Abstract
The high sulfide ion polarization is known to cause increased ionic conductivity in the solid sulfide-type electrolytes. Three groups of sulfide-based solid-state electrolytes, namely, Li-P-S, Li6PS5X (X: Cl, Br, and I), and Li x MP x S x (M: Sn, Si, and Al) were reviewed systematically from several aspects, such as conductivity, stability, and crystal structure. The advantages and disadvantages of each electrolyte were briefly considered and compared. The method of the preparation was presented with experimental and theoretical studies. The analysis that has been carried out showed that the solid electrolyte Li10GeP2S12 is superior to others with an ionic conductivity of 12 × 10−2 S cm−1. This conductivity is comparable to that of conventional liquid electrolytes. However, the availability and high price of Ge are the problems encountered. Furthermore, because sulfide-based solid electrolytes have low chemical stability in ambient humidity, their handling is restricted to inert gas environments. When solid sulfide electrolytes are hydrolyzed, structural changes occur and H2S gas is produced. The review’s objective includes presenting a complete knowledge of sulfide-solid electrolyte synthesis method, characteristics, such as conductivity, structure, and stability, as well as generating more efficient and targeted research in enhancing the performance of the chemical substance.
1 Introduction
The electrochemical energy storage device, such as rechargeable batteries with high power density and high energy are indispensable in their application to electric vehicles and portable electronic equipment [1]. Batteries are being extensively examined, in order to have sufficient capacity to be applied to electric vehicles. A lithium-ion (Li-ion) battery is one among the most popular commercial types as a source of electrochemical energy [2]. Li-ion batteries are superior to conventional types such as lead-acid and NiMH batteries, due to their high energy density and voltage. Li-ion battery was first coined in the 1960s and was invented in 1991 by the Sony company as an energy store in cell phones, notebook computers, and more recently for electric vehicles [3]. Liquid electrolyte is one of the most important components used in the construction of commercial lithium-ion batteries, such as lithium salt hexafluorophosphate (LiPF6) [4]. The liquid electrolytes in Li-ion batteries are less safe when used, especially at extreme temperatures, which can trigger an explosion. Complex chemical reactions occur triggered by the presence of high temperature and voltage in Li-ion batteries [5].
High energy density and power requirements can trigger complex reactions in Li-ion batteries that are harmful to users [6]. Lithium all-solid battery (ASSLB) is a solution to the Li-ion battery problem because ASSLB has higher stability and safety than Li-ion batteries [7]. The topic of ASSLB was less attractive in its development 30 years ago because researchers considered its ionic conductivity to be relatively lower than that of solid electrolytes [8,9]. However, in the last 10 years, tremendous progress has been achieved in enhancing the ionic conductivity of solid electrolytes. The development of sulfide-based solid electrolytes with an ionic conductivity value of 2.5 × 10−2 S cm−1, which is superior to that of liquid electrolytes, has been described [10,11].
ASSLB is depicted in Figure 1 below. A solid-state battery is composed of an anode and a cathode as negative and positive poles, respectively, as well as a solid electrolyte. This is different from Li-ion batteries which use a liquid electrolyte [12,13]. Solid-state electrolytes (SSEs) are used to replace liquid types. Li-ion media is used as a separator in traditional batteries, for deflection between the anode and cathode poles, and to prevent short circuits and electron conduction [14]. Mechanical contact is made by applying pressure to a solid-state battery array consisting of a lithium metal anode, a solid electrolyte, and a composite cathode. The SSE must have a large electrochemical stability window and high ionic conductivity [15].
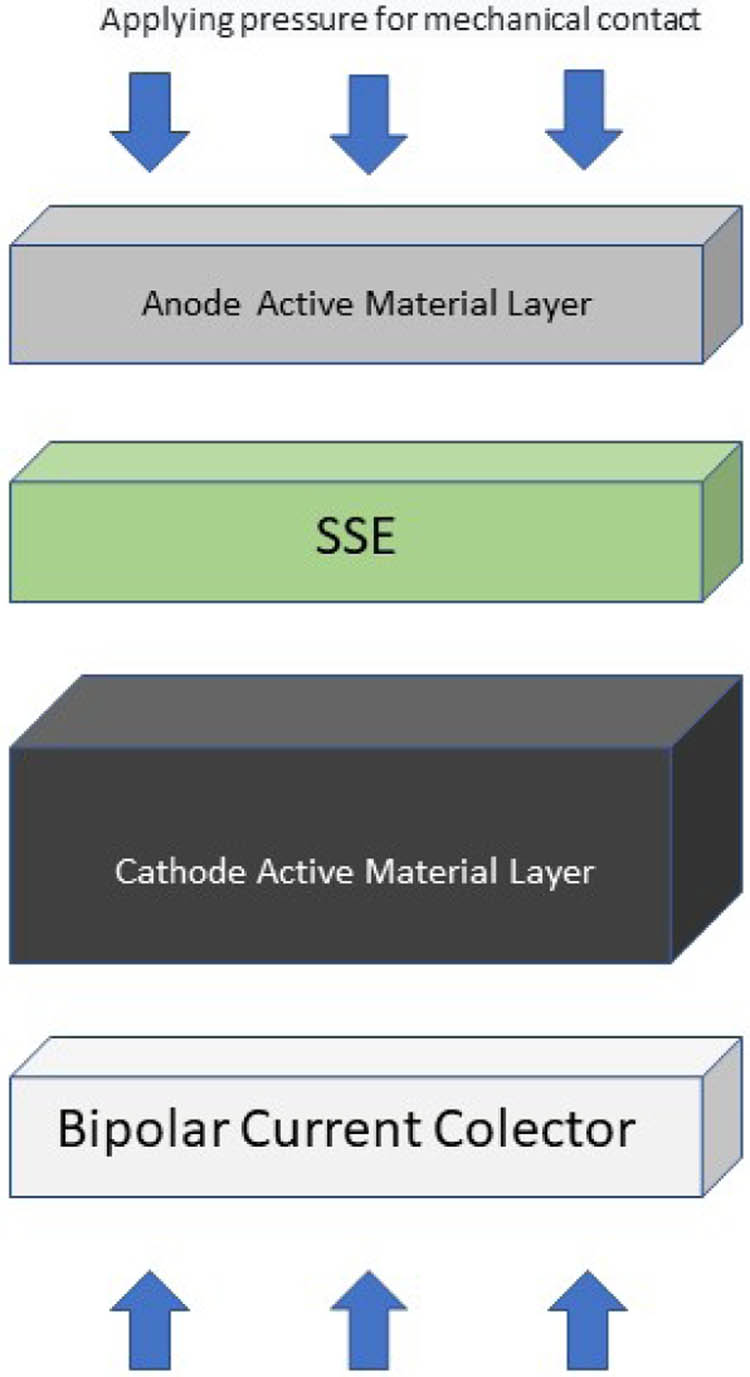
Arrangement of the components of a solid-state battery in which the cathode consists of a material containing an electrolyte. Composite cathodes are used to create ion pathways that are useful for increasing the voltage.
SSEs are divided into three categories, they are oxides, phosphates, and sulfides. The superior SSE that are commonly used and cover these three categories include phosphate-type Lithium Aluminum Titanium Phosphate (LATP), Lithium Lanthanum Zirconium Oxide (LLZO) oxide type, and Lithium Germanium Phosphorus Sulfide (LGPS) sulfide type. LATP has the advantage that the price of raw materials and production costs are low with the value of ionic conductivity being 0.7 mS cm−1 [16,17]. Although the LATP ionic conductivity value is quite high, it is not suitable for low potential anode materials, such as lithium. This is due to a reduction in Ti4+ ions at a voltage of 2.5 V against Li/Li+, allowing for a short circuit in the battery [18]. LLZO (oxide type) is another category with an ionic conductivity value of 0.774 mS cm−1. LLZO ionic conductivity value can still be increased with Ga-doping which causes the ionic conductivity value to be 2.06 mS cm−1 [19,20]. However, several challenges must be resolved before LLZO can be used in practical applications. At room temperature, LLZO reacts with ambient H2O and CO2, lowering the ionic conductivity. Therefore, an inert atmosphere or adding additives is required in the production process. In addition, LLZO has high rigidity, as a result of its high interfacial resistance, unstable solid electrolyte, and electrode interface contact. These variables have a major influence on the cycle stability and chargeability of the battery [20]. The last category is a sulfide-based SSEs. LGPS is a sulfide electrolyte that is one of the most widely utilized SSEs. The ionic conductivity of LGPS is very high, reaching 10−2 S cm−1 at a temperature of 50–80°C [10]. Sulfide-type electrolyte is one of the materials that is considered ideal for use in solid-state batteries. Several other types of sulfide-based SSEs are depicted in the schematic diagram below.
Sulfide-type SSEs that have been successfully synthesized include Li-P-S, Li6PS5X (X: Cl, Br, and I), and Li x MP x S x (M: Ge, Sn, Si, and Al) bases. A schematic diagram of a sulfide type SSE investigation is shown in Figure 2. The investigation began in 2005 when LPS batteries were first successfully synthesized. Furthermore, research is continued on new materials that have higher ionic conductivity. In 2008, research on Li6PS5X (X: Cl, Br, and I) was just started, and in 2011 research on Li x MP x S x (M: Ge, Sn, Si, and Al) was started and is still ongoing. Various kinds of research were carried out because solid-state batteries still have weaknesses and challenges such as poor chemical compatibility with electrodes, narrow electrochemical stability windows, and poor mechanical properties must also be considered. The discussion in this article will focus on sulfide-based solid electrolytes. We begin with the different categories of sulfide-based solid electrolytes, their electrochemical properties, the synthesis methods used, and the material structure of each type of sulfide-based solid electrolyte are discussed. The discussion in the article will end with a conclusion of perspectives and suggestions for the future improvement in sulfide-based ASSLB.

Schematic diagram of sulfide-based solid electrolytes.
2 Sulfide-type electrolyte
Various research on solid-state batteries have been presented and one of the materials that is considered as superior to others is the sulfide. This material is known to possess excellent electrochemical properties, in the form of high conductivity and wide potential window [10,21]. Sulfide showed a high-performance solid-state lithium metal battery [22], in comparison to the conventional liquid and oxide solid electrolytes. The sulfide solid electrolytes have better mechanical characteristics [23]. Several types of sulfide solid electrolyte are LGPS and similar compounds, argyrodite (Li6PS5X), Li-P-S (LPS) sulfides, and their derivatives, and thio-LISICONs [24]. This section explains three groups of sulfide-based SSEs: LPS, Li6PS5X (X: Cl, Br, and I), and Li x MP x S x (M: Ge, Sn, Si, and Al).
The explanation encompass synthesis, characterization, electrochemical performance, conductivity, and the method used to determine the best sulfide-based SSE as a lithium battery application. The synthesis of sulfide solid electrolyte is generally carried out by three methods: melt quenching, ball milling, and wet-chemical [25]. Melt quenching is done by heating and suddenly lowering the temperature. Ball milling is the most common method of mechanical high-energy milling involving complex processes, including mixing and solid-state reactions. A wet chemical is a synthesis through a reaction using a solution. The structure and properties of the material are determined through characterization. Furthermore, research on the electrochemical properties of sulfide solid electrolytes, like conductivity, stability, and performance, is required to decide which sulfide based solid electrolyte is most likely to be developed.
2.1 LPS
The lithium thiophosphate or LPS class consists of several high-conducting materials. Several sulfide crystalline phases have been found, of which the type of crystal formed depends on the heat treatment applied and the composition of the glass formed. The sulfide crystalline phases include: Li3PS4, Li7P3S11, and Li4P2S6 [24,26]. The composition of the glass in the LPS formed affects the ionic conductivity which relatively decreased due to the formation of individual crystals.
2.1.1 Synthesis methods
Synthetic methods that are often used in the manufacture of solid sulfide electrolytes are divided into melt quenching method, mechanical ball milling method, and wet-chemical method as illustrated in Figure 3. One of the most common methods for creating glass sulfides is the melt quenching process. The raw material mixture is sealed inside a carbon-coated quartz tube, which is subsequently heated in a furnace to a high temperature. The liquid sample was then quickly chilled using ice water. The materials experience a complex process in the mechanical ball-milling method, that includes blending, crushing, amorphization, and solid-state processes in high-energy grinding. This method offers a number of advantages, including the fact that it can be done at room temperature. Wet chemical approaches using liquid solvents as the medium are increasingly being investigated in the synthesis of solid sulfide electrolytes due to their low price, simple process, savings in time, and stability.
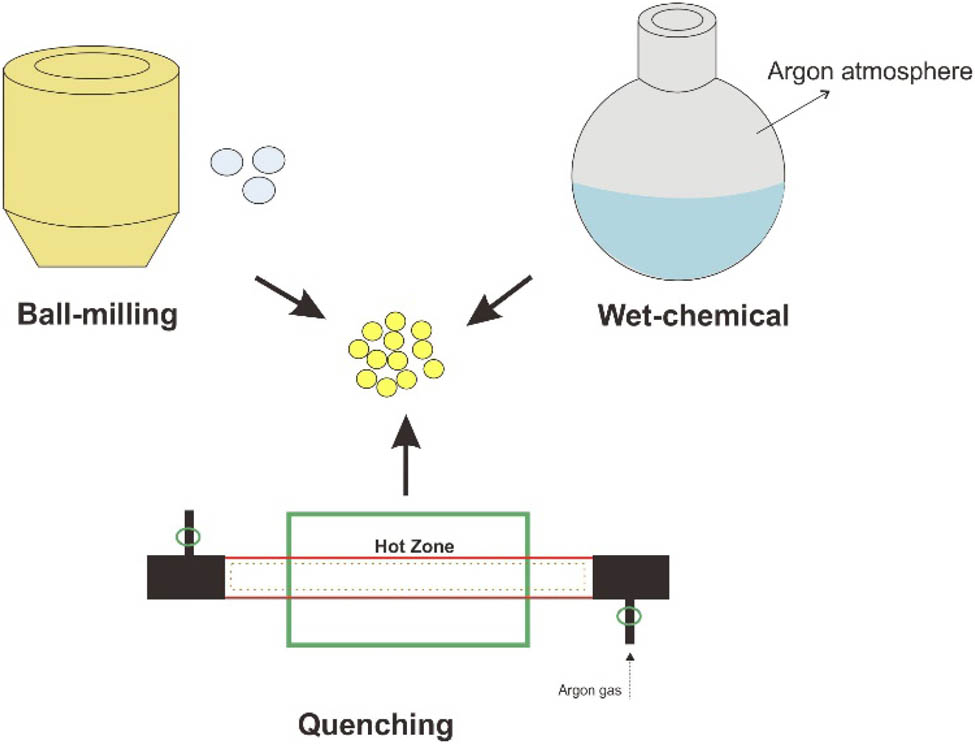
Synthesis methods of sulfide solid electrolyte.
In the LPS class, Li3PS4 is the most stable chemical. A wet chemical technique was used to make Li3PS4. The starting materials were Li2S and P2S5 mixed in appropriate molar ratios in a glove box filled with argon (Ar), put in a quartz tube and warmed at a fixed temperature of 700°C for 8 h. After the reaction at constant temperature, the tube was cooled slowly to room temperature [27]. The synthesis using the wet chemical method was also carried out by Liu et al. on Li3PS4 nanopores resulting in an ionic conductivity of up to 3 times that of Li3PS4 crystals, which is 1.6 × 10−4 S cm−1 [28]. In 2016, Puck et al. combined materials such as Li2S and P2S5 in a Li2S:P2S5 = 3:1 molar ratio with dimethyl carbonate and shook them with a zirconia ball for 5 h in a dry Ar atmosphere. The residue drying was carried out under low pressure at 90, 130, 150, and 190°C [29].
Another type of solid electrolyte in the form of LPS is Li7P3S11. This solid electrolyte is a glass ceramic with a stable phase Li7P3S11 which is newly formed at temperatures above 630°K [30]. The glass ceramics of Li7P3S11 is obtained from mixing materials in the form of 70 mol% Li2S and 30 mol% P2S5. For 40 h, mechanical milling was done in a planetary ball mill at 500 rpm. The whole process was performed in a glove box with H2O below 1 ppm because the resulting material is hygroscopic. The material was then warmed at 300°C for 2 h before being permitted to cool down to room temperature. After the heating process, the material is placed into a 10 mm tungsten-carbide die and then pressed with a pressure of 10 MPa [31]. Minami et al., in 2007, explored the local structure and conductivity of lithium glass Li7P3S11 crystallized by quenching melts at various temperatures. According to their findings,
The invention relating to LPS-type electrolytes with high conductivity, such as nanopores βLi3PS4, encourage research on Li4P2S6 which is still an LPS class. The solid electrolyte Li4P2S6 is known to be a product of synthesis and decomposition, obtained at high temperatures. [33, 34]. Solid electrolyte Li4P2S6 is produced by mixing the basic materials in the form of Li2S reagent level and P2S5 pounded using a mortar and pestle for 20 min [32, 35]. The obtained material is inserted into a quartz tube and synthesized at a high temperature. The synthesis temperature is found to be between 750 and 900°C. The powder is heated for 20 h at a temperature of 900°C and maintained for 24 h at 450°C. Anhydrous acetonitrile is used to remove the sulfur formed. After all these processes, the resulting powder was dried in a vacuum oven at 150°C for 2 h. The synthesis that occurred between 750 and 900°C produced the same characteristics [36]. Impedance and Arrhenius measurements were carried out by applying a pressure of 300 MPa to the material to be tested, in order for the sample to form pellets with a density of 2.23 g cm−3 [37,38].
2.1.2 Material characterization
The binary (100-x) Li2S-xP2S5 system, as a prominent member of solid sulfide-electrolytes, is a particularly attractive electrolyte choice for solid-state batteries due to its low price, high Li-ion conductivity, and large electrochemical window compared to Li/Li+. Between various compositions, Li3PS4, Li7P3S11, and Li4P2S6 have been studied extensively because Li3PS4 shows good compatibility with lithium metal, Li7P3S11 shows high electrical conductivity of greater than 10−3 S cm−1 at room temperature, and Li4P2S6 is really quite stable in preserving its structure crystals up to temperatures of 280°C in air and up to 950°C in vacuum.
Li3PS4 has a stoichiometry of 75% Li2S-25% P2S5. The Li3PS4 was reported to have a γ-Li3PO4-like structure with hexagonal closed packed sulfide ion ensembles in which the phosphorus ions are spread over the tetrahedral sites and the PS4 tetrahedra are separated from each other [40]. In 2010, Homma et al. modified the structure of Li3PS4 in γ, the low temperature phase, β, the middle temperature phase, and α, the high temperature phase. Figure 4 shows the arrangement of PS4 tetrahedral in the γ, β, and α phases in Li3PS4.The differences in the structure of γ and β phases are distinguished by the location of the PS4 tetrahedral. The arrangement of the PS4 tetrahedral affects the position of the Li ion. The γ phase shows an orderly arrangement with the top of the tetrahedral facing upwards. All of this indicates that the Li-ion is only in the tetrahedral site and the peak of the Li tetrahedral ion shows the same peak. In the β phase, the top of the tetrahedron has a zig–zag arrangement. This zig–zag arrangement causes the Li-ions to be positioned both on the octahedral side and on the tetrahedral side, which makes the Li-ions more mobile. In phase α, the distribution of Li-ions is not clearly found [27].
![Figure 4
Arrangements of PS4 tetrahedra in the γ, β, and α phase in Li3PS4. Reprinted with permission from [39]. Copyright 2011, Elsevier.](/document/doi/10.1515/eng-2022-0043/asset/graphic/j_eng-2022-0043_fig_004.jpg)
Arrangements of PS4 tetrahedra in the γ, β, and α phase in Li3PS4. Reprinted with permission from [39]. Copyright 2011, Elsevier.
Crystalline Li7P3S11 is formed for the 70% Li2S-30% P2S5 composition. The structure of Li7P3S11 depicted in triclinic space family P-1 with a relatively large cell (V/Z = 414.7; it is 3/unit formula) made up of anions
![Figure 5
The crystal structures of Li2S-P2S5 binary system. Reprinted with permission from [41]. Copyright 2018, Elsevier.](/document/doi/10.1515/eng-2022-0043/asset/graphic/j_eng-2022-0043_fig_005.jpg)
The crystal structures of Li2S-P2S5 binary system. Reprinted with permission from [41]. Copyright 2018, Elsevier.
The reaction that creates Li4P2S6 crystals is peculiar in that the composition is not perfectly positioned on the Li2S-P2S5 bond line. This can be calculated using the 67 mol% Li2S composition, which is applied to the Li4P2S7 stoichiometry.
2.1.3 Electrochemical properties
Li3PS4 is by far the most stable chemical in the LPS class, with an activation energy of 60–73 kJ mol−1 and a low ionic conductivity of 3 × 10−7 S cm−1 at room temperature. The tetrahedral arrangement of PS4 affects the position of the Li-ions. The γ phase displays an organized pattern with the top of the tetrahedral side up. All this indicates that the Li-ion is only in the tetrahedral site and that the peaks of the Li tetrahedral ion show the same peak. The γ phase shows an activation energy of 21.3 kJ mol−1 at room temperature with an ionic conductivity of 3.0 × 10−7 S cm−1. While in phase β, they are arranged in a zig zag fashion, which causes the Li-ions to be positioned both on the octahedral side and on the tetrahedral side, which makes the Li-ions more mobile. The phase shows an activation energy of 15.5 kJ mol−1 with a better ionic conductivity of 3.0 × 10−2 S cm−1 at 500 K [39].
The Li3PS4 nanostructure possesses a large electrochemical window and is chemically stable to lithium metal (5 V). The ionic conductivity of manipulated solid electrolytes has far-reaching ramifications for the synthesis of materials in battery applications [28]. The lithium metal anode also functions as a coating to prevent the formation of dendrites, improves electrochemical performance, and reduces parasitic side reactions [43]. The glass-ceramic material Li3PS4 (with polymorph, 7.5 × 10−4 S cm−1) [44] had a substantially greater ionic conductivity at ambient temperature (room temperature, RT) than the bulk crystal material (polymorph, 9 × 10−7 S cm−1) [45]. Although the reason for the higher conductivity in glass ceramics is unknown, the nanocrystalline βLi3PS4 produced using wet chemical method has a respectable ionic conductivity (1.6 × 10−4 S cm−1 at ambient temperature).
In terms of ionic conduction qualities, the material’s structure is crucial to the conductivity process. Due to the 3D conduction pathway generated by the crystal structure [30], the ionic conductivity of Li7P3S11 glass increases from 8 × 10−5 to 1.4 × 10−3 S cm−1 in the stoichiometric situation of 70% mol percent Li2S – 30% mol percent P2S5. The cold-pressed Li7P3S11 has an ionic conductivity of 1.3 × 10−3 mS cm−1 [46]. The deposition of superionic crystals reflected by Li7P3S11 [47] results in high ionic conductivity. As with other types of solid sulfide electrolytes, glass ceramics Li7P3S11 is found to be very sensitive to air and also contributes to gas formation (such as H2S, a poisonous gas) [48]. The electrochemical stability of Li7P3S11 with Li metal as the anode, carrying 5 V with the activation energy (E a), recovered from the theoretical estimated fitting is 187 meV [49].
Li4P2S6 is a relatively stable thiophosphate substance, maintaining its crystal structure at temperatures as high as 280°C in air and 950°C in vacuum. Despite having a low ionic conductivity of 2.38 × 10−7 S cm−1 at 25°C and 2.33 × 10−6 S cm−1 at 100°C, the crystalline conductivity of Li4P2S6 can be dramatically improved (from 2.9 × 10−11 to 10−6 S cm−1) by the addition of amorphous components at RT [42]. Similarly, despite the fact that the process of conduction is unknown, it is clear that high conductivity may be attained using a variety of designs [41]. For all materials in the LPS family, however, the high conductivity is not always generated from the crystalline phase.
2.2 Li6PS5X (X: Cl, Br, and I)
Lithium argyrodites Li6PS5X (X: Cl, Br, and I) are one type of sulfide solid electrolyte that has a rather high ionic conductivity at 298 K, with values ranging from 10−2 to 10−3 S cm−1 for Br and Cl [50,51]. The addition of anions to the solid electrolyte has been shown to increase the conductivity. The size and polarizability of the anions coordinated to the mobile cations are the most important factors that influence conductivity [40]. This section explains several types of Li-argyrodites sulfide-based solid electrolytes, such as Li6PS5Cl, Li6PS5Br, and Li6PS5I.
2.2.1 Synthesis methods
In 2008, Li6PS5X (X: Cl, Br, and I) crystals were successfully produced through a stoichiometric reaction involving Li2S, P2S5, and LiX in an inert gas. The synthesized material was pressed and heated for 7 days at a temperature of 823 K [52]. Subsequent studies have shown that the preparation time of Li6PS5X can be reduced, resulting in a solid electrolyte with an argyrodite phase with an appropriate crystallinity value. In 2011, Li6PS5X synthesis was successfully completed through a mechanical milling method for 24 h with annealing for 5 h at a temperature of 823 K [53]. Then, Li6PS5X is synthesized rapidly using high energy mechanical milling and annealed at 550°C for 5 h which produces an ionic conductivity of 0.74 mS cm−1 at 298 K for X = Cl and Br [54]. The annealing temperature of SSE materials greatly affects their performance and ionic conductivity [55]. It needed higher temperature treatment to reach large ionic conductivity value [56].
Milling time was shown to affect the Li-ion conductivity. The longer the grinding time, the smaller the resistance, resulting in an increase in Li-ion conductivity [55]. Therefore, the optimum milling time needs to be investigated. Due to the reactivity of the sample to moisture and oxygen in the air, all phases of solid electrolyte preparation were carried out in an Ar atmosphere.
In addition to using the solid-state method, the synthesis of Li6PS5X can be carried out using a wet chemical method with an anhydrous tetrahydrofuran (THF) solvent. Li2S and P2S5 were mixed in a mortar and then dissolved into THF, then mixed for 24 h at room temperature. Li2S and LiX dissolved in ethanol were added to the mixture. After mixing for 24 h, the solution was centrifuged at 8,000 rpm for 10 min. The result, in the form of a clear solution, is dried and pressed, then sintered for 6 h at 550oC. All synthesis steps were completed in an Ar filled glove box [57,58].
2.2.2 Material characterization
Li6PS5X (X: Cl, Br, and I), or argyrodite, has a high conductivity, and it is easily fabricated [59]. Li6PS5X is a halide-substituted from Li7PS6 derivative [60]. The Li7PS6 type and its variants, such as Li6PS5X, have a non-bcc type anion framework with a network of tetrahedral sites for mobile cations [11]. In the structure of Li6PS5X crystal, the unit cell in a completely ordered arrangement is a face centered cubic lattice of halide ions (Wyckoff 4a).
![Figure 6
Li6PS5X crystal structure (X: Cl, Br, and I). The X anions form a cubic close packed lattice with PS4 tetrahedra in the octahedral sites and free S2− in half of the tetrahedral sites. Reprinted with permission from [62]. Copyright 2018, American Chemical Society.](/document/doi/10.1515/eng-2022-0043/asset/graphic/j_eng-2022-0043_fig_006.jpg)
Li6PS5X crystal structure (X: Cl, Br, and I). The X anions form a cubic close packed lattice with PS4 tetrahedra in the octahedral sites and free S2− in half of the tetrahedral sites. Reprinted with permission from [62]. Copyright 2018, American Chemical Society.
2.2.3 Electrochemical properties
Deiseroth et al. found that the mobility of Li ions in Li6PS5X reaches 10−2 – 10−3 S cm−1, which approximates the mobility of Li-ions in the liquid electrolyte LiPF6 in carbonates [52]. Li6PS5X synthesis via ball milling method produced an ionic conductivity value of 10−3 S cm−1 at 298 K [53]. Because of their excellent ionic conductivity at RT, these compounds are considered one of the best solid electrolytes for high-energy all-solid-state battery applications.
The maximum ionic conductivity of Li6PS5Cl obtained by ball milling process and annealing at 250°C is 1.1 mS cm−1. In 2016, Zhou et al. did the synthesis of Li6PS5Cl with a solution-based preparative method, using THF/ethanol mixtures which resulted in a fairly large ionic conductivity value reaching 3.9 mS/cm of the formula Li6–y PS5–y Cl1+y with y = 0–0.5 [57]. Besides that, preparation of the composite using liquid-involved synthesis methods is more promising [64]. All solid-state batteries with the application of the Li6PS5Cl electrolyte using a composite cathode containing 1% by weight of ethyl cellulose has a capacity of 111.7 mA h g−1 and is quite stable after 100 cycles. This shows that Li6PS5Cl is capable enough to be used as a solid electrolyte [65].
Li6PS5Br has the highest ionic mobility of Li+ ions among others [52] with Li jump rate of 109 s−1 at 298 K [51]. Synthesis of Li6PS5Br was successfully carried out by ball milling and annealing for 5 h at 300oC, and milling further for 4 h at 450 rpm. The ionic conductivity of Li6PS5Br reached 1.38 mS cm−1 [55]. Li6PS5Br synthesized via liquid-phase method using THF and EtOH has ionic conductivity of 3.1 mS cm−1 at 298 K. [66]. The ionic conductivity was enhanced by various doping methods, such as substitution [25]. Substitution to Li6PS5Br formula affects the structure and ionic mobility properties. The substitution of Li6PS5Br with formula Li6.35P0.65 Si0,35S5Br result in ionic conductivity value of 2.4 mS cm−1 [67]. Furthermore, substitution of S using Se in Li6PS5Br with formula Li6PS5–x Se x Br results in higher ionic conductivity value of 3.9 mS cm−1 [68]. Batteries with solid electrolyte Li6PS5Br as a mixture in the composite cathode are proven to have good performance and stability [69,70]. Li6PS5Br has been successfully applied to battery and managed to achieve a high and fairly stable capacity [71,72]. Therefore, the Li6PS5Br is capable enough to be used as SSEs [69,70].
Ionic conductivity and interfacial kinetics were also improved by the addition of I [73,74]. The I-based conductors synthesized through the solvent-based method have a total ionic conductivity value of 0.12 mS cm−1 at 298 K and a bulk ionic conductivity of 1.3 mS cm−1 [75] at 500 K. The ionic conductivity was enhanced by various doping methods, such as substitution. The substitution of Ge into Li6PS5I with the formula Li6.6P0.4Ge0.6S5I was conducted and resulting ionic conductivity value was up to 18.4 × 10−3 S cm−1 with further sintering. [76].
Anion doping could potentially help to improve the stability of SSEs. The high electrochemical windows are found in sulfide electrolytes containing halogen components [77]. Li6PS5X also has a large chemical stability window for Li-ions which reaches 7 V [78]. Cyclic voltammetry under the observed conditions of the Li7P2S8I electrolyte revealed electrochemical stability of up to 10 V vs Li/Li+ [73]. This is high among other types of sulfide electrolytes [79, 80]. Therefore, with its high electrochemical stability and excellent value of ionic conductivity, Li6PS5X is acceptable to be used as a solid electrolyte in the Li-ion batteries [81].
2.3 Li x MP x S x (M: Ge, Sn, Si, and Al)
Li x MP x S x is a derivative of the ceramic thio-LISICON group, a group of solid electrolytes with good characteristics. Li x MP x S x is a derivative form of L-P-S system. Li10GeP2S12 (LGPS) is the first Li x MP x S x group developed, which is the result of the development of the LiS-PS form of the system, which was detected to have Li3PS4 and Li4GeS4 structures [82]. Ceramic-sulfide solids, such as Li10GeP2S12 (LGPS), have gotten a lot of interest because of their high ionic conductivity of 1–25 mS cm−1 [83]. The presence of Ge in Li10GeP2S12 have disadvantages in terms of availability and cost of materials. However, there are several alternatives that change the Ge component in Li10GeP2S12. LGPS has a chemical formula (Li x MP x S x ), where M represents Ge which is replaceable with M: Sn, Si, and Al. Li10GeP2S12 (LGPS) has been examined further and shows very high ionic conductivity values. However, the price of Ge is quite high, and its limited availability means that other alternatives are needed in order to be manufactured at lower prices [49]. Some alternatives that are used include Sn, Si, or Al because they maintain a similar polygon structure [31]. This section explains several types of sulfide-based solid electrolytes, such as Li10GeP2S12, Li10SnP2S12, Li10SiP2S12, as well as Li11AlP2S12.
2.3.1 Synthesis methods
The synthesis of Li10GeP2S12 was carried out by mixing starting materials, such as germanium sulfide (GeS2), lithium sulfide (Li2S), and phosphorus sulfide (P2S5) in a 5:1:1 molar ratio with agate mortar. The heat was given during the mixing process [84]. The sample is formed into pellets by applying pressure for the thickness to become 1 mm. The crystallization phase of Li10GeP2S12 is carried out by synthesis at above 400°C [85]. The material sintering process is carried out for 8 h at 700°C. The sintered material tends to be in the form of lumps of samples that need to be pulverized into powder for further processing [86]. The Li10GeP2S12 was synthesized using a solid-state method and carried out in glove boxes at an Ar atmosphere, because the material is very sensitive to moisture and to avoid the formation of H2S [84].
A series of investigations of glassy solid electrolytes, such as Li11AlP2S12 have been carried out previously with Al3+ replacing Ge4+ in Li10GeP2S12 and presenting high Li. Al3+ substitution was identified to remove non-bridging sulfur which limits the conduction of Li-ions [63]. The synthesis of Li11AlP2S12 was carried out by mixing Al2S3, P2S5, and Li2S. The molar ratio of each ingredient is 11:2:1. The mixing is carried out mechanically by ball milling for 10 h with a speed setting of 350 rpm. As a result of sintering mixing, the material is inserted into a glass tube. The sintering was carried out at various temperatures of 400, 500, and 600°C for 18 h. The synthetic variations were denoted by LAlPS400, LAlPS500, and LAlPS600 [87].
Synthesis using Si and Al is a problem because it is difficult to achieve the desired polygon structure. From the three alternatives for replacing Ge, the use of Sn is preferable. Li10SnP2S12 (LSnPS) was synthesized by mixing P2S5, Li2S, and nanocrystal SnS2 which had been synthesized before in a molar ratio of 5:1:1. This mixture was ball milled at 600 rpm for 30 min. Then, sintered with a vacuum quartz tube for 2 h at 500, 550, 600, and 650oC, and then cooled. All of the processes for preparing LSnPS should be performed in dry Ar gas atmosphere glove box which contains O2 and H2O under 1 ppm [33].
Li10SiP2S12 (LSiPS) was synthesized by mixing up P2S5, Li2S, and SiS2 with a molar ratio of 5:1:1, using ball milling for 20 h at 500 rpm in a 500 mL stainless steel tube. The powder was pressed to form pellets on a 375 MPa Ti-die with a diameter of 1.3 cm to a thickness of 2 mm. The pellets were heated for 8 h at 550oC and then crushed with a mortar and pestle.
Solid-state sulfide-based batteries are assembled to facilitate an Ar atmosphere. Besides, the challenge in using sulfide materials as SSE is the formation of H2S which should be prevented by the use of metal sulfide and metal oxide additives [88].
2.3.2 Material characterization
Analysis of the structure of the Li10GeP2S12 material has been reported previously. The structure is shown in the Figure 7(a) and (b). The structure of Li10GeP2S12 is a 3D framework composed of LiS4 tetrahedral, (Ge0,5P0,5)S4 tetrahedral, LiS6 octahedral, and PS4 tetrahedral [10], [89]. The structure is made up of an immobile framework of PS4 and (Ge/P)S4 tetrahedral, as well as a possibly immobile octahedral LiS6 complex. The average bond length for Li-S bond is 2.65, which is a respectable length. As a result, the LiS6 octahedron (also known as the Li2 site) has been included in the framework. The (Ge/P)S4 tetrahedral shared edges with the LiS6 octahedral, creating chains in the <001> direction that are linked by PS4 tetrahedral along the <110> location. Besides, the transport of Li-ions and the high conductivity of LGPS have been attributed to a 1D diffusion channel using two Li locations (Li1 and Li3, respectively) along the <001> direction, in between the chains produced by (Ge/P)S4 tetrahedral [90]. The analysis test using XRD on the Li10GeP2S12 material is shown in the Figure 8(a) which shows the typical spectral features of early P2S5, GeS2, and Li2S materials, while the band peaking at around 495 cm−1 was detected as Li10GeP2S12 material [91]. The main characteristic of the diffraction peak is at 2θ = 20°, 26.7°, and 29.4° for Li10GeP2S12, corresponding to a tetragonal of the P42/nmc space group [92]. The crystallite with a size of 723.21 Å has a high purity with an electrolyte impurity of less than 3.
![Figure 7
Crystal structure of Li10GeP2S12 (a and b). (a) Chains of (Ge/P)S4 tetrahedral and Li2S6 octahedral with shared edges formed a rigid structural framework in which chains are connected in the <110> direction by the PS4 tetrahedra. (b) The structural framework as a polyhedral representation in the <001> direction. Reprinted with permission from [90]. Copyright 2016, American Chemical Society. (c) Structure of Li10SnP2S12. Reprinted with permission from [95]. Copyright 2019, American Chemical Society.](/document/doi/10.1515/eng-2022-0043/asset/graphic/j_eng-2022-0043_fig_007.jpg)
Crystal structure of Li10GeP2S12 (a and b). (a) Chains of (Ge/P)S4 tetrahedral and Li2S6 octahedral with shared edges formed a rigid structural framework in which chains are connected in the <110> direction by the PS4 tetrahedra. (b) The structural framework as a polyhedral representation in the <001> direction. Reprinted with permission from [90]. Copyright 2016, American Chemical Society. (c) Structure of Li10SnP2S12. Reprinted with permission from [95]. Copyright 2019, American Chemical Society.
The characterization test of Li11AlP2S12 in the form of X-ray diffraction (XRD) analysis has been carried out and from the analysis results, the Li11AlP2S12 shows peaks and characterization similar to Li10GeP2S12 [93]. The crystal structure of Li11AlP2S12 is typical 3D thio-LISICON which has the arrangement of (Al/P) tetrahedral or and forms a 3D chain structure because it is connected by LiS4 tetrahedral and LiS6 octahedral [87].
Li10SnP2S12 (LSnPS) structure is a polygon crystal with a space group of P42/mc a = 8.854 Å, and c = 12.851 Å lattice parameters. The 3D percolating structure shows that ion conductivity Li+ is high [47]. Li10SnP2S12 structure is seen in Figure 7(c). The Li10SnP2S12 crystal structure was characterized by XRD. The XRD pattern shown in Figure 8 indicates comparison between annealing temperatures of 500, 550, 600, and 650oC. The diffraction peaks corresponding to the Li10SnP2S12 crystalline phase increased at 29.3°, 41.3°, 47.1° and 500°C strong temperature. The top of diffraction which are recognized as the crystalline phase of Li10SnP2S12 formed approximately at 20.1°, 23.8°, 26.7°, 36.2°, and 37.3° of the sample at the annealing temperature of 550°C. The multiple peak crystallization is identified and the strength of the XRD pattern is boosted greatly at 600°C as the optimum strong temperature. This indicates that the Li10SnP2S12 has a high crystallization, which improves the electrolyte’s ionic conductivity significantly. However, following strong temperature at 650°C, the diffraction peaks at 17.1°, 34.2°, 34.5°, and 34.8° of the Li10SnP2S12 disappeared. These results showed that the best annealing temperature to synthesize LSnPS is at 600oC. The LSnPS powder particles measure up to 1–3 µm and the diameter of LSnPS pellet is 10 mm and the thickness is 1.1 mm [33]. To measure crystallization level of the final LSiPS material, an XRD analysis was performed. The resulting XRD pattern shows the structural similarity between LSiPS and LGPS. Based on the crystal structure, LGPS and LSiPS have the same structure in the space group of P42/mc (no. 137) with a lower lattice parameter value than Li10GeP2S12 (LGPS), a = 8.6512 (5) Å and c = 12.5095 (8) Å as demonstrated by Le Bail refinements [94]. Interestingly, LSiPS and LGPS have a near identical structure. According to the Rietveld, the LSiPS and LGPS structures have a secondary phase of approximately 15% LISICON. This phase reduces the high level of pure super-ionic polygon conductivity. This is due to the fact that LGPS and LSiPS are extremely sensitive to heat and environmental conditions.
2.3.3 Electrochemical properties
SSEs Li10GeP2S12 (LGPS) is a type of sulfide with a high conductivity value at 298 K. Ionic conductivity of Li10GeP2S12 can be compared to liquid organic electrolytes in Li-ion. The Li10GeP2S12 is expected to have a high solid-state battery charge and discharge performance because it has high ionic conductivity [82]. The Li10GeP2S12 (LGPS) is an interesting material after the first discovery in 2011, which showed ionic conductivity value of 12 mS cm−1 at 298 K [10]. This value is obtained from the sum of bulk resistance and grain boundaries. The temperatures between 100 and 110°C show activated energy of 24 kJ mol−1 which identifies it as a super ionic conductor [10].
Li11AlP2S12 has ionic conductivity value of 0.802 mS cm−1 at 298 K, and has electrochemical stability up to 5.0 V with an activation energy of 25.4 kJ mol−1. The Li-ion conduction in Li11AlP2S12 has potential and important in solid-state battery development [87].
The synthesis of Li10SnP2S12 (LSnPS) has total ionic conductivity value of 3.2 mS cm−1 at 300 K. Meanwhile, the instability of Li10SnP2S12 electrolyte in the lithium anode becomes one of the disadvantages. However, no obvious cathodic and anodic peaks are seen in the SS| Li10SnP2S12 |Li-In cell across the voltage range of –0.5 to 5 V, suggesting redox reaction does not happen at the Li10SnP2S12/Li-In interface [33]. Apart from its superior properties, the price of the raw material for making Li10SnP2S12 is only about 1/3 of Li10GeP2S12 [34]. Moreover, Li10SnP2S12 (LSnPS) has been commercialized under the NANOMYTE brand in powder (SSE-10) and slurry (SSE-10D) was formed [49]. However, the Li10SnP2S12 electrolyte is not stable against the lithium anode [96].
Li10SiP2S12 (LSiPS) is an alternative electrolyte to substitute Ge in the LGPS electrolytes other than Li10SnP2S12 (LSnPS). To compare the performance among them, in terms of ionic conductivity, diffusivity, and activation energy values, LSiPS shows a better value than LSnPS. The Li diffusivity of LSiPS was higher than LGPS, while the Li diffusivity of LSnPS was slightly lower. LSiPS has a lower activation energy value than LGPS, while LSnPS has a slightly greater value. Besides, the value of bulk ionic conductivity, when it is compared to LiSnPS, which is derived from NMR diffusivity calculations, LSiPS has a higher value [40]. Li10SiP2S12 (LSiPS) showed a conductivity value of 2.3 × 10−3 mS cm−1 with 0.29 eV activation energy. This value indicates a high conductivity value for unsintered materials [36]. The combination of Sn and Si to replace Ge resulted in the significant enhancement of ionic conductivity. At ambient temperature, Li10Si0.3Sn0.7P2S12 have ionic conductivity value of 8 × 10−3 S cm−1, that is relatively high when compared to the materials with single Sn or Si [49]. This shows that the combination of Sn and Si is used in solid-state as an electrolyte material.
Based on the cyclic voltammetry test, the lithium sulfide-based solid electrolyte material shows a wide electrochemical stability window of 0–5 V, including LGPS [10], LAlPS [87], LSnPS, and LSiPS. However, based on calculations, the electrochemical window of LGPS is narrower than 5 V [97], only in the range of 1.7–2.1 V. Moreover, the stability window value is low compared to other solid electrolyte types, such as LLZO, LATP, and LISICON group [98]. The formation of new interfaces due to the degradation of electrolyte material is the basic problem in solid electrolyte stability. The results reveal that the electrochemical window for both solid electrolytes is substantially narrower than that reported previously based on electrode semiblocking. To overcome the high interfacial resistance, stabilizing the solid electrolyte is required. The key to the good performance of all solid-state Li-ion bulk type batteries is to expand the electrochemical stability window solid electrolyte through spontaneous formation or application of the artificial SEI layer [99] (Table 1).
Summary of properties of sulfide solid electrolytes
| Sulfide solid electrolytes | Ionic conductivity (mS cm−1) | Activation energy (kJ mol−1) | Electrochemical stability (V) vs Li+/Li | Ref. |
|---|---|---|---|---|
| Li3PS4 | 0.16 | 60–73 | — | [28,100] |
| Li7P3S11 | 1.3 | 0.29 × 10−13 | 5 | [30,49] |
| Li4P2S6 | 0.002 | 0.46 × 10−22 | — | [38] |
| Li10GeP2S12 | 120 | 23.156 | –0.5 to 5 | [10] |
| Li10SnP2S12 | 7 | 26.051 | — | [101] |
| 4 | 57.89 | — | [101] | |
| 2 | 29.91 | — | [102] | |
| ∼3 | — | 0.5 to 5 | [103] | |
| 5 | 25.086 | 0.5 | [104] | |
| 3.2 | 20.262 | –0.5–5 | [95] | |
| Li10SiP2S12 | 2.3 | 24.025 | 0–5 | [94] |
| — | 18.332 | — | [105] | |
| Li11AlP2S12 | 0.8 | 25.086 | 5 | [87] |
| Li6PS5X | 1–10 | — | — | [52] |
| Li6PS5Cl | 0.22 | 25.086 | — | [53] |
| 0.74 | 10.613 | — | [54] | |
| 1.33 | 28.95–38.59 | 0–7 | [78] | |
| 1.1 | 15.438 | — | [56] | |
| 1.3 | 30.875 | –0.5 to 5 V | [65] | |
| 0.74 | — | — | [57] | |
| Li6PS5Br | 1–10 | 19.3 | — | [51] |
| 0.72 | 16.403 | [54] | ||
| 1.38 | 14.473 | 0–4.2 V | [55] | |
| 3.1 | 29.1 | — | [66] | |
| Li6PS5I | 0.00046 | 24.121 | — | [54] |
| Li4PS4I | 0.12 | 41.489 | — | [75] |
3 Conclusion and future perspectives
Due to its low cost, strong Li-ion conductivity, and large electrochemical window relative to Li/Li+, the binary (100 – x) Li2S-xP2S5 system, as an important member of solid sulfide electrolytes, is a particularly desirable electrolyte choice for solid-state batteries. Li3PS4, Li7P3S11, and Li4P2S6 have been extensively studied among various compositions. Li3PS4 has good compatibility with lithium metal, Li7P3S11 has a high conductivity of greater than 1 mS cm−1 at 298 K, and Li4P2S6 is quite stable in maintaining its structure crystals up to temperatures as high as 950°C in vacuum and up to 280°C in air. Although solid electrolytes based on Li2S–P2S5 come with limitations in terms of chemical and electrochemical stability, they are promising candidates for the next generation of SSBs due to their high ionic conductivity (>10−4 S cm−1). To overcome these limitations, the stability of the materials should be assessed in a strict way. Likewise, with the synthesis method used, it is necessary to conduct a thorough examination of the relationship between the synthesis method and the properties of the resulting material.
The Li-argyrodite solid electrolyte is another sulfide-based solid electrolyte. At 298 K, argyrodite type solid electrolytes, Li6PS5X (X: Cl, Br, and I), have a high ionic conductivity, with values in the range from 10−2 to 10−3 S cm−1 for Cl and Br. Li6PS5X is generally synthesized by the ball milling method, but can also be synthesized by the wet chemical method using THF and EtOH as solvents. Apart from its high conductivity, Li6PS5X also has the largest chemical stability of any sulfide based solid electrolyte, up to 10 V vs Li/Li+. Therefore, with its high electrochemical stability and excellent ionic conductivity value, Li6PS5X is acceptable as an SSE in the Li-ion batteries. So, further research to improve the electrochemical performance of Li6PS5X still needs to be done to get the best performance from this solid electrolyte.
Based on a review of the types of sulfide-based solid electrolyte that have been carried out, LGPS is a material that has the highest ionic conductivity value. Li10GeP2S12 (LGPS) has the greatest conductivity value of Li+ at room temperature and excellent electrochemical performance. However, the price of Ge required for the synthesis of LGPS is relatively expensive, therefore, it is a big consideration in the use of LGPS as a solid electrolyte. A cheap and abundant source of Ge is needed to reduce the cost. One of the alternative sources is recovering Ge from coal and sphalerites [106]. Ge is an impurity in sphalerites [106]. In addition, recoverable Ge content is also found in coal. The discovery of the Ge content in coal was first made in 1935, when it was known that coal ash contained up to 1.1% Ge [107]. The use of coal in the world is still quite large, reaching 5,400 mtce in 2019 [108]. The estimated ash produced reaches 5% of the amount of coal used. Meanwhile, 75% of the coal ash produced has not been managed properly [109]. This creates opportunities for the utilization of the Ge content in coal ash, in order to reduce the cost of producing LGPS. Besides, the electrochemical stability window of LGPS is quite narrow, 0–5 V or even narrower than that. As a result, increasing the electrochemical stability of this type of solid electrolyte by forming a spontaneous SEI layer or applying an artificial SEI layer is required. Other classes, such as LPS and Li6PS5X (X: Cl, Br, and I) are also options for solid electrolyte applications: however, Li x MP x S x (M: Sn, Si, and Al) still outperformed them. Therefore, with its conductivity value, LGPS is the most promising solid electrolyte, but further research is needed to increase the stability window to support perfect electrochemical performance.
Assembling sulfide-based electrolytes in solid-state batteries should be under Ar atmospheric conditions, because of the sensitive nature of the material. The low chemical stability of sulfide-based solid electrolytes to environmental humidity should be overcome. The hydrolysis of solid electrolytes leads to the formation of toxic H2S gas. The solution offered for the H2S gas problem is the use of M x O y metal oxides, such as Fe2O3, Bi2O3, and ZnO which can act as H2S gas absorbers, by responding spontaneously to Gibbs energy from the reaction between M x O y and negative H2S gas to form metal sulfides [88]. This also opens up opportunities for further research on improving the stability of solid electrolyte materials in atmospheric conditions.
Acknowledgment
This article was supported by the Center of Excellence for Electrical Energy Storage, Sebelas Maret University as a provider of facilities and UMG Idealab as a provider of funds for this research.
-
Conflict of interest: Authors state no conflict of interest.
References
[1] Liang Y, Zhao C, Yuan H, Chen Y, Zhang W, Huang J, et al. A review of rechargeable batteries for portable electronic devices. InfoMat. 2019;1:1–27.10.1002/inf2.12000Search in Google Scholar
[2] Judez X, Martinez-Ibañez M, Santiago A, Armand M, Zhang H, Li C. Quasi-solid-state electrolytes for lithium sulfur batteries: Advances and perspectives. J Power Sources. 2019;438:226985.10.1016/j.jpowsour.2019.226985Search in Google Scholar
[3] Nishi Y, Power J. Lithium ion secondary batteries; past 10 years and the future. Sources. 2001;100:101–6.10.1016/S0378-7753(01)00887-4Search in Google Scholar
[4] Li Q, Chen J, Fan L, Kong X, Lu Y. Progress in electrolytes for rechargeable Li-based batteries and beyond. Green Energy Env. 2016;1:18–42.10.1016/j.gee.2016.04.006Search in Google Scholar
[5] Chen Y, Kang Y, Zhao Y, Wang L, Liu J, Li Y, et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J Energy Chem. 2021;59:83–99.10.1016/j.jechem.2020.10.017Search in Google Scholar
[6] Schmuch R, Wagner R, Hörpel G, Placke T, Winter M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat Energy. 2018;3:267–78.10.1038/s41560-018-0107-2Search in Google Scholar
[7] Janek J, Zeier WG. A solid future for battery development. Nat Energy. 2016;1:1.10.1038/nenergy.2016.141Search in Google Scholar
[8] Fan L, Wei S, Li S, Li Q, Lu Y. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv Energy Mater. 2018;8:1–31.10.1002/aenm.201702657Search in Google Scholar
[9] Gao Z, Sun H, Fu L, Ye F, Zhang Y, Luo W, et al. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv Mater. 2018;30:1–27.10.1002/adma.201705702Search in Google Scholar PubMed
[10] Kamaya N, Homma K, Yamakawa Y, Hirayama M, Kanno R, Yonemura M, et al. A lithium superionic conductor. Nat Mater. 2011;10:682–6.10.1038/nmat3066Search in Google Scholar PubMed
[11] Wang Y, Richards WD, Ong SP, Miara LJ, Kim JC, Mo Y, et al. Design principles for solid-state lithium superionic conductors. Nat Mater. 2015;14:1026–31.10.1038/nmat4369Search in Google Scholar PubMed
[12] Sun YK. Promising all-solid-state batteries for future electric vehicles. ACS Energy Lett. 2020;5:3221–3.10.1021/acsenergylett.0c01977Search in Google Scholar
[13] Varzi A, Raccichini R, Passerini S, Scrosati B. Challenges and prospects of the role of solid electrolytes in the revitalization of lithium metal batteries. J Mater Chem A. 2016;4:17251–9.10.1039/C6TA07384KSearch in Google Scholar
[14] Zheng F, Kotobuki M, Song S, Lai MO, Lu L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J Power Sources. 2018;389:198–213.10.1016/j.jpowsour.2018.04.022Search in Google Scholar
[15] Chen MY, Lee KL, Hsu PN, Wu CS, Wu CH. Is there an ethnic difference in the prevalence of lupus cystitis? A report of six cases. Lupus. 2004;167:263–9.10.1191/0961203304lu527crSearch in Google Scholar PubMed
[16] Xiao T, Xu Z, Zhang H, Geng J, Qiao Y, Liang Y, et al. TP53I11 suppresses epithelial-mesenchymal transition and metastasis of breast cancer cells. Energy Storage Mater. 2019;19:379–84.10.5483/BMBRep.2019.52.6.173Search in Google Scholar
[17] Yan G, Yu S, Nonemacher JF, Tempel H, Kungl H, Malzbender J, et al. Influence of sintering temperature on conductivity and mechanical behavior of the solid electrolyte LATP. Ceram Int. 2019;45:14697–703.10.1016/j.ceramint.2019.04.191Search in Google Scholar
[18] Aatiq A, Ménétrier M, Croguennec L, Suard E, Delmas C. On the structure of Li3Ti2(PO4)3. J Mater Chem. 2002;12:2971–8.10.1039/B203652PSearch in Google Scholar
[19] Murugan R, Thangadurai V, Weppner W. Fast lithium ion conduction in garnet-type Li(7)La(3)Zr(2)O(12). Angew Chemie – Int Ed. 2007;46:7778–81.10.1002/anie.200701144Search in Google Scholar PubMed
[20] Cao S, Song S, Xiang X, Hu Q, Zhang C, Xia Z, et al. Review modeling, preparation, and elemental doping of Li7La3Zr2O12 garnet-type solid electrolytes: A Review. J Korean Ceram Soc. 2019;56:111–29.10.4191/kcers.2019.56.2.01Search in Google Scholar
[21] Kato Y, Hori S, Saito T, Suzuki K, Hirayama M, Mitsui A, et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat Energy. 2016;1:1.10.1038/nenergy.2016.30Search in Google Scholar
[22] Su QC, Wang X, Deng C, Yun YL, Zhao Y, Peng Y. Transcriptome responses to elevated CO2 level and Wolbachia-infection stress in Hylyphantes graminicola (Araneae: Linyphiidae). Energy Env Sci. 2020;13:908–20.10.1039/C9EE04007BSearch in Google Scholar
[23] Lau J, DeBlock RH, Butts DM, Ashby DS, Choi CS, Dunn BS. Sulfide solid electrolytes for lithium battery applications. Adv Energy Mater. 2018;8:1.10.1002/aenm.201800933Search in Google Scholar
[24] Zhang W, Cai L, Cao S, Qiao L, Zeng Y, Zhu Z, et al. Electrode materials: interfacial lattice-strain-driven generation of oxygen vacancies in an aerobic-annealed TiO2(B) Electrode (Adv. Mater. 52/2019). Adv Mater. 2019;31:1.10.1002/adma.201970367Search in Google Scholar
[25] Chen S, Xie D, Liu G, Mwizerwa JP, Zhang Q, Zhao Y, et al. Sulfide solid electrolytes for all-solid-state lithium batteries: Structure, conductivity, stability and application. Energy Storage Mater. 2018;14:58–74.10.1016/j.ensm.2018.02.020Search in Google Scholar
[26] Dietrich C, Weber DA, Sedlmaier SJ, Indris S, Culver SP, Walter D, et al. Lithium ion conductivity in Li2S–P2S5 glasses – building units and local structure evolution during the crystallization of superionic conductors Li3PS4, Li7P3S11and Li4P2S7. J Mater Chem A. 2017;5:18111–9.10.1039/C7TA06067JSearch in Google Scholar
[27] Homma K, Yonemura M, Nagao M, Hirayama M, Kanno R. Crystal structure of high-temperature phase of lithium ionic conductor, Li3PS4. Journal of the Physical Society of Japan. 2010;79:90–3.10.1143/JPSJS.79SA.90Search in Google Scholar
[28] Liu Z, Fu W, Payzant EA, Yu X, Wu Z, Dudney NJ, et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J Am Chem Soc. 2013;135:975–8.10.1021/ja3110895Search in Google Scholar PubMed
[29] Phuc NHH, Morikawa K, Totani M, Muto H, Matsuda A. Chemical synthesis of Li3PS4 precursor suspension by liquid-phase shaking. Solid State Ion. 2016;285:2–5.10.1016/j.ssi.2015.11.019Search in Google Scholar
[30] Chu IH, Nguyen H, Hy S, Lin YC, Wang Z, Xu Z, et al. Insights into the Performance Limits of the Li7P3S11 Superionic Conductor: A Combined First-Principles and Experimental Study. ACS Appl Mater Interfaces. 2016;8:7843–53.10.1021/acsami.8b03438Search in Google Scholar PubMed
[31] Yamane H, Shibata M, Shimane Y, Junke T, Seino Y, Adams S, et al. Crystal structure of a superionic conductor, Li7P3S11. Solid State Ion. 2007;178:1163–7.10.1016/j.ssi.2007.05.020Search in Google Scholar
[32] Minami K, Mizuno F, Hayashi A, Tatsumisago M. Lithium ion conductivity of the Li2S–P2S5 glass-based electrolytes prepared by the melt quenching method. Solid State Ion. 2007;178:837–41.10.1016/j.ssi.2007.03.001Search in Google Scholar
[33] Minami K, Hayashi A, Tatsumisago M. Preparation and characterization of superionic conducting Li7P3S11 crystal from glassy liquids. J Ceram Soc Jpn. 2010;118:305–8.10.2109/jcersj2.118.305Search in Google Scholar
[34] Hayashi A, Minami K, Tatsumisago M. Development of sulfide glass-ceramic electrolytes for all-solid-state lithium rechargeable batteries. J Solid State Electrochem. 2010;14:1761–7.10.1007/s10008-010-1098-5Search in Google Scholar
[35] Mercier R, Malugani JP, Fahys B, Robert G, Douglade J. Structure du tetrathiophosphate de lithium. Acta Crystallogr Sect B Struct Crystallogr Cryst Chem. 1982;38:1887–90.10.1107/S0567740882007535Search in Google Scholar
[36] Hood ZD, Kates C, Kirkham M, Adhikari S, Liang C, Holzwarth N. Structural and electrolyte properties of Li4P2S6. Solid State Ion. 2016;284:61–70.10.1016/j.ssi.2015.10.015Search in Google Scholar
[37] Tatsumisago M, Hayashi A. Preparation of lithium ion conducting glasses and glass–ceramics for all-solid-state batteries. J Non Cryst Solids. 2008;354:1411–7.10.1016/j.jnoncrysol.2006.10.091Search in Google Scholar
[38] Mizuno F, Hayashi A, Tadanaga K, Tatsumisago M. High lithium ion conducting glass-ceramics in the system Li2S–P2S5. Solid State Ion. 2006;177:2721–5.10.1016/j.ssi.2006.04.017Search in Google Scholar
[39] Homma K, Yonemura M, Kobayashi T, Nagao M, Hirayama M, Kanno R. Crystal structure and phase transitions of the lithium ionic conductor Li3PS4. Solid State Ion. 2011;182:53–8.10.1016/j.ssi.2010.10.001Search in Google Scholar
[40] Mercier R, Malugani JP, Fahys B, Douglande J, Robert G. Synthese, structure cristalline et analyse vibrationnelle de l'hexathiohypodiphosphate de lithium Li4P2S6. J Solid State Chem. 1982;43:151–62.10.1016/0022-4596(82)90224-9Search in Google Scholar
[41] Kudu ÖU, Famprikis T, Fleutot B, Braida MD, Le Mercier T, Islam MS, et al. A review of structural properties and synthesis methods of solid electrolyte materials in the Li2S − P2S5 binary system. J Power Sources. 2018;407:31–43.10.1016/j.jpowsour.2018.10.037Search in Google Scholar
[42] Dietrich C, Sadowski M, Sicolo S, Weber DA, Sedlmaier SJ, Weldert KS, et al. Local Structural Investigations, Defect Formation, and Ionic Conductivity of the Lithium Ionic Conductor Li4P2S6. Chem Mater. 2016;28:8764–73.10.1021/acs.chemmater.6b04175Search in Google Scholar
[43] Liang J, Li X, Zhao Y, Goncharova LV, Wang G, Adair KR, et al. In situ Li3PS4 solid-state electrolyte protection layers for superior long-life and high-rate lithium-metal anodes. Adv Mater. 2018;30:1–9.10.1002/adma.201804684Search in Google Scholar
[44] Tsukasaki H, Mori S, Shiotani S, Yamamura H. Ionic conductivity and crystallization process in the Li2S–P2S5 glass electrolyte. Solid State Ion. 2018;317:122–6.10.1016/j.ssi.2018.01.010Search in Google Scholar
[45] Tachez M, Malugani J, Mercier R, Robert G. Ionic conductivity of and phase transition in lithium thiophosphate Li3PS4. Solid State Ion. 1984;14:181–5.10.1016/0167-2738(84)90097-3Search in Google Scholar
[46] Aoki Y, Ogawa K, Nakagawa T, Hasegawa Y, Sakiyama Y, Kojima T, et al. Chemical and structural changes of 70Li2S-30P2S5 solid electrolyte during heat treatment. Solid State Ion. 2017;310:50–5.10.1016/j.ssi.2017.08.006Search in Google Scholar
[47] Hayashi A, Hama S, Minami T, Tatsumisago M. Formation of superionic crystals from mechanically milled Li2S–P2S5 glasses. Electrochem Commun. 2003;5:111–4.10.1016/S1388-2481(02)00555-6Search in Google Scholar
[48] Khurram Tufail M, Ahmad N, Zhou L, Faheem M, Yang L, Chen R, et al. Insight on air-induced degradation mechanism of Li7P3S11 to design a chemical-stable solid electrolyte with high Li2S utilization in all-solid-state Li/S batteries. Chem Eng J. 2021;425:130535.10.1016/j.cej.2021.130535Search in Google Scholar
[49] Wenzel S, Weber DA, Leichtweiss T, Busche MR, Sann J, Janek J. Interphase formation and degradation of charge transfer kinetics between a lithium metal anode and highly crystalline Li7P3S11 solid electrolyte. Solid State Ion. 2016;286:24–33.10.1016/j.ssi.2015.11.034Search in Google Scholar
[50] Liu Y, Yang Y. Recent progress of TiO2-based anodes for Li ion batteries. J Nanomater. 2016;2:1–15.10.1155/2016/8123652Search in Google Scholar
[51] Epp V, Gün Ö, Deiseroth HJ, Wilkening M. Highly Mobile Ions: Low-Temperature NMR Directly Probes Extremely Fast Li+Hopping in Argyrodite-Type Li6PS5Br. J Phys Chem Lett. 2013;4:2118–23.10.1021/jz401003aSearch in Google Scholar
[52] Deiseroth H-J, Kong ST, Eckert H, Vannahme J, Reiner C, Zaiß T, et al. Li6PS5X: A Class of Crystalline Li-Rich Solids With an Unusually High Li+ Mobility. Angew Chem. 2008;120:767–70.10.1002/ange.200703900Search in Google Scholar
[53] Rao RP, Adams S. Studies of lithium argyrodite solid electrolytes for all-solid-state batteries. Phys Status Solidi A. 2011;208:1804–7.10.1002/pssa.201001117Search in Google Scholar
[54] Rayavarapu PR, Sharma N, Peterson VK, Adams S. Variation in structure and Li+-ion migration in argyrodite-type Li6PS5X (X = Cl, Br, I) solid electrolytes. J Solid State Electrochem. 2012;16:1807–13.10.1007/s10008-011-1572-8Search in Google Scholar
[55] Yu S, Siegel DJ, Yu L, Grace T, Batmunkh M, Dadkhah M, et al. Grain Boundary Contributions to Li-Ion Transport in the Solid Electrolyte Li7La3Zr2O12(LLZO. J Mater Chem A. 2017;29:9639–47.10.1021/acs.chemmater.7b02805Search in Google Scholar
[56] Rao RP, Sharma N, Peterson VK, Adams S. Formation and conductivity studies of lithium argyrodite solid electrolytes using in-situ neutron diffraction. Solid State Ion. 2013;230:72–6.10.1016/j.ssi.2012.09.014Search in Google Scholar
[57] Zhou L, Park KH, Sun X, Lalère F, Adermann T, Hartmann P, et al. Solvent-Engineered Design of Argyrodite Li6PS5X (X = Cl, Br, I) Solid Electrolytes with High Ionic Conductivity. ACS Energy Lett. 2019;4:265–70.10.1021/acsenergylett.8b01997Search in Google Scholar
[58] Duan H, Zheng H, Zhou Y, Xu B, Liu H. Stability of garnet-type Li ion conductors: An overview. Solid State Ion. 2018;318:45–53.10.1016/j.ssi.2017.09.018Search in Google Scholar
[59] Reddy MV, Julien CM, Mauger A, Zaghib K. Sulfide and Oxide Inorganic Solid Electrolytes for All-Solid-State Li Batteries: A Review. Nanomaterials. 2020;10:1.10.3390/nano10081606Search in Google Scholar PubMed PubMed Central
[60] Gautam A, Ghidiu M, Suard E, Kraft MA, Zeier WG. On the Lithium Distribution in Halide Superionic Argyrodites by Halide Incorporation in Li7–xPS6–xClx. ACS Appl Energy Mater. 2021;4:7309–15.10.1021/acsaem.1c01417Search in Google Scholar
[61] Bai X, Duan Y, Zhuang W, Yang R, Wang J. Research progress in Li-argyrodite-based solid-state electrolytes. J Mater Chem A. 2020;8:25663–86.10.1039/D0TA08472GSearch in Google Scholar
[62] Hanghofer I, Gadermaier B, Wilkening HMR. Fast Rotational Dynamics in Argyrodite-Type Li6PS5X (X: Cl, Br, I) as Seen by31P Nuclear Magnetic Relaxation—On Cation–Anion Coupled Transport in Thiophosphates. Chem Mater. 2019;31:4591–7.10.1021/acs.chemmater.9b01435Search in Google Scholar
[63] Arnold W, Buchberger DA, Li Y, Sunkara M, Druffel T, Wang H. Halide doping effect on solvent-synthesized lithium argyrodites Li6PS5X (X= Cl, Br, I) superionic conductors. J Power Sources. 2020;464:1.10.1016/j.jpowsour.2020.228158Search in Google Scholar
[64] Xu J, Liu L, Yao N, Wu F, Li H, Chen L. Liquid-involved synthesis and processing of sulfide-based solid electrolytes, electrodes, and all-solid-state batteries. Mater Today Nano. 2019;8:100048.10.1016/j.mtnano.2019.100048Search in Google Scholar
[65] Zhang J, Zhong H, Zheng C, Xia Y, Liang C, Huang H, et al. All-solid-state batteries with slurry coated LiNi0.8Co0.1Mn0.1O2 composite cathode and Li6PS5Cl electrolyte: Effect of binder content. J Power Sources. 2018;391:73–9.10.1016/j.jpowsour.2018.04.069Search in Google Scholar
[66] Yubuchi S, Uematsu M, Hotehama C, Sakuda A, Hayashi A, Tatsumisago M. An argyrodite sulfide-based superionic conductor synthesized by a liquid-phase technique with tetrahydrofuran and ethanol. J Mater Chem A. 2019;7:558–66.10.1039/C8TA09477BSearch in Google Scholar
[67] Minafra N, Culver SP, Krauskopf T, Senyshyn A, Zeier WG. Effect of Si substitution on the structural and transport properties of superionic Li-argyrodites. J Mater Chem A. 2018;6:645–51.10.1039/C7TA08581HSearch in Google Scholar
[68] Bernges T, Culver SP, Minafra N, Koerver R, Zeier WG. Competing Structural Influences in the Li Superionic Conducting Argyrodites Li6PS5- xSe xBr (0 ≤ x ≤ 1) upon Se Substitution. Inorg Chem. 2018;57:13920–8.10.1021/acs.inorgchem.8b02443Search in Google Scholar PubMed
[69] Chen M, Yin X, Reddy MV, Adams S. All-solid-state MoS2/Li6PS5Br/In–Li batteries as a novel type of Li/S battery. J Mater Chem A. 2015;3:10698–702.10.1039/C5TA02372FSearch in Google Scholar
[70] Chen M, Adams S. High performance all-solid-state lithium/sulfur batteries using lithium argyrodite electrolyte. J Solid State Electrochem. 2015;19:697–702.10.1007/s10008-014-2654-1Search in Google Scholar
[71] Chen M, Rao RP, Adams S. High capacity all-solid-state Cu–Li2S/Li6PS5Br/In batteries. Solid State Ion. 2014;262:183–7.10.1016/j.ssi.2013.10.057Search in Google Scholar
[72] Chen M, Prasada R, Rao S, Adams S. The unusual role of Li6PS5Br in all-solid-state CuS/Li6PS5Br/In–Li batteries. Solid State Ion. 2014;268:300–4.10.1016/j.ssi.2014.05.004Search in Google Scholar
[73] Rangasamy E, Liu Z, Gobet M, Pilar K, Sahu G, Zhou W, et al. An iodide-based Li7P2S8I superionic conductor. J Am Chem Soc. 2015;137:1384–7.10.1021/ja508723mSearch in Google Scholar PubMed
[74] Pecher O, Kong ST, Goebel T, Nickel V, Weichert K, Reiner C, et al. Atomistic characterisation of Li+ mobility and conductivity in Li(7-x)PS(6-x)Ix argyrodites from molecular dynamics simulations, solid-state NMR, and impedance spectroscopy. Chem – A Eur J. 2010;16:8347–54.10.1002/chem.201000501Search in Google Scholar PubMed
[75] Sedlmaier SJ, Indris S, Dietrich C, Yavuz M, Dräger C, von Seggern F, et al. Li4PS4I: A Li+ superionic conductor synthesized by a solvent-based soft chemistry approach. Chem Mater. 2017;29:1830–5.10.1021/acs.chemmater.7b00013Search in Google Scholar
[76] Kraft MA, Ohno S, Zinkevich T, Koerver R, Culver SP, Fuchs T, et al. Inducing high ionic conductivity in the lithium superionic argyrodites li6+ xp1- xge xs5i for all-solid-state batteries. J Am Chem Soc. 2018;140:16330–9.10.1021/jacs.8b10282Search in Google Scholar PubMed
[77] Ma Z, Xue HG, Guo SP. Recent achievements on sulfide-type solid electrolytes: crystal structures and electrochemical performance. J Mater Sci. 2018;53:3927–38.10.1007/s10853-017-1827-6Search in Google Scholar
[78] Boulineau S, Courty M, Tarascon JM, Viallet V. Mechanochemical synthesis of Li-argyrodite Li6PS5X (X=Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ion. 2012;221:1–5.10.1016/j.ssi.2012.06.008Search in Google Scholar
[79] Takada K, Power J. Progress in solid electrolytes toward realizing solid-state lithium batteries. Sources. 2018;394:74–85.10.1016/j.jpowsour.2018.05.003Search in Google Scholar
[80] Ohta S, Kobayashi T, Asaoka T. High lithium ionic conductivity in the garnet-type oxide Li7−XLa3(Zr2−X,NbX)O12 (X = 0–2). J Power Sources. 2011;196:3342–5.10.1016/j.jpowsour.2010.11.089Search in Google Scholar
[81] Deiseroth HJ, Maier J, Weichert K, Nickel V, Kong ST, Reiner C. Li7PS6 and Li6PS5X (X: Cl, Br, I): possible three-dimensional diffusion pathways for lithium ions and temperature dependence of the ionic conductivity by impedance measurements. Z Fur Anorg Und Allg Chem. 2011;637:1287–94.10.1002/zaac.201100158Search in Google Scholar
[82] Kato Y, Kawamoto K, Kanno R, Hirayama M. Discharge performance of all-solid-state battery using a lithium superionic conductor Li10GeP2S12. Electrochemistry. 2012;80:749–51.10.5796/electrochemistry.80.749Search in Google Scholar
[83] Lee YG, Fujiki S, Jung C, Suzuki N, Yashiro N, Omoda R, et al. High-energy long-cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes. Nat Energy. 2020;5:299–308.10.1038/s41560-020-0575-zSearch in Google Scholar
[84] Suh KS, Hojjaji A, Villenueve G, Ménétrier M, Levasseur A. 11B NMR studies of the local environment of boron in B2S3-Li2S-LiI glasses. J Non Cryst Solids. 1991;128:13–7.10.1016/0022-3093(91)90772-XSearch in Google Scholar
[85] Tsukasaki H, Otoyama M, Mori Y, Mori S, Morimoto H, Hayashi A, et al. Analysis of structural and thermal stability in the positive electrode for sulfide-based all-solid-state lithium batteries. J Power Sources. 2017;367:42–8.10.1016/j.jpowsour.2017.09.031Search in Google Scholar
[86] Zhao Y, Wu C, Peng G, Chen X, Yao X, Bai Y, et al. A new solid polymer electrolyte incorporating Li10GeP2S12 into a polyethylene oxide matrix for all-solid-state lithium batteries. J Power Sources. 2016;301:47–53.10.1016/j.jpowsour.2015.09.111Search in Google Scholar
[87] Zhou P, Wang J, Cheng F, Li F, Chen J. A solid lithium superionic conductor Li11AlP2S12 with a thio-LISICON analogous structure. Chem Commun. 2016;52:6091–4.10.1039/C6CC02131JSearch in Google Scholar PubMed
[88] Ohtomo T, Hayashi A, Tatsumisago M, Kawamoto K. Suppression of H2S gas generation from the 75Li2S·25P2S5 glass electrolyte by additives. J Mater Sci. 2013;48:4137–42.10.1007/s10853-013-7226-8Search in Google Scholar
[89] Kwon O, Hirayama M, Suzuki K, Kato Y, Saito T, Yonemura M, et al. Synthesis, structure, and conduction mechanism of the lithium superionic conductor Li10+δGe1+δP2−δS12. J Mater Chem A. 2015;3:438–46.10.1039/C4TA05231ESearch in Google Scholar
[90] Weber DA, Senyshyn A, Weldert KS, Wenzel S, Zhang W, Kaiser R, et al. Structural Insights and 3D Diffusion Pathways within the Lithium Superionic Conductor Li10GeP2S12. Chem Mater. 2016;28:5905–15.10.1021/acs.chemmater.6b02424Search in Google Scholar
[91] Hassoun J, Verrelli R, Reale P, Panero S, Mariotto G, Greenbaum S, et al. A structural, spectroscopic and electrochemical study of a lithium ion conducting Li10GeP2S12 solid electrolyte. J Power Sources. 2013;229:117–22.10.1016/j.jpowsour.2012.11.130Search in Google Scholar
[92] Yin J, Yao X, Peng G, Yang J, Huang Z, Liu D, et al. Influence of the Li–Ge–P–S based solid electrolytes on NCA electrochemical performances in all-solid-state lithium batteries. Solid State Ion. 2015;274:8–11.10.1016/j.ssi.2015.02.014Search in Google Scholar
[93] Kanno R, Murayama M. Lithium ionic conductor thio-LISICON: the Li2SGeS2P2S5 system. J Electrochem Soc. 2001;148:A742–6.10.1149/1.1379028Search in Google Scholar
[94] Whiteley JM, Woo JH, Hu E, Nam KW, Lee SH. Empowering the Lithium Metal Battery through a Silicon-Based Superionic Conductor. J Electrochem Soc. 2014;161:A1812–7.10.1149/2.0501412jesSearch in Google Scholar
[95] Yi J, Chen L, Liu Y, Geng H, Fan LZ. High capacity and superior cyclic performances of all-solid-state lithium-sulfur batteries enabled by high-conductivity Li10SnP2S12 solid electrolyte. ACS Appl Mater Interfaces. 2019;1:36674–81.10.1021/acsami.9b12846Search in Google Scholar PubMed
[96] Rettenwander D, Wagner R, Reyer A, Bonta M, Cheng L, Doeff MM, et al. Interface Instability of Fe-Stabilized Li7La3Zr2O12 versus Li Metal. J Phys Chem. 2018:122:3780–5.10.1021/acs.jpcc.7b12387Search in Google Scholar PubMed PubMed Central
[97] Mo Y, Ong SP, Ceder G. First principles study of the Li10GeP2S12 lithium super ionic conductor material. Chem Mater. 2012;24:15–7.10.1021/cm203303ySearch in Google Scholar
[98] Binninger T, Marcolongo A, Mottet M, Weber V, Laino T. Comparison of computational methods for the electrochemical stability window of solid-state electrolyte materials. J Mater Chem A. 2020;8:1347–59.10.1039/C9TA09401FSearch in Google Scholar
[99] Han F, Zhu Y, He X, Mo Y, Wang C. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv Energy Mater. 2016;6:1–9.10.1149/MA2016-03/2/663Search in Google Scholar
[100] Iikubo S, Shimoyama K, Kawano S, Fujii M, Yamamoto K, Matsushita M, et al. Novel stable structure of Li3PS4 predicted by evolutionary algorithm under high-pressure. AIP Adv. 2018;8:015008.10.1063/1.5011401Search in Google Scholar
[101] Bron P, Johansson S, Zick K, Gunne JS, Dehnen S, Rolling B. Li10SnP2S12: An affordable lithium superionic conductor. J Am Chem Soc. 2013;135:15694–7.10.1021/ja407393ySearch in Google Scholar PubMed
[102] Bron P, Dehnen S, Roling B. Li10Si0.3Sn0.7P2S12 – A low-cost and low-grain-boundary-resistance lithium superionic conductor. J Power Sources. 2016;329:530–5.10.1016/j.jpowsour.2016.08.115Search in Google Scholar
[103] Vinado C, Wang S, He Y, Xiao X, Li Y, Wang C, et al. Electrochemical and interfacial behavior of all solid state batteries using Li10SnP2S12 solid electrolyte. J Power Sources. 2018;396:824–30.10.1016/j.jpowsour.2018.06.038Search in Google Scholar
[104] Tarhouchi I, Viallet V, Vinatier P, Ménétrier M. Electrochemical characterization of Li10SnP2S12: An electrolyte or a negative electrode for solid state Li-ion batteries. Solid State Ion. 2016;296:18–25.10.1016/j.ssi.2016.08.016Search in Google Scholar
[105] Kuhn A, Gerbig O, Zhu C, Falkenberg F, Maier J, Lotsch BV. A new ultrafast superionic Li-conductor: ion dynamics in Li11Si2PS12 and comparison with other tetragonal LGPS-type electrolytes. Phys Chem Chem Phys. 2014;16:14669–74.10.1039/C4CP02046DSearch in Google Scholar PubMed
[106] Frenzel M, Ketris MP, Gutzmer J. On the geological availability of germanium. Min Depos. 2014;49:471–86.10.1007/s00126-013-0506-zSearch in Google Scholar
[107] Goldschmidt VM. Rare Elements in Coal Ashes. Ind Eng Chem. 1935;27:1100–2.10.1021/ie50309a032Search in Google Scholar
[108] Prime J, Martinez LM. Statistucs report coal information – Overview. Paris; 2020.Search in Google Scholar
[109] Sommerville R, Blissett R, Rowson N, Blackburn S. Producing a Synthetic Zeolite from Improved Fly Ash Residue. Int J Min Process. 2013;124:20–5.10.1016/j.minpro.2013.07.005Search in Google Scholar
© 2022 Windhu Griyasti Suci et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Performance of a horizontal well in a bounded anisotropic reservoir: Part I: Mathematical analysis
- Key competences for Transport 4.0 – Educators’ and Practitioners’ opinions
- COVID-19 lockdown impact on CERN seismic station ambient noise levels
- Constraint evaluation and effects on selected fracture parameters for single-edge notched beam under four-point bending
- Minimizing form errors in additive manufacturing with part build orientation: An optimization method for continuous solution spaces
- The method of selecting adaptive devices for the needs of drivers with disabilities
- Control logic algorithm to create gaps for mixed traffic: A comprehensive evaluation
- Numerical prediction of cavitation phenomena on marine vessel: Effect of the water environment profile on the propulsion performance
- Boundary element analysis of rotating functionally graded anisotropic fiber-reinforced magneto-thermoelastic composites
- Effect of heat-treatment processes and high temperature variation of acid-chloride media on the corrosion resistance of B265 (Ti–6Al–4V) titanium alloy in acid-chloride solution
- Influence of selected physical parameters on vibroinsulation of base-exited vibratory conveyors
- System and eco-material design based on slow-release ferrate(vi) combined with ultrasound for ballast water treatment
- Experimental investigations on transmission of whole body vibration to the wheelchair user's body
- Determination of accident scenarios via freely available accident databases
- Elastic–plastic analysis of the plane strain under combined thermal and pressure loads with a new technique in the finite element method
- Design and development of the application monitoring the use of server resources for server maintenance
- The LBC-3 lightweight encryption algorithm
- Impact of the COVID-19 pandemic on road traffic accident forecasting in Poland and Slovakia
- Development and implementation of disaster recovery plan in stock exchange industry in Indonesia
- Pre-determination of prediction of yield-line pattern of slabs using Voronoi diagrams
- Urban air mobility and flying cars: Overview, examples, prospects, drawbacks, and solutions
- Stadiums based on curvilinear geometry: Approximation of the ellipsoid offset surface
- Driftwood blocking sensitivity on sluice gate flow
- Solar PV power forecasting at Yarmouk University using machine learning techniques
- 3D FE modeling of cable-stayed bridge according to ICE code
- Review Articles
- Partial discharge calibrator of a cavity inside high-voltage insulator
- Health issues using 5G frequencies from an engineering perspective: Current review
- Modern structures of military logistic bridges
- Retraction
- Retraction note: COVID-19 lockdown impact on CERN seismic station ambient noise levels
- Special Issue: Trends in Logistics and Production for the 21st Century - Part II
- Solving transportation externalities, economic approaches, and their risks
- Demand forecast for parking spaces and parking areas in Olomouc
- Rescue of persons in traffic accidents on roads
- Special Issue: ICRTEEC - 2021 - Part II
- Switching transient analysis for low voltage distribution cable
- Frequency amelioration of an interconnected microgrid system
- Wireless power transfer topology analysis for inkjet-printed coil
- Analysis and control strategy of standalone PV system with various reference frames
- Special Issue: AESMT
- Study of emitted gases from incinerator of Al-Sadr hospital in Najaf city
- Experimentally investigating comparison between the behavior of fibrous concrete slabs with steel stiffeners and reinforced concrete slabs under dynamic–static loads
- ANN-based model to predict groundwater salinity: A case study of West Najaf–Kerbala region
- Future short-term estimation of flowrate of the Euphrates river catchment located in Al-Najaf Governorate, Iraq through using weather data and statistical downscaling model
- Utilization of ANN technique to estimate the discharge coefficient for trapezoidal weir-gate
- Experimental study to enhance the productivity of single-slope single-basin solar still
- An empirical formula development to predict suspended sediment load for Khour Al-Zubair port, South of Iraq
- A model for variation with time of flexiblepavement temperature
- Analytical and numerical investigation of free vibration for stepped beam with different materials
- Identifying the reasons for the prolongation of school construction projects in Najaf
- Spatial mixture modeling for analyzing a rainfall pattern: A case study in Ireland
- Flow parameters effect on water hammer stability in hydraulic system by using state-space method
- Experimental study of the behaviour and failure modes of tapered castellated steel beams
- Water hammer phenomenon in pumping stations: A stability investigation based on root locus
- Mechanical properties and freeze-thaw resistance of lightweight aggregate concrete using artificial clay aggregate
- Compatibility between delay functions and highway capacity manual on Iraqi highways
- The effect of expanded polystyrene beads (EPS) on the physical and mechanical properties of aerated concrete
- The effect of cutoff angle on the head pressure underneath dams constructed on soils having rectangular void
- An experimental study on vibration isolation by open and in-filled trenches
- Designing a 3D virtual test platform for evaluating prosthetic knee joint performance during the walking cycle
- Special Issue: AESMT-2 - Part I
- Optimization process of resistance spot welding for high-strength low-alloy steel using Taguchi method
- Cyclic performance of moment connections with reduced beam sections using different cut-flange profiles
- Time overruns in the construction projects in Iraq: Case study on investigating and analyzing the root causes
- Contribution of lift-to-drag ratio on power coefficient of HAWT blade for different cross-sections
- Geotechnical correlations of soil properties in Hilla City – Iraq
- Improve the performance of solar thermal collectors by varying the concentration and nanoparticles diameter of silicon dioxide
- Enhancement of evaporative cooling system in a green-house by geothermal energy
- Destructive and nondestructive tests formulation for concrete containing polyolefin fibers
- Quantify distribution of topsoil erodibility factor for watersheds that feed the Al-Shewicha trough – Iraq using GIS
- Seamless geospatial data methodology for topographic map: A case study on Baghdad
- Mechanical properties investigation of composite FGM fabricated from Al/Zn
- Causes of change orders in the cycle of construction project: A case study in Al-Najaf province
- Optimum hydraulic investigation of pipe aqueduct by MATLAB software and Newton–Raphson method
- Numerical analysis of high-strength reinforcing steel with conventional strength in reinforced concrete beams under monotonic loading
- Deriving rainfall intensity–duration–frequency (IDF) curves and testing the best distribution using EasyFit software 5.5 for Kut city, Iraq
- Designing of a dual-functional XOR block in QCA technology
- Producing low-cost self-consolidation concrete using sustainable material
- Performance of the anaerobic baffled reactor for primary treatment of rural domestic wastewater in Iraq
- Enhancement isolation antenna to multi-port for wireless communication
- A comparative study of different coagulants used in treatment of turbid water
- Field tests of grouted ground anchors in the sandy soil of Najaf, Iraq
- New methodology to reduce power by using smart street lighting system
- Optimization of the synergistic effect of micro silica and fly ash on the behavior of concrete using response surface method
- Ergodic capacity of correlated multiple-input–multiple-output channel with impact of transmitter impairments
- Numerical studies of the simultaneous development of forced convective laminar flow with heat transfer inside a microtube at a uniform temperature
- Enhancement of heat transfer from solar thermal collector using nanofluid
- Improvement of permeable asphalt pavement by adding crumb rubber waste
- Study the effect of adding zirconia particles to nickel–phosphorus electroless coatings as product innovation on stainless steel substrate
- Waste aggregate concrete properties using waste tiles as coarse aggregate and modified with PC superplasticizer
- CuO–Cu/water hybrid nonofluid potentials in impingement jet
- Satellite vibration effects on communication quality of OISN system
- Special Issue: Annual Engineering and Vocational Education Conference - Part III
- Mechanical and thermal properties of recycled high-density polyethylene/bamboo with different fiber loadings
- Special Issue: Advanced Energy Storage
- Cu-foil modification for anode-free lithium-ion battery from electronic cable waste
- Review of various sulfide electrolyte types for solid-state lithium-ion batteries
- Optimization type of filler on electrochemical and thermal properties of gel polymer electrolytes membranes for safety lithium-ion batteries
- Pr-doped BiFeO3 thin films growth on quartz using chemical solution deposition
- An environmentally friendly hydrometallurgy process for the recovery and reuse of metals from spent lithium-ion batteries, using organic acid
- Production of nickel-rich LiNi0.89Co0.08Al0.03O2 cathode material for high capacity NCA/graphite secondary battery fabrication
- Special Issue: Sustainable Materials Production and Processes
- Corrosion polarization and passivation behavior of selected stainless steel alloys and Ti6Al4V titanium in elevated temperature acid-chloride electrolytes
- Special Issue: Modern Scientific Problems in Civil Engineering - Part II
- The modelling of railway subgrade strengthening foundation on weak soils
- Special Issue: Automation in Finland 2021 - Part II
- Manufacturing operations as services by robots with skills
- Foundations and case studies on the scalable intelligence in AIoT domains
- Safety risk sources of autonomous mobile machines
- Special Issue: 49th KKBN - Part I
- Residual magnetic field as a source of information about steel wire rope technical condition
- Monitoring the boundary of an adhesive coating to a steel substrate with an ultrasonic Rayleigh wave
- Detection of early stage of ductile and fatigue damage presented in Inconel 718 alloy using instrumented indentation technique
- Identification and characterization of the grinding burns by eddy current method
- Special Issue: ICIMECE 2020 - Part II
- Selection of MR damper model suitable for SMC applied to semi-active suspension system by using similarity measures
Articles in the same Issue
- Regular Articles
- Performance of a horizontal well in a bounded anisotropic reservoir: Part I: Mathematical analysis
- Key competences for Transport 4.0 – Educators’ and Practitioners’ opinions
- COVID-19 lockdown impact on CERN seismic station ambient noise levels
- Constraint evaluation and effects on selected fracture parameters for single-edge notched beam under four-point bending
- Minimizing form errors in additive manufacturing with part build orientation: An optimization method for continuous solution spaces
- The method of selecting adaptive devices for the needs of drivers with disabilities
- Control logic algorithm to create gaps for mixed traffic: A comprehensive evaluation
- Numerical prediction of cavitation phenomena on marine vessel: Effect of the water environment profile on the propulsion performance
- Boundary element analysis of rotating functionally graded anisotropic fiber-reinforced magneto-thermoelastic composites
- Effect of heat-treatment processes and high temperature variation of acid-chloride media on the corrosion resistance of B265 (Ti–6Al–4V) titanium alloy in acid-chloride solution
- Influence of selected physical parameters on vibroinsulation of base-exited vibratory conveyors
- System and eco-material design based on slow-release ferrate(vi) combined with ultrasound for ballast water treatment
- Experimental investigations on transmission of whole body vibration to the wheelchair user's body
- Determination of accident scenarios via freely available accident databases
- Elastic–plastic analysis of the plane strain under combined thermal and pressure loads with a new technique in the finite element method
- Design and development of the application monitoring the use of server resources for server maintenance
- The LBC-3 lightweight encryption algorithm
- Impact of the COVID-19 pandemic on road traffic accident forecasting in Poland and Slovakia
- Development and implementation of disaster recovery plan in stock exchange industry in Indonesia
- Pre-determination of prediction of yield-line pattern of slabs using Voronoi diagrams
- Urban air mobility and flying cars: Overview, examples, prospects, drawbacks, and solutions
- Stadiums based on curvilinear geometry: Approximation of the ellipsoid offset surface
- Driftwood blocking sensitivity on sluice gate flow
- Solar PV power forecasting at Yarmouk University using machine learning techniques
- 3D FE modeling of cable-stayed bridge according to ICE code
- Review Articles
- Partial discharge calibrator of a cavity inside high-voltage insulator
- Health issues using 5G frequencies from an engineering perspective: Current review
- Modern structures of military logistic bridges
- Retraction
- Retraction note: COVID-19 lockdown impact on CERN seismic station ambient noise levels
- Special Issue: Trends in Logistics and Production for the 21st Century - Part II
- Solving transportation externalities, economic approaches, and their risks
- Demand forecast for parking spaces and parking areas in Olomouc
- Rescue of persons in traffic accidents on roads
- Special Issue: ICRTEEC - 2021 - Part II
- Switching transient analysis for low voltage distribution cable
- Frequency amelioration of an interconnected microgrid system
- Wireless power transfer topology analysis for inkjet-printed coil
- Analysis and control strategy of standalone PV system with various reference frames
- Special Issue: AESMT
- Study of emitted gases from incinerator of Al-Sadr hospital in Najaf city
- Experimentally investigating comparison between the behavior of fibrous concrete slabs with steel stiffeners and reinforced concrete slabs under dynamic–static loads
- ANN-based model to predict groundwater salinity: A case study of West Najaf–Kerbala region
- Future short-term estimation of flowrate of the Euphrates river catchment located in Al-Najaf Governorate, Iraq through using weather data and statistical downscaling model
- Utilization of ANN technique to estimate the discharge coefficient for trapezoidal weir-gate
- Experimental study to enhance the productivity of single-slope single-basin solar still
- An empirical formula development to predict suspended sediment load for Khour Al-Zubair port, South of Iraq
- A model for variation with time of flexiblepavement temperature
- Analytical and numerical investigation of free vibration for stepped beam with different materials
- Identifying the reasons for the prolongation of school construction projects in Najaf
- Spatial mixture modeling for analyzing a rainfall pattern: A case study in Ireland
- Flow parameters effect on water hammer stability in hydraulic system by using state-space method
- Experimental study of the behaviour and failure modes of tapered castellated steel beams
- Water hammer phenomenon in pumping stations: A stability investigation based on root locus
- Mechanical properties and freeze-thaw resistance of lightweight aggregate concrete using artificial clay aggregate
- Compatibility between delay functions and highway capacity manual on Iraqi highways
- The effect of expanded polystyrene beads (EPS) on the physical and mechanical properties of aerated concrete
- The effect of cutoff angle on the head pressure underneath dams constructed on soils having rectangular void
- An experimental study on vibration isolation by open and in-filled trenches
- Designing a 3D virtual test platform for evaluating prosthetic knee joint performance during the walking cycle
- Special Issue: AESMT-2 - Part I
- Optimization process of resistance spot welding for high-strength low-alloy steel using Taguchi method
- Cyclic performance of moment connections with reduced beam sections using different cut-flange profiles
- Time overruns in the construction projects in Iraq: Case study on investigating and analyzing the root causes
- Contribution of lift-to-drag ratio on power coefficient of HAWT blade for different cross-sections
- Geotechnical correlations of soil properties in Hilla City – Iraq
- Improve the performance of solar thermal collectors by varying the concentration and nanoparticles diameter of silicon dioxide
- Enhancement of evaporative cooling system in a green-house by geothermal energy
- Destructive and nondestructive tests formulation for concrete containing polyolefin fibers
- Quantify distribution of topsoil erodibility factor for watersheds that feed the Al-Shewicha trough – Iraq using GIS
- Seamless geospatial data methodology for topographic map: A case study on Baghdad
- Mechanical properties investigation of composite FGM fabricated from Al/Zn
- Causes of change orders in the cycle of construction project: A case study in Al-Najaf province
- Optimum hydraulic investigation of pipe aqueduct by MATLAB software and Newton–Raphson method
- Numerical analysis of high-strength reinforcing steel with conventional strength in reinforced concrete beams under monotonic loading
- Deriving rainfall intensity–duration–frequency (IDF) curves and testing the best distribution using EasyFit software 5.5 for Kut city, Iraq
- Designing of a dual-functional XOR block in QCA technology
- Producing low-cost self-consolidation concrete using sustainable material
- Performance of the anaerobic baffled reactor for primary treatment of rural domestic wastewater in Iraq
- Enhancement isolation antenna to multi-port for wireless communication
- A comparative study of different coagulants used in treatment of turbid water
- Field tests of grouted ground anchors in the sandy soil of Najaf, Iraq
- New methodology to reduce power by using smart street lighting system
- Optimization of the synergistic effect of micro silica and fly ash on the behavior of concrete using response surface method
- Ergodic capacity of correlated multiple-input–multiple-output channel with impact of transmitter impairments
- Numerical studies of the simultaneous development of forced convective laminar flow with heat transfer inside a microtube at a uniform temperature
- Enhancement of heat transfer from solar thermal collector using nanofluid
- Improvement of permeable asphalt pavement by adding crumb rubber waste
- Study the effect of adding zirconia particles to nickel–phosphorus electroless coatings as product innovation on stainless steel substrate
- Waste aggregate concrete properties using waste tiles as coarse aggregate and modified with PC superplasticizer
- CuO–Cu/water hybrid nonofluid potentials in impingement jet
- Satellite vibration effects on communication quality of OISN system
- Special Issue: Annual Engineering and Vocational Education Conference - Part III
- Mechanical and thermal properties of recycled high-density polyethylene/bamboo with different fiber loadings
- Special Issue: Advanced Energy Storage
- Cu-foil modification for anode-free lithium-ion battery from electronic cable waste
- Review of various sulfide electrolyte types for solid-state lithium-ion batteries
- Optimization type of filler on electrochemical and thermal properties of gel polymer electrolytes membranes for safety lithium-ion batteries
- Pr-doped BiFeO3 thin films growth on quartz using chemical solution deposition
- An environmentally friendly hydrometallurgy process for the recovery and reuse of metals from spent lithium-ion batteries, using organic acid
- Production of nickel-rich LiNi0.89Co0.08Al0.03O2 cathode material for high capacity NCA/graphite secondary battery fabrication
- Special Issue: Sustainable Materials Production and Processes
- Corrosion polarization and passivation behavior of selected stainless steel alloys and Ti6Al4V titanium in elevated temperature acid-chloride electrolytes
- Special Issue: Modern Scientific Problems in Civil Engineering - Part II
- The modelling of railway subgrade strengthening foundation on weak soils
- Special Issue: Automation in Finland 2021 - Part II
- Manufacturing operations as services by robots with skills
- Foundations and case studies on the scalable intelligence in AIoT domains
- Safety risk sources of autonomous mobile machines
- Special Issue: 49th KKBN - Part I
- Residual magnetic field as a source of information about steel wire rope technical condition
- Monitoring the boundary of an adhesive coating to a steel substrate with an ultrasonic Rayleigh wave
- Detection of early stage of ductile and fatigue damage presented in Inconel 718 alloy using instrumented indentation technique
- Identification and characterization of the grinding burns by eddy current method
- Special Issue: ICIMECE 2020 - Part II
- Selection of MR damper model suitable for SMC applied to semi-active suspension system by using similarity measures

![Figure 8
(a) X ray diffraction of the Li10GeP2S12 solid electrolyte. Reprinted with permission from [91]. Copyright 2013, Elsevier. (b) XRD pattern of Li10SnP2S12 at various annealing temperatures. Reprinted with permission from [95]. Copyright 2019, American Chemical Society.](/document/doi/10.1515/eng-2022-0043/asset/graphic/j_eng-2022-0043_fig_008.jpg)
