1016/j.jgr.2019.01.005.Suche in Google Scholar PubMed PubMed Central
© 2021 Qin Luo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

