Abstract
C14H14Br2Sn, monoclinic, P21/c (no. 14), a = 12.9848(2) Å, b = 18.9911(2) Å, c = 6.0550(1) Å, β = 99.136(1)°, V = 1474.19(4) Å3, Z = 4, R gt (F) = 0.0193, wR ref (F2) = 0.0490, T = 100 K.
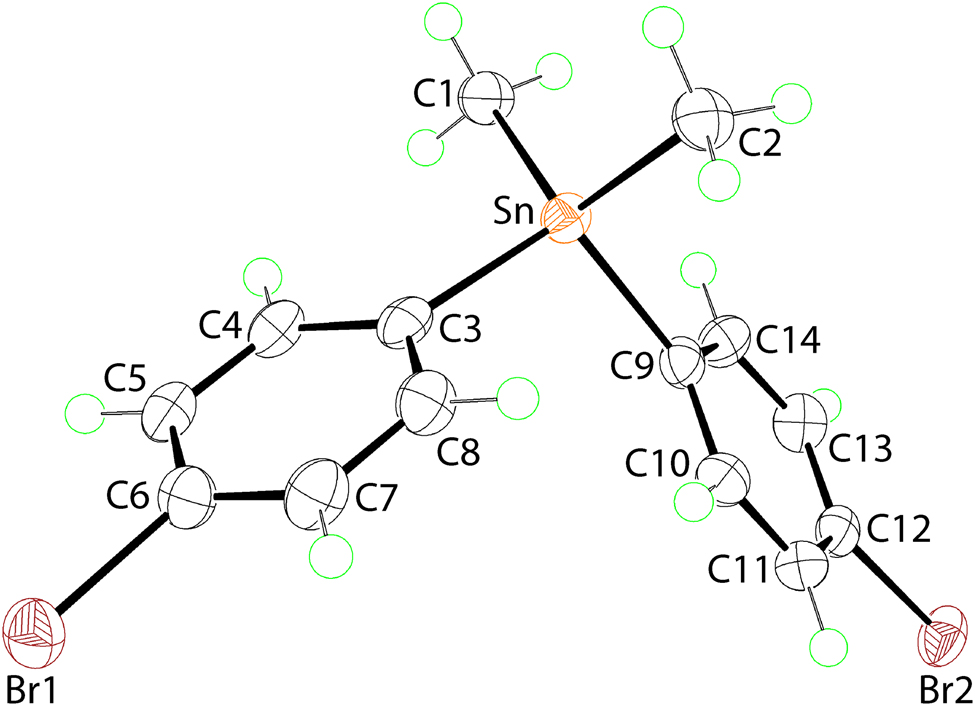
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless prism |
| Size: | 0.08 × 0.07 × 0.04 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 19.9 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy, ω |
| θmax, completeness: | 67.1°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 18,097, 2634, 0.036 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2553 |
| N(param)refined: | 156 |
| Programs: | CrysAlisPRO [1], Shelx [2, 3], WinGX/ORTEP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.2838 (2) | 0.68751 (14) | 1.1539 (4) | 0.0220 (5) |
| H1A | 0.266085 | 0.731926 | 1.074459 | 0.033* |
| H1B | 0.350087 | 0.692791 | 1.255230 | 0.033* |
| H1C | 0.228649 | 0.675204 | 1.240375 | 0.033* |
| C2 | 0.3176 (2) | 0.50108 (14) | 1.0459 (4) | 0.0224 (6) |
| H2A | 0.389460 | 0.494796 | 1.121796 | 0.034* |
| H2B | 0.302950 | 0.467407 | 0.922266 | 0.034* |
| H2C | 0.269316 | 0.492960 | 1.152390 | 0.034* |
| C3 | 0.1665 (2) | 0.60827 (13) | 0.6553 (4) | 0.0184 (5) |
| C4 | 0.1014 (2) | 0.66750 (14) | 0.6205 (4) | 0.0198 (5) |
| H4 | 0.111032 | 0.704779 | 0.726430 | 0.024* |
| C5 | 0.0234 (2) | 0.67328 (14) | 0.4358 (4) | 0.0202 (5) |
| H5 | −0.019947 | 0.713827 | 0.415086 | 0.024* |
| C6 | 0.0100 (2) | 0.61841 (15) | 0.2822 (5) | 0.0212 (5) |
| C7 | 0.0699 (2) | 0.55801 (15) | 0.3137 (5) | 0.0238 (6) |
| H7 | 0.058335 | 0.520313 | 0.209580 | 0.029* |
| C8 | 0.1474 (2) | 0.55325 (14) | 0.5004 (4) | 0.0218 (5) |
| H8 | 0.188339 | 0.511650 | 0.523196 | 0.026* |
| C9 | 0.4346 (2) | 0.62597 (13) | 0.7692 (4) | 0.0181 (5) |
| C10 | 0.4484 (2) | 0.59212 (15) | 0.5693 (4) | 0.0209 (5) |
| H10 | 0.394178 | 0.562922 | 0.495495 | 0.025* |
| C11 | 0.5388 (2) | 0.60013 (14) | 0.4767 (4) | 0.0212 (6) |
| H11 | 0.546345 | 0.577355 | 0.340460 | 0.025* |
| C12 | 0.6178 (2) | 0.64204 (14) | 0.5872 (4) | 0.0193 (5) |
| C13 | 0.6081 (2) | 0.67698 (14) | 0.7842 (5) | 0.0225 (6) |
| H13 | 0.662918 | 0.705789 | 0.857312 | 0.027* |
| C14 | 0.5159 (2) | 0.66877 (14) | 0.8719 (4) | 0.0208 (5) |
| H14 | 0.508060 | 0.692980 | 1.005534 | 0.025* |
| Br1 | −0.09056 (2) | 0.62811 (2) | 0.01873 (5) | 0.02498 (8) |
| Br2 | 0.74478 (2) | 0.64959 (2) | 0.46927 (5) | 0.02530 (9) |
| Sn | 0.29792 (2) | 0.60609 (2) | 0.91768 (3) | 0.01673 (7) |
Source of material
A solution of 1,4-dibromobenzene (Sigma, 23.6 g, 0.1 mol) in dry ether (50 mL) was added dropwise to magnesium turnings (Merck, 2.4 g, 0.1 mol) suspended in dry ether (150 mL) in a three necked flask equipped with a reflux condenser, a nitrogen supply source and a dropping funnel filled with the 1,4-dibromobenzene solution. After the addition was complete, the reaction mixture was refluxed with stirring for 2 h. This was followed by the addition of dimethyltin dichloride (Merck, 11 g, 0.05 mol). The reaction mixture was subsequently refluxed for 2 h. This was followed by hydrolysis with 20% aqueous ammonium chloride solution (200 mL). The organic layer was separated, washed with distilled water and dried over molecular sieves. The solution was then filtered and concentrated to obtain a white crystalline solid. The precipitate was recrystallised from chloroform and colourless crystals were obtained from the slow evaporation of the solvent. Yield: 8.05 g (34.9%). M.pt (Stuart SMP30 digital melting point apparatus; uncorrected): 524–526 K (dec). IR (Bruker Vertex 70v FTIR Spectrometer; cm−1): 2930 (s) ν(C–H), 1495 (m) ν(C=C). 1 H NMR (Bruker Ascend 400 MHz NMR spectrometer; CDCl3; ppm relative to Me4Si): δ 0.98 (s, 6H, CH3), 7.13–7.59 (m, 8H, Ph–H). 13 C{ 1 H} NMR (as for 1H NMR): 11.4 (CH3), 115.3, 123.7, 129.7, 137.5 (Ph–C).
Experimental details
The C-bound H atoms were geometrically placed (C–H = 0.95–0.98 Å) and refined as riding with Uiso(H) = 1.2–1.5Ueq(C).
Comment
The title compound, Me2Sn(C6H4Br-4)2, hereafter (I), is only the third crystal structure containing the Sn(C6H4Br-4) fragment, the others being Sn(C6H4Br-4)4 [5] and the anion in the salt [4-Me2NC5H4NH][Me2SnBr2(C6H4Br-4)] [6]. The structure determination of (I) was undertaken as part of recent and on-going investigations of related 4-halophenyltin species [7, 8].
The molecular structure of (I) is shown in the Figure (70% displacement ellipsoids). The molecule lacks symmetry with the tin atom within a C4 donor set defined by two methyl-C and two phenyl-C atoms. The range of angles subtended at the tin atom is a low 104.88(10)°, for C2–Sn–C9, to a wide 116.74(10)°, for C1–Sn–C2, consistent with a distorted tetrahedral geometry for the tin atom. The Sn–C bond lengths are indistinguishable, ranging from 2.134(3) Å, for Sn–C1, to 2.147(3) Å, for Sn–C9. The dihedral angle between the phenyl rings is 68.47(12)°.
In the crystal, molecules assemble largely devoid of directional interactions according to an analysis based on PLATON [9]. Thus, methyl-C–H⋯π(C6H4Br) contacts [C1–H1c⋯Cg(C3–C8) i : H1c⋯Cg(C3–C8) i = 2.74 Å with angle at H1c = 168° and C2–H2a⋯Cg(C9–C14) ii : H2a⋯Cg(C9–C14) ii = 2.87 Å with angle at H2a = 125° for symmetry operations (i): x, y, 1 + z and (ii): 1 − x, 1 − y, 2 − z] are apparent and occur between molecules arranged in a zig–zag chain aligned along the c-axis. The most prominent contacts between chains are type II [10] Br⋯Br halogen bonding interactions [Br1⋯Br2 iii = 3.6807(4) Å cf. the sum of the van der Waals radii = 3.70 Å [9]; C6–Br1⋯Br2 iii = 172.36(9)° and C12 iii –Br2 iii ⋯Br1 = 93.79(7)° for (iii) −1 + x, y, −1 + z]. Additional Br⋯Br contacts are noted, at separations just greater than the sum of the van der Waals radii [Br1⋯Br2 iv = 3.7459(4) Å for (iv): −1 + x, y, z].
Additional information on the molecular packing was obtained through an analysis of all surface contacts in the crystal of (I). This was accomplished by the calculation of the Hirshfeld surfaces and two-dimensional fingerprint plots (full and decomposed into individual contacts) using Crystal Explorer 17 [11] and literature protocols [12]. The Hirshfeld surface analysis indicated the dominance of hydrogen in the surface contacts, namely H⋯H [39.9%], Br⋯H/H⋯Br [26.8%] and C⋯H/H⋯C [26.2%]. The next most significant contacts are due to Br⋯Br [3.5%] and Br⋯C/C⋯Br [3.1%] contacts with a minor contribution from C⋯C [0.4%] contacts.
Funding source: Sunway University doi.org/10.13039/501100010798
Award Identifier / Grant number: GRTIN-IRG-08-2021
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: Crystallographic research at Sunway University is supported by Sunway University Sdn Bhd (Grant No. GRTIN-IRG-08-2021).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffraction. CrysAlisPRO; Rigaku Corporation: Oxford, UK, 2018.Suche in Google Scholar
2. Sheldrick, G. M. A short history of SHELXL. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Suche in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar PubMed PubMed Central
4. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Suche in Google Scholar
5. Beele, B., Guenther, J., Perera, M., Stach, M., Oeser, T., Blümel, J. New linker systems for superior immobilized catalysts. New J. Chem. 2010, 34, 2729–2731; https://doi.org/10.1039/c0nj00482k.Suche in Google Scholar
6. Yau, C. K., Lo, K. M., Ng, S. W. 4-(Dimethylamino)pyridinium dibromido(4-bromophenyl)dimethylstannate(IV). Acta Crystallogr. 2008, E64, m1391; https://doi.org/10.1107/s1600536808032248.Suche in Google Scholar
7. Lo, K. M., Lee, S. M., Tiekink, E. R. T. Crystal structure of trans-dichloridobis(4-chlorophenyl-κC1)-(1,10-phenanthroline-κ2N,N′)tin(IV) dimethylsulphoxide solvate, C26H22Cl4N2OSSn. Z. Kristallogr. N. Cryst. Struct. 2020, 235, 1327–1329; https://doi.org/10.1515/ncrs-2020-0302.Suche in Google Scholar
8. Lo, K. M., Lee, S. M., Tiekink, E. R. T. Crystal structure of dichloridobis(4-chlorophenyl-κC1)tin(IV), C12H8Cl4Sn. Z. Kristallogr. N. Cryst. Struct. 2021, 236, https://doi.org/10.1515/ncrs-2021-0271.Suche in Google Scholar
9. Spek, A. L. checkCIF validation ALERTS: what they mean and how to respond. Acta Crystallogr. 2020, E76, 1–11; https://doi.org/10.1107/s2056989019016244.Suche in Google Scholar
10. Desiraju, G. R., Parthasarathy, R. The nature of halogen⋯halogen interactions – are short halogen contacts due to specific attractive forces or due to close packing of nonspherical atoms. J. Am. Chem. Soc. 1989, 111, 8725–8726; https://doi.org/10.1021/ja00205a027.Suche in Google Scholar
11. Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D., Spackman, M. A. Crystal Explorer v17; The University of Western Australia: Australia, 2017.Suche in Google Scholar
12. Tan, S. L., Jotani, M. M., Tiekink, E. R. T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. 2019, E75, 308–318; https://doi.org/10.1107/s2056989019001129.Suche in Google Scholar
© 2021 See Mun Lee et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

