Abstract
C27H20Cl2F2N2O3S, triclinic,
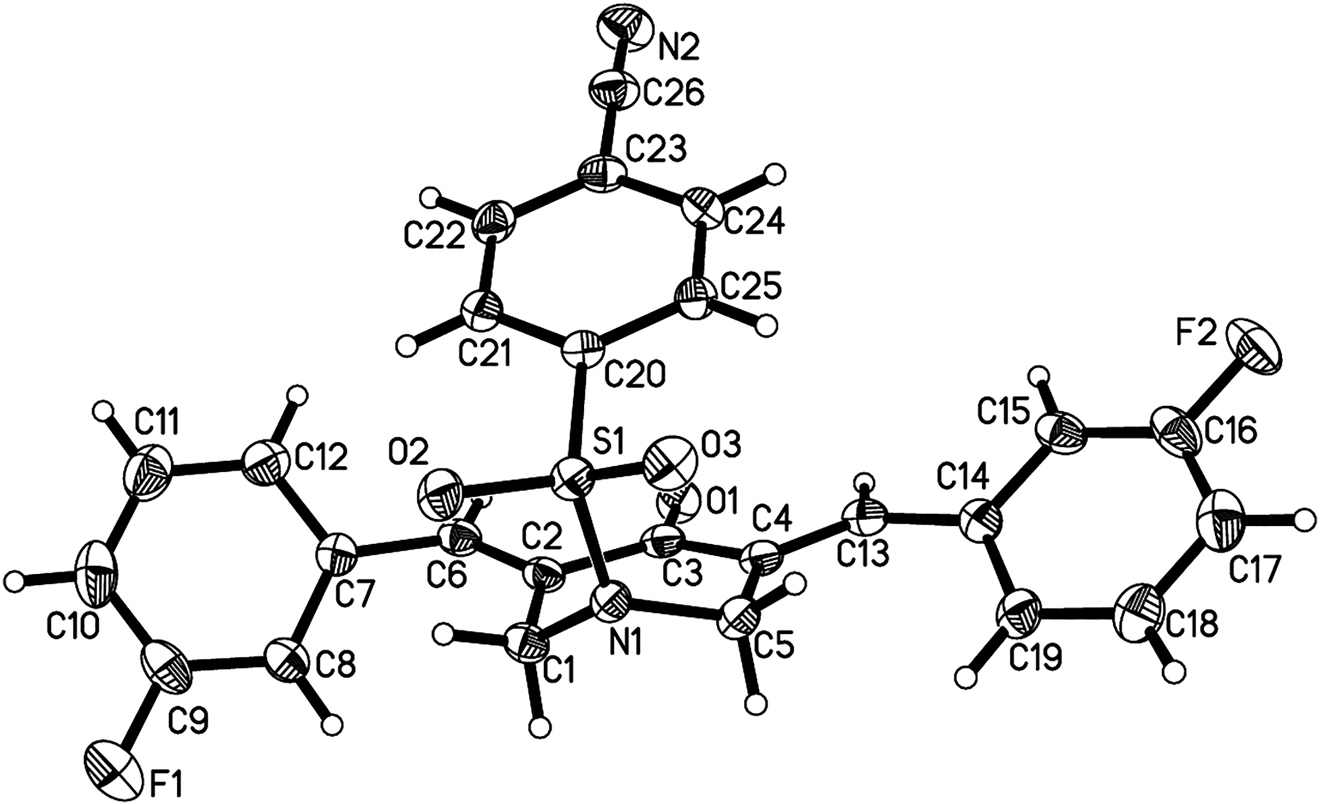
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.15 × 0.13 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.40 mm−1 |
| Diffractometer, scan mode: | SuperNova |
| θmax, completeness: | 29.6°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 11,635, 5784, 0.033 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4418 |
| N(param)refined: | 350 |
| Programs: | CrysAlisPRO [1], SHELX [2, 3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.1551 (3) | 0.9503 (2) | 0.0751 (2) | 0.0231 (5) |
| H1A | 0.053606 | 0.946082 | 0.113060 | 0.028* |
| H1B | 0.138444 | 1.035979 | 0.032045 | 0.028* |
| C2 | 0.2801 (3) | 0.9266 (2) | 0.14703 (19) | 0.0211 (5) |
| C3 | 0.4535 (3) | 0.8806 (2) | 0.11010 (19) | 0.0224 (5) |
| C4 | 0.4932 (3) | 0.8431 (2) | 0.01056 (19) | 0.0216 (5) |
| C5 | 0.3562 (3) | 0.8590 (3) | −0.05079 (19) | 0.0229 (5) |
| H5A | 0.337563 | 0.940722 | −0.099644 | 0.027* |
| H5B | 0.387138 | 0.791133 | −0.087740 | 0.027* |
| C6 | 0.2456 (3) | 0.9336 (2) | 0.2438 (2) | 0.0232 (5) |
| H6 | 0.333216 | 0.913422 | 0.281584 | 0.028* |
| C7 | 0.0857 (3) | 0.9695 (2) | 0.2973 (2) | 0.0238 (5) |
| C8 | −0.0427 (3) | 1.0723 (2) | 0.2554 (2) | 0.0262 (6) |
| H8 | −0.029744 | 1.123116 | 0.191414 | 0.031* |
| C9 | −0.1893 (3) | 1.0961 (3) | 0.3118 (2) | 0.0336 (7) |
| C10 | −0.2155 (4) | 1.0239 (3) | 0.4065 (3) | 0.0407 (8) |
| H10 | −0.316448 | 1.041989 | 0.441692 | 0.049* |
| C11 | −0.0890 (4) | 0.9245 (3) | 0.4474 (2) | 0.0413 (8) |
| H11 | −0.103849 | 0.874866 | 0.511599 | 0.050* |
| C12 | 0.0604 (4) | 0.8970 (3) | 0.3947 (2) | 0.0315 (6) |
| H12 | 0.145164 | 0.829689 | 0.424156 | 0.038* |
| C13 | 0.6496 (3) | 0.7909 (2) | −0.0172 (2) | 0.0224 (5) |
| H13 | 0.722333 | 0.782780 | 0.028225 | 0.027* |
| C14 | 0.7205 (3) | 0.7452 (2) | −0.1090 (2) | 0.0232 (5) |
| C15 | 0.8672 (3) | 0.6468 (2) | −0.1046 (2) | 0.0285 (6) |
| H15 | 0.916736 | 0.611916 | −0.044300 | 0.034* |
| C16 | 0.9377 (3) | 0.6020 (3) | −0.1897 (3) | 0.0334 (7) |
| C17 | 0.8708 (4) | 0.6518 (3) | −0.2816 (2) | 0.0354 (7) |
| H17 | 0.920296 | 0.620270 | −0.338356 | 0.043* |
| C18 | 0.7281 (4) | 0.7500 (3) | −0.2858 (2) | 0.0341 (7) |
| C19 | 0.6523 (3) | 0.7978 (3) | −0.2023 (2) | 0.0277 (6) |
| H19 | 0.556304 | 0.864538 | −0.207821 | 0.033* |
| C20 | 0.3020 (3) | 0.6245 (2) | 0.15259 (18) | 0.0201 (5) |
| C21 | 0.2511 (3) | 0.6259 (2) | 0.25291 (19) | 0.0240 (5) |
| H21 | 0.146134 | 0.671444 | 0.270636 | 0.029* |
| C22 | 0.3575 (3) | 0.5591 (2) | 0.3264 (2) | 0.0259 (6) |
| H22 | 0.324655 | 0.558935 | 0.393970 | 0.031* |
| C23 | 0.5147 (3) | 0.4918 (2) | 0.29827 (19) | 0.0238 (5) |
| C24 | 0.5653 (3) | 0.4900 (2) | 0.1975 (2) | 0.0241 (5) |
| H24 | 0.669891 | 0.443777 | 0.179727 | 0.029* |
| C25 | 0.4586 (3) | 0.5575 (2) | 0.12419 (19) | 0.0221 (5) |
| H25 | 0.491249 | 0.558129 | 0.056509 | 0.026* |
| C26 | 0.6296 (4) | 0.4243 (3) | 0.3735 (2) | 0.0301 (6) |
| C27 | 0.7397 (4) | 0.7305 (3) | 0.3522 (2) | 0.0403 (8) |
| H27A | 0.764444 | 0.810790 | 0.321723 | 0.048* |
| H27B | 0.727871 | 0.693610 | 0.298449 | 0.048* |
| Cl1 | 0.55934 (10) | 0.76047 (12) | 0.42894 (7) | 0.0659 (3) |
| Cl2 | 0.89824 (9) | 0.62350 (7) | 0.42406 (6) | 0.0384 (2) |
| F1 | −0.3130 (2) | 1.19405 (18) | 0.27309 (15) | 0.0469 (5) |
| F2a | 1.0812 (4) | 0.5073(3) | −0.1850 (3) | 0.0429 (10) |
| H18a | 0.687 (11) | 0.775 (9) | −0.349 (3) | 0.052* |
| F2′b | 0.6617 (8) | 0.8121 (6) | −0.3704 (4) | 0.0425 (18) |
| H16b | 1.027 (11) | 0.531 (9) | −0.173 (11) | 0.051* |
| N1 | 0.2053 (2) | 0.8547 (2) | 0.01211 (15) | 0.0203 (4) |
| N2 | 0.7232 (3) | 0.3712 (2) | 0.43143 (19) | 0.0394 (6) |
| O1 | 0.5608 (2) | 0.87332 (18) | 0.16111(13) | 0.0270(4) |
| O2 | 0.0085 (2) | 0.74401 (18) | 0.10387(14) | 0.0279(4) |
| O3 | 0.2126 (2) | 0.65024 (18) | −0.02404 (14) | 0.0279 (4) |
| S1 | 0.16951 (7) | 0.71565 (6) | 0.05705 (5) | 0.02108 (16) |
-
aOccupancy: 0.643(5), bOccupancy: 0.357(5).
Source of material
The title compound was obtained by the Claisen–Schmidt condensation of 4-piperidone and the appropriate aldehyde [4]. A mixture of 4-piperidone hydrochloride (0.68 g, 5 mmol), and 3-fluorobenzaldehyde (1.24 g, 10 mol) was dissolved in acetic acid (10 mL). The reaction was catalyzed by dry hydrogen chloride for 45 min. Then, the mixture was stirred continually at room temperature and monitored by thin layer chromatography (TLC). After the end of reaction, the solvent was removed by filtration and the residue was redissolved in water. The solution was adjusted to a neutral pH value with saturated Na2CO3 solution. Yellow precipitates were obtained and purified by silica gel column chromatography (methanol/petroleum ether/ethyl acetate = 1:10:10, v/v/v). The intermediate product (3,5-bis(3-fluorobenzylidene)piperidin-4-one) was dissolved in dichloromethane (100 mL). 4-Cyanobenzene-sulfonyl chloride (0.98 g, 5 mmol) and three drops of pyridine were added to the above solution. The mixture was stirred at room temperature (monitored by TLC). After 10 h, the reaction solution was concentrated under reduced pressure. The yellow solid was washed twice by distilled water and recrystallized from dichloromethane/methanol (1:1, v/v). Crystals were obtained by slow evaporation from the solution of dichloromethane and methanol (1:1, v/v) at room temperature.
Experimental details
The H atoms were positioned geometrically and treated as riding on their parent atoms, with d (C–H) = 0.97 Å (methylene) and Uiso(H) = 1.2Ueq(C); d(C–H) = 0.93 Å (aromatic) and Uiso(H) = 1.2Ueq(C).
Comment
As one of the most widespread tumors, colorectal cancer has become the second leading cause of death among all cancers [5]. The low incidence of colorectal cancer in South and Southeast Asia receives intensive attention. It was found that curcumin was generally utilized as an element of dietary supplements in those countries. Curcumin, known as 1,7-bis(4-hydroxy-3-meth-oxyphenyl)hepta-1,6-diene-3,5-dione, is a lipophilic polyphenol from the rhizome of Curcuma longa [6]. Due to the pharmacophore of α, β-unsaturated ketone, curcumin can work as anti-inflammatory, antibacterial and anti-oxidant agent [7, 8]. However, poor aqueous solubility, rapid metabolic degradation and low bioavailability limit its therapeutic usage. In order to ameliorate these defects, structural modification based on curcumin was necessary and large amounts of curcumin analogs have been synthesized. For example, 3,5-bis(arylidene)-4-piperidone derivatives exhibited anticancer, antioxidant and anticholinesterase properties [9]. 4-Boc-piperidone chalcones have the potential against highly-metastatic cancer cells [10]. 3,5-Bis(3-alkylamino-methyl-4-hydroxybenzylidene)-4-piperidones can be considered as a novel class of potent tumor-selective cytotoxins [11]. The 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophor of these curcumin analogs has a selective affinity for cellular thiols with little or no affinity for hydroxyl and amino groups in nucleic acids [12]. In the previous study, it was found that 3,5-bis(arylidene)-4-piperidones bearing strong electron-withdrawing substituents in the aromatic rings possess moderate antitumor properties [13]. Additionally, it was reported that many compounds containing phenylsulfonyl moiety had significant anti-inflammatory activity [14, 15]. Based on the above consideration, the title compound was synthesized by Claisen–Schmidt condensation reaction and N-benzenesulfonylation reaction.
X-ray crystallographic analysis shows that there are a 3,5-bis(arylidene)-4-piperidinone molecule and a dichloromethane in the asymmetric unit (cf. the figure). For clarity, the responding solvent molecule was omitted. All bond lengths and bond angles are all in the close agreement with those values reported previously [16], [17], [18], [19], [20]. In the title molecule, the 3-fluorobenzylidene moieties are symmetrically arranged on the both sides of the central piperidone scaffold. Due to the arrangement of the aromatic rings and carbonyl group around the C=C olefinic bonds, the title compound adopts the E stereochemistry. The piperidone ring shows a half-chair conformation, which can be attributed to the conjugated relationship of the carbonyl group with adjacent double bonds. Due to the rotation of C6–C7 and C13–C14 single bonds, the aromatic rings and the carbonyl group of the central piperidone are co-planar to each other. The dihedral angles between them are 55.24(13) and 34.60(18)°, respectively. For the further observation, it was found that the dihedral angle between the fluorophenyl rings is 38.40(9)°. The N-phenylsulfonyl substituent of the title molecule is extended in the direction of the carbonyl group, which can be attributed to the π–π interaction between the benzene ring of the phenylsulfonyl moiety and the carbonyl group. No classic hydrogen bonds were found in the crystal, but solvent CH2Cl2 molecules are connected to 3,5-bis(arylidene)-4-piperidinone molecules through weak C27–H27B⋯O1 hydrogen bonds. It’s worth noting that the heterocycle piperidonyl moiety and peripheric heteroatoms (such as N and F can serve as the potential hydrogen bonding donor/acceptor to improve binding affinity of target protein. Therefore, molecules containing these fragments may exhibit potential biological activity in the aspect of anticancer, anti-bacterial and antifungal activities [21].
Funding source: Project of the Shandong Province Higher Educational Science and Technology Program 10.13039/501100015642
Award Identifier / Grant number: J18KA092
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Project of the Shandong Province Higher Educational Science and Technology Program (No. J18KA092).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku OD. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Das, U., Alcorn, J., Shrivastav, A., Sharma, R. K., De Clercq, E., Balzarini, J., Dimmock, J. R. Design, synthesis and cytotoxic properties of novel 1-[4-(2-alkylaminoethoxy)phenylcarbonyl]-3,5-bis(arylidene)-4-piperidones and related compounds. Eur. J. Med. Chem. 2007, 42, 71–80; https://doi.org/10.1016/j.ejmech.2006.08.002.Search in Google Scholar PubMed
5. Ruiz de Porras, V., Layos, L., Martínez-Balibrea, E. Curcumin: a therapeutic strategy for colorectal cancer? Semin. Canc. Biol. 2021, 73, 321–330; https://doi.org/10.1016/j.semcancer.2020.09.004.Search in Google Scholar PubMed
6. Soleimani, V., Sahebkar, A., Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res. 2018, 32, 985–995; https://doi.org/10.1002/ptr.6054.Search in Google Scholar PubMed
7. Schmitt, F., Subramaniam, D., Anant, S., Padhye, S., Begemann, G., Schobert, R., Biersack, B. Halogenated bis(methoxybenzylidene)-4-piperidone curcuminoids with improved anticancer activity. ChemMedChem 2018, 13, 1115–1123; https://doi.org/10.1002/cmdc.201800135.Search in Google Scholar PubMed
8. Helal, M., Das, U., Bandy, B., Islam, A., Nazarali, A. J., Dimmock, J. R. Mitochondrial dysfunction contributes to the cytotoxicity of some 3,5-bis(benzylidene)-4-piperidone derivatives in colon HCT-116 cells. Bioorg. Med. Chem. Lett 2013, 23, 1075–1078; https://doi.org/10.1016/j.bmcl.2012.12.016.Search in Google Scholar PubMed
9. Sun, J., Zhang, S., Yu, C., Hou, G., Zhang, X., Li, K., Zhao, F. Design, synthesis and bioevaluation of novel N-substituted-3,5-bis(arylidene)-4-piperidone derivatives as cytotoxic and antitumor agents with fluorescent properties. Chem. Biol. Drug Des. 2014, 83, 392–400; https://doi.org/10.1111/cbdd.12254.Search in Google Scholar PubMed
10. Ocasio-Malave, C., Donate, M. J., Sanchez, M. M., Sosa-Rivera, J. M., Mooney, J. W., Pereles-De Leon, T. A., Carballeira, N. M., Zayas, B., Velez-Gerena, C. E., Martinez-Ferrer, M., Sanabria-Rios, D. J. Synthesis of novel 4-boc-piperidone chalcones and evaluation of their cytotoxic activity against highly-metastatic cancer cells. Bioorg. Med. Chem. Lett 2020, 30, 126760; https://doi.org/10.1016/j.bmcl.2019.126760.Search in Google Scholar PubMed PubMed Central
11. Karki, S. S., Das, U., Umemura, N., Sakagami, H., Iwamoto, S., Kawase, M., Balzarini, J., De Clercq, E., Dimmock, S. G., Dimmock, J. R. 3,5-Bis(3-alkylaminomethyl-4-hydroxybenzylidene)-4-piperidones: a novel class of potent tumor-selective cytotoxins. J. Med. Chem. 2016, 59, 763–769; https://doi.org/10.1021/acs.jmedchem.5b01706.Search in Google Scholar PubMed
12. Das, S., Das, U., Michel, D., Gorecki, D. K., Dimmock, J. R. Novel 3,5-bis(arylidene)-4-piperidone dimers: potent cytotoxins against colon cancer cells. Eur. J. Med. Chem. 2013, 64, 321–328; https://doi.org/10.1016/j.ejmech.2013.03.055.Search in Google Scholar PubMed
13. Makarov, M. V., Rybalkina, E. Y., Klemenkova, Z. S., Röschenthaler, G.-V. 3,5-Bis(arylidene)-4-piperidinones modified with bisphosphonate groups using a 1,2,3-triazole ring: synthesis and antitumor properties. Russ. Chem. Bull. Int. Ed. 2014, 63, 2388–2394; https://doi.org/10.1007/s11172-014-0752-y.Search in Google Scholar
14. Sun, Y., Gao, Z. F., Yan, W. B., Yao, B. R., Xin, W. Y., Wang, C. H., Meng, Q. G., Hou, G. G. Discovery of novel NF-κB inhibitor based on scaffold hopping: 1,4,5,6,7,8-hexahydropyrido[4,3-d]pyrimidine. Eur. J. Med. Chem. 2020, 198, 112366; https://doi.org/10.1016/j.ejmech.2020.112366.Search in Google Scholar PubMed
15. Li, N., Xin, W. Y., Yao, B. R., Cong, W., Wang, C. H., Hou, G. G. N-Phenylsulfonyl-3,5-bis(arylidene)-4-piperidone derivatives as activation NF-κB inhibitors in hepatic carcinoma cell lines. Eur. J. Med. Chem. 2018, 155, 531–544; https://doi.org/10.1016/j.ejmech.2018.06.027.Search in Google Scholar PubMed
16. Zhang, X. F., Wang, H. Y., Song, J., Liang, L. H., Xu, Y. R., Zhao, F. L., Meng, Q. G. Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 209–211; https://doi.org/10.1515/ncrs-2020-0492.Search in Google Scholar
17. Zhang, J. J., Chen, D. X., Lv, L. Y., Qi, C. H., Xu, W. C., Hou, G. G. Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one, C25H18F3NO3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1051–1053; https://doi.org/10.1515/ncrs-2020-0165.Search in Google Scholar
18. Yan, W. B., Liu, Y. J., Hou, G. G., Cong, W., Meng, Q. G. Crystal structure and anti-inflammatory activity of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)piperidin-4-one-dichloromethane (1/1), C26H20Cl2F3NO3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 947–949; https://doi.org/10.1515/ncrs-2020-0116.Search in Google Scholar
19. Wang, A. Q., Gao, R. N., Luan, Q. H., Wang, Z. P., Li, X. M., Hou, G. G. Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-acetamidophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one-methanol-hydrate (2/1/1), C53H50F2N6O10S2. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 381–384; https://doi.org/10.1515/ncrs-2019-0686.Search in Google Scholar
20. Sun, Y., Liu, Y. K., Li, J. D., Meng, Q. G., Hou, G. G. Crystal structure and anti-inflammatory activity of (3E,5E)-3-(2-fluorobenzylidene)-1-((4-fluorophenyl)sulfonyl)-5-(pyridin-3-ylmethylene)piperidin-4-one, C24H18F2N2O3S. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 377–379; https://doi.org/10.1515/ncrs-2019-0683.Search in Google Scholar
21. Goslinski, T., Piskorz, J. Fluorinated porphyrinoids and their biomedical applications. J. Photochem. Photobiol. C: Photochem. Rev. 2011, 12, 304–321; https://doi.org/10.1016/j.jphotochemrev.2011.09.005.Search in Google Scholar
© 2021 Zhong-Fei Gao et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

