Abstract
C22H16Cl4N2Sn, monoclinic, P21/n (no. 14), a = 10.0393(1) Å, b = 14.4490(2) Å, c = 14.1502(2) Å, β = 92.846(1)°, V = 2050.07(5) Å3, Z = 4, R gt (F) = 0.0224, wR ref (F 2) = 0.0590, T = 100 K.
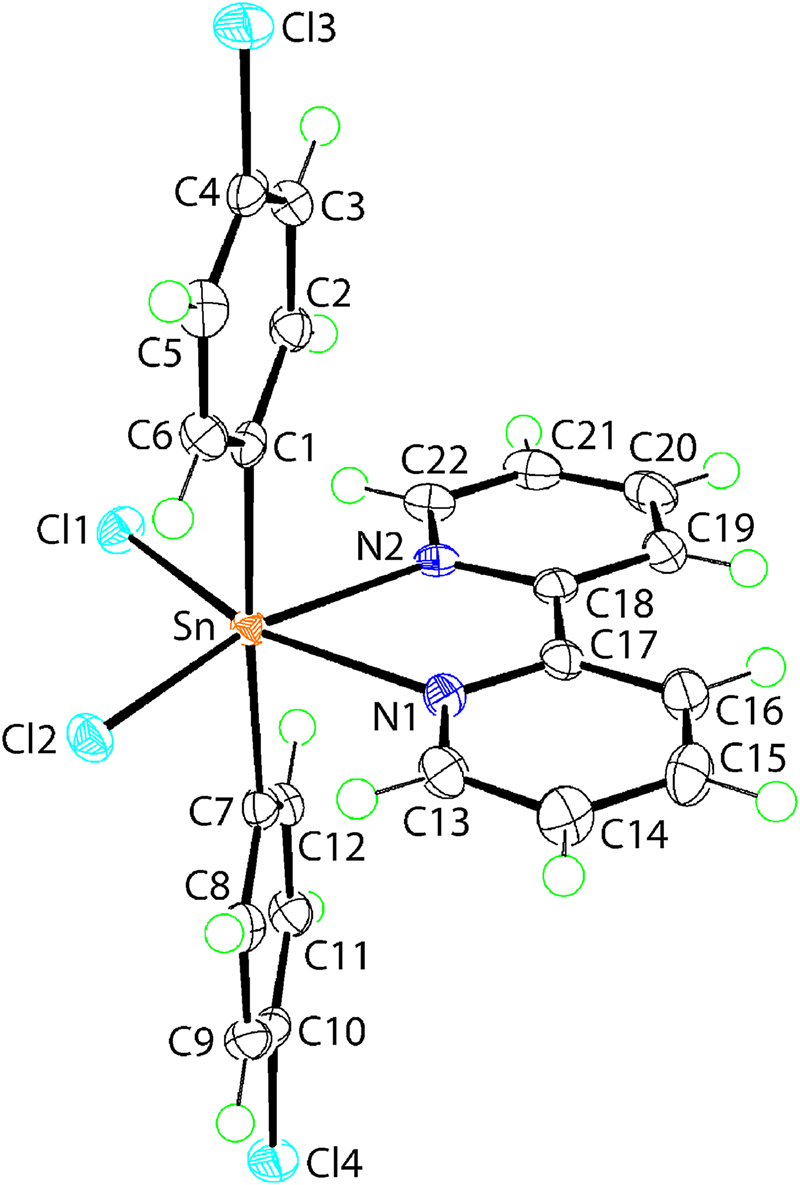
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.13 × 0.10 × 0.06 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 14.8 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy, ω |
| θ max, completeness: | 67.1°, >99% |
| N(hkl)measured, N(hkl)unique, R int: | 25,880, 3647, 0.036 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3497 |
| N(param)refined: | 262 |
| Programs: | Bruker [1], SHELX [2, 3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | U iso*/U eq |
|---|---|---|---|---|
| Sn | 0.48922 (2) | 0.34213 (2) | 0.34646 (2) | 0.00959 (7) |
| Cl1 | 0.35554 (6) | 0.44407 (4) | 0.44585 (4) | 0.01527 (14) |
| Cl2 | 0.73368 (6) | 0.36947 (5) | 0.38890 (4) | 0.01501 (14) |
| Cl3 | 0.49888 (7) | 0.66403 (5) | 0.00763 (5) | 0.01858 (15) |
| Cl4 | 0.48966 (7) | 0.00721 (5) | 0.67177 (5) | 0.01939 (15) |
| N1 | 0.5352 (2) | 0.22738 (16) | 0.23146 (15) | 0.0120 (5) |
| N2 | 0.2884 (2) | 0.28155 (16) | 0.27707 (15) | 0.0114 (4) |
| C1 | 0.4930 (3) | 0.44484 (19) | 0.23635 (18) | 0.0117 (5) |
| C2 | 0.3745 (3) | 0.48319 (19) | 0.19884 (18) | 0.0135 (5) |
| H2 | 0.292026 | 0.463369 | 0.222010 | 0.016* |
| C3 | 0.3748 (3) | 0.5503 (2) | 0.12777 (18) | 0.0144 (6) |
| H3 | 0.293527 | 0.575887 | 0.102478 | 0.017* |
| C4 | 0.4957 (3) | 0.57877 (19) | 0.09485 (18) | 0.0145 (6) |
| C5 | 0.6149 (3) | 0.5425 (2) | 0.13087 (19) | 0.0162 (6) |
| H5 | 0.697138 | 0.563035 | 0.107763 | 0.019* |
| C6 | 0.6131 (3) | 0.4756 (2) | 0.20149 (19) | 0.0142 (6) |
| H6 | 0.694846 | 0.450346 | 0.226428 | 0.017* |
| C7 | 0.4888 (3) | 0.23218 (19) | 0.44930 (18) | 0.0125 (5) |
| C8 | 0.6047 (3) | 0.1833 (2) | 0.47377 (19) | 0.0152 (6) |
| H8 | 0.684796 | 0.198237 | 0.444123 | 0.018* |
| C9 | 0.6055 (3) | 0.1131 (2) | 0.54082 (19) | 0.0158 (6) |
| H9 | 0.685144 | 0.079962 | 0.557020 | 0.019* |
| C10 | 0.4880 (3) | 0.09214 (19) | 0.58364 (18) | 0.0142 (6) |
| C11 | 0.3709 (3) | 0.1393 (2) | 0.56072 (19) | 0.0154 (6) |
| H11 | 0.291089 | 0.124002 | 0.590499 | 0.019* |
| C12 | 0.3720 (3) | 0.2093 (2) | 0.49344 (19) | 0.0144 (6) |
| H12 | 0.292007 | 0.242092 | 0.477280 | 0.017* |
| C13 | 0.6593 (3) | 0.2006 (2) | 0.21367 (19) | 0.0159 (6) |
| H13 | 0.732261 | 0.228985 | 0.247745 | 0.019* |
| C14 | 0.6852 (3) | 0.1330 (2) | 0.1473 (2) | 0.0189 (6) |
| H14 | 0.774118 | 0.114378 | 0.137105 | 0.023* |
| C15 | 0.5790 (3) | 0.0933 (2) | 0.0965 (2) | 0.0203 (6) |
| H15 | 0.593860 | 0.047696 | 0.049885 | 0.024* |
| C16 | 0.4506 (3) | 0.1207 (2) | 0.1143 (2) | 0.0180 (6) |
| H16 | 0.376438 | 0.094166 | 0.079883 | 0.022* |
| C17 | 0.4312 (3) | 0.1878 (2) | 0.18311 (18) | 0.0127 (5) |
| C18 | 0.2954 (3) | 0.21638 (19) | 0.20850 (18) | 0.0129 (5) |
| C19 | 0.1812 (3) | 0.1775 (2) | 0.1660 (2) | 0.0170 (6) |
| H19 | 0.187879 | 0.132879 | 0.117149 | 0.020* |
| C20 | 0.0579 (3) | 0.2040 (2) | 0.1951 (2) | 0.0196 (6) |
| H20 | −0.020962 | 0.177475 | 0.166768 | 0.024* |
| C21 | 0.0498 (3) | 0.2694 (2) | 0.2659 (2) | 0.0182 (6) |
| H21 | −0.034149 | 0.288067 | 0.287551 | 0.022* |
| C22 | 0.1674 (3) | 0.3069 (2) | 0.30448 (19) | 0.0152 (6) |
| H22 | 0.162202 | 0.352600 | 0.352434 | 0.018* |
Source of material
The (4-chlorophenyl)2SnCl2 precursor was prepared as per the literature procedure [5]. Equimolar quantities of (4-chlorophenyl)2SnCl2 (0.5 g, 1.2 mmol) and 2,2′-bipyridyl (Sigma, 0.2 g, 1.2 mmol) were dissolved separately in ethanol (50 mL). The solutions were mixed to give a white precipitate which was recrystallised from methanol to afford colourless crystals of the title compound. Yield: 0.41 g (60.1%). M.pt (Stuart SMP30 digital melting point apparatus; uncorrected): 509–511 K. IR (Bruker Vertex 70v FTIR Spectrometer; cm−1): 1600(s) ν(C=N), 1594 (m) ν(C=C), 1084 (m) ν(C–N). 1H NMR (Bruker Ascend 400 MHz NMR spectrometer; DMSO-d 6; ppm relative to Me4Si): δ 7.40–7.47 (m, 4H, Ph–H), 7.62–7.67 (m, 4H, Ph–H), 7.70–7.75 (m, 6H, Pyr–H), 8.21–8.28 (m, 2H, Pyr–H). 13 C{ 1 H} NMR (as for 1H NMR): 121.6, 123.4, 128.8, 129.8, 134.3, 136.9, 138.9, 148.9, 157.4.
Experimental details
The H atoms were geometrically placed (C–H = 0.95 Å) and refined as riding with U iso(H) = 1.2U eq(C). Owing to poor agreement, seven reflections (as detailed in the deposited CIF) were omitted from the final cycles of refinement.
Comment
Having the attributes of hydrogen-bonding, for example, directionality and strength, halogen-bonding is well-established in crystals of molecules containing halide atoms [6, 7]. In this context and during the course of structural studies of relatively halide-rich diorganotin dichlorides [5, 8, 9], crystals of the title adduct (4-chlorophenyl)2SnCl2(2,2′-bipyridyl), hereafter (I), were isolated.
The molecular structure of (I) is shown in the figure (70% displacement ellipsoids). The tin atom in (I) is located on a general position and is coordinated by two chloride atoms [Sn–Cl1, Cl2 = 2.4772(6) & 2.5285(6) Å], two nitrogen atoms [Sn–N1, N2 = 2.384(2) & 2.366(2) Å] and two ipso-carbon atoms [Sn–C1, C7 = 2.153(3) & 2.155(3) Å] of the 4-chlorophenyl groups. A significant discrepancy is noted in the Sn–Cl bond lengths with the longer Sn–Cl2 bond being trans to the N2 atom, forming the shorter of the Sn–N bonds; the 4-chlorophenyl groups are opposite each other. However, it is noted that previously reported DFT calculations indicated non-systematic variations in the geometric parameters involving tin atom may be generally ascribed to the influence of molecular packing effects, for example for compounds of the general formula R2SnCl2(bipyridyl-type ligand) [10, 11]. Although the C2Cl2N2 donor set approximates an octahedron, there are deviations from 180° noted for the trans angles, i.e. C1–Sn–C7 is 175.97(10)°, Cl1–Sn–N1 is 158.38(6)° and Cl2–Sn–N2 is 162.43(6)°. These distortions may be ascribed, at least in part, to the tight N1–Sn–N2 chelate angle of 69.47(8)°.
There are several crystal structures related to (I) available in the literature. Thus, the two polymorphs of Ph2SnCl2(2,2′-bipyridyl) [12, 13] and that of (4-tolyl)2SnCl2(2,2′-bipyridyl) [14] adopt the same structural motif, as does the compound closely related to (I) where 2,2′-bipyridyl is replaced by 1,10-phenanthroline [8].
The most notable interactions in the crystal are of the type chlorophenyl-C–Cl⃛π(pyridyl, chlorophenyl) and π(pyridyl)⃛π(chlorophenyl). For the former interactions, each chloride of the chlorophenyl groups is located in a bay defined by two rings [C4–Cl3⃛Cg(N1–C13–C17) i : Cl3⃛Cg(N1–C13–C17) i = 3.5402(13) Å with angle at Cl3 = 169.76(11)°; C4–Cl3⃛Cg(C1–C6) i : Cl3⃛Cg(C1–C6) i = 3.5353(13) Å with angle at Cl3 = 88.91(9)°; C10–Cl4⃛Cg(N1–C13–C17) ii : Cl4⃛Cg(N1–C13–C17) ii = 3.4013(13) Å with angle at Cl4 = 171.47(11)°; C10–Cl4⃛Cg(C7–C12) ii : Cl4⃛Cg(C7–C12) ii = 3.6311(13) Å with angle at Cl4 = 86.88(9)° for symmetry operations (i): 1−x, 1−y, −z and (ii): 1−x, −y, 1−z] with the result a supramolecular chain can be discerned along [0 −1 1]. Based on the C–Cl⃛Cg angles, each chloride atom forms an end-on C–Cl⃛π interaction with a pyridyl ring and a side-on C–Cl⃛π interaction with a 4-chlorophenyl ring. The connections between the chains to consolidate a three-dimensional architecture are the π(pyridyl)⃛π(chlorophenyl) contacts [Cg(N2, C18–C22)⃛Cg(C7–C12) iii = 3.7972(16) Å, angle between rings = 11.41(13)° and the slippage = 2.12 Å for (iii): −1/2+x, 1/2−y, −1/2+z].
Additional data on the molecular packing was achieved by the calculation of the Hirshfeld surfaces and two-dimensional fingerprint plots (full and delineated into individual contacts) with Crystal Explorer 17 [15] using literature procedures [16]. As is usually the case, H⃛H contacts dominate, at 33.1%. Other significant contributions to the surface contacts are due to Cl⃛H/H⃛Cl [29.0%], C⃛H/H⃛C [16.3%] and Cl⃛C/C⃛Cl [11.5%]. The next most significant contribution comes from Cl⃛Cl contacts, at 2.0%, but these, e.g. Cl1⃛Cl1 iv = 3.5962(8) Å [(iv): 1−x, 1−y, 1−z] are at distances longer than the van der Waals radii of 3.50 Å [17] and are unlikely to be structure directing. Similar lack of formation of Cl⃛Cl halogen-bonding interactions was noted for the other aforementioned chloride-rich diorganotin dichloride species [5, 8, 9].
Funding source: Sunway University
Award Identifier / Grant number: GRTIN-IRG-01-2021
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Sunway University Sdn Bhd (Grant No. GRTIN-IRG-01-2021).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CrysAlisPRO; Rigaku Corporation: Oxford, UK, 2018.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/S2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Suche in Google Scholar
5. Lo, K. M., Lee, S. M., Tiekink, E. R. T. Crystal structure of dichlorido-bis(4-chlorophenyl-κC1)tin(IV), C12H8Cl4Sn. Z. Kristallogr. N. Cryst. Struct. 2021, 236.10.1515/ncrs-2021-0271Suche in Google Scholar
6. Cavallo, G., Metrangolo, P., Milani, R., Pilati, T., Priimagi, A., Resnati, G., Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601; https://doi.org/10.1021/acs.chemrev.5b00484.Suche in Google Scholar PubMed PubMed Central
7. Tiekink, E. R. T. Characterising supramolecular architectures in crystals featuring I…Br halogen bonding: persistence of X…X′ secondary-bonding in their congeners. Crystals 2021, 11, 433; https://doi.org/10.3390/cryst11040433.Suche in Google Scholar
8. Lo, K. M., Lee, S. M., Tiekink, E. R. T. Crystal structure of dichloridobis(4-chlorophenyl-κC1)-(1,10-phenanthroline-κ2N,N′)tin(IV), C24H16Cl4N2Sn. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 695–697; https://doi.org/10.1515/ncrs-2019-0903.Suche in Google Scholar
9. Lo, K. M., Lee, S. M., Tiekink, E. R. T. Crystal structure of trans-dichloridobis(4-chlorophenyl-κC1)-(1,10-phenanthroline-κ2N,N′)tin(IV) dimethylsulphoxide solvate, C26H22Cl4N2OSSn. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1327–1329; https://doi.org/10.1515/ncrs-2020-0302.Suche in Google Scholar
10. Buntine, M. A., Hall, V. J., Kosovel, F. J., Tiekink, E. R. T. Influence of crystal packing on molecular geometry: a crystallographic and theoretical investigation of selected diorganotin systems. J. Phys. Chem. 1998, 102, 2472–2482; https://doi.org/10.1021/jp9728722.Suche in Google Scholar
11. Buntine, M. A., Hall, V. J., Tiekink, E. R. T. The crystal and molecular structures of R2SnCl2(1,10-phenanthroline), R = iPr, Cy, CH2Ph and R2 = Me, Ph: a comparison between solid state and theoretical structures. Z. Kristallogr. - Cryst. Mater. 1998, 213, 669–678; https://doi.org/10.1524/zkri.1998.213.12.669.Suche in Google Scholar
12. Harrison, P. G., King, T. J., Richards, J. A. Structural studies in main-group chemistry. Part VI. Crystal and molecular structure of 2,2′-bipyridyldichlorodiphenyltin. J. Chem. Soc., Dalton Trans. 1974, 1723–1726.10.1039/DT9740001723Suche in Google Scholar
13. Cox, M. J., Tiekink, E. R. T. Crystal structure of 2,2′-bipyridyldichlorodiphenyltin (tetragonal form), C22H18Cl2N2Sn. Z. Kristallogr. - Cryst. Mater. 1994, 209, 291–292; https://doi.org/10.1524/zkri.1994.209.3.291.Suche in Google Scholar
14. Kumar Das, V. G., Wei, C., Keong, Y. C., Mak, T. C. W. Crystal and molecular structure of 2,2′-bipyridyldichlorodi(p-tolyl)tin(IV). J. Organomet. Chem. 1986, 299, 41–49; https://doi.org/10.1016/0022-328x(86)84032-3.Suche in Google Scholar
15. Turner, M. J., Mckinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D., Spackman, M. A. Crystal Explorer (v17); The University of Western Australia: Australia, 2017.Suche in Google Scholar
16. Tan, S. L., Jotani, M. M., Tiekink, E. R. T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. 2019, E75, 308–318; https://doi.org/10.1107/s2056989019001129.Suche in Google Scholar
17. Spek, A. L. checkCIF validation ALERTS: what they mean and how to respond. Acta Crystallogr. 2020, E76, 1–11; https://doi.org/10.1107/s2056989019016244.Suche in Google Scholar
© 2021 Kong Mun Lo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

