Abstract
C26H18F5NO3S, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.14 × 0.12 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.22 mm−1 |
| Diffractometer, scan mode: | SuperNova, |
| θmax, completeness: | 29.6°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12,356, 5294, 0.032 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 4144 |
| N(param)refined: | 325 |
| Programs: | CrysAlisPRO [1], Shelx [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.6245 (3) | 0.49568 (19) | 0.32766 (16) | 0.0204 (4) |
| H1A | 0.672869 | 0.547280 | 0.266911 | 0.025* |
| H1B | 0.714554 | 0.456990 | 0.387854 | 0.025* |
| C2 | 0.4638 (3) | 0.5783 (2) | 0.35755 (16) | 0.0219 (4) |
| C3 | 0.3133 (3) | 0.52704 (19) | 0.39938 (15) | 0.0208 (4) |
| C4 | 0.3200 (2) | 0.40041 (19) | 0.40027 (15) | 0.0193 (4) |
| C5 | 0.4951 (3) | 0.32090 (19) | 0.38201 (16) | 0.0210 (4) |
| H5A | 0.570449 | 0.279806 | 0.448999 | 0.025* |
| H5B | 0.474953 | 0.256714 | 0.359422 | 0.025* |
| C6 | 0.4407 (3) | 0.6995 (2) | 0.34183 (16) | 0.0234 (4) |
| H6 | 0.331586 | 0.740863 | 0.358513 | 0.028* |
| C7 | 0.5623 (3) | 0.7758 (2) | 0.30232 (17) | 0.0244 (5) |
| C8 | 0.4897 (3) | 0.9051 (2) | 0.24935 (18) | 0.0299 (5) |
| H8 | 0.367195 | 0.939410 | 0.241474 | 0.036* |
| C9 | 0.5950 (3) | 0.9826 (2) | 0.20869 (18) | 0.0324 (5) |
| H9 | 0.545673 | 1.068166 | 0.172639 | 0.039* |
| C10 | 0.7745 (3) | 0.9294 (2) | 0.22313 (17) | 0.0282 (5) |
| C11 | 0.8548 (3) | 0.8037 (2) | 0.27762 (16) | 0.0240 (4) |
| H11 | 0.977686 | 0.771826 | 0.287092 | 0.029* |
| C12 | 0.7481 (3) | 0.7261 (2) | 0.31796 (16) | 0.0228 (4) |
| H12 | 0.799282 | 0.640971 | 0.355439 | 0.027* |
| C13 | 0.1691 (3) | 0.36798 (19) | 0.40819 (15) | 0.0201 (4) |
| H13 | 0.066478 | 0.425800 | 0.414602 | 0.024* |
| C14 | 0.1518 (2) | 0.24925 (19) | 0.40759 (16) | 0.0204 (4) |
| C15 | 0.2630 (3) | 0.13494 (19) | 0.47520 (17) | 0.0247 (5) |
| H15 | 0.348292 | 0.133267 | 0.523102 | 0.030* |
| C16 | 0.2480 (3) | 0.0242 (2) | 0.47189 (19) | 0.0296 (5) |
| H16 | 0.321826 | −0.052124 | 0.517421 | 0.036* |
| C17 | 0.1222 (3) | 0.0288 (2) | 0.40016 (19) | 0.0289 (5) |
| C18 | 0.0073 (3) | 0.1393 (2) | 0.33258 (18) | 0.0278 (5) |
| H18 | −0.077816 | 0.139791 | 0.285263 | 0.033* |
| C19 | 0.0231 (3) | 0.2497 (2) | 0.33755 (17) | 0.0236 (4) |
| H19 | −0.053694 | 0.325551 | 0.293321 | 0.028* |
| C20 | 0.3392 (3) | 0.53275 (19) | 0.12335 (15) | 0.0194 (4) |
| C21 | 0.3311 (3) | 0.6585 (2) | 0.08351 (16) | 0.0245 (5) |
| H21 | 0.433541 | 0.682656 | 0.083388 | 0.029* |
| C22 | 0.1691 (3) | 0.7477 (2) | 0.04396 (17) | 0.0265 (5) |
| H22 | 0.161566 | 0.832345 | 0.017175 | 0.032* |
| C23 | 0.0178 (3) | 0.7096 (2) | 0.04463 (16) | 0.0229 (4) |
| C24 | 0.0264 (3) | 0.5838 (2) | 0.08493 (16) | 0.0236 (4) |
| H24 | −0.075989 | 0.559595 | 0.085210 | 0.028* |
| C25 | 0.1881 (3) | 0.4946 (2) | 0.12467 (16) | 0.0219 (4) |
| H25 | 0.195541 | 0.409979 | 0.151978 | 0.026* |
| C26 | −0.1567 (3) | 0.8044 (2) | −0.00493 (17) | 0.0285 (5) |
| F1 | 0.88070 (19) | 1.00437 (13) | 0.18209 (11) | 0.0390 (3) |
| F2 | 0.10867 (18) | −0.08073 (12) | 0.39666 (12) | 0.0396 (4) |
| F3 | −0.16273 (17) | 0.91979 (13) | −0.01286 (12) | 0.0398 (3) |
| F4 | −0.19243 (18) | 0.81126 (15) | −0.10532 (11) | 0.0454 (4) |
| F5 | −0.29304 (16) | 0.77733 (14) | 0.05012 (11) | 0.0399 (4) |
| N1 | 0.5844 (2) | 0.39745 (15) | 0.29951 (13) | 0.0180 (3) |
| O1 | 0.18551 (18) | 0.58662 (14) | 0.43178 (12) | 0.0250 (3) |
| O2 | 0.67987 (18) | 0.47214 (14) | 0.11741 (11) | 0.0249 (3) |
| O3 | 0.53295 (19) | 0.30282 (14) | 0.17191 (12) | 0.0261 (3) |
| S1 | 0.54701 (6) | 0.41882 (5) | 0.17298 (4) | 0.01909 (13) |
Source of material
The synthesis of 3,5-bis(4-fluorobenzylidene)piperidin-4-one was carried out by the Claisen–Schmidt condensation reaction [4]. 4-Piperidone hydrochloride (0.68 g, 5 mmol) and 4-fluorobenzaldehyde (1.24 g, 10 mmol) were added into glacial acetic acid (10 mL). Under a dry HCl flow, the mixture was stirred to obtain a clear solution. Then, the reaction continued to be stirred at room temperature for 24 h. The endpoint of which was determined by thin-layer chromatography (TLC). After the reaction was complete, the above mixture was poured to 100 mL water and adjusted to neutral pH by an aqueous Na2CO3 solution. The obtained yellow solids were filtered and washed twice by water. The intermediate 3,5-bis(4-fluorobenzylidene)piperidin-4-one was obtained by the recrystallization of methanol solution. The curcumin analogue and 4-trifluoromethylbenzene-sulfonyl chloride (1.22 g, 5 mmol) were dissolved in dichloromethane (100 mL). The solution was stirred under the catalysis of organic pyridine (two drop) and monitored by TLC. After 24 h, the precipitate was collected and dried under vacuum conditions. The resulting product was recrystallized from dichloromethane/petroleum ether (1:1, v/v). Single crystals of the title compound were prepared by slow evaporation of dichloromethane/methanol solutions at room temperature.
Experimental details
The H atoms were positioned geometrically and treated as riding on their parent atoms, with d (C–H) = 0.97 Å (methylene) and Uiso(H) = 1.2Ueq(C); d(C–H) = 0.93 Å (aromatic) and Uiso(H) = 1.2Ueq(C).
Comment
Curcumin, acknowledged as 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, has attracted extensive attentions because of their potential applications in the aspect of anti-inflammatory, anti-proliferative and antiangiogenic therapies [5], [6], [7]. As an α,β-unsaturated polyphenol compound, it can be considered as one kind of chemopreventive and anticancer agent [8, 9]. Just because of the presence of a diketone group, curcumin becomes unstable at physiological pH. Rapid metabolism and deficient absorption affect its pharmacokinetics and hinder its clinical application [10]. In order to overcome the defect, it is important to modify its structure with mono ketone instead of the β-diketone group. Liu’s group synthesized novel curcumin analogues by the deletion of a methylene and the β-diketone moiety groups. Among these compounds, 5,7-dimethoxy-3-(3-(2-((1E,4E)-3-oxo-5-(pyridin-2-yl)penta-1,4-dien-1-yl)phenoxy)propoxy)-2-(3,4,5-trimethoxyphenyl)-4H-chromen-4-one could induce gastric cancer cells apoptosis by inhibiting the TrxR activity [11]. Li’s group reported that curcumin analogues based on a cyclohexanone moiety possess anti-inflammatory properties [12]. It was found that heterocyclic compounds often exhibit a wide range of biological activities such as antimicrobial, antitumor and antimalarial properties [13]. A piperidone ring was introduced to curcumin analogues and a set of 3,5-bis(arylidene)-4-piperidones were designed [14, 15]. Previous results indicated that fluorine-containing substituents in the aromatic rings usually imparted some distinctive properties to medicines including enhanced binding interactions, metabolic stability, and selective reactivity of compounds [16]. Additionally, it was reported that the phenylsulfonyl moiety in 3,5-bis(arylidene)-4-piperidones could significantly improve the bioactivity of anti-inflammatory [17]. As a part of our continuing study on anti-neuroinflammatory agents, one new curcumin analogue containing the piperidone ring, a fluorine atom and phenylsulfonyl moiety was synthesized by the Claisen–Schmidt condensation reaction and N-benzenesulfonylation reaction.
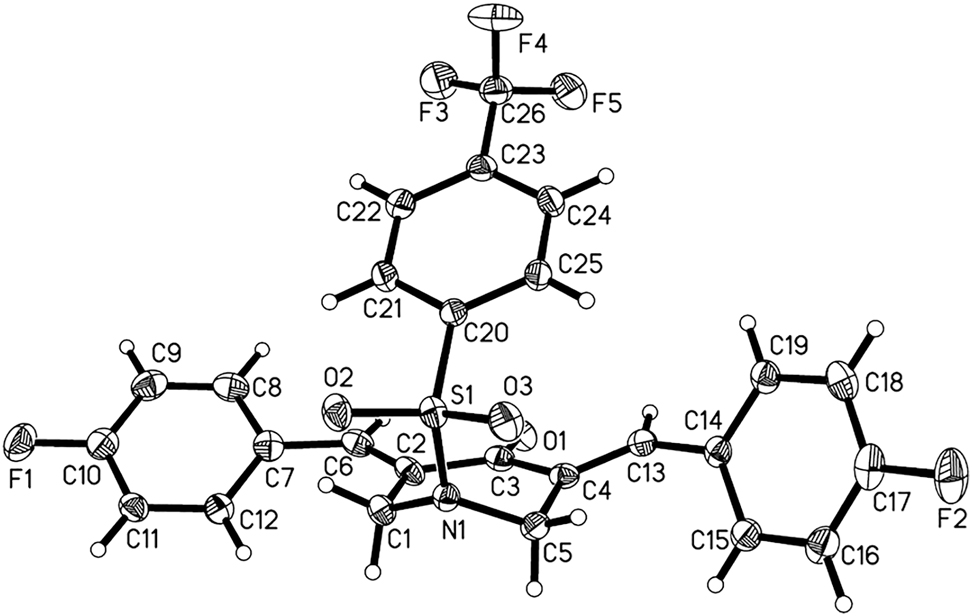
Single-crystal structure analysis shows that the asymmetric unit consists of a 3,5-bis(arylidene)-4-piperidinone molecule (cf. Figure). All bond lengths and bond angles were found to be consistent with the values reported in the literature [18], [19], [20], [21]. Both 4-fluorobenzylidene moieties are symmetrically arranged on the both sides of the central piperidone scaffold. In the title molecule, the torsion angles of C3–C2–C6–C7 and C3–C4–C13–C14 bonds are 179.84(19)° and 178.45(17)°, respectively. It means that the title molecule is in the E stereochemistry of the exocyclic olefinic double bonds. The piperidone ring displays a half-chair conformation with the N1 atom deviating by 0.594(2) Å from the least-squares plane through the ring. The title compound is obviously not planar with the dihedral angle between the fluorophenyl and piperidone rings being 31.97(6)° and 63.87(6)°, respectively. The dihedral angle between fluorophenyl rings is 48.21(7)°. The phenylsulfonyl moiety adopts a pseudo-axial conformation with respect to the piperidone ring, meaning that the molecule as a whole forms a “organic click” in the direction of the carbonyl group. No classic hydrogen bonds were found. Neighbouring molecules are linked via weak C–H⋯F hydrogen bonds to a two-dimensional sheet in the ab plane. Molecules containing as a group the α,β-unsaturated ketone and peripheric heteroatoms (such as O and F) may have anticancer, anti-bacterial and antifungal activities [22].
Award Identifier / Grant number: J18KA092
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Project of the Shandong Province Higher Educational Science and Technology Program (No. J18KA092).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Suche in Google Scholar
2. Sheldrick, G. M. A short history of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Osman, H., Idris, N. H., Kamarulzaman, E. E., Wahab, H. A., Hassan, M. Z. 3,5-Bis(arylidene)-4-piperidones as potential dengue protease inhibitors. Acta Pharm. Sin. B 2017, 7, 479–484; https://doi.org/10.1016/j.apsb.2017.04.009.Suche in Google Scholar PubMed PubMed Central
5. Schmitt, F., Subramaniam, D., Anant, S., Padhye, S., Begemann, G., Schobert, R., Biersack, B. Halogenated bis(methoxybenzylidene)-4-piperidone curcuminoids with improved anticancer activity. ChemMedChem 2018, 13, 1115–1123; https://doi.org/10.1002/cmdc.201800135.Suche in Google Scholar PubMed
6. Sun, J., Zhang, S., Yu, C., Hou, G., Zhang, X., Li, K., Zhao, F. Design, synthesis and bioevaluation of novel N-substituted-3,5-bis(arylidene)-4-piperidone derivatives as cytotoxic and antitumor agents with fluorescent properties. Chem. Biol. Drug Des. 2014, 83, 392–400; https://doi.org/10.1111/cbdd.12254.Suche in Google Scholar PubMed
7. Ocasio-Malave, C., Donate, M. J., Sanchez, M. M., Sosa-Rivera, J. M., Mooney, J. W., Pereles-De Leon, T. A., Carballeira, N. M., Zayas, B., Velez-Gerena, C. E., Martinez-Ferrer, M., Sanabria-Rios, D. J. Synthesis of novel 4-boc-piperidone chalcones and evaluation of their cytotoxic activity against highly-metastatic cancer cells. Bioorg. Med. Chem. Lett 2020, 30, 126760; https://doi.org/10.1016/j.bmcl.2019.126760.Suche in Google Scholar PubMed PubMed Central
8. Kalai, T., Kuppusamy, M. L., Balog, M., Selvendiran, K., Rivera, B. K., Kuppusamy, P., Hideg, K. Synthesis of N-substituted 3,5-bis(arylidene)-4-piperidones with high antitumor and antioxidant activity. J. Med. Chem. 2011, 54, 5414–5421; https://doi.org/10.1021/jm200353f.Suche in Google Scholar PubMed
9. Pricci, M., Girardi, B., Giorgio, F., Losurdo, G., Ierardi, E., Di Leo, A. Curcumin and colorectal cancer: from basic to clinical evidences. Int. J. Mol. Sci. 2020, 21, 2364; https://doi.org/10.3390/ijms21072364.Suche in Google Scholar PubMed PubMed Central
10. Kotha, R. R., Luthria, D. L. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930; https://doi.org/10.3390/molecules24162930.Suche in Google Scholar PubMed PubMed Central
11. Wang, J. Q., Wang, X., Wang, Y., Tang, W. J., Shi, J. B., Liu, X. H. Novel curcumin analogue hybrids: synthesis and anticancer activity. Eur. J. Med. Chem. 2018, 156, 493–509; https://doi.org/10.1016/j.ejmech.2018.07.013.Suche in Google Scholar PubMed
12. Liang, G., Yang, S., Zhou, H., Shao, L., Huang, K., Xiao, J., Huang, Z., Li, X. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur. J. Med. Chem. 2009, 44, 915–919; https://doi.org/10.1016/j.ejmech.2008.01.031.Suche in Google Scholar PubMed
13. Damayanti, P. N., Ritmaleni, S. E. P. Synthesis and antibacterial activity of 4-piperidone curcumin analogues against gram-positive and gram-negative bacteria. Res. J. Pharm. Technol. 2020, 13, 4765–4769; https://doi.org/10.5958/0974-360x.2020.00838.0.Suche in Google Scholar
14. Liu, G.-Y., Jia, C.-C., Han, P.-R., Yang, J. 3,5-Bis(2-fluorobenzy-lidene)-4-piperidone induce reactive oxygen species-mediated apoptosis in A549 cells. Med. Chem. Res. 2017, 27, 128–136; https://doi.org/10.1007/s00044-017-2056-x.Suche in Google Scholar
15. Zhang, L., Chen, Q., Hou, G., Zhao, W., Hou, Y. Hydroxyl-substituted double Schiff-base condensed 4-piperidone/cyclohexanones as potential anticancer agents with biological evaluation. J. Enzym. Inhib. Med. Chem. 2019, 34, 264–271; https://doi.org/10.1080/14756366.2018.1501042.Suche in Google Scholar PubMed PubMed Central
16. Hagmann, W. K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008, 51, 4359–4369; https://doi.org/10.1021/jm800219f.Suche in Google Scholar PubMed
17. Li, N., Xin, W. Y., Yao, B. R., Cong, W., Wang, C. H., Hou, G. G. N-Phenylsulfonyl-3,5-bis(arylidene)-4-piperidone derivatives as activation NF-κB inhibitors in hepatic carcinoma cell lines. Eur. J. Med. Chem. 2018, 155, 531–544; https://doi.org/10.1016/j.ejmech.2018.06.027.Suche in Google Scholar PubMed
18. Zhang, J. J., Chen, D. X., Lv, L. Y., Qi, C. H., Xu, W. C., Hou, G. G. Crystal structure and anti-inflammatory activity of (3E, 5E)-3,5-bis(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C25H18F3NO3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1051–1053; https://doi.org/10.1515/ncrs-2020-0165.Suche in Google Scholar
19. Yan, W. B., Liu, Y. J., Hou, G. G., Cong, W., Meng, Q. G. Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 947–949; https://doi.org/10.1515/ncrs-2020-0116.Suche in Google Scholar
20. Wang, A. Q., Gao, R. N., Luan, Q. H., Wang, Z. P., Li, X. M., Hou, G. G. Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 381–384; https://doi.org/10.1515/ncrs-2019-0686.Suche in Google Scholar
21. Sun, Y., Liu, Y. K., Li, J. D., Meng, Q. G., Hou, G. G. Crystal structure and anti-inflammatory activity of (3E, 5E)-3-(2-fluorobenzy-lidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 377–379; https://doi.org/10.1515/ncrs-2019-0683.Suche in Google Scholar
22. Goslinski, T., Piskorz, J. Fluorinated porphyrinoids and their biomedical applications. J. Photochem. Photobiol. C 2011, 12, 304–321; https://doi.org/10.1016/j.jphotochemrev.2011.09.005.Suche in Google Scholar
© 2021 Zhong-Fei Gao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

