Abstract
C20H19NO10S, triclinic,
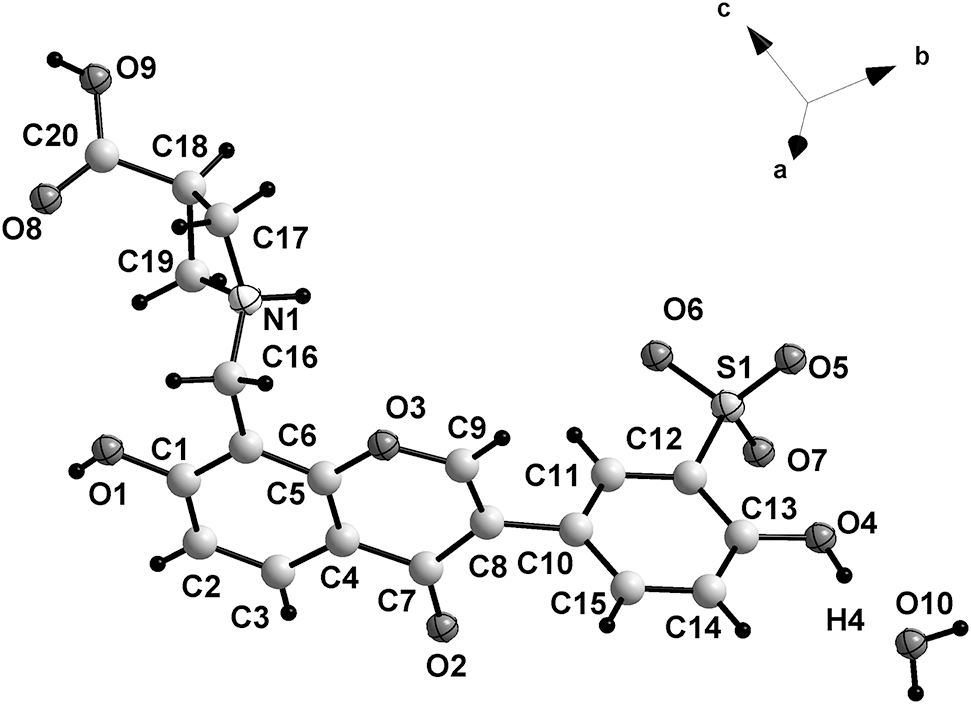
The asymmetric unit of the title structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless plate |
| Size: | 0.30 × 0.20 × 0.20 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 2.05 mm−1 |
| Diffractometer, scan mode: | ROD, ω |
| θmax, completeness: | 75.8°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 10313, 3921, 0.029 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3522 |
| N(param)refined: | 309 |
| Programs: | CrysAlisPRO [1], Diamond [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| S1 | 0.32952 (6) | 1.02505 (6) | 0.27507 (4) | 0.02944 (15) |
| O1 | 0.8547 (2) | 0.31935 (17) | 0.70992 (13) | 0.0349 (4) |
| H1 | 0.812691 | 0.230914 | 0.686191 | 0.052* |
| O2 | 0.7064 (2) | 0.53247 (16) | 0.27267 (12) | 0.0345 (4) |
| O3 | 0.91535 (18) | 0.74483 (15) | 0.59367 (11) | 0.0276 (3) |
| O4 | 0.54484 (19) | 1.10244 (18) | 0.13564 (13) | 0.0350 (4) |
| H4 | 0.606955 | 1.120779 | 0.095664 | 0.052* |
| O5 | 0.36072 (19) | 1.18161 (17) | 0.31074 (14) | 0.0354 (4) |
| O6 | 0.27614 (19) | 0.95781 (17) | 0.35597 (13) | 0.0336 (3) |
| O7 | 0.21524 (19) | 0.96833 (18) | 0.16621 (13) | 0.0353 (4) |
| C1 | 0.8307 (3) | 0.3832 (2) | 0.62611 (17) | 0.0298 (4) |
| C2 | 0.7558 (3) | 0.3033 (2) | 0.51718 (18) | 0.0303 (4) |
| H2 | 0.720157 | 0.201624 | 0.501020 | 0.036* |
| C3 | 0.7340 (3) | 0.3724 (2) | 0.43402 (18) | 0.0296 (4) |
| H3 | 0.683211 | 0.318161 | 0.360512 | 0.035* |
| C4 | 0.7866 (2) | 0.5232 (2) | 0.45705 (17) | 0.0267 (4) |
| C5 | 0.8598 (2) | 0.5986 (2) | 0.56547 (17) | 0.0266 (4) |
| C6 | 0.8830 (2) | 0.5328 (2) | 0.65227 (17) | 0.0281 (4) |
| C7 | 0.7621 (3) | 0.5994 (2) | 0.37095 (17) | 0.0279 (4) |
| C8 | 0.8017 (2) | 0.7557 (2) | 0.40900 (17) | 0.0274 (4) |
| C9 | 0.8767 (3) | 0.8169 (2) | 0.51582 (17) | 0.0280 (4) |
| H9 | 0.904785 | 0.919306 | 0.538307 | 0.034* |
| C10 | 0.7430 (3) | 0.8490 (2) | 0.33547 (17) | 0.0273 (4) |
| C11 | 0.5911 (3) | 0.8909 (2) | 0.33709 (17) | 0.0283 (4) |
| H11 | 0.530742 | 0.861269 | 0.385058 | 0.034* |
| C12 | 0.5252 (3) | 0.9753 (2) | 0.27009 (17) | 0.0281 (4) |
| C13 | 0.6136 (3) | 1.0209 (2) | 0.19941 (17) | 0.0288 (4) |
| C14 | 0.7673 (3) | 0.9801 (2) | 0.19900 (17) | 0.0296 (4) |
| H14 | 0.829442 | 1.011125 | 0.152384 | 0.036* |
| C15 | 0.8308 (3) | 0.8950 (2) | 0.26545 (18) | 0.0291 (4) |
| H15 | 0.935378 | 0.867537 | 0.263397 | 0.035* |
| C16 | 0.9590 (3) | 0.6194 (3) | 0.76862 (18) | 0.0328 (5) |
| H16Aa | 1.044340 | 0.702711 | 0.771243 | 0.039* |
| H16Ba | 1.014112 | 0.558952 | 0.813030 | 0.039* |
| H16Cb | 1.071882 | 0.596747 | 0.791700 | 0.039* |
| H16Db | 0.976312 | 0.722136 | 0.767019 | 0.039* |
| C17 | 0.8602 (3) | 0.7436 (2) | 0.93544 (18) | 0.0337 (5) |
| H17Aa | 0.868785 | 0.849548 | 0.952386 | 0.040* |
| H17Ba | 0.958451 | 0.717166 | 0.980506 | 0.040* |
| H17Cb | 0.945466 | 0.769669 | 1.007065 | 0.040* |
| H17Db | 0.851131 | 0.828605 | 0.904998 | 0.040* |
| C18 | 0.6900 (3) | 0.6598 (3) | 0.93670 (19) | 0.0366 (5) |
| H18c | 0.599331 | 0.717743 | 0.934275 | 0.044* |
| H18Ad | 0.612944 | 0.724971 | 0.914152 | 0.044* |
| C19 | 0.6853 (3) | 0.5632 (3) | 0.82590 (19) | 0.0351 (5) |
| H19Aa | 0.718724 | 0.469796 | 0.830475 | 0.042* |
| H19Ba | 0.577189 | 0.548190 | 0.770036 | 0.042* |
| H19Cb | 0.623800 | 0.594047 | 0.762558 | 0.042* |
| H19Db | 0.645980 | 0.459292 | 0.818317 | 0.042* |
| O8c | 0.7990 (3) | 0.4901 (2) | 1.03187 (17) | 0.0393 (5) |
| O9c | 0.6237 (2) | 0.6199 (2) | 1.09650 (15) | 0.0320 (4) |
| H9Ac | 0.665273 | 0.600332 | 1.155863 | 0.048* |
| N1a | 0.8202 (3) | 0.6712 (2) | 0.81336 (18) | 0.0294 (5) |
| H1Aa | 0.768690 | 0.735728 | 0.770122 | 0.035* |
| C20c | 0.7088 (3) | 0.5797 (3) | 1.0269 (2) | 0.0292 (5) |
| O8Ad | 0.7696 (12) | 0.5924 (11) | 1.0976 (9) | 0.0393 (5) |
| H8Ad | 0.733365 | 0.553297 | 1.141914 | 0.059* |
| O9Ad | 0.5176 (11) | 0.6564 (10) | 1.0654 (7) | 0.0320 (4) |
| N1Ab | 0.8817 (10) | 0.6091 (9) | 0.8531 (7) | 0.0294 (5) |
| H1AAb | 0.934801 | 0.544207 | 0.895186 | 0.035* |
| C20Ad | 0.6481 (14) | 0.6395 (15) | 1.0394 (10) | 0.0292 (5) |
| O10 | 0.7320 (2) | 1.19437 (18) | 0.01927 (13) | 0.0365 (4) |
| H10A | 0.734750 | 1.144244 | −0.044482 | 0.055* |
| H10B | 0.724942 | 1.280205 | 0.010318 | 0.055* |
-
aOccupancy: 0.805(4), bOccupancy: 0.195(4), cOccupancy: 0.833(2), dOccupancy: 0.167(2).
Source of material
The title compound 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxy-benzenesulfonate was synthesized via a Mannich reaction. Formaldehyde (solution, 10 mL, 37%), water (10 mL), azetidine-3-carboxylic acid (1.52 g, 0.015 mol) and sodium 2-hydroxy-5-(7-hydroxy-4-oxo-4H-chromen-3-yl)benzenesulfonate(3.56 g, 0.01 mol) were added to ethanol (150 mL, 99.5%) and stirred for 24 h at 328 K. Then the mixture was filtered and the residue was collected. The residue was dried at 383 K. Sodium 5-(8-((3-carboxyazetidin-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate (2.93 g) was obtained. 1H NMR (500 MHz, DMSO-d6) δ: 8.34 (s, 1H, H9), 7.92 (d, J = 8.8 Hz, 1H, H3), 7.71 (d, J = 2.3 Hz, 1H, H11), 7.39 (dd, J = 8.4, 2.3 Hz, 1H, H15), 6.95 (d, J = 8.8 Hz, 1H, H2), 6.83 (d, J = 8.4 Hz, 1H, H14), 4.03 (s, 2H, H16A, H16B), 3.63 (t, J = 8.2 Hz, 2H, H17A, H19B), 3.51 (t, J = 7.4 Hz, 2H, H17B, H19A), 3.32–3.18 (m, 1H, H18). 13C NMR (125 MHz, DMSO-d6) δ:174.67 (C7), 173.98 (C20), 162.49 (C1), 155.42 (C5), 153.19 (C13), 152.81 (C9), 131.41 (C15), 130.60 (C12), 127.73 (C3), 126.06 (C11), 122.89 (C10), 122.28 (C8), 116.20 (C4), 116.17 (C14), 115.00 (C2), 108.51 (C6), 56.38 (C17, C19), 50.32 (C16), 33.41 (C18). A mixture of sodium salt described before (0.024 g), ammonium iron(II) sulfate hexahydrate (0.039 g), concentrated nitric acid (50 μL) and water (10 mL) was sealed in a 20 mL vial and sonicated for 5 min. Then the mixture was heated at 363 K for 30 min. Colorless plate-shaped crystals of the title compound were obtained. IR spectra (potassium bromide pellet) were recorded on a Nicolet 6700. IR (v/cm−1): 3382, 3021, 2635, 1703, 1632, 1570, 1510, 1445, 1417, 1351, 1290, 1230, 1201, 1170, 1094, 1080, 1049, 1024, 916, 898, 845, 837, 814, 798, 780, 737, 727, 650, 639, 627, 557, 540, 496.
Experimental details
Carbon-bound H atoms were placed in calculated positions and were included in the refinement in the riding model approximation, with Uiso(H) set to 1.2 Ueq(C). The oxygen-bound and nitrogen-bond H atoms were located on a difference Fourier map. The carboxylic acid group and N1 atoms were disordered.
Comment
Azetidine-3-carboxylic acids are a kind of strained four-membered heterocyclic amino acid. In the past decade, azetidine-3-carboxylic acid has become an important pharmaceutical intermediate for the preparation of various medicines [5], [6], [7], [8]. Our previous investigations showed that sodium 2-hydroxy-5-(7-hydroxy-4-oxo-4H-chromen-3-yl)benzenesulfonate reacts with amino acids by Mannich reaction [9], [10], [11], [12], [13]. The reaction takes place without a catalyst. As a continuation, we synthesized the anazetidine-3-carboxylic acid derivative, sodium 5-(8-((3-carboxyazetidin-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate. In this paper, the title compound was obtained by reaction of the aforementioned sodium salt. Studies of the pharmacological activities of the title compound are in progress. The title compound contains one zwitterion and one water molecule in the asymmetric unit (cf. the figure). The bond distances and bond angles are in normal ranges [9], [10], [11], [12], [13]. The dihedral angle between planar rings B (C10–C15) and C (C7–C9/O3/C5/C4) is 85.89°. The nitrogen atom N1 is protonated. There exist various O–H⋯O and N–H⋯O hydrogen bonds. A two dimensional layer along the bc plane is formed by intermolecular hydrogen bonds. There are π⋯π stacking interactions between C1–C6 phenyl rings from adjacent layers. The centroid-centroid distance is 3.6746(13) Å. Together with the π⋯π stacking interactions a three-dimensional network is obtained.
Funding source: Foundation of Hechi University
Award Identifier / Grant number: XJ2018GKQ012
Funding source: Guangxi Natural Science Foundation of China
Award Identifier / Grant number: 2020GXNSFBA297138
Funding source: 2021 High-level Talents Scientific Research Startup Fund of Hechi University
Award Identifier / Grant number: 2021GCC021
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was financially supported by the Foundation of Hechi University (No. XJ2018GKQ012), Guangxi Natural Science Foundation of China (No. 2020GXNSFBA297138) and 2021 High-level Talents Scientific Research Startup Fund of Hechi University (No. 2021GCC021).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku. CrysAlisPRO; Rigaku Corporation: Yarnton: Oxfordshire, England, 2020.Search in Google Scholar
2. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. ver. 3.2; Crystal Impact: Bonn, Germany, 2012.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
5. Hobson, A. D., Harris, C. M., van der Kam, E. L., Turner, S. C., Abibi, A., Aguirre, A. L., Bousquet, P., Kebede, T., Konopacki, D. B., Gintant, G., Kim, Y., Larson, K., Maull, J. W., Moore, N. S., Shi, D., Shrestha, A., Tang, X., Zhang, P., Sarris, K. K. Discovery of A-971432, an orally bioavailable selective sphingosine-1-phosphate receptor 5 (S1P5) agonist for the potential treatment of neurodegenerative disorders. J. Med. Chem. 2015, 58, 9154–9170; https://doi.org/10.1021/acs.jmedchem.5b00928.Search in Google Scholar PubMed
6. Saha, A. K., Yu, X., Lin, J., Lobera, M., Sharadendu, A., Chereku, S., Schutz, N., Segal, D., Marantz, Y., McCauley, D., Middleton, S., Siu, J., Bürli, R. W., Buys, J., Horner, M., Salyers, K., Schrag, M., Vargas, H. M., Xu, Y., McElvain, M., Xu, H. Benzofuran derivatives as potent, orally active S1P1 receptor agonists: a preclinical lead molecule for MS. ACS Med. Chem. Lett. 2011, 2, 97–101; https://doi.org/10.1021/ml100227q.Search in Google Scholar PubMed PubMed Central
7. Pan, S., Gray, N. S., Gao, W., Mi, Y., Fan, Y., Wang, X., Tuntland, T., Che, J., Lefebvre, S., Chen, Y., Chu, A., Hinterding, K., Gardin, A., End, P., Heining, P., Bruns, C., Cooke, N. G., Nuesslein-Hildesheim, B. Discovery of BAF312 (Siponimod), a potent and selective S1P receptor modulator. ACS Med. Chem. Lett. 2013, 4, 333–337; https://doi.org/10.1021/ml300396r.Search in Google Scholar PubMed PubMed Central
8. Xiao, H.‑Y., Watterson, S. H., Langevine, C. M., Srivastava, A. S., Ko, S. S., Zhang, Y., Cherney, R. J., Guo, W.‑W., Gilmore, J. L., Sheppeck, J. E., Wu, D.‑R., Li, P., Ramasamy, D., Arunachalam, P., Mathur, A., Taylor, T. L., Shuster, D. J., McIntyre, K. W., Shen, D.‑R., Yarde, M., Cvijic, M. E., Marino, A. M., Balimane, P. V., Yang, Z., Banas, D. M., Cornelius, G., DArienzo, C. J., Warrack, B. M., Lehman-McKeeman, L., Salter-Cid, L. M., Xie, J., Barrish, J. C., Carter, P. H., Dyckman, A. J., Dhar, T. G. M. Identification of tricyclic agonists of sphingosine-1-phosphate receptor 1 (S1P1) employing ligand-based drug design. J. Med. Chem. 2016, 59, 9837–9854; https://doi.org/10.1021/acs.jmedchem.6b01099.Search in Google Scholar PubMed
9. Chen, H.‑L., Lai, H.‑F., Qin, Y.‑L., Guo, Y.‑N., Chen, T., Huang, X.‑Y. Synthesis and crystal structure poly[aqua(μ3-2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo- 4H-chromen-8-yl)methyl)ammonio)acetate-κ4O, O′:O″: O‴) sodium] monohydrate, C18H18NNaO11S. Z. Kristallogr. NCS 2018, 233, 417–419; https://doi.org/10.1515/ncrs-2017-0321.Search in Google Scholar
10. Chen, H.‑L., Pan, L.‑W., Zhang, H.‑Y., Yin, X.‑J. Synthesis and crystal structure of poly[aqua{μ3-(1S,2S)-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo- 4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylato-κ4O, O′:O″: O‴}sodium(I)] monohydrate, C21H22NNaO11S. Z. Kristallogr. NCS 2018, 233, 469–471; https://doi.org/10.1515/ncrs-2017-0360.Search in Google Scholar
11. Chen, H.‑L., Lai, H.‑F., Wei, L.‑Q., Jiang, L.‑R., Su, Y.‑X. Synthesis and crystal structure of trans-tetraqua-bis(2-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4- oxo-4H-chromen-8-yl)methyl)ammonio)acetato-κO)cobalt(II) hexahydrate, C36H48CoN2O28S2. Z. Kristallogr. NCS 2017, 232, 1025–1027; https://doi.org/10.1515/ncrs-2017-0153.Search in Google Scholar
12. Chen, H.‑L., Yin, X.‑J. Synthesis and crystal structure of 5-(8-(((2-carboxyethyl)ammonio)methyl)-7-hydroxy-4-oxo-4H-chromen-3- yl)-2-hydroxybenzenesulfonate trihydrate, C19H23NO12S. Z. Kristallogr. NCS 2019, 234, 265–267; https://doi.org/10.1515/ncrs-2018-0307.Search in Google Scholar
13. Chen, H.‑L., Pan, L.‑W., Qin, Y.‑L., Xie, Y.‑J., Zhang, P. Synthesis and crystal structure of trans-tetraaqua-bis(3-(((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4- oxo-4H-chromen-8-yl)methyl)ammonio)propanoato-κO)zinc(II) tetrahydrate, C38H48N2O26S2Zn. Z. Kristallogr. NCS 2019, 234, 217–219; https://doi.org/10.1515/ncrs-2018-0250.Search in Google Scholar
© 2021 Hai-Lin Chen et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

