Abstract
C23H41KN2O6, monoclinic, P21/c (no. 14), a = 10.9817(6) Å, b = 23.8330(15) Å, c = 9.7379(7) Å, β = 94.705(5)°, V = 2540.1(3) Å3, Z = 4, R gt (F) = 0.0327, wR ref(F 2) = 0.0682, T = 120 K.
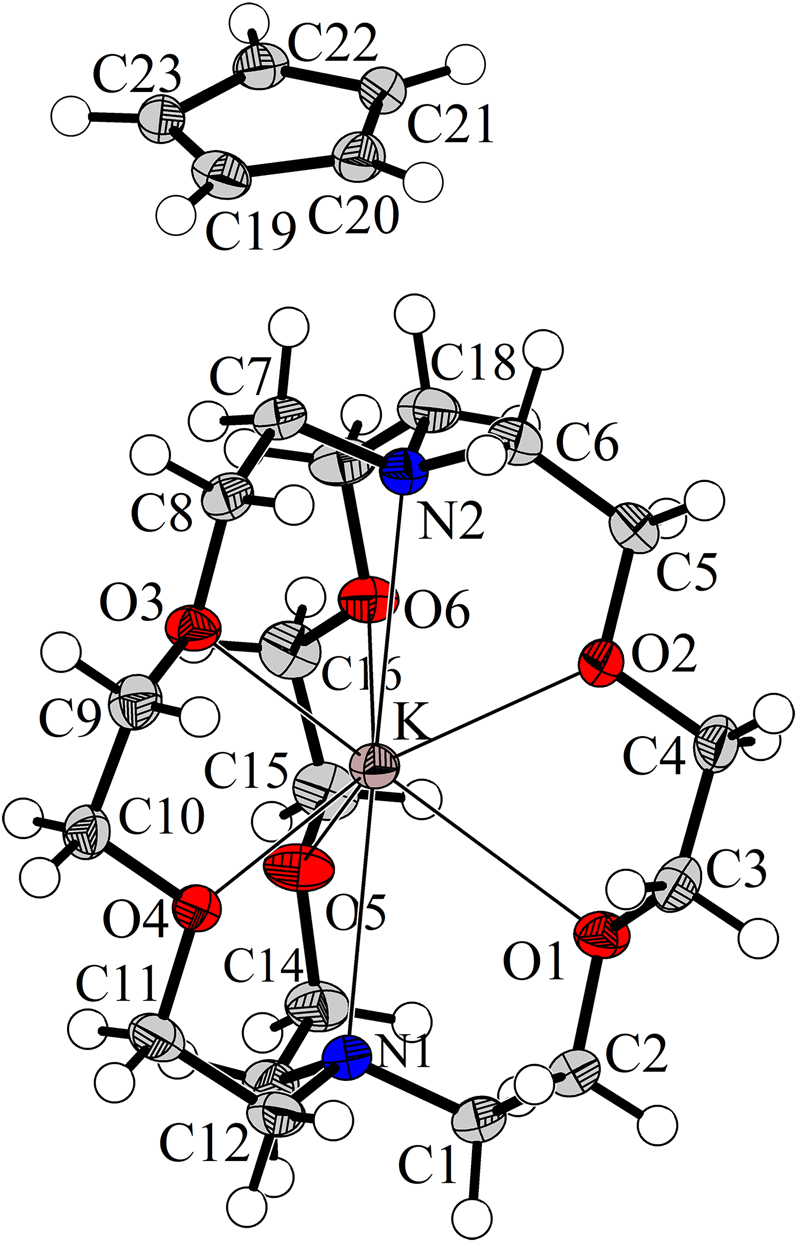
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.25 × 0.20 × 0.15 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.25 mm−1 |
| Diffractometer, scan mode: | Oxford Xcalibur 3, φ and π |
| θ max, completeness: | 28.0°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 32,316, 6125, 0.047 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 4029 |
| N(param)refined: | 453 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| K | 0.37019 (3) | 0.13897 (2) | 0.60803 (3) | 0.01955 (8) |
| N1 | 0.12350 (10) | 0.11496 (4) | 0.47325 (11) | 0.0203 (2) |
| N2 | 0.62089 (10) | 0.16191 (5) | 0.74401 (11) | 0.0203 (2) |
| O1 | 0.34765 (8) | 0.08353 (4) | 0.34195 (9) | 0.0231 (2) |
| O2 | 0.57483 (8) | 0.08797 (4) | 0.50517 (9) | 0.0224 (2) |
| O3 | 0.42672 (8) | 0.10438 (4) | 0.88158 (9) | 0.0222 (2) |
| O4 | 0.20947 (8) | 0.06480 (4) | 0.73713 (9) | 0.0227 (2) |
| O5 | 0.21257 (8) | 0.22836 (4) | 0.54725 (10) | 0.0268 (2) |
| O6 | 0.45760 (8) | 0.25150 (4) | 0.63071 (9) | 0.0239 (2) |
| C1 | 0.13196 (13) | 0.10268 (6) | 0.32584 (15) | 0.0253 (3) |
| H1A | 0.0514 (15) | 0.0896 (6) | 0.2814 (15) | 0.035 (4)* |
| H1B | 0.1518 (12) | 0.1381 (6) | 0.2783 (14) | 0.024 (4)* |
| C2 | 0.22976 (13) | 0.06073 (6) | 0.30041 (16) | 0.0257 (3) |
| H2A | 0.2197 (12) | 0.0250 (6) | 0.3553 (13) | 0.024 (4)* |
| H2B | 0.2237 (12) | 0.0533 (6) | 0.1985 (15) | 0.027 (4)* |
| C3 | 0.44048 (13) | 0.04239 (6) | 0.33627 (16) | 0.0236 (3) |
| H3A | 0.4284 (13) | 0.0121 (6) | 0.4015 (14) | 0.032 (4)* |
| H3B | 0.4361 (12) | 0.0253 (5) | 0.2427 (14) | 0.020 (4)* |
| C4 | 0.56307 (13) | 0.06878 (7) | 0.36642 (15) | 0.0255 (3) |
| H4A | 0.6258 (13) | 0.0398 (6) | 0.3550 (14) | 0.028 (4)* |
| H4B | 0.5711 (13) | 0.0991 (6) | 0.3060 (15) | 0.031 (4)* |
| C5 | 0.69057 (13) | 0.11418 (7) | 0.53806 (15) | 0.0265 (3) |
| H5A | 0.6958 (12) | 0.1511 (6) | 0.4873 (13) | 0.023 (4)* |
| H5B | 0.7539 (14) | 0.0903 (6) | 0.5092 (14) | 0.033 (4)* |
| C6 | 0.70998 (13) | 0.12309 (6) | 0.69139 (15) | 0.0253 (3) |
| H6A | 0.7027 (12) | 0.0879 (6) | 0.7346 (13) | 0.018 (3)* |
| H6B | 0.7931 (13) | 0.1362 (6) | 0.7119 (14) | 0.026 (4)* |
| C7 | 0.61742 (13) | 0.15441 (6) | 0.89417 (14) | 0.0230 (3) |
| H7A | 0.6971 (14) | 0.1559 (6) | 0.9402 (14) | 0.031 (4)* |
| H7B | 0.5708 (12) | 0.1858 (5) | 0.9326 (12) | 0.016 (3)* |
| C8 | 0.55280 (12) | 0.10190 (6) | 0.93247 (15) | 0.0232 (3) |
| H8A | 0.5583 (12) | 0.0970 (5) | 1.0314 (15) | 0.022 (4)* |
| H8B | 0.5894 (13) | 0.0667 (6) | 0.8941 (14) | 0.030 (4)* |
| C9 | 0.36129 (13) | 0.05691 (6) | 0.92401 (16) | 0.0247 (3) |
| H9A | 0.3746 (13) | 0.0527 (6) | 1.0248 (16) | 0.029 (4)* |
| H9B | 0.3914 (14) | 0.0219 (6) | 0.8835 (14) | 0.033 (4)* |
| C10 | 0.22805 (13) | 0.06385 (6) | 0.88335 (14) | 0.0232 (3) |
| H10A | 0.1978 (12) | 0.0985 (6) | 0.9228 (13) | 0.021 (4)* |
| H10B | 0.1846 (12) | 0.0320 (5) | 0.9218 (13) | 0.019 (3)* |
| C11 | 0.08261 (13) | 0.06706 (7) | 0.69296 (15) | 0.0264 (3) |
| H11A | 0.0423 (14) | 0.0330 (6) | 0.7285 (15) | 0.036 (4)* |
| H11B | 0.0457 (13) | 0.1011 (6) | 0.7342 (14) | 0.028 (4)* |
| C12 | 0.06761 (13) | 0.06713 (6) | 0.53823 (15) | 0.0247 (3) |
| H12A | −0.0221 (15) | 0.0653 (6) | 0.5095 (15) | 0.036 (4)* |
| H12B | 0.1020 (12) | 0.0322 (6) | 0.5092 (13) | 0.019 (4)* |
| C13 | 0.04758 (13) | 0.16510 (6) | 0.48871 (16) | 0.0250 (3) |
| H13A | 0.0283 (12) | 0.1672 (5) | 0.5859 (15) | 0.022 (4)* |
| H13B | −0.0294 (14) | 0.1626 (6) | 0.4304 (14) | 0.030 (4)* |
| C14 | 0.10737 (13) | 0.21931 (6) | 0.45306 (17) | 0.0271 (3) |
| H14A | 0.0484 (12) | 0.2497 (6) | 0.4633 (13) | 0.022 (4)* |
| H14B | 0.1326 (13) | 0.2213 (6) | 0.3564 (15) | 0.032 (4)* |
| C15 | 0.26416 (14) | 0.28219 (6) | 0.52912 (17) | 0.0280 (3) |
| H15A | 0.2030 (13) | 0.3105 (6) | 0.5380 (14) | 0.030 (4)* |
| H15B | 0.2964 (14) | 0.2842 (6) | 0.4360 (16) | 0.035 (4)* |
| C16 | 0.36374 (13) | 0.29203 (6) | 0.64038 (16) | 0.0268 (3) |
| H16A | 0.3984 (13) | 0.3289 (6) | 0.6307 (13) | 0.031 (4)* |
| H16B | 0.3312 (12) | 0.2890 (5) | 0.7334 (14) | 0.021 (4)* |
| C17 | 0.55787 (13) | 0.26237 (6) | 0.72976 (16) | 0.0275 (3) |
| H17A | 0.5278 (12) | 0.2630 (6) | 0.8257 (14) | 0.025 (4)* |
| H17B | 0.5884 (13) | 0.3009 (6) | 0.7127 (14) | 0.031 (4)* |
| C18 | 0.65696 (13) | 0.21978 (6) | 0.71538 (17) | 0.0267 (3) |
| H18A | 0.6809 (13) | 0.2224 (6) | 0.6222 (15) | 0.028 (4)* |
| H18B | 0.7269 (13) | 0.2308 (6) | 0.7787 (14) | 0.025 (4)* |
| C19 | 0.82887 (12) | 0.08625 (6) | 0.14593 (15) | 0.0248 (3) |
| H19 | 0.7900 (13) | 0.0546 (6) | 0.1859 (14) | 0.025 (4)* |
| C20 | 0.90191 (12) | 0.08470 (6) | 0.03450 (15) | 0.0241 (3) |
| H20 | 0.9220 (13) | 0.0529 (6) | −0.0155 (14) | 0.026 (4)* |
| C21 | 0.94360 (12) | 0.13917 (6) | 0.01261 (14) | 0.0217 (3) |
| H21 | 0.9954 (13) | 0.1499 (6) | −0.0544 (14) | 0.025 (4)* |
| C22 | 0.89716 (12) | 0.17456 (6) | 0.11033 (14) | 0.0230 (3) |
| H22 | 0.9106 (12) | 0.2145 (6) | 0.1172 (13) | 0.021 (4)* |
| C23 | 0.82596 (12) | 0.14175 (6) | 0.19253 (14) | 0.0226 (3) |
| H23 | 0.7849 (12) | 0.1547 (5) | 0.2659 (14) | 0.020 (4)* |
Source of material
The Zintl phase K4Sn9 was prepared from stoichiometric mixtures of the elements in steel containers, which were encapsulated in an evacuated fused silica tube. The mixture was heated to 550 °C for 46 h and slowly cooled to room temperature with a rate of 1 °C/min. Bis-cyclopentadienyl-germanium (GeCp2) was prepared as described in literature [5], [6], [7]. 91.9 mg K4Sn9 (0.075 mmol), 15.2 mg 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane ([2.2.2]cryptand; 0.3 mmol; Merck, p. a.), and 15.2 mg GeCp2 (0.075 mmol) were given into a dry Schlenk vessel and ca. 2 ml NH3 (Westfalen, 99.999%, stored over elemental Na) were condensed on this. The red solution was stored at −70 °C for 11 months, after this time colourless crystals of the title compound were obtained. A crystal appropriate for X-ray diffraction measurements was selected under perfluoroalkylether in a stream of cold nitrogen gas.
Experimental details
All H atoms have been located from the difference Fourier map and refined with free atomic coordinates and isotropic displacement parameters [3].
Comment
For the study of behaviour and reactivity of tetrel element Zintl compounds in solution, liquid ammonia has been found to be highly suitable [8, 9]. To support the dissolution of the solid Zintl compounds often sequestering agents like crown ethers or cryptands are necessary. During our investigations with the goal to extend or interconnect such clusters via additional tetrel or transition metal atoms [9, 10], [2.2.2]cryptand has been used to dissolve K4Sn9 in liquid ammonia in presence of GeCp2. As found in several examples where the Cp component forms well-crystallised side products [9, 11], [12], [13] the title compound has been obtained as a crystallised product of a partial metathesis reaction, while the remaining ingredients, Ge as well as [Sn9]4− cluster anions, were not found as parts of crystalline phases after this experiment.
[K(crypt-222)]Cp crystallises in space group P21/c with one formula unit as the asymmetric unit and all atoms are at general positions. The cyclopentadienyl counter anion is formed by a regular pentagon of C atoms with C–C distances in a very narrow range of 1.399(2) and 1.402(2) Å and C–C–C angles are between 107.67(12) and 108.29(12)°, all atoms are perfectly planar (angle sum 540.00°). The H atoms of the anion, which are freely refined without any constraints, are in plane, too, and are found in C–H distances between 0.928(13) and 0.965(14) Å. The potassium cation is coordinated by one [2.2.2]cryptand molecule with K–O distances between 2.7781(9) and 2.9009(9) Å and K–N distances of 2.9675(11) and 3.0056(11) Å. Separated this way, no close contacts between K+ and Cp are observed. The shortest intermolecular distances between the K atom and atoms belonging to the anion are K–H19 (5.38 Å) and K–C19 (6.34 Å). This is in contrast to pure KCp which is found to crystallise in polymeric “multidecker” strands [14, 15], while the presence of crown ether often induces the formation of molecular [(18-crown-6)K(Cp)] [16] or even double-sandwich-like [(18-crown-6)K(Cp)K(18-crown-6)]+ units [17], all including η 5 coordination of the K atoms with K–C distances around 3 Å. In the title compound the (K[2.2.2]crypt) complex and the Cp anion are packed in an 1:1 ion packing with identical ten-membered polyhedra of three equally charged ions and seven oppositely charged ions. The shortest C–C distances between different Cp− ions are 4.65 Å and possible π–π interactions are not observed. Hydrogen bonds to electronegative O or N atoms of the cryptand which are sometimes formed e.g. with crown ether molecules [13], can also be neglected as the shortest intermolecular C–O distance is 3.81 Å (H⋅⋅⋅O 2.94 Å, C–H⋅⋅⋅O 150°). Thus, in absence of any noteworthy interactions to neighbouring entities the anion in the title compound can be considered as an example of a “naked” cyclopentadienyl group.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CrysAlis CCD, CrysAlis RED (Version 1.171.33.34d); Oxford Diffraction Ltd.: Yarnton, Oxfordshire, UK, 2009.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System (Version 3.2i); Crystal Impact: Bonn, Germany, 2012.Search in Google Scholar
5. Grenz, M., Hahn, E., du Mont, W. W., Pickardt, J. Neue Strukturen mit Germanium(II): Germanocen und dimeres Tricarbonyl(di-tert-butoxygermylen)nickel(0). Angew. Chem. 1984, 69, 69–70; https://doi.org/10.1002/ange.19840960125.Search in Google Scholar
6. Mathur, S., Shen, H., Sivakov, V., Werner, U. Germanium nanowires and core-shell nanostructures by chemical vapor deposition of [Ge(C5H5)2]. Chem. Mater. 2004, 16, 2449–2456; https://doi.org/10.1021/cm031175l.Search in Google Scholar
7. Fjeldberg, T., HaalandSchilling, A. B. E. R., Lappert, M. F., Thorne, A. J. Subvalent group 4B metal alkyls and amides. part 8. germanium and tin carbene analogues MR2, [M = Ge or Sn, R = CH(SiMe3)2]: syntheses and structures in the gas phase (electron diffraction); molecular-orbital calculations for MH2 and GeMe2. Dalton Trans. 1986, 1986, 1551–1556; https://doi.org/10.1039/dt9860001551.Search in Google Scholar
8. Henneberger, T., Klein, W., Fässler, T. F. Silicon containing nine atom clusters from liquid ammonia solution: crystal structures of the first protonated clusters [HSi9]3− and [H{2Si/Ge}]2. Z. Anorg. Allg. Chem. 2018, 644, 1018–1027; https://doi.org/10.1002/zaac.201800227.Search in Google Scholar
9. Benda, C. B., Waibel, M., Fässler, T. F. On the formation of intermetalloid clusters: titanocene(III)diammin as a versatile reactant toward nonastannide zintl clusters. Angew. Chem. 2015, 127, 532–536; https://doi.org/10.1002/ange.201407855.Search in Google Scholar
10. Bentlohner, M. M., Jantke, L.-A., Henneberger, T., Fischer, C., Mayer, K., Klein, W., Fässler, T. F. On the nature of bridging metal atoms in intermetalloid clusters: synthesis and structure of the metal-atom-bridged zintl clusters [Sn(Ge9)2]4− and [Zn(Ge9)2]6−. Chem. Eur J. 2016, 22, 13946–13952; https://doi.org/10.1002/chem.201601706.Search in Google Scholar PubMed
11. Benda, C. B., Klein, W., Fässler, T. F. Crystal structure of 1,2,3,4,5- pentamethyl-1,3-cyclopentadiene, C10H16. Z. Kristallogr. N. Cryst. Struct. 2017, 232, 511–512; https://doi.org/10.1515/ncrs-2016-0402.Search in Google Scholar
12. Henneberger, T., Klein, W., Fässler, T. F. Crystal structure of [(1,2-η)-1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl] (1,4,10,13-tetraoxa-7,16-diazacyclooctadecane- κ6N2,O4) rubidium(I), [Rb(diaza-18-crown-6)]Cp*, C22H41N2O4Rb. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 165–167; https://doi.org/10.1515/ncrs-2019-0368.Search in Google Scholar
13. Henneberger, T., Klein, W., Fässler, T. F. Crystal structure of (1,4,7,10,13,16-hexaoxacyclooctadecane-κ6O6) 1,2,3,4,5-pentamethyl-cyclopenta-2,4-dien-1-yl(potassium, rubidium)-ammonia (1/2), [K0.3Rb0.7(18-crown-6)]Cp*2̇ NH3, C22H45K0.3N2O6Rb0.7. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 1241–1243; https://doi.org/10.1515/ncrs-2019-0368.Search in Google Scholar
14. Dinnebier, R. E., Behrens, U., Olbrich, F. Solid State Structures of Cyclopentadienyllithium, -sodium, and -potassium. determination by high-resolution powder diffraction. Organometallics 1997, 16, 3855–3858; https://doi.org/10.1021/om9700122.Search in Google Scholar
15. Jordan, V., Behrens, U., Olbrich, F., Weiss, E. Über Metallalkyl- und Arylverbindungen LV. Cyclopentadienyl- und Indenyl-Verbindungen des Kaliums und Natriums. J. Organomet. Chem. 1996, 517, 81–88; https://doi.org/10.1016/0022-328x(95)05988-2.Search in Google Scholar
16. Neander, S., Tio, F. E., Buschmann, R., Behrens, U., Olbrich, F. Cyclopentadienyl, indenyl, fluorenyl, and pentamethylcyclopentadienyl complexes of potassium with 18-crown-6. J. Organomet. Chem. 1999, 582, 58–65; https://doi.org/10.1016/s0022-328x(98)01186-3.Search in Google Scholar
17. Brennessel, W. W., Ellis, J. E. Napthalene and anthracene cobaltates(1-): useful storable sources of an atomic cobalt anion. Inorg. Chem. 2012, 51, 9076–9094; https://doi.org/10.1021/ic301240u.Search in Google Scholar PubMed
© 2021 Christina Fischer et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

