Abstract
C18H12O, triclinic,
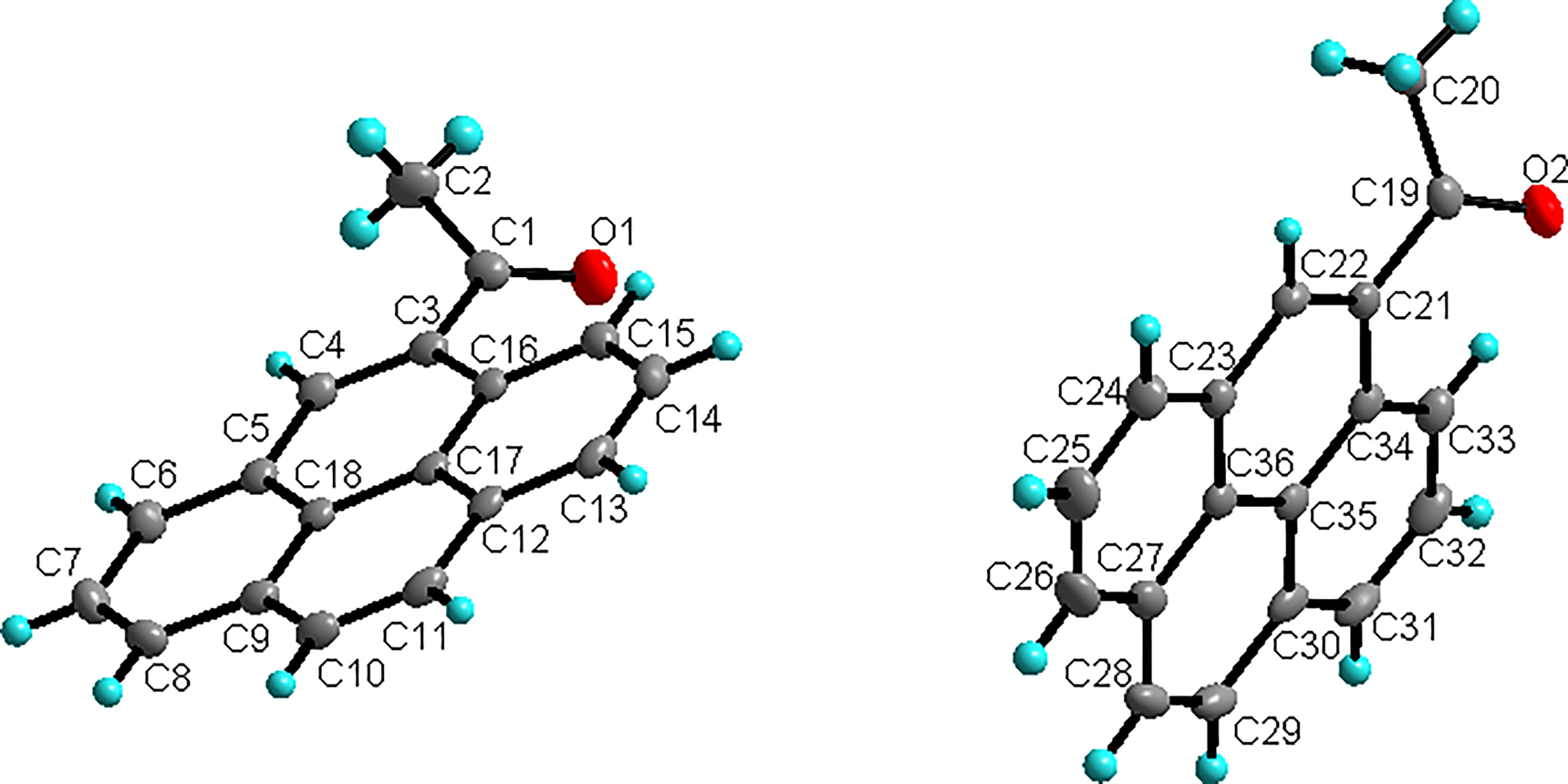
The asymmetric unit of the title crystal structure is shown in Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.10 × 0.08 × 0.08 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Bruker Apex II, φ and ω |
| θmax, completeness: | 27.5°, 97% |
| N(hkl)measured, N(hkl)unique, Rint: | 9761, 5251, 0.027 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 3742 |
| N(param)refined: | 345 |
| Programs: | Bruker [1], Shelx [2], Platon [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.59444 (9) | 0.16236 (9) | 0.05203 (8) | 0.0343 (2) |
| C1 | 0.66692 (12) | 0.19276 (11) | −0.01931 (10) | 0.0234 (3) |
| C2 | 0.60684 (13) | 0.23671 (14) | −0.13607 (11) | 0.0337 (3) |
| H2A | 0.5078 | 0.2239 | −0.1451 | 0.051* |
| H2B | 0.6239 | 0.1870 | −0.1972 | 0.051* |
| H2C | 0.6504 | 0.3273 | −0.1405 | 0.051* |
| C3 | 0.81763 (11) | 0.19112 (11) | 0.00927 (9) | 0.0193 (3) |
| C4 | 0.91177 (11) | 0.28638 (11) | −0.03050 (9) | 0.0200 (3) |
| H4 | 0.8785 | 0.3441 | −0.0808 | 0.024* |
| C5 | 1.05921 (11) | 0.30121 (11) | 0.00188 (9) | 0.0185 (2) |
| C6 | 1.15481 (12) | 0.40113 (11) | −0.03725 (10) | 0.0233 (3) |
| H6 | 1.1225 | 0.4617 | −0.0845 | 0.028* |
| C7 | 1.29674 (12) | 0.41111 (12) | −0.00663 (11) | 0.0267 (3) |
| H7 | 1.3591 | 0.4781 | −0.0335 | 0.032* |
| C8 | 1.34681 (12) | 0.32185 (12) | 0.06384 (10) | 0.0246 (3) |
| H8 | 1.4426 | 0.3294 | 0.0833 | 0.029* |
| C9 | 1.25569 (11) | 0.22064 (11) | 0.10624 (9) | 0.0197 (3) |
| C10 | 1.30359 (12) | 0.12492 (12) | 0.17701 (10) | 0.0228 (3) |
| H10 | 1.3990 | 0.1309 | 0.1979 | 0.027* |
| C11 | 1.21295 (12) | 0.02645 (12) | 0.21375 (10) | 0.0226 (3) |
| H11 | 1.2473 | −0.0344 | 0.2592 | 0.027* |
| C12 | 1.06446 (12) | 0.01286 (11) | 0.18461 (9) | 0.0193 (3) |
| C13 | 0.96960 (12) | −0.08923 (11) | 0.22113 (10) | 0.0231 (3) |
| H13 | 1.0024 | −0.1508 | 0.2667 | 0.028* |
| C14 | 0.82800 (12) | −0.10031 (11) | 0.19074 (10) | 0.0247 (3) |
| H14 | 0.7664 | −0.1692 | 0.2162 | 0.030* |
| C15 | 0.77563 (12) | −0.01004 (11) | 0.12252 (10) | 0.0222 (3) |
| H15 | 0.6795 | −0.0198 | 0.1023 | 0.027* |
| C16 | 0.86579 (11) | 0.09525 (10) | 0.08394 (9) | 0.0178 (2) |
| C17 | 1.01284 (11) | 0.10661 (10) | 0.11494 (9) | 0.0162 (2) |
| C18 | 1.10954 (11) | 0.21003 (10) | 0.07529 (9) | 0.0171 (2) |
| O2 | −0.37033 (9) | 0.14448 (9) | 0.55836 (8) | 0.0350 (2) |
| C19 | −0.29545 (12) | 0.14518 (11) | 0.48936 (10) | 0.0226 (3) |
| C20 | −0.35154 (12) | 0.06341 (12) | 0.37635 (10) | 0.0285 (3) |
| H20A | −0.3068 | −0.0061 | 0.3781 | 0.043* |
| H20B | −0.3329 | 0.1162 | 0.3126 | 0.043* |
| H20C | −0.4508 | 0.0278 | 0.3660 | 0.043* |
| C21 | −0.14643 (11) | 0.22500 (11) | 0.51765 (9) | 0.0187 (2) |
| C22 | −0.04921 (12) | 0.17927 (11) | 0.47795 (9) | 0.0201 (3) |
| H22 | −0.0796 | 0.1039 | 0.4287 | 0.024* |
| C23 | 0.09716 (12) | 0.24236 (11) | 0.50888 (10) | 0.0200 (3) |
| C24 | 0.19561 (12) | 0.19259 (12) | 0.47034 (10) | 0.0266 (3) |
| H24 | 0.1662 | 0.1157 | 0.4233 | 0.032* |
| C25 | 0.33696 (13) | 0.25644 (14) | 0.50134 (12) | 0.0334 (3) |
| H25 | 0.4017 | 0.2222 | 0.4752 | 0.040* |
| C26 | 0.38188 (13) | 0.37103 (14) | 0.57113 (11) | 0.0326 (3) |
| H26 | 0.4770 | 0.4126 | 0.5917 | 0.039* |
| C27 | 0.28734 (12) | 0.42572 (12) | 0.61139 (10) | 0.0254 (3) |
| C28 | 0.32979 (14) | 0.54629 (13) | 0.68121 (11) | 0.0314 (3) |
| H28 | 0.4243 | 0.5901 | 0.7022 | 0.038* |
| C29 | 0.23609 (13) | 0.59723 (12) | 0.71692 (10) | 0.0292 (3) |
| H29 | 0.2672 | 0.6758 | 0.7616 | 0.035* |
| C30 | 0.08917 (13) | 0.53329 (11) | 0.68770 (9) | 0.0236 (3) |
| C31 | −0.00893 (14) | 0.58545 (12) | 0.72430 (10) | 0.0286 (3) |
| H31 | 0.0205 | 0.6649 | 0.7676 | 0.034* |
| C32 | −0.14908 (14) | 0.52042 (12) | 0.69705 (10) | 0.0292 (3) |
| H32 | −0.2130 | 0.5561 | 0.7230 | 0.035* |
| C33 | −0.19622 (13) | 0.40255 (12) | 0.63146 (10) | 0.0244 (3) |
| H33 | −0.2913 | 0.3606 | 0.6139 | 0.029* |
| C34 | −0.10292 (12) | 0.34554 (11) | 0.59116 (9) | 0.0193 (3) |
| C35 | 0.04227 (12) | 0.41232 (11) | 0.61981 (9) | 0.0188 (3) |
| C36 | 0.14259 (11) | 0.35991 (11) | 0.58090 (9) | 0.0195 (3) |
Source of material
All chemicals were purchased from commercial sources and used as received without further purification. The title compound was prepared by two steps using 1,2,3,6,7,8-hexahydropyrene as the starting material. 0.427 g (3.2 mmol) of anhydrous AlCl3 and 30 mL of dichloromethane were added into a 100 mL three port flask at 0 °C. An amount of 0.251 g (3.2 mmol) acetyl chloride was dissolved in 10 mL dichloromethane, and the solution was added drop by drop into the flask under the protection of nitrogen and magnetic stirring. After the addition, the mixed solution was cooled to room temperature until it became clear, and then transferred to the low temperature bath. An amount of 0.56 g (2.7 mmol) of 1,2,3,6,7,8-hexahydropyrene dissolved in 20 mL dichloromethane was added drop by drop into the reaction solution. After the addition, it was moved to room temperature for 2 h. Then, the reaction solution was poured into 50 mL 10% hydrochloric acid solution for hydrolysis. After 2 h, the solution was separated. The aqueous layer was extracted three times with 20 mL dichloromethane and the organic phase was combined. The organic phase was washed with saturated NaCl solution and deionized water for three times, dried with anhydrous MgSO4, and dried to obtain light yellow solid. Using petroleum ether and dichloromethane as eluent, 0.5535 g of white solid was obtained through silica gel column. Yield: 81%, melting point: 71–73 °C. FTIR (KBr): 3045, 1675, 1597, 1558, 1419, and 1351 cm−1. 1 H NMR (400 MHz, CDCl3): 7.40 (s, 1H), 7.22 (q, J = 7.1 Hz, 2H), 3.30 (t, J = 6.1 Hz, 2H), 3.10 (dt, J = 9.2, 6.1 Hz, 6H), 2.67 (s, 3H), 2.17–1.95 (m, 4H). GC–MS: (C18H18O) m/z 250(M + , 100), 235(85), 207(70), 191(40), 179(42), 101(18).
The title compound was synthesized by dehydrogenation of the above intermediate 4-acetyl-1,2,3,6,7,8-hexahydropyrene as following: 0.29 g (1.16 mmol) of 4-acetyl-1,2,3,6,7,8-hexahydropyrene, 0.9216 g (4.06 mmol) of DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) and 30 mL of toluene were added into a 50 mL double flask with reflux device, the reaction mixture was heated and refluxed for 6 h under the protection of nitrogen. After the reaction, the filtrate was filtered, collected and dried. The solid was dissolved in 50 mL dichloromethane, washed with 5% NaOH solution and deionized water for three times, respectively, dried with anhydrous MgSO4, and rotary evaporated to obtain brown solid. Using dichloromethane and petroleum ether as eluent, 0.2182 g yellow plate crystals were was obtained by silica gel column separation and purification. Yield: 77%, melting point: 135–138 °C. Structural analysis: FTIR 3045, 1675, 1597, 1558, 1419, and 1351 cm−1. 1 H NMR (400 MHz, CDCl3): 7.40(s, 1H), 7.22 (q, J = 7.1 Hz, 2H), 3.30 (t, J = 6.1 Hz, 2H), 3.10 (dt, J = 9.2, 6.1 Hz, 6H), 2.67 (s, 3H), 2.17–1.95 (m, 4H). The yellow block crystals of the title compound were obtained by slow evaporation of ethanol/CHCl2 solution (v:v = 2/10).
Experimental details
All H atoms were introduced using the HFIX command in the Shelxl program [2], with the value of 0.93 Å or 0.96 Å for C–H bonds distances, respectively. All H atoms were allowed for as riding atoms with Uiso(H) = 1.2 Ueq(C) and Uiso(H) = 1.5 Ueq(C) for hydrogen atoms, respectively. The structure was checked using Platon [3].
Comment
The development of efficient optoelectronic materials based on polycyclic aromatic hydrocarbons has been extensively investigated in the past decades [4], [5], [6]. Indeed, various polycyclic aromatic hydrocarbons have been used in organic light-emitting diodes (OLEDs) [7], [8], [9], organic field effect transistor (OFET) [10, 11], organic lasers [12], chemosensors [13, 14], solar cells [15] and fluorescence probes [16, 17]. Pyrene and its derivatives are important members of polycyclic aromatic hydrocarbons which have displayed several advantages such as pure blue fluorescence with high quantum yield, exceptionally long fluorescence lifetime, excellent thermal stability and high charge carrier mobility [18, 19]. However, many pyrenes tend to form excimers in solid state or in concentrated solution through π–π stacking because of the flat structure of pyrene core, resulting in a red-shifted emission, the decrease of fluorescence quantum yield and the degrade of colour purity. To suppress the aggregation and improve performance, the most effective strategies involve both the control of the supramolecular order and the optimization of functional units through the introduction of peripheral attachments into the suitable positions of pyrene. Generally, the 1-, 3-, 6- and 8-positions of pyrene core preferentially undergo electrophilic aromatic substitution reactions. Thus, various 1-substituted pyrenes, 1,6-disubstituted, 1,8-disubstituted and 1,3,6,8-tetrasubstituted pyrenes with same group can be easily obtained [18, 20], [21], [22], [23]. There are very limited studies on 2- or 4-substituted pyrene because the position is not directly accessible by electrophilic substitution of pyrene itself, and only a few 2- or 4-substituted pyrenes have been obtained up to date [24, 25]. Recently, our group focused on the synthesis of new pyrene-based compounds with substituents on the non-active position of pyrene through indirect method via hydrogenation derivatives of pyrene [26], [27], [28]. As a continuation of our work, we synthesized one important pyrene-based compound 4-acetylpyrene using 1,2,3,6,7,8-hexahydropyrene as the starting material.
Similar to its derivative 2-acetylpyrene [28], there are two independent 4-acetylpyrene molecules in the crystal structure of the title compound. The acetyl functional group locates at the non-active 4-position of pyrene. The C–O bond length is 1.2203(14) Å for C1–O1 and 1.2211(14) Å for C19–O2 in the two 4-acetyl pyrene molecules, which is the typical double bond distance of an acetyl group. Different from its derivative 2-acetylpyrene for both independent title molecules, the methyl carbon and oxygen atoms from acetyl group are significantly deviate from the mean plane of carbon atoms of pyrene ring (see the Figure). One reason may be the space resistance of hydrogen atom at 1-position of pyrene. In one of the independent 4-acetylpyrene units, the three bond angles around the carbonyl group are 120.71(11)° for C3–C1–O1, 120.24(11)° for C2–C1–O1 and 119.02(11)° for C2–C1–C3, respectively. In the other unit, the corresponding angles are 120.50(11)° for C11–C19–O2, 120.09(11)° for C20–C19–O2 and 119.39(11)° for C21–C19–C20, respectively. The shortest distance between the adjacent pyrene planes of the two 4-acetylpyrene units is 3.362(2) and 3.390(2) Å, indicating that there are a relatively strong π–π interactions between the adjacent molecules of the title structure. The adjacent pyrene rings are almost completely parallel, forming a dimeric supramolecular structure. These dimeric supramolecular structures are linked together by relatively weak C–H⋯π interactions and C–H⋯O hydrogen bonds involving pyrene rings and carbonyl oxygen atoms, forming a three-dimensional supramolecular structure. The distances and angles of the typical bonds are similar to its derivative 2-acetylpyrene. The general supramolecular interactions is also similar to 2-acetylpyrene.
Funding source: China Postdoctoral Science Foundation
Award Identifier / Grant number: 13013655
Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (No. 13013655).
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: China Postdoctoral Science Foundation (No. 13013655).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SAINT, APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Spek, A. L. Single-crystal structure validation with the program Platon. J. Appl. Crystallogr. 2003, 36, 7–13; https://doi.org/10.1107/s0021889802022112.Search in Google Scholar
4. Richter, M. M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036; https://doi.org/10.1021/cr020373d.Search in Google Scholar PubMed
5. Jacob, J., Karcher, W., Belliardo, J. J., Wagstaffe, P. J. Polycyclic aromatic compounds of environmental and occupational importance. Fresenius Z. Anal. Chem. 1986, 323, 1–10; https://doi.org/10.1007/bf00531122.Search in Google Scholar
6. Yang, X., Xu, X., Zhou, G. Recent advances of the emitters for high performance deep-blue organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 913–944; https://doi.org/10.1039/c4tc02474e.Search in Google Scholar
7. Farinola, G. M., Ragni, R. Electroluminescent materials for white organic light emitting diodes. Chem. Soc. Rev. 2011, 40, 3467–3482; https://doi.org/10.1039/c0cs00204f.Search in Google Scholar PubMed
8. Tao, Y. T., Yang, C. L., Qin, J. G. Organic host materials for phosphorescent organic light-emitting diodes. Chem. Soc. Rev. 2011, 40, 2943–2970; https://doi.org/10.1039/c0cs00160k.Search in Google Scholar PubMed
9. Sasabe, H., Kido, J. J. Development of high performance OLEDs for general lighting. J. Mater. Chem. C 2013, 1, 1699–1707; https://doi.org/10.1039/c2tc00584k.Search in Google Scholar
10. Mei, J. G., Diao, Y., Appleton, A. L., Fang, L., Bao, Z. N. Integrated materials design of organic semiconductors for field-effect transistors. J. Am. Chem. Soc. 2013, 135, 6724–6746; https://doi.org/10.1021/ja400881n.Search in Google Scholar PubMed
11. Torsi, L., Magliulo, M., Manoli, K., Palazzo, G. Organic field-effect transistor sensors: a tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628; https://doi.org/10.1039/c3cs60127g.Search in Google Scholar PubMed
12. Samuel, I. D. W., Turnbull, G. A. Organic semiconductor lasers. Chem. Rev. 2007, 107, 1272–1295; https://doi.org/10.1021/cr050152i.Search in Google Scholar PubMed
13. Wu, J. S., Liu, W. M., Ge, J. C., Zhang, H. Y., Wang, P. F. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495; https://doi.org/10.1039/c0cs00224k.Search in Google Scholar PubMed
14. Duke, R. M., Veale, E. B., Pfeffer, F. M., Kruger, P. E., Gunnlaugsson, T. Colorimetric and fluorescent anion sensors: an overview of recent developments in the use of 1,8-naphthalimide-based chemosensors. Chem. Soc. Rev. 2010, 39, 3936–3953; https://doi.org/10.1039/b910560n.Search in Google Scholar PubMed
15. Ouared, I., Rekis, M., Trari, M. Phenothiazine based organic dyes for dye sensitized solar cells: a theoretical study on the role of pi-spacer. Dyes Pigments 2021, 190, 109330; https://doi.org/10.1016/j.dyepig.2021.109330.Search in Google Scholar
16. Ni, X. L., Zeng, X., Redshaw, C., Yamato, T. Ratiometric fluorescent receptors for both Zn2+ and H2PO−4 ions based on a pyrenyl-linked triazole-modified homooxacalix[3]arene: a potential molecular traffic signal with an R–S latch logic circuit. J. Org. Chem. 2011, 76, 5696–5702; https://doi.org/10.1021/jo2007303.Search in Google Scholar PubMed
17. Ahmed, N., Shirinfar, B., Geronimo, I., Kim, K. S. Fluorescent imidazolium-based cyclophane for detection of guanosine-5′-triphosphate and I− in aqueous solution of physiological pH. Org. Lett. 2011, 13, 5476–5479; https://doi.org/10.1021/ol202183t.Search in Google Scholar PubMed
18. Figueira-Duarte, T. M., Mullen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314; https://doi.org/10.1021/cr100428a.Search in Google Scholar PubMed
19. Zhang, R., Zhao, Y., Zhang, L.-F., Xu, L., Ni, Z.-H. A series of short axially symmetrically 1,3,6,8-tetrasubstituted pyrene-based green and blue emitters with 4-tert-butylphenyl and aryamine attachments. Dyes Pigments 2016, 130, 106–115; https://doi.org/10.1016/j.dyepig.2016.03.020.Search in Google Scholar
20. Wen, B., Xu, L.-H., Liu, N., Ni, Z.-H. Crystal structure and photochemical property of 1,8-bis (p-tolylthio) pyrene, C30H22S2. Z. Kristallogr. N. Cryst. Struct 2019, 234, 275–278.10.1515/ncrs-2018-0318Search in Google Scholar
21. Nie, J., Wei, J.-H., Ni, Z., Synthesis, H. Crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2. Z. Kristallogr. N. Cryst. Struct 2021, 236, 21–23.10.1515/ncrs-2020-0466Search in Google Scholar
22. Niko, Y., Sasaki, S., Narushima, K., Sharma, D. K., Vacha, M., Konishi, G. 1-, 3-, 6-, and 8–Tetrasubstituted asymmetric pyrene derivatives with electron donors and acceptors: high photostability and regioisomer-specific photophysical properties. J. Org. Chem. 2015, 80, 10794–10805; https://doi.org/10.1021/acs.joc.5b01987.Search in Google Scholar PubMed
23. Muangpaisal, R., Ho, M. C., Huang, T. H., Chen, C. H., Shen, J. Y., Ni, J. S., Lin, J. T., Ke, T. H., Chen, L. Y., Wu, C. C., Tsai, C. Tetrasubstituted-pyrene derivatives for electroluminescent application. Org. Electron. 2014, 15, 2148–2157; https://doi.org/10.1016/j.orgel.2014.06.003.Search in Google Scholar
24. Harvey, R. G., Schmolka, S., Cortez, C., Lee, H. Syntheses of 2-bromopyrene and 2-hydroxypyrene. Synth. Commun. 1988, 18, 2207–2209; https://doi.org/10.1080/00397918808082362.Search in Google Scholar
25. Kreyenschmidt, M., Baumgarten, M., Tyutyulko, N., Müllen, K. 2,2′-Bipyrenyl and para-terpyrenyl-a new type of electronically decoupled oligoarylene. Angew. Chem. Int. Ed. 1994, 33, 1957–1959; https://doi.org/10.1002/anie.199419571.Search in Google Scholar
26. Zhang, R., Han, F. F., Zhang, L. F. Crystal structure of 2-(4-methylbenzoyl)pyrene, C24H16O. Z. Kristallogr. N. Cryst. Struct 2016, 231, 855–857; https://doi.org/10.1515/ncrs-2016-0022.Search in Google Scholar
27. Xu, Y.-H., Miao, B.-X., Zhang, R. Crystal structure of 2-acetyl pyrene, C18H12O. Z. Kristallogr. N. Cryst. Struct 2019, 234, 369–371; https://doi.org/10.1515/ncrs-2018-0432.Search in Google Scholar
28. Li, J.-Y., Miao, B.-X., Zhao, Y., Zhang, L.-F. Crystal structure of 2-benzoylpyrene, C23H14O. Z. Kristallogr. N. Cryst. Struct 2020, 235, 547–549; https://doi.org/10.1515/ncrs-2019-0790.Search in Google Scholar
© 2021 Zhang Ran et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Redetermination of the crystal structure of 3-bromonitrobenzene at 200 K, C6H4BrNO2 – temperature effects on cell constants
- Crystal structure of (E)-ethyl 2-((4-oxo-4H-chromen-3-yl)methyleneaminooxy)acetate, C14H13NO5
- Crystal structure of (8R,10R,14R, Z)-2-((3–Fluoropyridin-4-yl) methylene)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6, 6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-3H-cyclopenta[a] phenanthren-3-one, C36H52FNO3
- Crystal structure of [6,6′-((1E,1′E)-(propane-1,3- diylbis(azaneylylidene))bis(methaneylylidene)) bis(3-chlorophenol)-κ4N,N′,O,O′] copper(II), C17H14Cl2CuN2O2
- The crystal structure of 6-amino-2-carboxypyridin-1-ium bromide, C6H7BrN2O2
- Redetermination of the crystal structure of bis[N,N′-ethylenebis(acetylacetoniminato)nickel(II)] sodium perchlorate, C24H36ClN4NaNi2O8
- The crystal structure of 3-methyl-2,6-dinitrophenol, C7H6N2O5
- The crystal structure of 5-chloro-2-(quinolin-8-yl)isoindoline-1,3-dione, C17H9ClN2O2
- Crystal structure of trans-tetraaqua-bis{2-carboxy-4-((5-carboxypyridin-3-yl)oxy)benzoato-κ1 N}cobalt(II) dihydrate C28H28O20N2Co
- Crystal structure of 3-allyl-4-(2-bromoethyl)-5-(4-methoxyphenyl)-2-(p-tolyl)furan, C23H23BrO2
- The crystal structure of 6,6′-(((2-(dimethylamino)ethyl)azanediyl)bis(methylene))bis(benzo[d][1,3]dioxol-5-ol ato-κ4N,N′,O,O′)-(pyridine-2,6-dicarboxylato-N,O,O′)-titanium(IV)-dichloromethane(1/1), C27H25N3O10Ti
- Crystal structure of (((1E,1′E)-1,2-phenylenebis(methaneylylidene))bis(hydrazin-1-yl-2-ylidene))bis(aminomethaniminium) dinitrate C10H16N10O6
- Crystal structure of catena-poly[triaqua-(μ 2-1,3-di(1H-imidazol-1-yl)propane-κ 2 N:N′)-(4,4′-(1H-1,2,4-triazole-3,5-diyl)dibenzoato-κ 1 O)nickel(II)]N,N′-dimethylformamide (1/1), C28H35N8O8Ni
- The crystal structure of 3,3′-[1,4-phenylenebis(methylene)]bis(1-ethenyl-1H-imidazol-3-ium) dichloride – dichloromethane – water (1/1/1), C19H24Cl4N4O1
- Crystal structure of 1,1′-(methane-1,1-diyl)bis(3-propyl-1H-imidazol-3-ium) bis(hexafluoridophosphate), C13H22F12N4P2
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)tin(IV), C12H8Cl4Sn
- Synthesis and crystal structure of 4-acetylpyrene, C18H12O
- Crystal structure of 2,2′-(butane-1,4-diylbis(azanylylidene))bis(methanylylidene))bis(4-methoxyphenol), C20H24N2O4
- The crystal structure of (E)-2-(((5-((triphenylstannyl)thio)-1,3,4-thiadiazol-2-yl)imino)methyl)phenol, C27H21N3OS2Sn
- Crystal structure of diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)lead(II) – N,N-dimethylformamide – water (1/2/4), C54H58N6O16Pb2
- Crystal structure of methyl 4-acetoxy-3-methoxybenzoate, C11H12O5
- Crystal structure of 2,2′-(propane-1,3-dilylbis(azaneylylidene))bis(methanylylidene)bis(4-methylphenol), C19H22N2O2
- Crystal structure of dichlorido-bis(4-methylphenyl-κC1)tin(IV), C14H14Cl2Sn
- Crystal structure of methyl (E)-3-(4-acetoxyphenyl)acrylate, C12H12O4
- The crystal structure of bis(benzoato-κ2 O,O′)-(2,9-dimethyl-1,10-phenanthroline-κ2 N,N′)-copper(II), C28H22CuN2O4
- Crystal structure of (8R,10R,14R,Z)-12-hydroxy-2-((6-methoxypyridin-2-yl)methylene)-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)hexadecahydro-3H-cyclopenta[a]phenanthren-3-one–water (2/1), C37H56NO4.5
- Crystal structure of dimethyl-bis(4-bromophenyl-κC1)tin(IV), C14H14Br2Sn
- The crystal structure of the cocrystal di-μ2-chlorido-octamethyl-di-μ3-oxido-bis(2,3,4,5-tetrafluorobenzoato-κ2 O,O′)tetratin(IV) ─ octamethyl-di-μ3-oxido-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O′)-bis(μ2-2,3,4,5-tetrafluorobenzoato-κ2 O:O;O′)tetratin(IV) C58H54Cl2F24O16Sn8
- Crystal structure of 3-iodo-N 2-(2-methyl-1-(methylsulfonyl)propan-2-yl)-N 1-(2-methyl-4-(perfluoropropan-2-yl)phenyl)phthalamide, C23H22F7I1N2O4S1
- Crystal structure of 1-(2-(4-bromophenyl)-2,3-dihydro-1H-benzo[e]indol-1-yl)-naphthalen-2-ol – dichloromethane – dimethyl sulfoxide (1/1/1), C28H18BrNO·CH2Cl2·C2H6SO
- Crystal structure of [meso-5,7,7,12,14,14,-hexamethyl-1,4,8,11-tetraazacyclotetradecane]nickel(II) diperchlorate – dimethylsulphoxide (1/2), C20H48Cl2N4NiO10S2
- Crystal structure of 1,1′-(1,3-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S:S) palladium(II), C26H18N6PdS4
- The crystal structure of bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C24H16N2O4Cu
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC)-bis(triphenylarsine oxide-κO)tin(IV), C48H38As2Cl4O2Sn
- Crystal structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane-κ 8 N 2, O 6) potassium cyclopentadienide, [K([2.2.2]crypt)]Cp, C23H41KN2O6
- The crystal structure of bis(2-oxidopyridin-1-ium-3-carboxylato-κ2O,O′)-(phenantroline-κ2N,N′)manganese(II) - methanol (1/3), C27H28N4O9Mn
- Crystal structure of 4-(dimethylamino)pyridinium dibromido-tris(4-chlorophenyl-κC)stannate(IV), C25H23Br2Cl3N2Sn
- Crystal structure of (3E,5E)-1-(4-cyanobenzenesulfonyl)-3,5-bis(3-fluorobenzylidene)piperidin-4-one-dichloromethane (1/1), C27H20Cl2F2N2O3S
- Crystal structure of (3E,5E)-3,5-bis(4-fluorobenzylidene)-1-((4-trifluoromethyl)benzenesulfonyl)piperidin-4-one, C26H18F5NO3S
- Crystal structure of chlorido-(4-methyl-2-((phenylimino)methyl)phenolato-κ2 N,O)-(pyridine-κ1 N)platinum(II), C19H17ClN2OPt
- Crystal structure of (4-methylbenzyl)(triphenyl)phosphonium chloride dihydrate, C26H28ClO2P
- The crystal structure of poly[μ2-chlorido-(μ2-1,2-bis(4-pyridyl)ethane-κ2N:N′silver(I)], C12H12AgClN2
- Crystal structure of poly[(μ4-benzene-1,2,4,5-tetracarboxylato)-bis(μ2-adipohydrazide)dicadmium], C11H15N4O6Cd
- The crystal structure of (E)-N′-(butan-2-ylidene)isonicotinohydrazide 0.5 hydrate C10H13N3O·0.5H2O
- The crystal structure of bis(6-phenylpyridine-2-carboxylate-κ2 N,O)-(2,2′-bipyridine-κ2 N,N′)zinc(II) monohydrate, C34H26N4O5Zn
- The crystal structure of (1R *,2S *)-1,2-bis(2-fluorophenyl)-3,8-dimethoxyacenaphthene-1,2-diol, C26H20F2O4
- Crystal structure of catena-poly[(μ2-1-((2-ethyl-4-methyl-1H-imidazol-1-yl)methyl)-1H-benzotriazole-κ2N:N′)-(nitrato-κ2O,O′)silver (I)], C13H15Ag1N6O3
- The crystal structure of [(phenantroline-κ2 N,N′)-bis(6-phenylpyridine-2-carboxylate-κ2 N,O)cobalt(II)]monohydrate, C36H26N4O5Co
- Crystal structure of (1E)-N′-[(1E)-1-(4-chlorophenyl)ethylidene]-2-[1-(4-chlorophenyl)ethylidene]hydrazine-1-carbohydrazonamide, C 17 H 17 Cl 2 N 5
- The crystal structure of (E)-2-((tert-butylimino)methyl)-4-chlorophenol, C11H14ClNO
- Crystal structure of all-cis-2,4,6-trihydroxycyclohexane- 1,3,5-triaminium chloride sulfate, C6H18ClN3O7S
- Crystal structure of dichlorido-bis(dimethyl sulfoxide-κO)bis(4-methylphenyl-κC 1)tin(IV), C18H26Cl2O2S2Sn
- Crystal structure of dichlorido-bis(4-chlorophenyl-κC 1)(2,2′-bipyridyl-κ 2 N,N′)tin(IV), C22H16Cl4N2Sn
- Redetermination of the crystal structure of (E)-5-bromo-2-hydroxybenzaldehyde oxime, C 7 H 6 BrNO 2
- The crystal structure of (E)-amino(2-(4-methylbenzylidene)hydrazineyl)methaniminium 4-methylbenzoate, C9H13N4 + C8H7O2 −
- Crystal structure of 2-chloro-3-(isopentylamino)naphthalene-1,4-dione, C 15 H 16 ClNO 2
- The crystal structure of bis(2-acetyl-5-methoxyphenyl)carbonate 1.5 hydrate, C19H18O7
- The crystal structure of poly[(μ 4-4,4′-(azanediylbis(methylene))dibenzoato-κ 4 O:N:O′:Oʺ)zinc(II)], C16H13NO4Zn
- The crystal structure of catena-poly[(1,10-phenanthroline-k2N,N′)-(μ3-tetraoxidomoybdato(VI)-k3O:O′:O″)manganese(II)] C12H8N2O4MoMn
- Crystal structure of catena-poly[(4-hydroxyl-5-(methoylcarbonyl)thiophene-2-carboxylato-κ1 O)-(μ2-piperazine-1,4-diylbis(pyridin-4-ylmethanone)-κ2 N:N′)silver(I)] monohydrate, C23H23AgN4O8S
- Crystal structure of bis(4-bromo-2-(((3-bromopropyl)imino)methyl)phenolato-κ2N,O)-oxido-vanadium(IV), C20H20Br4N2O3V
- The crystal structure of (2a′S,2a1′S,3R,5a′S,7′R)-5-(furan-3-yl)-2a′,2a1′-dihydroxy-7′-methyldecahydro-2H-spiro[furan-3,6′-naphtho[1,8-bc]furan]-2,2′(2a′H)-dione, C19H22O7
- The crystal structure of 3-bromopicolinic acid, C6H4BrNO2
- Crystal structure of 1,1′-(1,4-phenylenebis(methylene))bis(pyridin-1-ium) bis(1,2-dicyanoethene-1,2-dithiolato-κ2 S,S) platinum(II), C26H18N6PtS4
- Synthesis and crystal structure of 5-(8-((3-carboxyazetidin-1-ium-1-yl)methyl)-7-hydroxy-4-oxo-4H-chromen-3-yl)-2-hydroxybenzenesulfonate monohydrate, C20H19NO10S
- The crystal structure of 3-amino-5-carboxypyridin-1-ium bromide, C6H7BrN2O2
- The crystal structure of (2-hydroxy-5-methyl-phenyl)-(1H-pyrazol-4-yl)-methanone hemihydrate, C11H10.5N2O2.5
- Crystal structure of tetraaqua-(2-(4-formylphenoxy)acetato-k1O)cadmium(II), C18H22O12Cd
- Crystal structure of diethyl 6,12-dimethyl-3,9-di-p-tolyl-3,9-diazapentacyclo[6.4.0.02,7.04,11.05,10]dodecane-1,5-dicarboxylate, C32H38N2O4
- Crystal structure of (E)-N′-(1-(3-chloro-4-fluorophenyl)ethylidene)-4-hydroxy – tetrahydrofuran (2/1), C17H16ClFN2O2.5

