Abstract
C13H22F12N4P2, monoclinic, P21/c (no. 14), a = 25.252(3) Å, b = 8.3297(9) Å, c = 23.268(2) Å, β = 116.271(1)°, V = 4388.7(8) Å3, Z = 8, R gt (F) = 0.0661, wRref(F2) = 0.2191, T = 296(2) K.
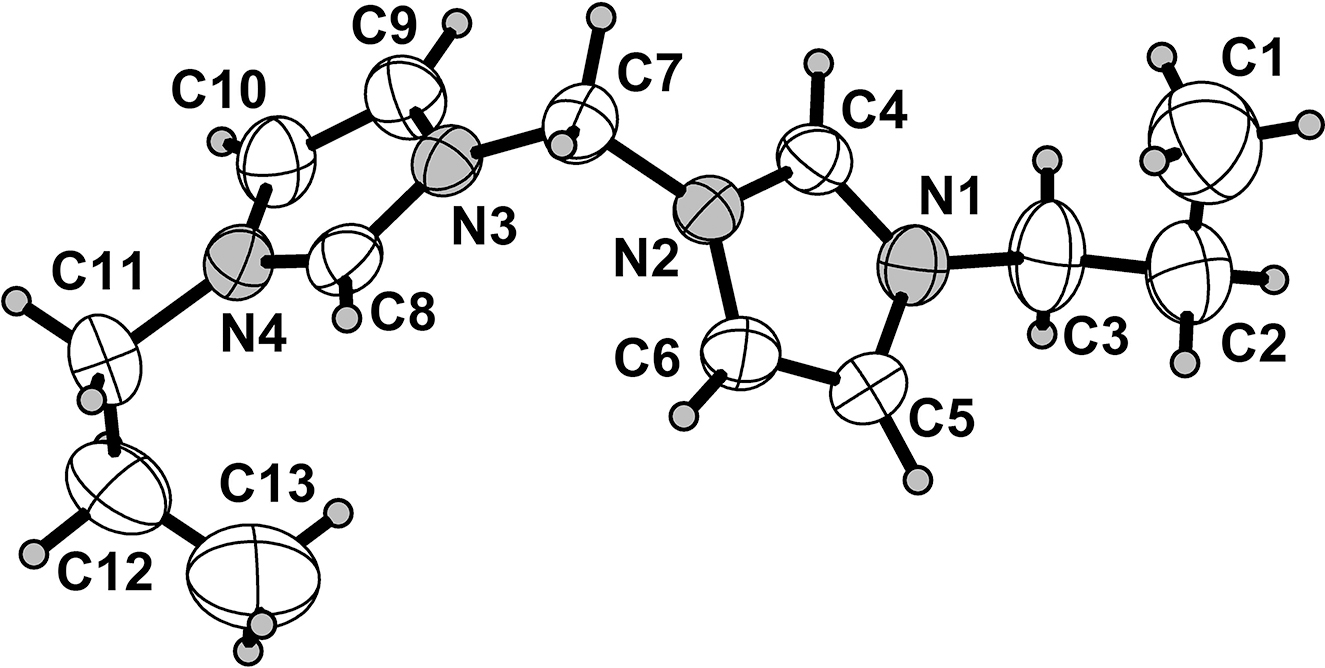
One of the two crystallographically independent cations of the title structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.21 × 0.13 × 0.11 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.31 mm−1 |
| Diffractometer, scan mode: | Bruker APEXII, φ and ω |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 31,566, 7703, 0.042 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4784 |
| N(param)refined: | 820 |
| Programs: | Bruker [1], SHELX [2, 3], Diamond [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 1.0233 (4) | 0.8614 (11) | 0.9429 (4) | 0.172 (3) |
| H1A | 1.045501 | 0.832878 | 0.919980 | 0.258* |
| H1B | 1.048723 | 0.913759 | 0.982282 | 0.258* |
| H1C | 0.991781 | 0.932652 | 0.917208 | 0.258* |
| C2 | 0.9988 (3) | 0.7176 (10) | 0.9569 (3) | 0.142 (3) |
| H2A | 0.962792 | 0.743509 | 0.960221 | 0.170* |
| H2B | 1.026756 | 0.672069 | 0.997400 | 0.170* |
| C3 | 0.9859 (3) | 0.5989 (8) | 0.9038 (2) | 0.112 (2) |
| H3A | 1.021212 | 0.582425 | 0.897996 | 0.135* |
| H3B | 0.975015 | 0.496822 | 0.915622 | 0.135* |
| C4 | 0.94188 (18) | 0.6862 (5) | 0.7902 (2) | 0.0626 (10) |
| H4 | 0.976303 | 0.680672 | 0.785052 | 0.075* |
| C5 | 0.8807 (2) | 0.6746 (6) | 0.8319 (2) | 0.0770 (13) |
| H5 | 0.865371 | 0.659753 | 0.861228 | 0.092* |
| C6 | 0.85070 (19) | 0.7198 (6) | 0.7710 (2) | 0.0739 (12) |
| H6 | 0.810482 | 0.741909 | 0.749869 | 0.089* |
| C7 | 0.8763 (2) | 0.7748 (5) | 0.68021 (19) | 0.0708 (12) |
| H7A | 0.849239 | 0.865082 | 0.667383 | 0.085* |
| H7B | 0.912365 | 0.808277 | 0.678539 | 0.085* |
| C8 | 0.7930 (2) | 0.6175 (5) | 0.6018 (2) | 0.0656 (11) |
| H8 | 0.763104 | 0.681539 | 0.602427 | 0.079* |
| C9 | 0.8799 (2) | 0.5235 (6) | 0.6226 (2) | 0.0785 (13) |
| H9 | 0.920686 | 0.511897 | 0.639862 | 0.094* |
| C10 | 0.8394 (2) | 0.4272 (6) | 0.5795 (2) | 0.0820 (13) |
| H10 | 0.846830 | 0.335211 | 0.561697 | 0.098* |
| C11 | 0.7272 (2) | 0.4209 (7) | 0.5218 (2) | 0.1019 (18) |
| H11A | 0.727840 | 0.394372 | 0.481529 | 0.122* |
| H11B | 0.696775 | 0.501273 | 0.513176 | 0.122* |
| C12 | 0.7122 (4) | 0.2729 (10) | 0.5487 (4) | 0.169 (4) |
| H12A | 0.672004 | 0.242702 | 0.520017 | 0.203* |
| H12B | 0.737677 | 0.186841 | 0.547722 | 0.203* |
| C13 | 0.7169 (4) | 0.2807 (12) | 0.6127 (4) | 0.200 (4) |
| H13A | 0.756553 | 0.309470 | 0.642173 | 0.300* |
| H13B | 0.707409 | 0.177857 | 0.624235 | 0.300* |
| H13C | 0.689983 | 0.359941 | 0.614189 | 0.300* |
| N1 | 0.93778 (15) | 0.6541 (4) | 0.84307 (16) | 0.0663 (9) |
| N2 | 0.88943 (14) | 0.7277 (4) | 0.74541 (14) | 0.0544 (8) |
| N3 | 0.85003 (15) | 0.6419 (4) | 0.63617 (15) | 0.0575 (8) |
| N4 | 0.78511 (16) | 0.4880 (5) | 0.56651 (15) | 0.0681 (9) |
| C14a | 0.8230 (6) | 0.0291 (19) | 0.8847 (7) | 0.156 (5) |
| H14Aa | 0.815940 | −0.044163 | 0.912347 | 0.234* |
| H14Ba | 0.833942 | −0.030015 | 0.856135 | 0.234* |
| H14Ca | 0.854409 | 0.101101 | 0.910005 | 0.234* |
| C15a | 0.7690 (6) | 0.1219 (19) | 0.8471 (8) | 0.135 (5) |
| H15Aa | 0.758140 | 0.175904 | 0.877289 | 0.162* |
| H15Ba | 0.737793 | 0.045561 | 0.823736 | 0.162* |
| C14′b | 0.7618 (7) | 0.049 (2) | 0.8683 (9) | 0.132 (5) |
| H14Db | 0.784876 | −0.008941 | 0.907230 | 0.198* |
| H14Eb | 0.731089 | 0.107133 | 0.872862 | 0.198* |
| H14Fb | 0.744407 | −0.026094 | 0.833470 | 0.198* |
| C15′b | 0.8007 (6) | 0.163 (2) | 0.8549 (9) | 0.147 (5) |
| H15Cb | 0.818697 | 0.237933 | 0.890389 | 0.176* |
| H15Db | 0.832038 | 0.104481 | 0.850931 | 0.176* |
| C16 | 0.7684 (2) | 0.2480 (8) | 0.7990 (3) | 0.114 (2) |
| H16Aa | 0.802510 | 0.317970 | 0.818184 | 0.137* |
| H16Ba | 0.768349 | 0.197276 | 0.761411 | 0.137* |
| H16Cb | 0.760498 | 0.179132 | 0.762439 | 0.137* |
| H16Db | 0.796271 | 0.328141 | 0.798981 | 0.137* |
| C17 | 0.7074 (2) | 0.4724 (6) | 0.8079 (2) | 0.0761 (12) |
| H17 | 0.738302 | 0.527764 | 0.840028 | 0.091* |
| C18 | 0.6584 (2) | 0.2953 (6) | 0.7371 (2) | 0.0786 (13) |
| H18 | 0.649047 | 0.204864 | 0.711005 | 0.094* |
| C19 | 0.6205 (2) | 0.4070 (6) | 0.7378 (2) | 0.0828 (14) |
| H19 | 0.579942 | 0.408718 | 0.712066 | 0.099* |
| C20 | 0.6288 (2) | 0.6606 (6) | 0.7985 (2) | 0.0836 (14) |
| H20A | 0.594849 | 0.698609 | 0.760711 | 0.100* |
| H20B | 0.658551 | 0.744262 | 0.812386 | 0.100* |
| C21 | 0.5565 (2) | 0.6177 (5) | 0.8410 (2) | 0.0699 (12) |
| H21 | 0.523151 | 0.624970 | 0.801747 | 0.084* |
| C22 | 0.6470 (2) | 0.6110 (6) | 0.9134 (2) | 0.0824 (14) |
| H22 | 0.688053 | 0.612743 | 0.933480 | 0.099* |
| C23 | 0.6117 (2) | 0.5916 (6) | 0.9414 (2) | 0.0789 (13) |
| H23 | 0.623766 | 0.577504 | 0.985085 | 0.095* |
| C24 | 0.5021 (3) | 0.5878 (11) | 0.9065 (3) | 0.147 (3) |
| H24Ac | 0.471960 | 0.643352 | 0.870088 | 0.176* |
| H24Bc | 0.490852 | 0.475423 | 0.900887 | 0.176* |
| H24Cd | 0.503016 | 0.494716 | 0.931969 | 0.176* |
| H24Dd | 0.467088 | 0.582012 | 0.865762 | 0.176* |
| C25d | 0.5016 (6) | 0.7467 (15) | 0.9406 (8) | 0.118 (4) |
| H25Ad | 0.536603 | 0.748307 | 0.981471 | 0.141* |
| H25Bd | 0.505408 | 0.834161 | 0.915205 | 0.141* |
| C26d | 0.4491 (5) | 0.7829 (15) | 0.9534 (5) | 0.124 (4) |
| H26Ad | 0.454764 | 0.884628 | 0.974718 | 0.186* |
| H26Bd | 0.413888 | 0.786586 | 0.913549 | 0.186* |
| H26Cd | 0.445232 | 0.700320 | 0.980134 | 0.186* |
| C25′c | 0.4975 (9) | 0.8161 (17) | 0.9494 (11) | 0.109 (4) |
| H25Cc | 0.492556 | 0.873449 | 0.982521 | 0.164* |
| H25Dc | 0.535349 | 0.841429 | 0.951369 | 0.164* |
| H25Ec | 0.466937 | 0.846860 | 0.908350 | 0.164* |
| C26′c | 0.4938 (9) | 0.6378 (17) | 0.9589 (8) | 0.136 (4) |
| H26Dc | 0.524936 | 0.600550 | 0.999226 | 0.163* |
| H26Ec | 0.455623 | 0.606052 | 0.955641 | 0.163* |
| N5 | 0.71300 (16) | 0.3392 (5) | 0.78178 (17) | 0.0711 (10) |
| N6 | 0.65214 (16) | 0.5170 (4) | 0.78272 (16) | 0.0646 (9) |
| N7 | 0.61133 (16) | 0.6278 (4) | 0.84918 (15) | 0.0615 (8) |
| N8 | 0.55593 (16) | 0.5959 (4) | 0.89612 (17) | 0.0695 (9) |
| F1e | 0.6093 (10) | 0.706 (3) | 0.6353 (10) | 0.139 (6) |
| F2e | 0.7246 (7) | 0.901 (2) | 0.6735 (8) | 0.136 (5) |
| F3e | 0.6922 (6) | 0.8243 (18) | 0.7305 (5) | 0.131 (4) |
| F4e | 0.6587 (8) | 0.7785 (19) | 0.5873 (6) | 0.145 (4) |
| F5e | 0.6405 (6) | 0.9704 (11) | 0.6423 (9) | 0.121 (4) |
| F6e | 0.6991 (10) | 0.638 (2) | 0.6720 (9) | 0.129 (5) |
| F1′f | 0.6114 (7) | 0.739 (2) | 0.6580 (7) | 0.124 (4) |
| F2′f | 0.7238 (5) | 0.8658 (15) | 0.6497 (6) | 0.104 (3) |
| F3′f | 0.6642 (6) | 0.9012 (18) | 0.7032 (6) | 0.171 (4) |
| F4′f | 0.6597 (7) | 0.6967 (15) | 0.5914 (7) | 0.158 (4) |
| F5′f | 0.6257 (4) | 0.9306 (14) | 0.5993 (5) | 0.120 (3) |
| F6′f | 0.7047 (6) | 0.6669 (17) | 0.6961 (6) | 0.127 (5) |
| P1 | 0.66619 (5) | 0.80208 (14) | 0.65239 (6) | 0.0683 (4) |
| F7g | 0.9228 (2) | 0.1680 (6) | 0.7031 (2) | 0.1183 (15) |
| F8g | 0.9298 (3) | 0.2390 (8) | 0.8382 (2) | 0.1333 (18) |
| F9g | 0.88119 (17) | 0.3454 (4) | 0.7441 (2) | 0.1093 (14) |
| F10g | 0.9718 (3) | 0.0603 (8) | 0.7968 (3) | 0.140 (2) |
| F11g | 0.9792 (2) | 0.3228 (6) | 0.7852 (4) | 0.131 (2) |
| F12g | 0.8731 (2) | 0.0855 (5) | 0.7545 (3) | 0.1161 (18) |
| F7′h | 0.8755 (11) | 0.142 (3) | 0.7191 (14) | 0.136 (7) |
| F8′h | 0.9817 (11) | 0.247 (3) | 0.8387 (11) | 0.149 (6) |
| F9′h | 0.8992 (11) | 0.316 (3) | 0.8052 (16) | 0.124 (6) |
| F10′h | 0.9640 (12) | 0.066 (4) | 0.7629 (14) | 0.110 (6) |
| F11′h | 0.9492 (12) | 0.311 (3) | 0.7399 (14) | 0.123 (6) |
| F12′h | 0.9113 (11) | 0.071 (3) | 0.8121 (13) | 0.120 (5) |
| P2 | 0.92685 (5) | 0.20252 (13) | 0.77207 (7) | 0.0711 (4) |
| F13i | 0.5595 (17) | 0.157 (6) | 0.7765 (15) | 0.124 (7) |
| F14i | 0.5868 (17) | 0.065 (5) | 0.9123 (14) | 0.120 (7) |
| F15i | 0.6141 (19) | 0.242 (5) | 0.8845 (18) | 0.116 (8) |
| F16i | 0.534 (2) | −0.027 (6) | 0.806 (2) | 0.135 (9) |
| F17i | 0.6238 (13) | 0.015 (4) | 0.845 (2) | 0.118 (6) |
| F18i | 0.5156 (14) | 0.196 (5) | 0.8303 (19) | 0.126 (7) |
| F13′j | 0.5807 (2) | 0.0497 (8) | 0.7857 (2) | 0.1219 (17) |
| F14′j | 0.5630 (2) | 0.1804 (8) | 0.9025 (2) | 0.1348 (18) |
| F15′j | 0.6276 (2) | 0.2289 (7) | 0.8642 (2) | 0.1068 (16) |
| F16′j | 0.5171 (2) | 0.0001 (8) | 0.8234 (3) | 0.128 (2) |
| F17′j | 0.6126 (3) | −0.0216 (5) | 0.8882 (3) | 0.145 (2) |
| F18′j | 0.5325 (3) | 0.2504 (5) | 0.8003 (2) | 0.132 (2) |
| P3 | 0.57248 (5) | 0.11384 (14) | 0.84525 (5) | 0.0674 (4) |
| F19k | 0.7961 (3) | 0.6555 (9) | 0.9238 (3) | 0.134 (3) |
| F20k | 0.8686 (3) | 0.4915 (14) | 1.0575 (3) | 0.153 (3) |
| F21k | 0.8765 (4) | 0.5108 (12) | 0.9676 (4) | 0.149 (3) |
| F22k | 0.7891 (3) | 0.6422 (13) | 1.0153 (5) | 0.144 (3) |
| F23k | 0.7910 (6) | 0.4223 (15) | 0.9654 (8) | 0.119 (4) |
| F24k | 0.8701 (6) | 0.7226 (15) | 1.0142 (5) | 0.164 (4) |
| F19Al | 0.8416 (8) | 0.554 (2) | 0.9355 (6) | 0.132 (4) |
| F20Al | 0.8151 (8) | 0.557 (2) | 1.0520 (6) | 0.134 (4) |
| F21Al | 0.8651 (6) | 0.4034 (14) | 1.0163 (9) | 0.135 (4) |
| F22Al | 0.7889 (6) | 0.7081 (16) | 0.9717 (10) | 0.144 (5) |
| F23Al | 0.7746 (9) | 0.454 (4) | 0.9575 (16) | 0.104 (5) |
| F24Al | 0.8858 (8) | 0.662 (3) | 1.0380 (10) | 0.138 (6) |
| P4 | 0.83213 (5) | 0.56915 (16) | 0.99287 (5) | 0.0728 (4) |
-
aOccupancy: 0.533(11), bOccupancy: 0.467(11), cOccupancy: 0.422(10), dOccupancy: 0.578(10), eOccupancy: 0.447(11), fOccupancy: 0.553(11), gOccupancy: 0.824(4), hOccupancy: 0.176(4), iOccupancy: 0.135(6), jOccupancy: 0.865(6), kOccupancy: 0.654(7), lOccupancy: 0.346(7).
Source of material
1-Propylimidazole (4.40 g, 0.04 mol) was dissolved in acetonitrile (25 mL), dibromomethane (3.47 g, 0.02 mol) was quickly added under stirring. The mixture was vigorously stirred at 85 °C for about 12 h. After the reaction completed (monitored by thin layer chromatography (TLC)), a white solid was produced after cooling. The white solid was filtered, crushed, and washed with ethylacetate and diethyl ether three times respectively. Then residual solvent was removed, and the white powder intermediate (C1PM-Br) was dried in vacuo (7.6 g, yield 96.57%). Then the intermediates (C1PM-Br) (1.97 g, 0.005 mol), potassium hexafluoro phosphate (2.48 g, 0.012 mol) was dissolved in water (30 mL). The mixture stirred well for 8 h at 105 °C and then cooled slowly. The crystals suitable for X-ray analysis were obtained (yield 78.90%).
Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.90–0.97 Å with Uiso(H) = 1.5 Ueq(C) for methyl H atoms and 1.2 Ueq(C) for all other H atoms.
Comment
Ionic liquids are composed of organic cations and inorganic or organic anions [5]. Its melting point is 100 °C or lower [6], and it has the advantages of low vapor pressure, non-flammability, good thermal and chemical stability, and great structural adjustability [7, 8]. Carrying out research on ionic liquids is in line with the needs of the development of green chemistry, and is also a topic of common concern for researchers from all over the world in recent years [9, 10]. It is worth mentioning that in recent years, our research group has been devoted to the research of ionic liquids, especially imidazole ionic liquids, and applied them to the catalytic conversion process of biodiesel [11], [12], [13], [14], [15]. And reported four crystal structures of 1,1′-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), 3-(2-ethoxy-2-oxoethyl)-1-methyl-1H-imidazol-3-ium hexafluoridophosphate(V), 3,3′-(1,2-phenylenebis(methylene))bis(1-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), and 3,3′-(1,2-phenylene-bis(methylene)) bis(1-ethyl-1H-imidazol-3-ium) bis(hexafluoro-phosphate) [16], [17], [18], [19]. In order to find an ionic liquid with better catalytic effects and a higher recycling efficiency to catalyze the reaction, we synthesized a new type of imidazole ionic liquid catalyst.
There are two crystallographic independent cations and four crystallographic independent hexafluoridophosphate anions in the asymmetric unit (only one cation is shown in the figure). In the molecule of the title compound bond lengths and angles are very similar to those given in the literature [17–20]. The atoms of imidazole ring are coplanar, and the dihedral angles between the two imidazole rings are 69 and 66°. The torsion angles of C1–C2–C3–N1, C2–C3–N1–C4, C13–C12–C11–N4, C12–C11–N4–C8, C14–C15–C16–N5, C15–C16–N5–C17, C26–C25–C24–N8, and C25–C24–N8–C21 are −67.4(8), 117.2(6), 50.4(9), 70.0(7), −167.9(12), 94.5(8), 175.0(10), and −107.9(8)°, respectively. In the molecule, both propyl groups of 1,1′-(methane-1,1-diyl) bis(3-propyl-1H-imidazol-3-ium) cation and four hexafluoridophosphate anions are disordered to some extent. Never the less the structure determination verifies the existence of the title compound.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 31760193
Funding source: Natural Science Foundation of Jiangxi Province of China
Award Identifier / Grant number: 20202BABL205003
Funding source: Education Department of Jiangxi Province
Award Identifier / Grant number: GJJ190181
Award Identifier / Grant number: GJJ200404
Award Identifier / Grant number: GJJ200462
Award Identifier / Grant number: 202110410028
Acknowledgments
X-ray data were collected at Instrumental Analysis Center Nanchang Hangkong University, Nanchang, 330063, People's Republic of China.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the National Natural Science Foundation of China (No. 31760193), the Natural Science Foundation of Jiangxi Province of China (No. 20202BABL205003), the Key Research Foundation of Education Department of Jiangxi Province of China (No. GJJ190181, GJJ200404, GJJ200462) and National College Students Innovation and Entrepreneurship Training Program (No. 202110410028).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS. Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
4. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System (Ver. 4.0); Crystal Impact: Bonn, Germany, 2015.Suche in Google Scholar
5. Zhang, D. C., Zhang, L. P., Yu, X. J., Chen, E. Z., Dong, T., Li, H. Synthesis and characterization of ionic liquids 1-butyl-3-methylimidazolium hexafluorophosphate. Chem. Ind. Times 2012, 26, 5–8.Suche in Google Scholar
6. Sandip, K. S., Anthony, W. S. Ionic liquids synthesis and applications: an overview. J. Mol. Liq. 2020, 297, 112038.10.1016/j.molliq.2019.112038Suche in Google Scholar
7. Huang, T., Zhao, W., Zhang, X. H., Nie, X. L., Chen, J., Xiong, W. M. Synthesis and characterization of diimidazole-based hexafluorophosphate ionic liquids. J. Mol. Liq. 2020, 320, 114465; https://doi.org/10.1016/j.molliq.2020.114465.Suche in Google Scholar
8. Haifa, B. S., Paul, N., Amani, A. O. Ionic liquid-assisted refinery processes-a review and industrial perspective. Fuel 2021, 302, 121195.10.1016/j.fuel.2021.121195Suche in Google Scholar
9. Majeda, K., Fares, A. M., Mehreen, I., Mohammad, K. H., Mohammad, A. G. Ionic liquids application for wastewater treatment and biofuel production: a mini review. J. Mol. Liq. 2021, 337, 116421.10.1016/j.molliq.2021.116421Suche in Google Scholar
10. Samahe, S. Magnetic(poly) ionic liquids: a promising platform for green chemistry. J. Mol. Liq. 2021, 323, 114994.10.1016/j.molliq.2020.114994Suche in Google Scholar
11. Shailey, S., Shilpi, A., Manjeet, S., Sravendra, R., Shefali, A., Naveen, S. Ionic liquids: green catalysts for alkene-isoalkane alkylation. J. Mol. Liq. 2019, 285, 299–313.10.1016/j.molliq.2019.03.145Suche in Google Scholar
12. Xia, Y. Y., Zhao, F. Q., Zeng, B. Z. A molecularly imprinted copolymer based electrochemical sensor for the highly sensitive detection of L-tryptophan. Talanta 2020, 206, 120245; https://doi.org/10.1016/j.talanta.2019.120245.Suche in Google Scholar PubMed
13. Zhu, Z. Y., Bai, W. T., Xu, Y., Gong, H. Z., Wang, Y. L., Xu, D. M., Gao, J. Liquid-liquid extraction of methanol from its mixtures with hexane using three imidazolium-based ionic liquids. J. Chem. Thermodyn. 2019, 138, 189–195; https://doi.org/10.1016/j.jct.2019.06.024.Suche in Google Scholar
14. Hwai, C. O., Yong, W. T., Brandon, H. H. G., Yong, Y. G. Recent advances in biodiesel production from agricultural products and microalgae using ionic liquids: opportunities and challenges. Energy Convers. Manag. 2021, 228, 113647.10.1016/j.enconman.2020.113647Suche in Google Scholar
15. Zahoor, U., Amir, S. K., Nawshad, M., Riaz, U., Ali, S. A. A review on ionic liquids as perspective catalysts in transesterification of different feedstock oil into biodiesel. J. Mol. Liq. 2018, 266, 673–686.10.1016/j.molliq.2018.06.024Suche in Google Scholar
16. Kong, J. H., Lan, Y. D., Chen, J., Huang, C. G., Xiong, W. M. Preparation and component analysis of biodiesel catalyzed by functionalized dication ionic liquid. Acta Agric. Univ. Jiangxiensis 2016, 38, 386–390.Suche in Google Scholar
17. Zhao, W., Chen, J., Xiong, W. M., Lan, Y. D., Nie, X. L. Crystal structure of 1,1'-(hexane-1,6-diyl)bis(3-ethyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C16H28F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 609–611.10.1515/ncrs-2018-0527Suche in Google Scholar
18. Huang, T., Chen, J. Z., Nie, X. L., Chen, J., Xiong, W. M. Crystal structure of 3,3'-(1,2-phenylene-bis(methylene))bis(1-vinyl-1H-imidazol-3-ium)bis(hexafluorophosphate)(V), C18H20F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 369–371.10.1515/ncrs-2020-0555Suche in Google Scholar
19. Zhou, Y. H., Huang, T., Nie, X. L., Chen, J., Xiong, W. M. Crystal structure of 3,3'-(1,2-phenylenebis(methylene))bis(1-methyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C16H20F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1217–1219.10.1515/ncrs-2020-0268Suche in Google Scholar
20. Zhao, W., Liu, X. T., Wu, S. Q., Xiong, W. M., Nie, X. L. Crystal structure of 3,3'-(1,2-phenylene-bis(methylene))bis(1-ethyl- 1H-imidazol-3-ium)bis(hexafluorophosphate), C18H24F12N4P2. Z. Kristallogr. N. Cryst. Struct. 2021, 236, 545–547; https://doi.org/10.1515/ncrs-2020-0639.Suche in Google Scholar
© 2021 Xin-Ting Liu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

