Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
Abstract
C36H52FNO3, orthorhombic, P212121 (no. 19), a = 7.8016(7) Å, b = 13.1609(10) Å, c = 30.536(2) Å, V = 3135.3(4) Å3, Z = 4, R gt (F) = 0.0657, wR ref (F2) = 0.1435, T = 100 K.
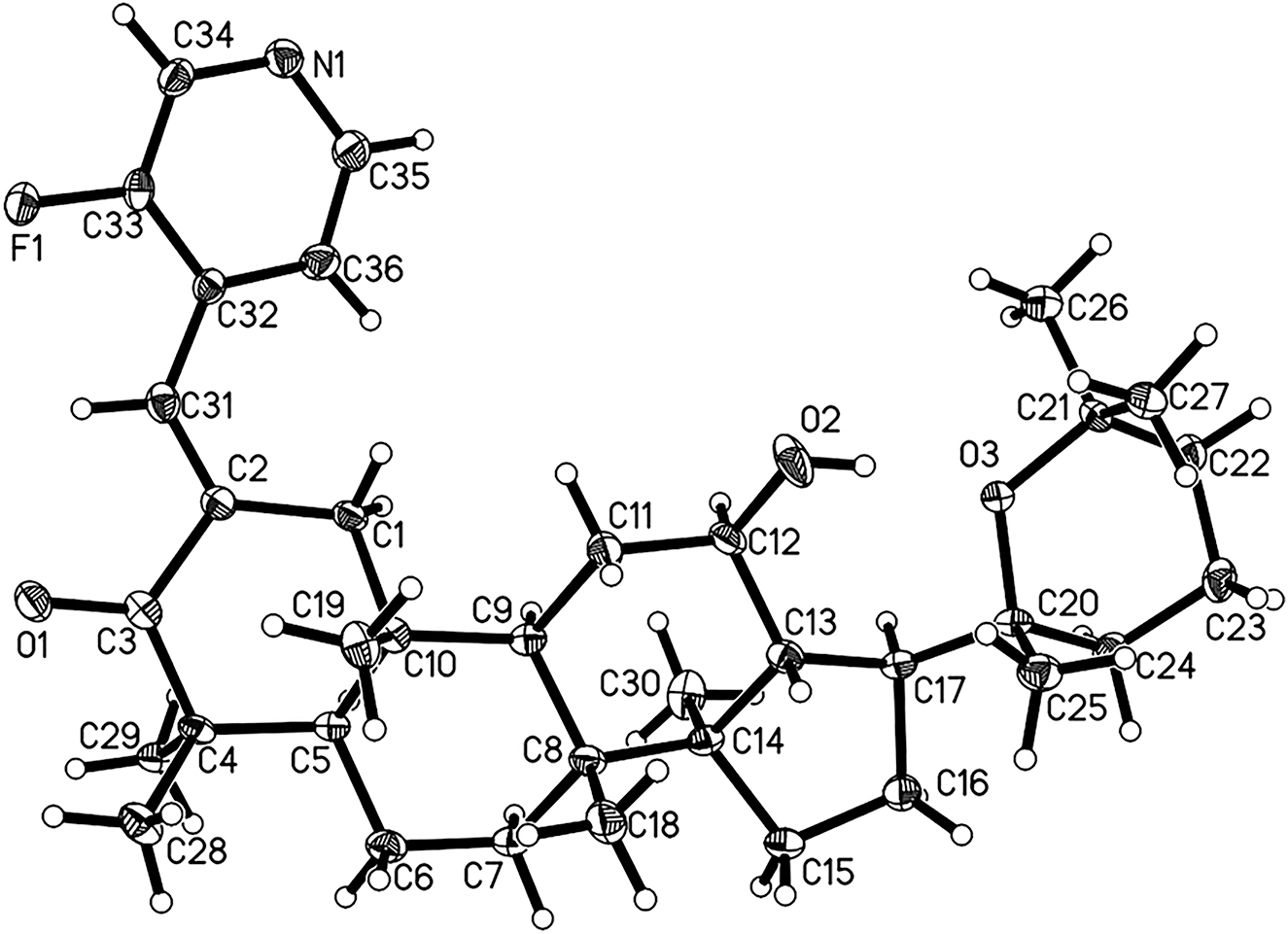
The molecular structure is shown in the figure. Displacement ellipsoids are drawn at the 30% probability level. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.13 × 0.12 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | SuperNova, |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 14226, 5509, 0.095 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3824 |
| N(param)refined: | 379 |
| Programs: | CrysAlisPRO [1], SHELX [2], [3], [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.2876 (6) | 0.7168 (3) | 0.60218 (14) | 0.0267 (11) |
| H1A | 0.391895 | 0.711210 | 0.585065 | 0.032* |
| H1B | 0.201292 | 0.674928 | 0.588127 | 0.032* |
| C2 | 0.2280 (6) | 0.8262 (3) | 0.60106 (14) | 0.0271 (11) |
| C3 | 0.1648 (6) | 0.8737 (3) | 0.64248 (15) | 0.0270 (11) |
| C4 | 0.1101 (6) | 0.8075 (3) | 0.68105 (13) | 0.0245 (11) |
| C5 | 0.1568 (6) | 0.6943 (3) | 0.67499 (13) | 0.0241 (10) |
| H5 | 0.063715 | 0.666078 | 0.657200 | 0.029* |
| C6 | 0.1523 (7) | 0.6339 (4) | 0.71757 (15) | 0.0344 (12) |
| H6A | 0.247982 | 0.654206 | 0.735862 | 0.041* |
| H6B | 0.047317 | 0.649423 | 0.733223 | 0.041* |
| C7 | 0.1616 (7) | 0.5208 (4) | 0.70934 (15) | 0.0367 (13) |
| H7A | 0.061956 | 0.500185 | 0.692562 | 0.044* |
| H7B | 0.157977 | 0.485487 | 0.737197 | 0.044* |
| C8 | 0.3249 (6) | 0.4888 (3) | 0.68446 (14) | 0.0275 (11) |
| C9 | 0.3439 (6) | 0.5559 (3) | 0.64300 (14) | 0.0237 (10) |
| H9 | 0.248135 | 0.535487 | 0.624201 | 0.028* |
| C10 | 0.3222 (6) | 0.6738 (3) | 0.64821 (14) | 0.0234 (10) |
| C11 | 0.5050 (6) | 0.5247 (3) | 0.61719 (17) | 0.0342 (12) |
| H11A | 0.512165 | 0.565863 | 0.590916 | 0.041* |
| H11B | 0.605323 | 0.539177 | 0.634859 | 0.041* |
| C12 | 0.5085 (7) | 0.4125 (4) | 0.60404 (16) | 0.0360 (13) |
| H12 | 0.420024 | 0.400024 | 0.581915 | 0.043* |
| C13 | 0.4765 (6) | 0.3444 (3) | 0.64334 (15) | 0.0272 (11) |
| H13 | 0.572895 | 0.354512 | 0.663464 | 0.033* |
| C14 | 0.3121 (6) | 0.3757 (3) | 0.66842 (14) | 0.0262 (11) |
| C15 | 0.3062 (8) | 0.2928 (4) | 0.70371 (16) | 0.0412 (14) |
| H15A | 0.381189 | 0.309854 | 0.727964 | 0.049* |
| H15B | 0.190522 | 0.284310 | 0.714747 | 0.049* |
| C16 | 0.3676 (6) | 0.1958 (4) | 0.68072 (15) | 0.0355 (13) |
| H16A | 0.271161 | 0.151548 | 0.674560 | 0.043* |
| H16B | 0.447942 | 0.159411 | 0.699215 | 0.043* |
| C17 | 0.4566 (6) | 0.2288 (3) | 0.63713 (14) | 0.0248 (11) |
| H17 | 0.373820 | 0.218237 | 0.613419 | 0.030* |
| C18 | 0.4772 (7) | 0.5014 (4) | 0.71648 (16) | 0.0406 (14) |
| H18A | 0.484252 | 0.570963 | 0.725801 | 0.061* |
| H18B | 0.581699 | 0.482664 | 0.701979 | 0.061* |
| H18C | 0.460133 | 0.458459 | 0.741503 | 0.061* |
| C19 | 0.4816 (6) | 0.7281 (4) | 0.66655 (17) | 0.0336 (12) |
| H19A | 0.581300 | 0.706522 | 0.650655 | 0.050* |
| H19B | 0.494925 | 0.711423 | 0.696960 | 0.050* |
| H19C | 0.467863 | 0.800246 | 0.663459 | 0.050* |
| C20 | 0.6169 (6) | 0.1648 (3) | 0.62624 (14) | 0.0259 (11) |
| C21 | 0.7709 (6) | 0.1363 (3) | 0.55358 (14) | 0.0278 (11) |
| C22 | 0.7212 (7) | 0.0240 (3) | 0.55536 (15) | 0.0334 (12) |
| H22A | 0.614124 | 0.014163 | 0.539798 | 0.040* |
| H22B | 0.808645 | −0.015991 | 0.540749 | 0.040* |
| C23 | 0.7014 (7) | −0.0131 (3) | 0.60216 (17) | 0.0370 (13) |
| H23A | 0.664489 | −0.083511 | 0.602121 | 0.044* |
| H23B | 0.810835 | −0.009248 | 0.617123 | 0.044* |
| C24 | 0.5703 (6) | 0.0516 (3) | 0.62608 (16) | 0.0314 (12) |
| H24A | 0.560877 | 0.027979 | 0.656078 | 0.038* |
| H24B | 0.459356 | 0.042988 | 0.612253 | 0.038* |
| C25 | 0.7660 (6) | 0.1871 (4) | 0.65689 (15) | 0.0360 (13) |
| H25A | 0.852674 | 0.136031 | 0.653322 | 0.054* |
| H25B | 0.726081 | 0.186868 | 0.686630 | 0.054* |
| H25C | 0.813223 | 0.252583 | 0.650055 | 0.054* |
| C26 | 0.7344 (7) | 0.1796 (4) | 0.50828 (15) | 0.0427 (14) |
| H26A | 0.614838 | 0.171870 | 0.501664 | 0.064* |
| H26B | 0.801332 | 0.143779 | 0.486873 | 0.064* |
| H26C | 0.764126 | 0.250339 | 0.507754 | 0.064* |
| C27 | 0.9583 (6) | 0.1531 (4) | 0.56523 (16) | 0.0371 (13) |
| H27A | 0.981809 | 0.224626 | 0.566207 | 0.056* |
| H27B | 1.029718 | 0.121637 | 0.543501 | 0.056* |
| H27C | 0.981740 | 0.123517 | 0.593348 | 0.056* |
| C28 | 0.1773 (7) | 0.8549 (4) | 0.72367 (15) | 0.0385 (13) |
| H28A | 0.299030 | 0.844937 | 0.725551 | 0.058* |
| H28B | 0.122654 | 0.822938 | 0.748248 | 0.058* |
| H28C | 0.152381 | 0.926278 | 0.723871 | 0.058* |
| C29 | −0.0879 (6) | 0.8160 (4) | 0.68132 (15) | 0.0298 (11) |
| H29A | −0.120492 | 0.884406 | 0.688456 | 0.045* |
| H29B | −0.134178 | 0.770291 | 0.702755 | 0.045* |
| H29C | −0.131715 | 0.798728 | 0.652905 | 0.045* |
| C30 | 0.1515 (6) | 0.3583 (4) | 0.63932 (18) | 0.0409 (13) |
| H30A | 0.138679 | 0.287031 | 0.633559 | 0.061* |
| H30B | 0.165081 | 0.394166 | 0.612156 | 0.061* |
| H30C | 0.051609 | 0.383047 | 0.654253 | 0.061* |
| C31 | 0.2241 (6) | 0.8849 (4) | 0.56523 (16) | 0.0308 (12) |
| H31 | 0.183236 | 0.950435 | 0.569761 | 0.037* |
| C32 | 0.2741 (6) | 0.8621 (3) | 0.52005 (14) | 0.0264 (11) |
| C33 | 0.3199 (6) | 0.9395 (3) | 0.49219 (16) | 0.0283 (11) |
| C34 | 0.3694 (6) | 0.9244 (4) | 0.44913 (16) | 0.0331 (12) |
| H34 | 0.399698 | 0.980146 | 0.432114 | 0.040* |
| C35 | 0.3272 (8) | 0.7547 (4) | 0.45781 (16) | 0.0429 (14) |
| H35 | 0.328042 | 0.689437 | 0.446141 | 0.051* |
| C36 | 0.2773 (7) | 0.7658 (4) | 0.50100 (16) | 0.0366 (12) |
| H36 | 0.245824 | 0.709137 | 0.517320 | 0.044* |
| F1 | 0.3172 (4) | 1.03671 (18) | 0.50736 (9) | 0.0384 (7) |
| N1 | 0.3748 (5) | 0.8318 (3) | 0.43148 (13) | 0.0357 (10) |
| O1 | 0.1510 (4) | 0.9668 (2) | 0.64495 (11) | 0.0365 (8) |
| O2 | 0.6732 (6) | 0.3964 (3) | 0.58506 (16) | 0.0782 (16) |
| H2 | 0.692325 | 0.335271 | 0.583501 | 0.117* |
| O3 | 0.6564 (4) | 0.1952 (2) | 0.58156 (9) | 0.0262 (7) |
Source of material
The title compound was synthesized by three-step chemical reactions in turn. First, ginsenoside of stems and leaves (GSL) was degraded by 10% sulfuric acid ethanol solution and purified by silica gel column chromatography. After recrystallization in ethyl acetate and CH2Cl2, the pure white crystalline product panaxadiol (PD; systematic name: (3S, 5R,8R,9R,10R,12R,13R,14R,17S)-4,4,8,10,14-pentamethyl-17 -((R)-2,6,6-trimethyltetra-hydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a] phen-anthrene-3,12-diol)-was obtained. Second, panaxadiol and PCC were dissolved in 25 ml dichloromethane(methylene chloride) solution and stirred for 4 h at room temperature. The product 3-oxo-panaxadiol was purified by silica gel column chromatography. Finally, in a representative experiment 0.72 mL (25%) of sodium hydroxide aqueous solution was added dropwise to the mixture of 3-oxo-panaxadiol (100 mg; 0.218 mmol) and 3-fluoropyridine-4-formaldehyde (0.022 ml; 0.218 mmol) in 1.4 mL methanol and stirred at room temperature for 2.5 h. The in process-control was monitored by silica gel thin layer chromatography (TLC, 254 nm), the developing solvent is petroleum ether and ethyl acetate. When the reaction was finished, the reaction system was extracted twice with appropriate amount of water and ethyl acetate, and the third time with brine and ethyl acetate. The crude yellow oil was obtained by vacuum distillation of the organic phase and the title compound was purified by silica gel column chromatography. Suitable crystals of the title compound were obtained by recrystallization in ethyl acetate system and dried under vacuo at 65 °C for 5 h.
Experimental details
The H atoms were placed in idealized positions and treated as riding on their parent atoms, with d(C–H) = 0.97 Å (methylene), d(C–H) = 0.98 Å (aromatic), d(C–H) = 0.96 Å (methyl), d(C–H) = 0.93 Å (alkenyl) and d(O–H) = 0.82 Å (–OH). The absolute configuration was derived from the synthesis and the configuration of the educts.
Comment
Ginsenoside are divided into two groups according to their glycosidic structures: dammarane and oleanane. There are two types of dammaranes: protopanaxadiol type [5, 6] and protopanaxatriol type sharing a tetrahydrofuran ring and a dammarane skeleton. Because these ginsenosides and their derivatives have a wide range of biological activities, such as anticancer, enhancing myocardial function [7], anti-inflammatory [8] and analgesic, their synthesis and biological activities have attracted extensive attention. At the same time the conformation of the compound will have a certain influence on the biological activity [9]. Our laboratory has done a lot of research work on panaxadiol derivatives [10, 11] and panaxatriol derivatives [12], and obtained crystal structures [13] with good data. The title compound is a PD derivative. It is possible to modify the structure of ginsenoside which is an important saponin in ginsenoside [14].
Single crystal X-ray structure analysis of the title compound revealed that the substituents are at on C(2), the structure of the title compound is similar to that of panaxadiol. In the crystal structure, the pyridine ring is planar, and the other six membered rings except the ring with the keto group have chair conformation. The bond lengths and angles are all in the expected ranges [12].
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81473104, 81773563, 81573585
Funding source: Science Foundation
Award Identifier / Grant number: 13ZJZ06
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the National Natural Science Foundation of China (No. 81473104, 81773563). Meanwhile, this work was also supported by National Natural Science Foundation of China (Grant no. 81573585) and other Science Foundation (No. 13ZJZ06).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku OD. CrysAlisP̂RO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
5. Liu, J., Xu, Y. R., Yang, J. J., Wang, W. Z., Zhang, J. Q., Zhang, R. M., Meng, Q. G. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J. Ginseng Res. 2017, 41, 373–378; https://doi.org/10.1016/j.jgr.2017.01.001.Search in Google Scholar PubMed PubMed Central
6. Zhang, S. N., Zhao, Y. Q. Crystallization separation of anti-tumor constituent 20(R) 25–OCH3–PPD. Chin. Tradit. Herb. Drugs 2014, 45, 770–773.Search in Google Scholar
7. Wang, J. Z., Wang, H. Y., Mou, X. D., Luan, M. Z., Zhang, X. F., He, X. T., Zhao, F. L., Meng, Q. G. The advances on the protective effects of ginsenosides on myocardial ischemia and ischemia-reperfusion injury. Mini Rev. Med. Chem. 2020, 20, 1610–1618; https://doi.org/10.2174/1389557520666200619115444.Search in Google Scholar PubMed
8. Zhang, J. Q., Zhang, Q., Xu, Y. R., Li, H. X., Zhao, F. L., Wang, C. M., Liu, Z., Liu, P., Liu, Y. N., Meng, Q. G., Zhao, F. Synthesis and in vitro anti-inflammatory activity of C20 epimeric ocotillol-type triterpenes and protopanaxadiol. Planta Med. 2019, 85, 292–301; https://doi.org/10.1055/a-0770-0994.Search in Google Scholar PubMed
9. Yang, Q. W., Wang, N., Zhang, J., Chen, G., Xu, H., Meng, Q. G., Du, Y., Yang, X., Fan, H. Y. In vitro and in silico evaluation of stereoselective effect of ginsenosideisomers on platelet P2Y12 receptor. Phytomedicine 2019, 64, 152889; https://doi.org/10.1016/j.phymed.2019.152899.Search in Google Scholar PubMed
10. Deng, J. Q., Mu, X. D., Zhao, R. L., Liu, Z., Tang, H. J., He, M., Meng, Q. G. Crystal structure of (20R)-20,25-epoxydammaran-3,12- dione, C30H48O3. Z. Kristallogr. N. Cryst. Struct 2019, 234, 145–147.10.1515/ncrs-2018-0237Search in Google Scholar
11. Wang, J. Z., Weng, W. Z., Ma, Y., He, X. T., Meng, Q. G. Crystal structure of (1S, 3aR, 3bR, 10aR, 10bR, 12aR)-8-amino-3a, 3b, 6, 6, 10a-pentamethyl-1- ((S)-2, 6, 6-trimethyltetrahydro-2H-pyran-2-yl)-2, 3, 3a, 3b, 4, 5, 5a, 6, 10, 10a, 10b, 11, 12, 12a-tetradecahydro-1H- cyclopenta[7, 8] phenanthro[2, 3-d]thiazol-12-ol a panaxadiol dervative, C31H50N2O2S. Z. Kristallogr. N. Cryst. Struct 2019, 234, 397–400; https://doi.org/10.1515/ncrs-2018-0238.Search in Google Scholar
12. Sun, K. W., Wang, X. H., Ma, G. Q., Luo, Q., Wang, Y. H., Meng, Q. G. The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H- pyran-2-yl) tetradecahydro-3H-cyclopenta[a] phenanthrene- 3,6(2H)-dione, C30H48O4. Z. Kristallogr. N. Cryst. Struct 2021, 236, 17–20; https://doi.org/10.1515/ncrs-2020-0459.Search in Google Scholar
13. Liu, J., Xu, Y. R., An, X. S., Hou, G. G., Meng, Q. G. Synthesis and crystal structures of a 3-acetylated (20S,24S)-ocotillol-type saponin and its C-24 epimer. Acta Crystallogr. 2017, C73, 464–469; https://doi.org/10.1107/s2053229617006507.Search in Google Scholar
14. Wang, C. M., Liu, J., Deng, J. Q., Wang, J. Z., Weng, W. Z., Chu, H. X., Meng, Q. G. Advances in the chemistry, pharmacological diversity, and metabolism of 20(R)-ginseng saponins. J. Ginseng Res. 2020, 44, 14–23; https://doi.org/10.1016/j.jgr.2019.01.005.Search in Google Scholar PubMed PubMed Central
© 2021 Mei Zhang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

