Abstract
C23H23BrO2, monoclinic, P21/c (no. 14), a = 24.193(4) Å, b = 5.8614(10) Å, c = 13.873(3) Å, β = 93.168(3)∘, V = 1964.3(6) Å3, Z = 4, R gt (F) = 0.0316, wR ref (F2) = 0.0915, T = 296(2) K.
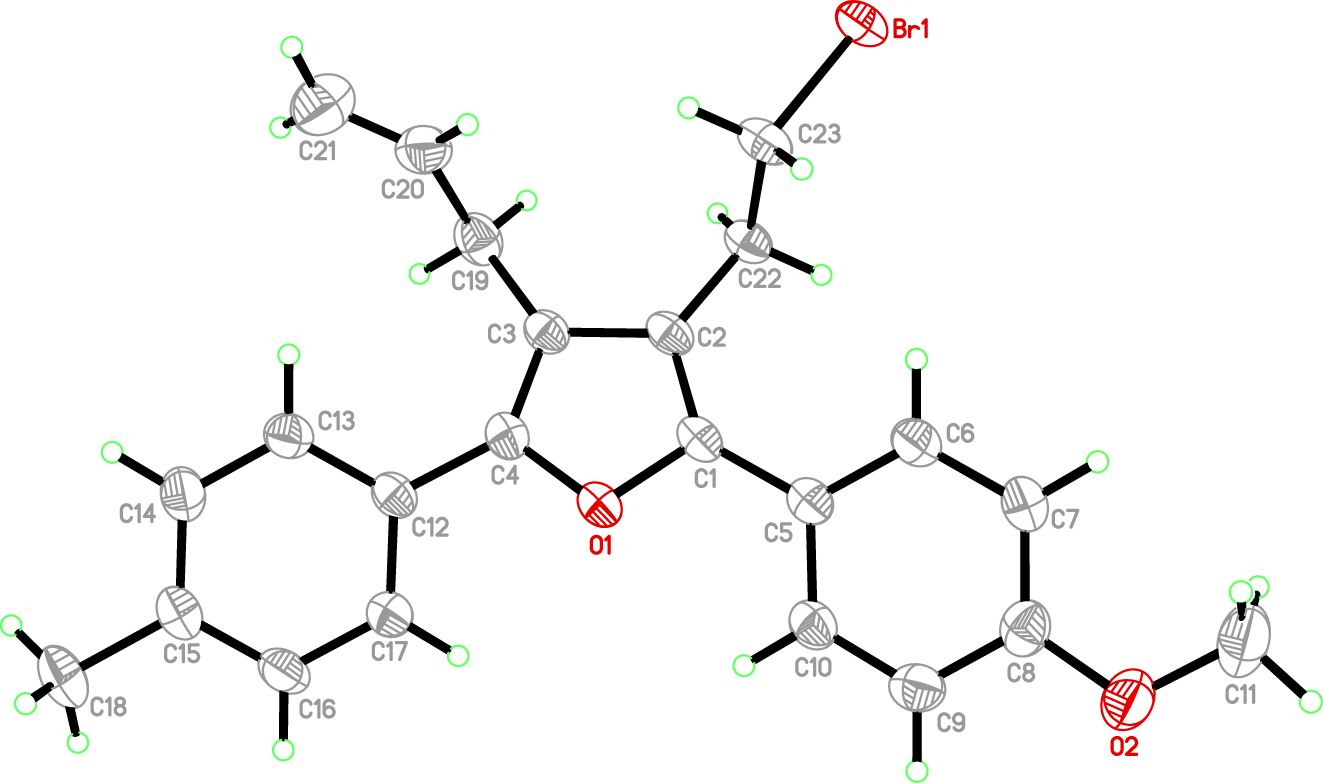
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.23 × 0.20 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.11 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 11421, 3446, 0.025 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2735 |
| N(param)refined: | 237 |
| Programs: | Bruker [1], SHELX [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Br1 | 0.80538 (2) | 1.43349 (4) | 0.48348 (2) | 0.05837 (13) |
| C1 | 0.79563 (10) | 0.7833 (4) | 0.73815 (16) | 0.0444 (6) |
| C2 | 0.76754 (10) | 0.8672 (4) | 0.65802 (17) | 0.0444 (6) |
| C3 | 0.71187 (10) | 0.7817 (4) | 0.65917 (17) | 0.0443 (6) |
| C4 | 0.70978 (10) | 0.6509 (4) | 0.73957 (17) | 0.0446 (6) |
| C5 | 0.85237 (10) | 0.7944 (4) | 0.77971 (17) | 0.0442 (6) |
| C6 | 0.88782 (12) | 0.9727 (5) | 0.7614 (2) | 0.0590 (7) |
| H6 | 0.875137 | 1.090814 | 0.721215 | 0.071* |
| C7 | 0.94177 (12) | 0.9807 (5) | 0.8012 (2) | 0.0614 (7) |
| H7 | 0.964986 | 1.100834 | 0.786586 | 0.074* |
| C8 | 0.96056 (11) | 0.8094 (5) | 0.86247 (19) | 0.0586 (7) |
| C9 | 0.92545 (12) | 0.6305 (5) | 0.8828 (2) | 0.0609 (7) |
| H9 | 0.937952 | 0.514510 | 0.924245 | 0.073* |
| C10 | 0.87257 (11) | 0.6236 (5) | 0.84238 (18) | 0.0522 (6) |
| H10 | 0.849606 | 0.502614 | 0.856966 | 0.063* |
| C11 | 1.04803 (14) | 0.9908 (7) | 0.8911 (3) | 0.0909 (11) |
| H11A | 1.055427 | 0.999513 | 0.823958 | 0.136* |
| H11B | 1.082181 | 0.970264 | 0.928745 | 0.136* |
| H11C | 1.030582 | 1.129354 | 0.910380 | 0.136* |
| C12 | 0.66853 (11) | 0.5015 (4) | 0.77978 (17) | 0.0451 (6) |
| C13 | 0.61223 (11) | 0.5440 (5) | 0.7682 (2) | 0.0569 (7) |
| H13 | 0.599808 | 0.673532 | 0.734945 | 0.068* |
| C14 | 0.57443 (12) | 0.3972 (5) | 0.8053 (2) | 0.0625 (8) |
| H14 | 0.536855 | 0.429549 | 0.796664 | 0.075* |
| C15 | 0.59108 (11) | 0.2030 (5) | 0.85518 (19) | 0.0567 (7) |
| C16 | 0.64687 (12) | 0.1608 (5) | 0.8672 (2) | 0.0602 (7) |
| H16 | 0.658970 | 0.031381 | 0.900903 | 0.072* |
| C17 | 0.68540 (11) | 0.3058 (5) | 0.83044 (18) | 0.0531 (6) |
| H17 | 0.722922 | 0.272755 | 0.839533 | 0.064* |
| C18 | 0.54915 (14) | 0.0422 (6) | 0.8964 (3) | 0.0786 (10) |
| H18A | 0.564734 | −0.108086 | 0.902175 | 0.118* |
| H18B | 0.516369 | 0.037798 | 0.854175 | 0.118* |
| H18C | 0.539854 | 0.095433 | 0.958928 | 0.118* |
| C19 | 0.66637 (11) | 0.8182 (5) | 0.58225 (18) | 0.0605 (7) |
| H19A | 0.644691 | 0.679356 | 0.575172 | 0.073* |
| H19B | 0.682870 | 0.846961 | 0.521274 | 0.073* |
| C20 | 0.62741 (14) | 1.0176 (6) | 0.6038 (2) | 0.0694 (8) |
| H20 | 0.643218 | 1.147781 | 0.631967 | 0.083* |
| C21 | 0.57506 (16) | 1.0176 (7) | 0.5859 (3) | 0.0918 (11) |
| H21A | 0.557757 | 0.890465 | 0.557761 | 0.110* |
| H21B | 0.554234 | 1.144762 | 0.601016 | 0.110* |
| C22 | 0.79033 (11) | 1.0073 (4) | 0.57863 (18) | 0.0466 (6) |
| H22A | 0.774486 | 0.954221 | 0.516896 | 0.056* |
| H22B | 0.830102 | 0.985830 | 0.579059 | 0.056* |
| C23 | 0.77790 (12) | 1.2574 (4) | 0.58950 (19) | 0.0578 (7) |
| H23A | 0.794968 | 1.312255 | 0.650027 | 0.069* |
| H23B | 0.738215 | 1.278718 | 0.591334 | 0.069* |
| O1 | 0.76068 (7) | 0.6490 (3) | 0.78880 (11) | 0.0459 (4) |
| O2 | 1.01226 (8) | 0.8026 (4) | 0.90646 (15) | 0.0781 (6) |
Source of material
(4-Methoxyphenyl)(1-(p-tolylethynyl)cyclopropyl)methanone (0.2 mmol, 1.0 eq.), allyl bromide (0.4 mmol, 2.0 eq.), PdBr2 (0.03 eq.) and acetonitrile (5 mL) were added to a screw-capped Schlenk tube containing a magnetic stir bar, which was evacuated and refilled with nitrogen for three times. The resulting mixture was stirred at room temperature for 3 h. After the reaction was complete, the reaction mixture was concentrated under reduced pressure, and the residue was purified with petroleum ether-ethyl acetate (15:1, v/v) as eluent by silica gel chromatography column to obtain a colourless solid. Crystals were obtained by slow evaporation of a tetrahydrofuran solution at room temperature after three days.
Experimental details
All hydrogen atoms were placed in calculated positions (C–H = 0.93–0.97 Å) and refined as riding atoms. The Uiso values were set to be 1.5Ueq of the carrier atom for methyl H atoms and 1.2Ueq for the remaining H atoms.
Comment
In recent years, furans as an important class of heterocycles have always been a hot topic due to their wide use in drug science, material science, and synthetic chemistry [3], [4], [5], [6], [7], [8], [9]. Now, we report the synthesis and crystal structure of the title compound (cf. Figure).
In the crystal structure, the asymmetric unit consists of one molecule with bonding parameters in the normal ranges [10, 11]. The title compound is a non-planar molecule. The dihedral angles of A/B, A/C, B/B are 21.091(1), 22.889(1), and 30.229(1)°, respectively, in which A, B, C representing aromatic rings [C5/C6/C7/C8/C9/C10], [C12/C13/C14/C15/C16/C17], and furan ring [C1/C2/C3/C4/O1], respectively. The intermolecular and weak C–H⃛Br hydrogen bonds link molecules into chains.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2008.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Aziz, M. A., Serya, R. A. T., Lasheen, D. S., Abouzid, K. A. M. Furo [2,3- d] pyrimidine based derivatives as kinase inhibitors and anticancer agents. J. Future Pharm. Sci. 2016, 2, 1–8; https://doi.org/10.1016/j.fjps.2015.12.001.Search in Google Scholar
4. Zhu, M., Fu, W.-J., Xu, C., Zou, G.-L., Wang, Z.-Q., Ji, B.-M. Highly efficient synthesis of multisubstituted furans through cupric halide-mediated intramolecular halocyclization of 1-(1-alkynyl)cyclopropyl ketones. Eur. J. Org Chem. 2012, 2012, 4609–4615; https://doi.org/10.1002/ejoc.201200601.Search in Google Scholar
5. Komogortsev, A. N., Melekhina, V. G., Lichitsky, B. V., Minyaev, M. E. Novel one-pot approach to 2-aminofuran derivatives via multicomponent reaction of 3-hydroxy-4H-pyran-4-ones, α-ketoaldehydes and methylene active nitriles. Tetrahedron Lett. 2020, 61, 152384; https://doi.org/10.1016/j.tetlet.2020.152384.Search in Google Scholar
6. Su, Z., Xie, Z., Wang, S., Luo, N., Wang, C. Direct synthesis of highly functionalized furans from donor-acceptor cyclopropanes via DBU-mediated ring expansion reactions. Org. Biomol. Chem. 2019, 17, 7342–7351; https://doi.org/10.1039/c9ob01308c.Search in Google Scholar PubMed
7. Nie, Y.-L., Wu, Y.-D., Wang, C.-X., Lin, R., Xie, Y., Fang, D.-S., Jiang, H., Lian, Y.-Y. Compounds from marine-derived Verrucosispora sp. FIM06054 and their potential antitumour activities. Nat. Prod. Res. 2014, 28, 2134–2139.10.1080/14786419.2014.926350Search in Google Scholar PubMed
8. Tsuji, H., Nakamura, E. Design and functions of semiconducting fused polycyclic furans for optoelectronic applications. Acc. Chem. Res. 2017, 50, 396–406; https://doi.org/10.1021/acs.accounts.6b00595.Search in Google Scholar PubMed
9. Mishra, A., Ulaganathan, M., Edison, E., Borah, P., Mishra, A., Sreejith, S., Madhavi, S., Stuparu, M. C. Polymeric nanomaterials based on the buckybowl motif: synthesis through ring- opening metathesis polymerization and energy storage applications. ACS Macro Lett. 2017, 6, 1212–1216; https://doi.org/10.1021/acsmacrolett.7b00746.Search in Google Scholar PubMed
10. Wang, R.-L., Shi, J.-X., Zheng, X.-F., Zhou, Y. 2-(3–Bromo-4-butoxyphenyl)- 3,4-dimethyl5-(3,4,5-trimethoxyphenyl)furan. Acta Crystallogr. 2005, E61, o2619–o2620; https://doi.org/10.1107/s1600536805022543.Search in Google Scholar
11. Hu, S.-L., Xu, C.-H., Zhang, S.-S., Sun, W.-B., Gao, Q. Synthesis, crystal structure and binding properties of a new fluorescent molecular clip based on 2,5-diphenylfuran. J. Chem. Res. 2013, 37, 210–212.10.3184/174751913X13626742215922Search in Google Scholar
© 2021 Xue-Li Xu and Jihong Li, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

