Abstract
Gel polymer electrolyte (GPE) membranes of polyvinylidene fluoride-based polymer matrix of different filler types such as nano-clay, ZnO, and SiO2 extracted from fly ash is produced using the non-solvent induced phase separation method. Moreover, the electrochemical properties, electrode compatibility, and the batteries performance are investigated using these gel electrolyte membranes. It is observed that gel electrolytes with nano-clay filler exhibited more stable ionic conductivity and charge–discharge performance than those containing ZnO and SiO2 extracted from fly ash. A maximum ionic conductivity of 5.62 × 10–3 S cm−1 was obtained with the addition of 8 wt% nano-clay filler at room temperature. The LiFePO4 performance assembled with these membranes is examined using coin cells (LiFePO4/GPE/graphite). After 50 cycles with a rate of 0.2 C, the battery with a different filler retained 97.7% of its initial capacity. It should be noted that this type of filler influences the electrolyte absorption, electrochemical properties, and performance of the LiFePO4.
1 Introduction
In the last two decades, there is increasing demand for portable and lightweight electronic devices, thereby creating a need for energy storage compatible with the design of these devices. The lithium ion battery (LIB) is an energy storage device adequately suitable for mobile systems applications. Additionally, lithium ion has an advantage over existing energy storage systems because they have a fast-charging design, long life cycle, and a higher power density in a simple battery pack module [1]. However, the battery system disadvantages include high cost, low compaction density, and battery safety, mainly when utilized in high-powered electric motors such as electric vehicles that require high energy density. The most critical safety factor is the generation of internal heat during electrochemical reactions in the battery cell [2]. Thus, the resulting heat includes a reversible heat generated from electrochemical reactions during the charging/discharging process, irreversible heat from internal ohmic resistance, polarization heat production, and side reaction heat [3,4]. To ensure batteries are kept safe, the rate of heat build-up and the use of solid or semi-solid electrolytes must be maintained.
Properties of polymer electrolytes such as safety factors, design flexibility, and suitability make them a suitable replacement for liquid electrolytes used in energy storage devices [5]. Generally, there are two types of electrolyte polymers, namely solid electrolyte polymer (SPE) and gel polymer electrolyte (GPE). The SPE is a salt of Lithium (Li) dissolved by a high molecular weight polymer chain. In addition, polymers serve as hosts for ion transfer through the movement of polymer segments [6]. However, practical use of the SPE is limited because its ionic conductivity is lower than the conductivity required in battery applications (10−3 S cm−1), while the GPE has advantages compared to SPE such as the ionic conductivity of GPE is higher than the SPE. The GPE also mitigates the risk of leakage since it entraps liquid electrolytes of polymer structures and performs a dual role of acting as a separator between the electrodes of the battery and electrolyte storage.
Furthermore, the GPE consists of a gel system and a heterogeneous phase, the heterogeneous phase is the polymer host matrix with interrelated pores filled with an electrolyte solution. In heterogeneous GPE, lithium-ion transport occurs in the quasi-solid state electrolyte phase. The common GPEs exhibit excellent conductivity of Li+ in the order of 10−3 S cm−1, this has made GPE one of the most attractive alternatives amongst various electrolyte systems with enhanced safety and flexibility.
The ionic conductivity and a stable porous structure of the membrane can be improved by combining the phase inversion process and the presence of filler in the membrane [7]. The Montmorillonite filler addition has the effect of increasing the charge and mobility for Li-ion battery applications [8]. The crystallinity of the polymer material can be reduced by the incorporation of several types of filler. In this case, the filler acts as a solid plasticizer and will increase the ion transfer characteristics and also interface characteristics to the anode [9]. Wachtler et al., [10] showed that the PVDF-based GPE membrane with the addition of SiO2 had the same electrochemical capacity when compared to that without filler. Costa et al. [11] reported that the best ionic conductivity is obtained by adding MgO, ZnO, and MCM-41 filler. ZnO particles are inert oxide ceramics that can alter polymer chain dynamics and increase the lithium transference number.
This research examines the influence of filler on the characterization and performance of the polymer electrolyte for LIB for PVDF-based polymer matrix. Additionally, this complements previous work carried out on GPEs using pore-forming materials and filler additives based on PVDF polymer matrix [12,13]. The results showed that fillers play a significant role in increasing the ionic conductivity and the life cycle of the battery in order to achieve optimum performance.
2 Materials and methods
2.1 Materials
Polyvinylidene fluoride (PVDF) powder (MW 534000, Sigma-Aldrich) and polyvinylpyrrolidone (PVP) (MW 25,000 g mol−1, Merck) were dried at 60°C under a vacuum for 24 h. N,N-Dimethylacetamide (DMAc, Merck) was used as received. Also, surface modification of nano-clay (Sigma Aldrich Nanomer® I.31PS, Nanomer® clay), nano zinc oxide, ZnO (20–30 nm, MTI Corporation, USA), and silicon oxide, SiO2, were extracted from fly ash waste. The electrolyte solution was 1.0 M LiPF6 lithium hexafluorophosphate (LiPF6) in EC/DMC/DEC = 4/2/4 (v/v/v) (MTI Corporation, USA).
2.2 Methodology
2.2.1 Preparation of gel polymer electrolyte (GPE) membranes
Different membranes were prepared by modifying the previously used procedure for nano-clay [13] and ZnO [12]. Modifications were made by allowing the nascent membrane to be in contact with air for 10 min and then immersed in demin water for 24 h with a water replacement process of 2 h periodically. The types of filler added were nano-clay, ZnO, and SiO2 from fly ash with concentrations of 6, 8, and 10 wt%, respectively. Each filler was dispersed in n,n-dimethylacetamide solvent at 35°C for 2 h using an ultrasonic sonicator. After that, 10wt% of PVDF polymer was added to the membranes solution and continued to be mixed at 45°C (20 h). Then, PVP pore-forming agent was added and stirred again until completely mixed (4 h). The next step is a membrane degassing process for 4 h to remove bubbles in the casting solution. The solution is then printed on flat glass using a casting machine with a membrane thickness of 150 μm. The wet membranes were then immersed in a non-solvent (deionized water) at 25°C for 24 h. The membrane formed was dried under air for 24 h. Finally, to remove the remaining water and solvent still in the membrane matrix, a final drying process was carried out at 70°C under vacuum for 12 h. The membrane obtained will be ready to be activated with an electrolyte solution.
2.2.2 Characterization method
The Fourier Transform Infrared Spectroscopy (FTIR) was used to analyze the surface chemistry of the membrane. The characterization was done on a Shimadzu Prestige-21. The porosity (P) of membranes was evaluated using equation (1).
where M m is the membrane mass in dry condition and M BuOH is the membrane mass after membranes were immersed in n-butanol solution. ρ BuOH and ρ P are the density of n-butanol and membranes, respectively. The amount of electrolyte trapped in the membrane pores is determined according to:
where M 0 is the membrane mass in dry condition and M is the membrane mass in the wet condition, respectively. Degree of crystallinity of the polymer membrane is determined using the differential scanning calorimetry (DSC) analysis. Equation (3) is used to estimate the value of the degree of crystallinity of membranes:
where X
C is the degree of crystallinity,
2.2.3 Electrochemical properties and battery performance
Ionic conductivity measurements were performed by Hi-Tester Model HIOKI LCR 3532. The characterization were done on the range of frequency of 42–5 MHz with an amplitude of 10 mV [15]. The membrane sample was immersed in electrolyte (1 M LiPF6) and then sandwiched with stainless steel (SS) electrodes (SS/GPEs/SS) in CR2032 coin battery cell. The ionic conductivity (σ) expression described by equation (4).
In this study, R b is the bulk resistance (Ω), d is the thickness of the membranes after swelling, and S is the contact area with SS electrode. The battery with a cell configuration consisting of LiFePO4/GPE/graphite was used in the electrochemical performance test of the battery, and battery analyzer (0.02–10 mA, MTI corp.) was CT-1008-S1(Neware), in the voltage range between 2.5 and 3.65 V, with current densities 0.2 C for 50 cycles.
3 Results and discussion
3.1 Thermal stability
The functions of GPE membrane are an electrolyte for ion transfer media and a separator between the electrodes in the battery cell. In the battery cell, an exothermic reaction takes place which releases heat during charging or discharging process. This thermal effect can cause deformation of the GPEs, which will result in reduced efficiency during the charging and discharging processes, and can cause short circuits in the battery cells. The thermal fusion ratio of PVDF obtained from the DSC analysis with thermal the fusion of pure PVDF critical materials is the value of the degree of crystallinity of the PVDF membranes [16]. The characteristics of the melting point and degree of crystallinity of PVDF membranes with PVP additives on different types of fillers are summarized in Table 1.
Thermal Properties and Crystallinity of PVDF membranes
| Composition | Degree of crystallinity, X C (%) | Melting point, T m (°C) | |||
|---|---|---|---|---|---|
| PVDF | PVP | Filler (wt%) | |||
| 10 | 0 | — | 37.26 | 162.71 | |
| 10 | 7 | Clay | 8 | 14.71 | 163.2 |
| 10 | 7 | ZnO | 8 | 31.26 | 164.12 |
| 10 | 7 | SiO2 | 8 | 34.23 | 164.97 |
The crystallinity values (Table 1) range from 14.71 to 37.26% for various types of fillers. The inhibition of crystallization during the membrane solidification process due to the presence of filler reduces the volume of the crystal fraction in the membrane, resulting in a decreased degree of crystallinity [17]. In the polymer, the increase in amorphous phase correlates with the increase in the rate of nucleation during the membrane solidification as reported by ref. [18]. The degree of crystallinity (X C) of this modified membrane was lower than the PVDF membrane when graphene/PVP (44%) was added [19].
3.2 Surface chemistry
Qualitative analysis of the chemical properties of a substance identified through specific absorption peaks for certain groups is achieved using infrared (IR) spectroscopy. The chemical bonds formed in the GPE membrane were used to determine the appearance of β-PVDF. Furthermore, the data in Figure 1 show the surface chemistry of PVDF GPE-modified membranes. Vibrational spectroscopy is the most important tool for studying the interactions between the different components of polymer electrolytes. FTIR Spectroscopy on the PVDF membrane was also used to determine the PVDF polymer’s beta phase (β-phase). The FTIR spectrum for the PVDF membrane is shown in Figure 1. The peak characteristics of pure PVDF membrane were identified at wavelengths of 1,414 cm−1 (CH2 functional group), 1,233 cm−1 (–CF-stretching), 1,176 cm−1 (–CF2-stretching), and 881 cm−1 (the vinylidene functional group of PVDF). The results of Deka and Kumar’s research showed that the peak characteristics of α-phase PVDF were 615 cm−1 (–CF2-bending and CCC skeletal bending) and 763 cm−1 (–CH2-rocking), while β-phase PVDF was observed at wavelengths of 840 and 510 cm−1 [20]. The wavelength of 840 cm−1 represents –CF2-stretching and –CH2-rocking, while the wavelength of 510 cm−1 represents the CF2 group. From the FTIR spectrum analysis of the PVDF/nano-clay/PVP membrane, the combined contribution of the nano-clay and carbonyl groups of PVP was observed at a wavelength of 1,661 cm−1 (C═O) due to the formation of hydrogen bonds (C═O…H═O). In the phase inversion process, the synergy between PVP and nano-clay influences the separation rate [21].
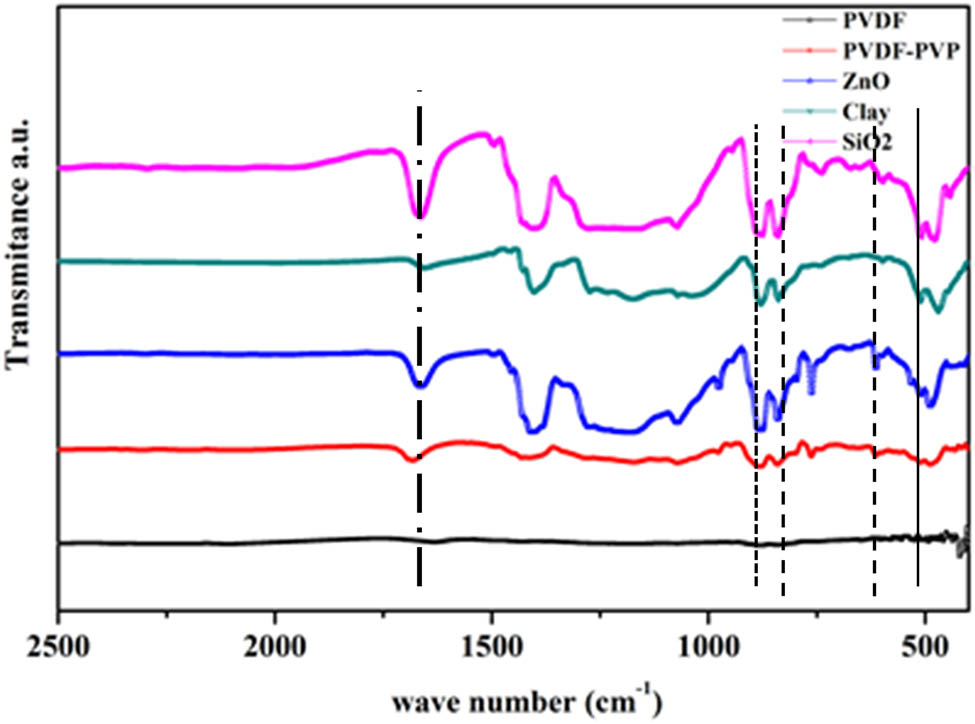
FTIR spectrum of PVDF GPE membranes with different fillers (nano-clay, ZnO, and SiO2 from fly ash waste).
The effect of the addition of the filler particles is shown in Figure 1. The visible peaks at wavelengths of 763 and 615 cm−1, which are the characteristics of the α-phase polymer PVDF, decreased, while the peaks at wavelengths of 834 and 512 cm−1, which are the characteristics of the β-phase PVDF, increased. The characteristics of the crystalline phase after the addition of filler exhibited the same tendency as the addition of nano-clay. Therefore, the accumulation of filler particles affects the change in the crystalline phase of the PVDF polymer from both α-phase and β-phase. This result is in line with Thakur’s research, which reported that adding clay particles into PVDF polymer can alter the phase’s crystalline properties, thereby changing the phase and increasing its concentration [22]. The addition of PVP did not show any significant difference in the characteristics of the α-phase and β-phase. From Figure 1, it is shown that adding any filler will change the PVDF to a crystal phase. These changes are observed in the increased F(β) value on the membrane. In addition, the change in the crystalline phase of PVDF decreased the degree of crystallinity of the PVDF polymer (Table 1). Therefore, the lower the crystallinity, the more the amorphous phases in the polymer increase the absorption of the membrane electrolyte.
3.3 Electrolyte uptake and ionic conductivity of gel polymer electrolyte
The porous membrane, activated with liquid electrolyte, has three phases: solid, gel, and adsorbed electrolyte liquid. The solid phase gives the structural membrane mechanical strength. Ion conductivity was significantly affected by the other two phases. The value of electrolyte absorption in the membrane is strongly influenced by the porosity and pore structure of the membrane [23]. Therefore, the design of pore structure of the membrane is the main factor in improving the ionic conductivity characteristics of GPEs. The type and concentration of fillers used to modify the membrane influence the porosity and absorption of ionic conductivity. In addition, the use of certain types of fillers enhances some specific characteristics of the separator [24,25,26]. Figures 2 and 3 show the relationship between the value of porosity (%), electrolyte uptake (%), and the ionic conductivity (mS cm−1) of GPEs in different filler types.

Porosity (%) and electrolyte uptake (%) of PVDF membrane with various types of filler.

Porosity (%) and ionic conductivity (mS cm−1) of PVDF membrane with various types of filler.
The GPEs with nano-clay filler particles have the porosity (87.03%), electrolyte uptake (801.69%), and ionic conductivity (5.62 mS cm−1) and showed the best at the addition of 8% by weight of nano-clay. When compared with nano-zinc oxide (ZnO) particles with the same concentration, it gave porosity value (82.74%), electrolyte absorption value (660.00%), and ionic conductivity (5.60 mS cm−1).
The addition of silicon oxide (SiO2) filler extracted from coal fly ash waste with the co-precipitation process in the same concentration (8 wt%) resulted in a porosity value of 82.74%, electrolyte uptake value of 660.00%, and ionic conductivity of 5.53 mS cm−1, which are lower than the results obtained with the other filler types. The superior properties of nano-clay particles are due to their layered structure.
The layered particle structure, which causes polymeric chain intercalation between the layers, is suitable for battery electrodes because it reduces the crystallinity of the membrane and increases its mechanical strength and heat resistance [27]. Furthermore, Meneghetti et al. found that nano-clay, which is well distributed in a polymer matrix, acts as a Lewis acid-base center because nano-clay forms complexes with lithium ions due to the negative charges on the surface [28]. Hence, the ion conductivity of lithium is increased due to the large number of free charge carriers generated from the interaction between lithium cations and polymer anions [29,30,31].
3.4 The electrochemical performance of the LiFePO4 batteries
A CR2032-type coin cell battery determined the effect of filler types and concentration on the electrochemical performance of the electrolytic separator membrane. Figure 4 shows the charge–discharge cycle of PVDF/PVP membranes of different filler types (nano-clay, ZnO, and SiO2) in LiFePO4/GPE/graphite batteries with a (C-rate) 0.2 C (0.4 mA). The flat second cycle charge–discharge graph shows the active cathode material’s reversible charge/discharge cycle.

(a) Specific capacity and (b) Coulombic efficiency of LiFePO4/GPE/graphite full cells at a charge–discharge rate 0.2 C (0.4 mA).
The specific discharge capacities at 0.2 C for the different filler types are nano-clay: 127.9094 mA h g−1; ZnO: 126.0627 mA h g−1; SiO2: 122.85 mA h g−1; celgard: 97.4256 mA h g−1, and for pure PVDF: 90.2056 mA h g−1. The specific discharge capacity is proportional to the ionic conductivity and electrolyte uptake of the membrane of different filler types. Out of the three fillers (nano clay, ZnO, and SiO2), the nano-clay has the best lithium-ion transfer rate between electrodes and the lowest interface resistance value, resulting in the highest discharge rate. Thus, indicating that GPEs with nano-clay fillers have better compatibility with electrodes and lower reactivity with lithium metal electrolytes [32,33].
Figures 5 and 6 show performance test results of the specific discharge capacity of the battery for different filler types at different discharge rates between 0.2 and 4 C. The figure demonstrates the cyclical behavior of recoverable charge and discharge rates of the LiFePO4 (LFP) cathode material for battery cells displayed in the following order: graphite-anode/separator/LFP-cathode. As depicted in the graph, the progressive decrease in potential discharge voltage results from the polarization of the battery electrode material. This behavior is due to the electrode’s increased resistance, which causes a slow lithium ion transfer rate [34].

The charge–discharge profiles of LiFePO4/GPE/graphite with current densities at a charge rate of 0.2 C and discharge rate of 0.2 C.

The discharge profiles of LiFePO4/GPE/graphite full cells at a different discharge rate condition.
4 Conclusion
GPE-based PVDF electrolyte membranes of different filler types have been fabricated with the phase inversion process of the non-solvent induced phase separation method. Different filler types were used in investigating the morphological characteristics, thermal structure, surface chemistry, membrane ionic conductivity, and electrochemical performance of GPE. The degree of porosity of GPEs with the addition of SiO2, ZnO, and nano-clay fillers was 82.05, 82.74, and 87.03%, respectively. The best porosity was with the addition of nano-clay filler (8 wt%). Furthermore, the PVDF/PVP membrane produced the highest ionic conductivity value of 5.62 mS cm−1 with the addition of a nano-clay filler. The full battery cell composed of graphite/separator/LiFePO4 provided the best performance using GPEs. The addition of nano-clay (8 wt%) resulted in a specific capacity of 127.9094 mA h g−1 at a rate of 0.2 C. Similarly, using celgard and PVDF as separators resulted in specific capacities of 97.4256 and 90.2056 mA h g−1, respectively.
GPE PVDF membranes with the different filler types showed good speed and cycle stability after 50 cycles. Therefore, all filler types used led to a targeted increase in the performance of GPE. The thermal characteristics, good ionic conductivity values, and high specific capacity performance results make nano-clay powder the best choice for filler in GPEs for lithium-ion batteries compared to ZnO and SiO2.
Acknowledgments
The authors express great appreciation to the Kemendikbudristek for the research grant awarded through the Penelitian Dasar Unggulan Perguruan Tinggi grant No. 11/E1/KP.PTNBH/2021 and No: 221.1/UN27.22/HK.07.00/2021. The authors also thank the Centre of Excellence for Electrical Energy Storage Technology, Universitas Sebelas Maret, for their help and support of this research work.
-
Author contributions: Conceptualization, ERD; data curation, ERD, TKP and ARF; Investigation, TKP; methodology: AGP and ARF; writing – original draft, TKA; writing – review and editing, ERD and ARF. All authors have read and accepted the published version of the manuscript.
-
Conflict of interest: The authors declare no competing financial interests.
-
Data availability statement: The datasets of this study are available on reasonable request from corresponding author.
References
[1] Perzyna K, Borkowska R, Syzdek J, Zalewska A, Wieczorek W. The effect of additive of Lewis acid type on lithium–gel electrolyte characteristics. Electrochim Acta. 2011;57:58–65.10.1016/j.electacta.2011.06.014Suche in Google Scholar
[2] Wang E, Chiu C-H, Chou P-H. Safety assessment of polyolefin and nonwoven separators used in lithium-ion batteries. J Power Sources. 2020;461:228148.10.1016/j.jpowsour.2020.228148Suche in Google Scholar
[3] Bandhauer TM, Garimella S, Fuller TF. A critical review of thermal issues in lithium-ion batteries. J Electrochem Soc. 2011;158:R1.10.1149/1.3515880Suche in Google Scholar
[4] Nazari A, Farhad S. Heat generation in lithium-ion batteries with different nominal capacities and chemistries. Appl Therm Eng. 2017;125:1501–17.10.1016/j.applthermaleng.2017.07.126Suche in Google Scholar
[5] Wang Y, Zhong W. Development of electrolytes towards achieving safe and high‐performance energy‐storage devices: a review. ChemElectroChem. 2015;2:22–36.10.1002/celc.201402277Suche in Google Scholar
[6] Mindemark J, Lacey MJ, Bowden T, Brandell D. Beyond PEO – alternative host materials for Li + -conducting solid polymer electrolytes. Prog Polym Sci. 2018;81:114–43.10.1016/j.progpolymsci.2017.12.004Suche in Google Scholar
[7] Du Pasquier A. Plastic PVDF-HFP electrolyte laminates prepared by a phase-inversion process. Solid State Ion. 2000;135:249–57.10.1016/S0167-2738(00)00371-4Suche in Google Scholar
[8] Wang M, Zhao F, Guo Z, Dong S. Poly(vinylidene fluoride-hexafluoropropylene)/organo-montmorillonite clays nanocomposite lithium polymer electrolytes. Electrochim Acta. 2004;49:3595–602.10.1016/j.electacta.2004.03.028Suche in Google Scholar
[9] Stephan AM, Nahm KS, Anbu Kulandainathan M, Ravi G, Wilson J. Poly(vinylidene fluoride-hexafluoropropylene) (PVdF-HFP) based composite electrolytes for lithium batteries. Eur Polym J. 2006;42:1728–34.10.1016/j.eurpolymj.2006.02.006Suche in Google Scholar
[10] Wachtler M, Ostrovskii D, Jacobsson P, Scrosati B. A study on PVdF-based SiO2-containing composite gel-type polymer electrolytes for lithium batteries. Electrochim Acta. 2004;50:357–61.10.1016/j.electacta.2004.01.103Suche in Google Scholar
[11] Costa CM, Silva MM, Lanceros-Méndez S. Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium ion battery applications. RSC Adv. 2013;3:11404.10.1039/c3ra40732bSuche in Google Scholar
[12] Dyartanti ER, Widiasa IN, Purwanto A, Susanto H. Nanocomposite polymer electrolytes in PVDF/ZnO membranes modified with PVP for use in LiFePo4 batteries. Evergreen. 2018;5:19–25.10.5109/1936213Suche in Google Scholar
[13] Dyartanti E, Purwanto A, Widiasa I, Susanto H. Ionic conductivity and cycling stability improvement of PVdF/nano-clay using PVP as polymer electrolyte membranes for LiFePO4 batteries. Membranes. 2018;8:36.10.3390/membranes8030036Suche in Google Scholar
[14] Raghavan P, Choi J-W, Ahn J-H, Cheruvally G, Chauhan GS, Ahn H-J, et al. Novel electrospun poly (vinylidene fluoride-co-hexafluoropropylene)–in situ SiO2 composite membrane-based polymer electrolyte for lithium batteries. J Power Sources. 2008;184:437–43.10.1016/j.jpowsour.2008.03.027Suche in Google Scholar
[15] Syifaurrahma A, Arnelli A, Astuti Y. LiOH/Coconut shell activated carbon ratio effect on the electrical conductivity of lithium ion battery anode active material. Molekul. 2021;16:235–43.10.20884/1.jm.2021.16.3.805Suche in Google Scholar
[16] Nawi NIM, Bilad MR, Nordin NAHM, Mavukkandy MO, Putra ZA, Wirzal MDH, et al. Exploiting the interplay between liquid-liquid demixing and crystallization of the pvdf membrane for membrane distillation. 2018;2018:1–10. Article ID 1525014.10.1155/2018/1525014Suche in Google Scholar
[17] Leo CJ, Thakur AK, Rao GVS, Chowdari BVR. Effect of glass–ceramic filler on properties of polyethylene oxide–LiCF3SO3 complex. J Power Sources. 2003;115:295–304.10.1016/S0378-7753(03)00007-7Suche in Google Scholar
[18] Sun H, Sohn H, Yamamoto O, Takeda Y, Imanishi N. Enhanced lithium‐ion transport in PEO‐based composite polymer electrolytes with ferroelectric BaTiO3. J Electrochem Soc. 1999;146:1672–6.10.1149/1.1391824Suche in Google Scholar
[19] Liu J, Wu X, He J, Li J, Lai Y. Preparation and performance of a novel gel polymer electrolyte based on poly (vinylidene fluoride)/graphene separator for lithium ion battery. Electrochim Acta. 2017;235:500–7.10.1016/j.electacta.2017.02.042Suche in Google Scholar
[20] Deka M, Kumar A. Electrical and electrochemical studies of poly(vinylidene fluoride)–clay nanocomposite gel polymer electrolytes for Li-ion batteries. J Power Sources. 2011;196:1358–64.10.1016/j.jpowsour.2010.09.035Suche in Google Scholar
[21] Chang X, Wang Z, Quan S, Xu Y, Jiang Z, Shao L. Exploring the synergetic effects of graphene oxide (GO) and polyvinylpyrrodione (PVP) on poly(vinylylidenefluoride) (PVDF) ultrafiltration membrane performance. Appl Surf Sci. 2014;316:537–48.10.1016/j.apsusc.2014.07.202Suche in Google Scholar
[22] Thakur P, Kool A, Bagchi B, Das S, Nandy P. Enhancement of β phase crystallization and dielectric behavior of kaolinite/halloysite modified poly (vinylidene fluoride) thin films. Appl Clay Sci. 2014;99:149–59.10.1016/j.clay.2014.06.025Suche in Google Scholar
[23] Liang S, Yan W, Wu X, Zhang Y, Zhu Y, Wang H. Gel polymer electrolytes for lithium ion batteries: Fabrication, characterization and performance. Solid State Ion. 2017;318:2–18.10.1016/j.ssi.2017.12.023Suche in Google Scholar
[24] Luo J, Fang C, Wu N. High polarity poly (vinylidene difluoride) thin coating for dendrite‐free and high‐performance lithium metal anodes. Adv Energy Mater. 2018;8:1701482.10.1002/aenm.201701482Suche in Google Scholar
[25] Arya A, Sharma AL. Polymer electrolytes for lithium ion batteries: a critical study. Ionics. 2017;23:497–540.10.1007/s11581-016-1908-6Suche in Google Scholar
[26] Lagadec MF, Zahn R, Wood V. Characterization and performance evaluation of lithium-ion battery separators. Nat Energy. 2019;4:16–25.10.1038/s41560-018-0295-9Suche in Google Scholar
[27] Shubha N, Prasanth R, Hoon HH, Srinivasan M. Dual phase polymer gel electrolyte based on non-woven poly(vinylidenefluoride-co-hexafluoropropylene)-layered clay nanocomposite fibrous membranes for lithium ion batteries. Mater Res Bull. 2013;48:526–37.10.1016/j.materresbull.2012.11.002Suche in Google Scholar
[28] Meneghetti P, Qutubuddin S, Webber A. Synthesis of polymer gel electrolyte with high molecular weight poly(methyl methacrylate)-clay nanocomposite. Electrochim Acta. 2004;49:4923–31.10.1016/j.electacta.2004.06.023Suche in Google Scholar
[29] He C, Liu J, Cui J, Li J, Wu X. A gel polymer electrolyte based on Polyacrylonitrile/organic montmorillonite membrane exhibiting dense structure for lithium ion battery. Solid State Ion. 2018;315:102–10.10.1016/j.ssi.2017.12.014Suche in Google Scholar
[30] Liu J, Liu M, He C, Li J, Li Q, Wang C, et al. Blending-based poly (vinylidene fluoride)/polymethyl methacrylate membrane for rechargeable lithium-ion batteries. Ionics. 2019;25:5201–11.10.1007/s11581-019-03060-ySuche in Google Scholar
[31] Chen H-W, Lin T-P, Chang F-C. Ionic conductivity enhancement of the plasticized PMMA/LiClO4 polymer nanocomposite electrolyte containing clay. Polymer. 2002;43:5281–8.10.1016/S0032-3861(02)00339-7Suche in Google Scholar
[32] Fang C, Yang S, Zhao X, Du P, Xiong J. Electrospun montmorillonite modified poly (vinylidene fluoride) nanocomposite separators for lithium-ion batteries. Mater Res Bull. 2016;79:1–7.10.1016/j.materresbull.2016.02.015Suche in Google Scholar
[33] Nunes-Pereira J, Kundu M, Gören A, Silva MM, Costa CM, Liu L, et al. Optimization of filler type within poly(vinylidene fluoride-co-trifluoroethylene) composite separator membranes for improved lithium-ion battery performance. Compos Part B Eng. 2016;96:94–102.10.1016/j.compositesb.2016.04.041Suche in Google Scholar
[34] Choi D, Kumta PN. Surfactant based sol–gel approach to nanostructured LiFePO4 for high rate Li-ion batteries. J Power Sources. 2007;163:1064–9.10.1016/j.jpowsour.2006.09.082Suche in Google Scholar
© 2022 Endah R. Dyartanti et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Performance of a horizontal well in a bounded anisotropic reservoir: Part I: Mathematical analysis
- Key competences for Transport 4.0 – Educators’ and Practitioners’ opinions
- COVID-19 lockdown impact on CERN seismic station ambient noise levels
- Constraint evaluation and effects on selected fracture parameters for single-edge notched beam under four-point bending
- Minimizing form errors in additive manufacturing with part build orientation: An optimization method for continuous solution spaces
- The method of selecting adaptive devices for the needs of drivers with disabilities
- Control logic algorithm to create gaps for mixed traffic: A comprehensive evaluation
- Numerical prediction of cavitation phenomena on marine vessel: Effect of the water environment profile on the propulsion performance
- Boundary element analysis of rotating functionally graded anisotropic fiber-reinforced magneto-thermoelastic composites
- Effect of heat-treatment processes and high temperature variation of acid-chloride media on the corrosion resistance of B265 (Ti–6Al–4V) titanium alloy in acid-chloride solution
- Influence of selected physical parameters on vibroinsulation of base-exited vibratory conveyors
- System and eco-material design based on slow-release ferrate(vi) combined with ultrasound for ballast water treatment
- Experimental investigations on transmission of whole body vibration to the wheelchair user's body
- Determination of accident scenarios via freely available accident databases
- Elastic–plastic analysis of the plane strain under combined thermal and pressure loads with a new technique in the finite element method
- Design and development of the application monitoring the use of server resources for server maintenance
- The LBC-3 lightweight encryption algorithm
- Impact of the COVID-19 pandemic on road traffic accident forecasting in Poland and Slovakia
- Development and implementation of disaster recovery plan in stock exchange industry in Indonesia
- Pre-determination of prediction of yield-line pattern of slabs using Voronoi diagrams
- Urban air mobility and flying cars: Overview, examples, prospects, drawbacks, and solutions
- Stadiums based on curvilinear geometry: Approximation of the ellipsoid offset surface
- Driftwood blocking sensitivity on sluice gate flow
- Solar PV power forecasting at Yarmouk University using machine learning techniques
- 3D FE modeling of cable-stayed bridge according to ICE code
- Review Articles
- Partial discharge calibrator of a cavity inside high-voltage insulator
- Health issues using 5G frequencies from an engineering perspective: Current review
- Modern structures of military logistic bridges
- Retraction
- Retraction note: COVID-19 lockdown impact on CERN seismic station ambient noise levels
- Special Issue: Trends in Logistics and Production for the 21st Century - Part II
- Solving transportation externalities, economic approaches, and their risks
- Demand forecast for parking spaces and parking areas in Olomouc
- Rescue of persons in traffic accidents on roads
- Special Issue: ICRTEEC - 2021 - Part II
- Switching transient analysis for low voltage distribution cable
- Frequency amelioration of an interconnected microgrid system
- Wireless power transfer topology analysis for inkjet-printed coil
- Analysis and control strategy of standalone PV system with various reference frames
- Special Issue: AESMT
- Study of emitted gases from incinerator of Al-Sadr hospital in Najaf city
- Experimentally investigating comparison between the behavior of fibrous concrete slabs with steel stiffeners and reinforced concrete slabs under dynamic–static loads
- ANN-based model to predict groundwater salinity: A case study of West Najaf–Kerbala region
- Future short-term estimation of flowrate of the Euphrates river catchment located in Al-Najaf Governorate, Iraq through using weather data and statistical downscaling model
- Utilization of ANN technique to estimate the discharge coefficient for trapezoidal weir-gate
- Experimental study to enhance the productivity of single-slope single-basin solar still
- An empirical formula development to predict suspended sediment load for Khour Al-Zubair port, South of Iraq
- A model for variation with time of flexiblepavement temperature
- Analytical and numerical investigation of free vibration for stepped beam with different materials
- Identifying the reasons for the prolongation of school construction projects in Najaf
- Spatial mixture modeling for analyzing a rainfall pattern: A case study in Ireland
- Flow parameters effect on water hammer stability in hydraulic system by using state-space method
- Experimental study of the behaviour and failure modes of tapered castellated steel beams
- Water hammer phenomenon in pumping stations: A stability investigation based on root locus
- Mechanical properties and freeze-thaw resistance of lightweight aggregate concrete using artificial clay aggregate
- Compatibility between delay functions and highway capacity manual on Iraqi highways
- The effect of expanded polystyrene beads (EPS) on the physical and mechanical properties of aerated concrete
- The effect of cutoff angle on the head pressure underneath dams constructed on soils having rectangular void
- An experimental study on vibration isolation by open and in-filled trenches
- Designing a 3D virtual test platform for evaluating prosthetic knee joint performance during the walking cycle
- Special Issue: AESMT-2 - Part I
- Optimization process of resistance spot welding for high-strength low-alloy steel using Taguchi method
- Cyclic performance of moment connections with reduced beam sections using different cut-flange profiles
- Time overruns in the construction projects in Iraq: Case study on investigating and analyzing the root causes
- Contribution of lift-to-drag ratio on power coefficient of HAWT blade for different cross-sections
- Geotechnical correlations of soil properties in Hilla City – Iraq
- Improve the performance of solar thermal collectors by varying the concentration and nanoparticles diameter of silicon dioxide
- Enhancement of evaporative cooling system in a green-house by geothermal energy
- Destructive and nondestructive tests formulation for concrete containing polyolefin fibers
- Quantify distribution of topsoil erodibility factor for watersheds that feed the Al-Shewicha trough – Iraq using GIS
- Seamless geospatial data methodology for topographic map: A case study on Baghdad
- Mechanical properties investigation of composite FGM fabricated from Al/Zn
- Causes of change orders in the cycle of construction project: A case study in Al-Najaf province
- Optimum hydraulic investigation of pipe aqueduct by MATLAB software and Newton–Raphson method
- Numerical analysis of high-strength reinforcing steel with conventional strength in reinforced concrete beams under monotonic loading
- Deriving rainfall intensity–duration–frequency (IDF) curves and testing the best distribution using EasyFit software 5.5 for Kut city, Iraq
- Designing of a dual-functional XOR block in QCA technology
- Producing low-cost self-consolidation concrete using sustainable material
- Performance of the anaerobic baffled reactor for primary treatment of rural domestic wastewater in Iraq
- Enhancement isolation antenna to multi-port for wireless communication
- A comparative study of different coagulants used in treatment of turbid water
- Field tests of grouted ground anchors in the sandy soil of Najaf, Iraq
- New methodology to reduce power by using smart street lighting system
- Optimization of the synergistic effect of micro silica and fly ash on the behavior of concrete using response surface method
- Ergodic capacity of correlated multiple-input–multiple-output channel with impact of transmitter impairments
- Numerical studies of the simultaneous development of forced convective laminar flow with heat transfer inside a microtube at a uniform temperature
- Enhancement of heat transfer from solar thermal collector using nanofluid
- Improvement of permeable asphalt pavement by adding crumb rubber waste
- Study the effect of adding zirconia particles to nickel–phosphorus electroless coatings as product innovation on stainless steel substrate
- Waste aggregate concrete properties using waste tiles as coarse aggregate and modified with PC superplasticizer
- CuO–Cu/water hybrid nonofluid potentials in impingement jet
- Satellite vibration effects on communication quality of OISN system
- Special Issue: Annual Engineering and Vocational Education Conference - Part III
- Mechanical and thermal properties of recycled high-density polyethylene/bamboo with different fiber loadings
- Special Issue: Advanced Energy Storage
- Cu-foil modification for anode-free lithium-ion battery from electronic cable waste
- Review of various sulfide electrolyte types for solid-state lithium-ion batteries
- Optimization type of filler on electrochemical and thermal properties of gel polymer electrolytes membranes for safety lithium-ion batteries
- Pr-doped BiFeO3 thin films growth on quartz using chemical solution deposition
- An environmentally friendly hydrometallurgy process for the recovery and reuse of metals from spent lithium-ion batteries, using organic acid
- Production of nickel-rich LiNi0.89Co0.08Al0.03O2 cathode material for high capacity NCA/graphite secondary battery fabrication
- Special Issue: Sustainable Materials Production and Processes
- Corrosion polarization and passivation behavior of selected stainless steel alloys and Ti6Al4V titanium in elevated temperature acid-chloride electrolytes
- Special Issue: Modern Scientific Problems in Civil Engineering - Part II
- The modelling of railway subgrade strengthening foundation on weak soils
- Special Issue: Automation in Finland 2021 - Part II
- Manufacturing operations as services by robots with skills
- Foundations and case studies on the scalable intelligence in AIoT domains
- Safety risk sources of autonomous mobile machines
- Special Issue: 49th KKBN - Part I
- Residual magnetic field as a source of information about steel wire rope technical condition
- Monitoring the boundary of an adhesive coating to a steel substrate with an ultrasonic Rayleigh wave
- Detection of early stage of ductile and fatigue damage presented in Inconel 718 alloy using instrumented indentation technique
- Identification and characterization of the grinding burns by eddy current method
- Special Issue: ICIMECE 2020 - Part II
- Selection of MR damper model suitable for SMC applied to semi-active suspension system by using similarity measures
Artikel in diesem Heft
- Regular Articles
- Performance of a horizontal well in a bounded anisotropic reservoir: Part I: Mathematical analysis
- Key competences for Transport 4.0 – Educators’ and Practitioners’ opinions
- COVID-19 lockdown impact on CERN seismic station ambient noise levels
- Constraint evaluation and effects on selected fracture parameters for single-edge notched beam under four-point bending
- Minimizing form errors in additive manufacturing with part build orientation: An optimization method for continuous solution spaces
- The method of selecting adaptive devices for the needs of drivers with disabilities
- Control logic algorithm to create gaps for mixed traffic: A comprehensive evaluation
- Numerical prediction of cavitation phenomena on marine vessel: Effect of the water environment profile on the propulsion performance
- Boundary element analysis of rotating functionally graded anisotropic fiber-reinforced magneto-thermoelastic composites
- Effect of heat-treatment processes and high temperature variation of acid-chloride media on the corrosion resistance of B265 (Ti–6Al–4V) titanium alloy in acid-chloride solution
- Influence of selected physical parameters on vibroinsulation of base-exited vibratory conveyors
- System and eco-material design based on slow-release ferrate(vi) combined with ultrasound for ballast water treatment
- Experimental investigations on transmission of whole body vibration to the wheelchair user's body
- Determination of accident scenarios via freely available accident databases
- Elastic–plastic analysis of the plane strain under combined thermal and pressure loads with a new technique in the finite element method
- Design and development of the application monitoring the use of server resources for server maintenance
- The LBC-3 lightweight encryption algorithm
- Impact of the COVID-19 pandemic on road traffic accident forecasting in Poland and Slovakia
- Development and implementation of disaster recovery plan in stock exchange industry in Indonesia
- Pre-determination of prediction of yield-line pattern of slabs using Voronoi diagrams
- Urban air mobility and flying cars: Overview, examples, prospects, drawbacks, and solutions
- Stadiums based on curvilinear geometry: Approximation of the ellipsoid offset surface
- Driftwood blocking sensitivity on sluice gate flow
- Solar PV power forecasting at Yarmouk University using machine learning techniques
- 3D FE modeling of cable-stayed bridge according to ICE code
- Review Articles
- Partial discharge calibrator of a cavity inside high-voltage insulator
- Health issues using 5G frequencies from an engineering perspective: Current review
- Modern structures of military logistic bridges
- Retraction
- Retraction note: COVID-19 lockdown impact on CERN seismic station ambient noise levels
- Special Issue: Trends in Logistics and Production for the 21st Century - Part II
- Solving transportation externalities, economic approaches, and their risks
- Demand forecast for parking spaces and parking areas in Olomouc
- Rescue of persons in traffic accidents on roads
- Special Issue: ICRTEEC - 2021 - Part II
- Switching transient analysis for low voltage distribution cable
- Frequency amelioration of an interconnected microgrid system
- Wireless power transfer topology analysis for inkjet-printed coil
- Analysis and control strategy of standalone PV system with various reference frames
- Special Issue: AESMT
- Study of emitted gases from incinerator of Al-Sadr hospital in Najaf city
- Experimentally investigating comparison between the behavior of fibrous concrete slabs with steel stiffeners and reinforced concrete slabs under dynamic–static loads
- ANN-based model to predict groundwater salinity: A case study of West Najaf–Kerbala region
- Future short-term estimation of flowrate of the Euphrates river catchment located in Al-Najaf Governorate, Iraq through using weather data and statistical downscaling model
- Utilization of ANN technique to estimate the discharge coefficient for trapezoidal weir-gate
- Experimental study to enhance the productivity of single-slope single-basin solar still
- An empirical formula development to predict suspended sediment load for Khour Al-Zubair port, South of Iraq
- A model for variation with time of flexiblepavement temperature
- Analytical and numerical investigation of free vibration for stepped beam with different materials
- Identifying the reasons for the prolongation of school construction projects in Najaf
- Spatial mixture modeling for analyzing a rainfall pattern: A case study in Ireland
- Flow parameters effect on water hammer stability in hydraulic system by using state-space method
- Experimental study of the behaviour and failure modes of tapered castellated steel beams
- Water hammer phenomenon in pumping stations: A stability investigation based on root locus
- Mechanical properties and freeze-thaw resistance of lightweight aggregate concrete using artificial clay aggregate
- Compatibility between delay functions and highway capacity manual on Iraqi highways
- The effect of expanded polystyrene beads (EPS) on the physical and mechanical properties of aerated concrete
- The effect of cutoff angle on the head pressure underneath dams constructed on soils having rectangular void
- An experimental study on vibration isolation by open and in-filled trenches
- Designing a 3D virtual test platform for evaluating prosthetic knee joint performance during the walking cycle
- Special Issue: AESMT-2 - Part I
- Optimization process of resistance spot welding for high-strength low-alloy steel using Taguchi method
- Cyclic performance of moment connections with reduced beam sections using different cut-flange profiles
- Time overruns in the construction projects in Iraq: Case study on investigating and analyzing the root causes
- Contribution of lift-to-drag ratio on power coefficient of HAWT blade for different cross-sections
- Geotechnical correlations of soil properties in Hilla City – Iraq
- Improve the performance of solar thermal collectors by varying the concentration and nanoparticles diameter of silicon dioxide
- Enhancement of evaporative cooling system in a green-house by geothermal energy
- Destructive and nondestructive tests formulation for concrete containing polyolefin fibers
- Quantify distribution of topsoil erodibility factor for watersheds that feed the Al-Shewicha trough – Iraq using GIS
- Seamless geospatial data methodology for topographic map: A case study on Baghdad
- Mechanical properties investigation of composite FGM fabricated from Al/Zn
- Causes of change orders in the cycle of construction project: A case study in Al-Najaf province
- Optimum hydraulic investigation of pipe aqueduct by MATLAB software and Newton–Raphson method
- Numerical analysis of high-strength reinforcing steel with conventional strength in reinforced concrete beams under monotonic loading
- Deriving rainfall intensity–duration–frequency (IDF) curves and testing the best distribution using EasyFit software 5.5 for Kut city, Iraq
- Designing of a dual-functional XOR block in QCA technology
- Producing low-cost self-consolidation concrete using sustainable material
- Performance of the anaerobic baffled reactor for primary treatment of rural domestic wastewater in Iraq
- Enhancement isolation antenna to multi-port for wireless communication
- A comparative study of different coagulants used in treatment of turbid water
- Field tests of grouted ground anchors in the sandy soil of Najaf, Iraq
- New methodology to reduce power by using smart street lighting system
- Optimization of the synergistic effect of micro silica and fly ash on the behavior of concrete using response surface method
- Ergodic capacity of correlated multiple-input–multiple-output channel with impact of transmitter impairments
- Numerical studies of the simultaneous development of forced convective laminar flow with heat transfer inside a microtube at a uniform temperature
- Enhancement of heat transfer from solar thermal collector using nanofluid
- Improvement of permeable asphalt pavement by adding crumb rubber waste
- Study the effect of adding zirconia particles to nickel–phosphorus electroless coatings as product innovation on stainless steel substrate
- Waste aggregate concrete properties using waste tiles as coarse aggregate and modified with PC superplasticizer
- CuO–Cu/water hybrid nonofluid potentials in impingement jet
- Satellite vibration effects on communication quality of OISN system
- Special Issue: Annual Engineering and Vocational Education Conference - Part III
- Mechanical and thermal properties of recycled high-density polyethylene/bamboo with different fiber loadings
- Special Issue: Advanced Energy Storage
- Cu-foil modification for anode-free lithium-ion battery from electronic cable waste
- Review of various sulfide electrolyte types for solid-state lithium-ion batteries
- Optimization type of filler on electrochemical and thermal properties of gel polymer electrolytes membranes for safety lithium-ion batteries
- Pr-doped BiFeO3 thin films growth on quartz using chemical solution deposition
- An environmentally friendly hydrometallurgy process for the recovery and reuse of metals from spent lithium-ion batteries, using organic acid
- Production of nickel-rich LiNi0.89Co0.08Al0.03O2 cathode material for high capacity NCA/graphite secondary battery fabrication
- Special Issue: Sustainable Materials Production and Processes
- Corrosion polarization and passivation behavior of selected stainless steel alloys and Ti6Al4V titanium in elevated temperature acid-chloride electrolytes
- Special Issue: Modern Scientific Problems in Civil Engineering - Part II
- The modelling of railway subgrade strengthening foundation on weak soils
- Special Issue: Automation in Finland 2021 - Part II
- Manufacturing operations as services by robots with skills
- Foundations and case studies on the scalable intelligence in AIoT domains
- Safety risk sources of autonomous mobile machines
- Special Issue: 49th KKBN - Part I
- Residual magnetic field as a source of information about steel wire rope technical condition
- Monitoring the boundary of an adhesive coating to a steel substrate with an ultrasonic Rayleigh wave
- Detection of early stage of ductile and fatigue damage presented in Inconel 718 alloy using instrumented indentation technique
- Identification and characterization of the grinding burns by eddy current method
- Special Issue: ICIMECE 2020 - Part II
- Selection of MR damper model suitable for SMC applied to semi-active suspension system by using similarity measures


