Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
-
Lucky Dey
, Tsugiko Takase
, Edward R.T. Tiekink
and Tapashi Ghosh Roy
Abstract
C24H36ClN4NaNi2O8, monoclinic, C2/c (no. 15), a = 19.5909(11) Å, b = 10.8023(6) Å, c = 14.5722(8) Å, β = 112.032(1)°, V = 2858.7(3) Å3, Z = 4, R gt (F) = 0.0260, wR ref (F 2) = 0.0701, T = 93(2) K.
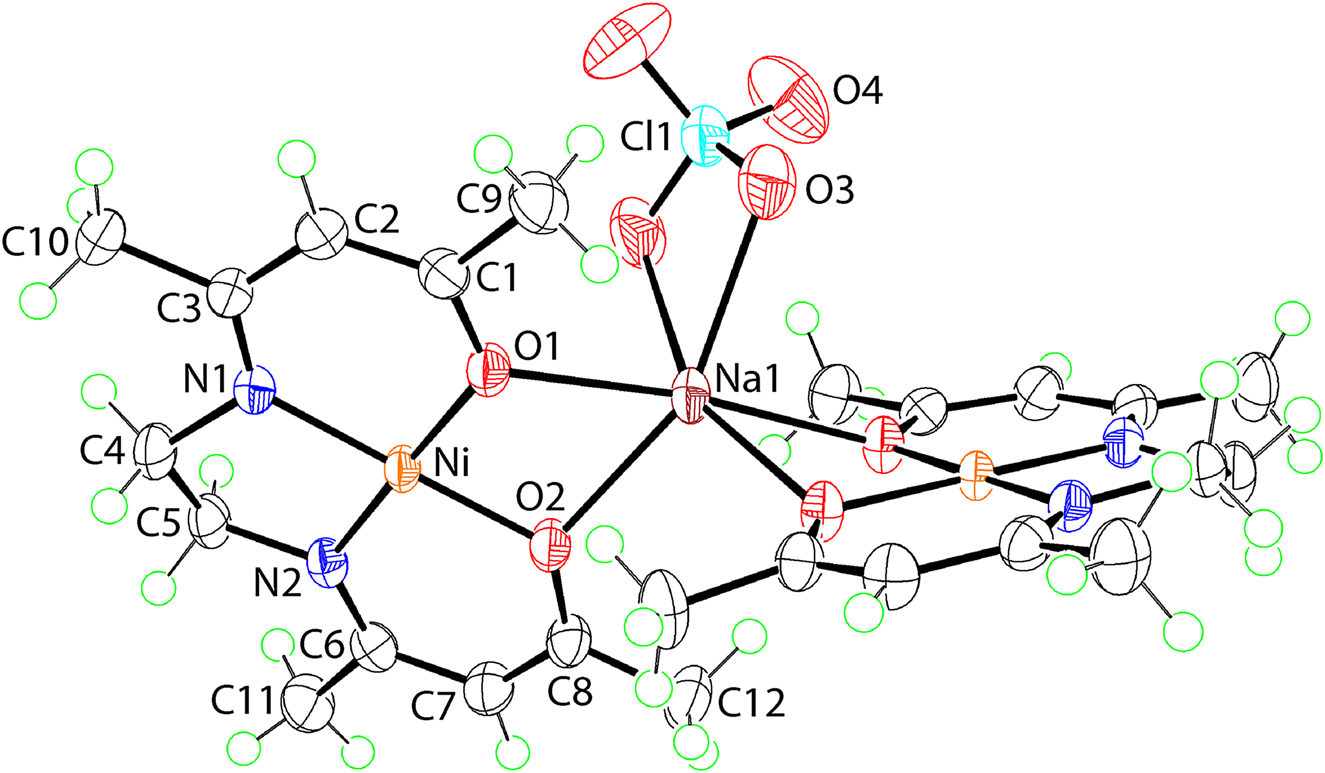
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Red block |
| Size: | 0.20 × 0.20 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.48 mm−1 |
| Diffractometer, scan mode: | Rigaku Saturn724, ω |
| θ max, completeness: | 27.5°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 14,344, 3261, 0.033 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3129 |
| N(param)refined: | 186 |
| Programs: | REQAB [1], CrystalClear [2], SHELX [3, 4], WinGX/ORTEP [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Ni | 0.67462 (2) | 0.65612 (2) | 0.82671 (2) | 0.01590 (8) |
| O1 | 0.60788 (5) | 0.72456 (9) | 0.71032 (7) | 0.0207 (2) |
| O2 | 0.58999 (5) | 0.59640 (9) | 0.83993 (7) | 0.0207 (2) |
| N1 | 0.75757 (6) | 0.71569 (10) | 0.80915 (9) | 0.0194 (2) |
| N2 | 0.73819 (6) | 0.58926 (10) | 0.94563 (8) | 0.0184 (2) |
| C1 | 0.62408 (8) | 0.79199 (13) | 0.64739 (10) | 0.0202 (3) |
| C2 | 0.69423 (8) | 0.82287 (13) | 0.65729 (11) | 0.0239 (3) |
| H2 | 0.699881 | 0.874073 | 0.607606 | 0.029* |
| C3 | 0.75898 (8) | 0.78446 (13) | 0.73576 (11) | 0.0219 (3) |
| C4 | 0.82698 (8) | 0.66642 (14) | 0.88110 (12) | 0.0252 (3) |
| H4A | 0.866150 | 0.729908 | 0.896655 | 0.030* |
| H4B | 0.842624 | 0.593109 | 0.853181 | 0.030* |
| C5 | 0.81466 (7) | 0.63091 (14) | 0.97357 (11) | 0.0215 (3) |
| H5A | 0.848873 | 0.563636 | 1.008442 | 0.026* |
| H5B | 0.824057 | 0.702938 | 1.018655 | 0.026* |
| C6 | 0.72007 (8) | 0.52347 (13) | 1.00862 (10) | 0.0208 (3) |
| C7 | 0.64664 (8) | 0.48441 (14) | 0.98815 (11) | 0.0238 (3) |
| H7 | 0.638224 | 0.430687 | 1.034448 | 0.029* |
| C8 | 0.58714 (8) | 0.51861 (13) | 0.90652 (10) | 0.0212 (3) |
| C9 | 0.55915 (9) | 0.83626 (14) | 0.55946 (12) | 0.0277 (3) |
| H9A | 0.520992 | 0.772111 | 0.539704 | 0.042* |
| H9B | 0.574860 | 0.853528 | 0.504330 | 0.042* |
| H9C | 0.539327 | 0.911954 | 0.577096 | 0.042* |
| C10 | 0.83117 (9) | 0.82580 (15) | 0.73190 (14) | 0.0325 (4) |
| H10A | 0.855965 | 0.881642 | 0.787464 | 0.049* |
| H10B | 0.822188 | 0.869280 | 0.669411 | 0.049* |
| H10C | 0.862402 | 0.753415 | 0.736296 | 0.049* |
| C11 | 0.77744 (8) | 0.48404 (15) | 1.10601 (11) | 0.0289 (3) |
| H11A | 0.810566 | 0.423580 | 1.094259 | 0.043* |
| H11B | 0.753314 | 0.446412 | 1.147139 | 0.043* |
| H11C | 0.805829 | 0.556444 | 1.140091 | 0.043* |
| C12 | 0.51235 (8) | 0.46434 (16) | 0.88615 (12) | 0.0301 (3) |
| H12A | 0.475258 | 0.530180 | 0.865340 | 0.045* |
| H12B | 0.511615 | 0.425025 | 0.946377 | 0.045* |
| H12C | 0.501329 | 0.402391 | 0.833417 | 0.045* |
| Na1 | 0.500000 | 0.73770 (7) | 0.750000 | 0.02234 (17) |
| Cl1 | 0.500000 | 1.02255 (5) | 0.750000 | 0.02701 (12) |
| O3 | 0.54812 (6) | 0.94232 (12) | 0.82627 (9) | 0.0382 (3) |
| O4 | 0.54217 (9) | 1.09586 (15) | 0.70970 (12) | 0.0587 (4) |
Source of material
Preparation of 5,7,12,14-tetramethyl-1,4,8,11-tetraazacyclotetradeca-4,6,11,13-tetraene (LH2): Ethylenediamine (6.7 mL, 0.1 mmol) was added to acetylacetone (10.29 mL, 0.1 mmol) in methanol (50 mL) taken in a 100 mL volumetric flask. The solution was stirred on a magnetic stirrer while 70% perchloric acid (8.9 mL, 0.1 mmol) was added slowly from a dropping funnel. The temperature of the reaction mixture was maintained at 333–338 K, at which stage the solution turned yellow. The resulting mixture was refluxed for 15 h. The reaction mixture was then allowed to stand for 2–3 days at room temperature, after which the solid product was filtered off, washed with methanol and finally with diethyl ether. The white crystalline product, LH2, was dried in vacuo.
Preparation of nickel(II) complex of L: Nickel acetate tetrahydrate (0.25 g, 1.0 mmol) and LH2 (0.449 g, 1.0 mmol) were dissolved separately in 30 mL hot methanol and mixed. After heating on a water bath for a few minutes, the solution immediately turned brown. The solution was then heated for 30 min to reduce the volume to 20 mL. Sodium perchlorate (0.368 g, 3.0 mmol) was added to the cooled solution. After standing overnight, a deep-brown precipitate, anticipated to be [NiL](ClO4)2, was separated by filtration, washed with methanol followed by diethyl ether and stored in a vacuum desiccator.
Preparation of crystals: Crystals were grown by the slow crystallization from its acetonitrile/n-hexane (1:1, v/v) solution. X-ray crystallography proved to the formulation to be [NiL]2Na(ClO4), a known species [6], so no further characterisation was performed. The melting point of the sample was not determined as perchlorates are explosive at elevated temperatures.
Experimental details
The C-bound H atoms were geometrically placed (C–H = 0.95–0.99 Å) and refined as riding with U iso(H) = 1.2–1.5U eq(C). Owing to poor agreement, one reflection, i.e. (−1 1 6), was omitted from the final cycles of refinement.
Comment
In continuation of recent and on-going studies of N4-donor macrocycles and their transition metal complexes [7, 8], an attempt was made to synthesise the nickel(II) complex derived from 5,7,12,14-tetramethyl-1,4,8,11-tetraazacyclotetradeca-4,6,11,13-tetraene, prepared from the 1:1 condensation reaction of ethylenediamine and acetylacetone. However, under the reaction conditions employed, cyclisation did not occur/persist and the title complex [NiL]2Na(ClO4), hereafter (I), was obtained. While the crystal structure of (I) has been reported previously [6], the new data enables a more detailed discussion of geometric parameters and the absence of disorder facilitates the discussion of supramolecular association in the crystal.
The molecular structure of two-fold symmetric (I) is shown in the figure (70% probably displacement ellipsoids); the Na1 and Cl1 atoms lie on the two-fold axis of symmetry. The nickel(II) centre lies within a square-planar geometry defined by a N2O2 donor set with the r.m.s. deviation for the five atoms being 0.0175 Å; the maximum deviation from the least-squares plane being 0.0208(5) Å for atom O1. There is no pattern in the Ni–O and Ni–N bond lengths with Ni–N1 [1.8527(11) Å], Ni–N2 [1.8592(12) Å] and Ni–O2 [1.8552(9) Å] being equal within experimental error and shorter than Ni–O1 [1.8625(10) Å]. Greater ranges of Ni–O [1.8570(18) and 1.8663(18) Å] and Ni–N [1.8454(18) and 1.8553(18) Å] bond lengths are noted in the structure of NiL [9]. These observations suggest the interactions formed between the O1 and O2 atoms and the sodium cation [Na1⃛O1 = 2.3953(9) Å and Na1⃛O2 = 2.3285(11) Å] do not exert a significant influence upon the Ni–O bonds. In the N1–C3–C2–C1–O1 residue, the sequence of bond lengths, i.e. 1.3110(18), 1.414(2), 1.368(2) and 1.3003(16) Å, in particular the lengthening and shortening of the formal imine and alkoxy bonds, suggest considerable delocalisation of π-electron density; the equivalent values for the N2–C6–C7–C8–O2 residue are 1.3107(18), 1.419(2), 1.367(2) and 1.3006(17) Å, respectively.
The Na1 cation provides the link between the two NiL complexes and also interacts with symmetry equivalent perchlorate–O3 atoms [2 × 2.4948(15) Å]. This results in a disparity in the Cl–O bonds, with Cl1–O3 [1.4454(12) Å] being longer than Cl1–O4 [1.4208(14) Å], in keeping with expectation.
The structure of (I) is not the only example where a second residue links two NiL complexes. In another example, Zn(NCS)2 mediates the formation of a three-molecule aggregate, with the zinc centre attaining a N2O4 donor set [10]. With each of Cd(NCS)2 and Cd(N3)2, NiL2 is an ancillary ligand in one-dimensional coordination polymers formulated as {Cd(NCS)2NiL2}n and {Cd(N3)2NiL2}n, respectively [11].
In the crystal, methylene-C–H⃛O(perchlorate) [C5–H5a⃛O3i: H5a⃛O3i = 2.50 Å, C5⃛O3i = 3.2369(19) Å with angle at H5a = 131° for symmetry operation (i): 3/2−x, 3/2−y, 2−z] and methyl-C–H⃛O(perchlorate) [C10–H10c⃛O4ii: H10c⃛O4ii = 2.43 Å, C10⃛O4ii = 3.386(2) Å with angle at H10c = 165° for (ii): 3/2−x, −1/2+y, 3/2−z] interactions combine with methylene- and methyl-C–H⃛π(chelate ring) interactions, well-known in coordination chemistry [12], within a three-dimensional architecture. While each ring participates in two C–H⃛π(chelate ring) contacts, one to either side of the plane, only data for the shortest contact for each are given [C11–H11c⃛Cg(O1,N1,C1–C3)i: H11c⃛Cg(O1,N1,C1–C3)i = 2.78 Å with angle at H11c = 145°; C10–H10b…Cg(O2,N2,C6–C8)iii: H10b…Cg(O2,N2,C6–C8)iii = 2.54 Å with angle at H10b = 149° for (iii): 3/2−x, 1/2+y, 3/2−z].
An analysis of the calculated Hirshfeld surfaces and of the full and delineated two-dimensional fingerprint plots was also conducted, being calculated with Crystal Explorer 17 [13] employing literature methods [14]. The calculations show all surface contacts involve hydrogen and over half of these are H⃛H contacts, contributing 52.5%. The next most significant contributions to the Hirshfeld surface are O⃛H/H⃛O contacts at 23.2%. The remaining contributions to the surface are from C⃛H/H⃛C [14.2%], N⃛H/H⃛N [5.9%] and Ni⃛H/H⃛Ni [4.2%].
Funding source: Japan Society for the Promotion of Science doi.org/10.13039/501100001691
Award Identifier / Grant number: 21K12287
Funding source: Japan Science and Technology Agency doi.org/10.13039/501100002241
Funding source: Japan International Cooperation Agency doi.org/10.13039/501100004532
Award Identifier / Grant number: JPMJSA1603
Funding source: Sunway University doi.org/10.13039/501100010798
Award Identifier / Grant number: GRTIN-IRG-01–2021
Funding source: Environmental Radioactivity Research Network Center
Award Identifier / Grant number: I-21-09
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: (a) Science and Technology Research Partnership for Sustainable Development (SATREPS) grant in collaboration with the Japan Science and Technology Agency (JST) and the Japan International Cooperation Agency (JICA) (Grant No. JPMJSA1603), and (b) Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS; Grant No. 21K12287). In addition, the Environmental Radioactivity Research Network Center at Fukushima University, Japan, is thanked for analytical support through Grant No. I-21-09. Sunway University Sdn Bhd is thanked for financial support of crystallographic work through Grant No. GRTIN-IRG-01–2021.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku. REQAB; Rigaku Corporation: Tokyo, Japan, 1998.Search in Google Scholar
2. Rigaku. CrystalClear; Rigaku Corporation: Tokyo, Japan, 2010.Search in Google Scholar
3. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
6. Armstrong, L. G., Lip, H. C., Lindoy, L. F., McPartlin, M., Tasker, P. A. Alkali-metal salt adducts of nickel(II) complexes of quadridentate Schiff-base ligands. The X-ray structure of N,N′- ethylenebis(acetylacetoneiminato)nickel(II). J. Chem. Soc., Dalton Trans. 1977, 1771–1774.10.1002/chin.197752332Search in Google Scholar
7. Dey, L., Rabi, S., Palit, D., Hazari, S. K. S., Begum, Z. A., Rahman, I. M. M., Roy, T. G. Syntheses, characterization, and antimicrobial studies of Ni(II), Cu(II), and Co(III) complexes with an N-pendant azamacrocyclic chelator. J. Mol. Struct. 2021, 1240, 130579; https://doi.org/10.1016/j.molstruc.2021.130579.Search in Google Scholar
8. Dey, L., Rabi, S., Begum, Z. A., Takase, T., Rahman, I. M. M., Tiekink, E. R. T., Roy, T. G. Redetermination of the crystal structure of (2E,4Z,13E,15Z)-3,5,14,16-tetramethyl- 2,6,13,17-tetraazatricyclo[16.4.0.07,12]docosa- 1(22),2,4,7,9,11,13,15,18,20-decaene, C22H24N4. Z. Kristallogr. NCS 2021, 236.10.1515/ncrs-2021-0244Search in Google Scholar
9. Cariati, F., Morazzoni, F., Busetto, C., Del Piero, G., Zazzetta, A. Paramagnetic anisotropy in cobalt(II) Schiff-base complexes. X-Ray crystal structure and electron spin resonance of N,N′-ethylenebis-(acetylacetoneiminato)cobalt(II)-doped N,N′-ethylenebis(acetylacetoneiminato)nickel(II). J. Chem. Soc., Dalton Trans. 1976, 342–347.10.1039/DT9760000342Search in Google Scholar
10. Li, G.-B., Fang, H.-C., Cai, Y.-P., Zhou, Z.-Y., Thallapally, P. K., Tian, J. Construction of a novel Zn–Ni trinuclear Schiff base and a Ni2+ chemosensor. Inorg. Chem. 2010, 49, 7241–7243; https://doi.org/10.1021/ic101036m.Search in Google Scholar PubMed
11. Ge, Y.-Y., Li, G.-B., Fang, H.-C., Zhan, X.-L., Gu, Z.-G., Chen, J.-H., Sun, F., Cai, Y.-P., Thallapally, P. K. Auxiliary ligand-dependent assembly of several Ni/Ni–Cd compounds with N2O2 donor tetradentate symmetrical Schiff base ligand. Cryst. Growth Des. 2010, 10, 4987–4994; https://doi.org/10.1021/cg101082t.Search in Google Scholar
12. Tiekink, E. R. T. Supramolecular assembly based on “emerging” intermolecular interactions of particular interest to coordination chemists. Coord. Chem. Rev. 2017, 345, 209–228; https://doi.org/10.1016/j.ccr.2017.01.009.Search in Google Scholar
13. Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D., Spackman, M. A.. Crystal Explorer (v17); The University of Western Australia: Australia, 2017.Search in Google Scholar
14. Tan, S. L., Jotani, M. M., Tiekink, E. R. T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. 2019, E75, 308–318; https://doi.org/10.1107/s2056989019001129.Search in Google Scholar
© 2021 Lucky Dey et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

