Abstract
The main purpose of this paper is to demonstrate the potential of time-domain nuclear magnetic resonance (TD-NMR) technology for monitoring the concentrations of metal ions in water-based solutions. The main focus of this work was paramagnetic ions, such as Mn2+, Cu2+, Fe3+, Fe2+, Zn2+ and Ni2+, which are often the principal metal components in mining waters. Laboratory samples of different concentrations of single metals and mixtures of them and samples of real mining water were used in the relaxation rate (R2) measurements. The measurements of single metal ions were used for the determination of the relaxivities of those ions. The concentrations of the ions in the mining water as a function of pH were also estimated by means of the X-ray fluorescence (XRF) method and ChemEQL software for calculating chemical speciation equilibria. Using these concentration values and the relaxivities of the metal ions, the total relaxation rate (R2) results were then calculated. Principally, the results of these three different determinations are in relatively good agreement. It can be concluded that TD-NMR has great potential for monitoring metal ion concentrations during water treatment.
1 Introduction
Tightening requirements for the environmental quality of mining and process waters require more efficient purification methods. However, today’s commercially available water quality measurements are not able to cope with certain significant contaminants such as metal and sulfate in real time. In addition, the presently used water quality monitoring systems require regular maintenance and calibration, which reduces their cost efficiency.
Chemical precipitation is a conventional technology used to treat mining waters[1]. Chemical precipitation processes involve the addition of chemical reagents, followed by the separation of precipitated solids from clean water. Typically, separation occurs in a clarifier, although separation by filtration or membranes is also possible. Chemical precipitation can also be applied in water pools, in which case the precipitated solids can simply be left at the bottom of the pool. Precipitation can be induced by the addition of an alkali, sulfide, coagulant, or other reagent that will bond with dissolved metal ions. Raising the pH with the use of alkaline reagents, such as sodium hydroxide, causes certain dissolved metals to precipitate as hydroxides.
Control of the effectiveness of heavy metal removal is essential in different applications [2, 3, 4]. It is important and necessary to use fast and accurate analytical methods that can be performed in real time. X-ray fluorescence (XRF) and atomic absorption spectrometry are typically used for heavy metal estimation. The nuclear magnetic resonance (NMR) technique could be an alternative to these methods. It was demonstrated that the limit of quantification of time-domain nuclear magnetic resonance (TD-NMR) is one order of magnitude larger than that of atomic absorption spectrometry; however, TD-NMR is a more robust and less expensive technique [5]. The main advantages of NMR are that it does not destroy the sample, it can be automated, and it is a non-fouling method.
High-resolution NMR has been extensively used in analytical chemistry, but in practice, it is difficult to apply to online process control due to its open magnetic field, large area of influence, high price and required cryogen use. On the other hand, the simpler TD-NMR method is becoming attractive for industrial applications due to its relatively low price, mobility, ease of operation, and simple sample preparation procedure [6, 7, 8].
TD-NMR is broadly applicable in practical use, and it could potentially be utilized for intensive water process characterization, as NMR relaxation rates are sensitive to paramagnetic ions present in solutions. For example,NMR relaxation times were used to determine the concentrations of various paramagnetic ions (Co2+, Cr3+, Cu2+, Fe3+ and Mn2+) [9, 10] and the solubility product of paramagnetic cations (Fe3+, Cu2+ and Mn2+) [11] and to investigate the precipitation of mixtures of paramagnetic ions as a function of pH [10, 11]. Several investigations were performed by in situ TD-NMR [9, 12] by placing the sample on the NMR probe, and the NMR parameters were measured continuously during the reaction or process.
Despite the mentioned advantages and broad applicability,TD-NMRis still rarely applied online [13].Monitoring of mining water during its processing was tested by online TD-NMR for the first time in a real mine [14]. TD-NMR was also recently applied to the online analysis of several liquid materials during different processes, such as fatty acid mixing [15, 16], starch gelatinization [17] and black liquor evaporation [18].Moreover, there is a high demand on process analytical tools in various industrial areas nowadays, and novel sensors, developed on the basis of NMR technology, could be beneficial [19, 20, 21].
In this paper, TD-NMR technology has been utilized for monitoring the concentrations of paramagnetic metal ions, such as Mn2+, Cu2+, Fe2+, Fe3+ and Zn2+, in water-based solutions. Different concentrations of single metals and mixtures of them and real mining water samples were used in the tests. First, the relaxivities of separate metal ions were determined by NMR. Then, the concentrations of the single metals were estimated by NMR during the precipitation reactions. Furthermore, the approach was demonstrated in the laboratory during the precipitation reactions of multiple ions in a simple mixture of metals and in real mining water. Additionally, simulation of the metal concentrations during this process was performed using ChemEQL software for calculating chemical speciation equilibria. Monitoring of the Mn2+ concentration in the output mining water was also demonstrated.
2 Materials and methods
2.1 Metal ion samples
Measurements were performed using samples made in a laboratory and samples of real mining water. The laboratory samples for the relaxivity measurements of single metal ions were made by adding compounds, including appropriate metal ions to drinking water. First, the pH of the water samples was adjusted to pH 1. Then, the concentrations of metal ions were varied so that their relaxivities could be estimated by a linear function. The measurements were performed for the following ions: Mn2+, Fe3+, Fe2+, Cu2+ and Ni2+. In addition, the relaxivities of Cu2+ were determined using two different Cu compounds, namely, CuSO4 and CuCl2.
For the mining water,the concentrations of metal ions were studied as a function of pH, which was increased by adding sodium hydroxide (NaOH). In the experiment, the pH level was increased from pH 4.6 to pH 12. The metal concentrations of the samples (inmg/l) were measured by the XRF method. Because the XRF method has certain problems detecting light atoms (e.g., Mg and Al), the concentrations were determined for Mn, Fe, Cu and Zn. In addition, the effects of Mg and Al ions on our NMR measurements are very small compared to those of other metal ions, and their concentrations are constant as a function of pH, which also supports the decision to leave these ions out of the analysis. The pH values and the metal concentrations of the samples are given in Table 1 and Figure 1.
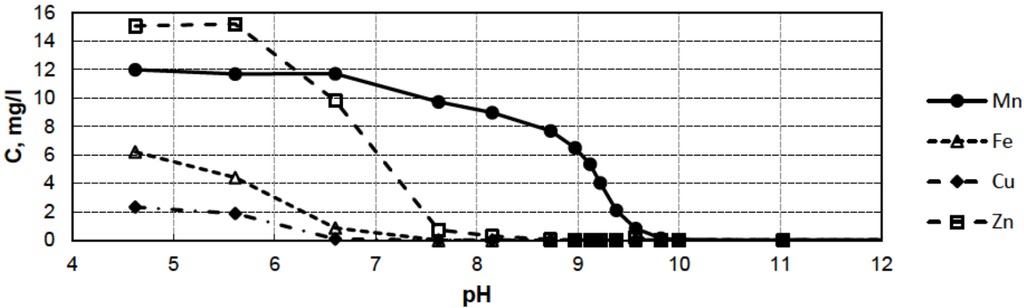
Concentrations of metal ions (Mn2+, Fe3+, Cu2+ and Zn2+) in mine water samples as a function of pH (based on the data from Table 1).
Concentrations of metals (Mn, Fe, Cu and Zn) in mine water samples (in mg/l) measured by XRF.
| pH | Mn mg/l | Fe mg/l | Cu mg/l | Zn mg/l |
|---|---|---|---|---|
| 4.62 | 11.98 | 6.206 | 2.325 | 15.051 |
| 5.61 | 11.689 | 4.399 | 1.873 | 15.194 |
| 6.6 | 11.705 | 0.853 | 0.081 | 9.812 |
| 7.62 | 8.971 | 0 | 0.008 | 0.294 |
| 8.15 | 7.678 | 0 | 0.005 | 0.045 |
| 8.73 | 6.49 | 0 | 0.006 | 0.018 |
| 8.97 | 5.349 | 0.015 | 0.014 | 0.013 |
| 9.12 | 4.021 | 0 | 0.009 | 0.013 |
| 9.22 | 2.1 | 0 | 0.007 | 0.005 |
| 9.38 | 0.82 | 0 | 0.005 | 0.203 |
| 9.57 | 0.149 | 0.008 | 0.007 | 0.007 |
| 9.82 | 0.036 | 0.013 | 0.01 | 0.017 |
| 10 | 0.011 | 0.018 | 0.006 | 0.007 |
| 11.03 | 0.01 | 0.011 | 0.005 | 0.01 |
2.2 TD-NMR
NMR is based on the absorption of energy by nuclei placed in a constant magnet field, which occurs under the resonance of the frequency of nuclei precession and the frequency of their excitation. In the NMR experiments, the registration of interactions of the magnetic moments of atomic nuclei with each other and their surroundings takes place. The main parameters measured by NMR are the spin-spin R2 and spin-lattice R1 relaxation rates, diffusion coefficients and signal magnitudes in the time domain and the Fourier spectra in the frequency domain. The measured parameters characterize the molecular properties and structure of the materials.
TD-NMR is sensitive to the presence of paramagnetic ions [22], which makes it a potentially applicable technology for measuring metal concentrations in water-intensive processes. In this study, the 1H resonance frequency of the system was 26 MHz, and the temperature of the magnet was 30∘C.
Transverse magnetization decays were measured by applying the Carr–Purcell–Meiboom–Gill (CPMG) pulse sequence [23, 24]. The echo time was 6 ms, and the number of 180∘ pulses in the sequence was 600. The relaxation delay was 6 s, and the number of scans was 4. The durations of the 90∘ and 180∘ RF pulses were 6.5 and 15 μs, respectively. The transverse magnetization decays of solutions with different Cu2+ concentrations are shown in Figure 2.
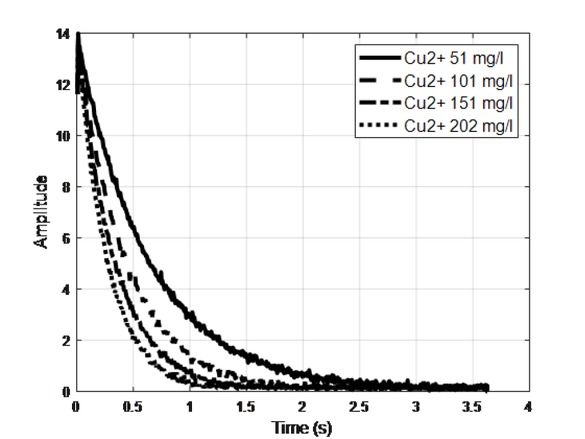
Four transverse magnetization decays of samples with Cu2+.
The relaxivity of metal ions reflects how the relaxation rates of a solution change as a function of its concentration C. As described in Bloembergen-Purcell-Pound (BPP) theory [22], the effect of paramagnetic ions is strong because their magnetic moments are higher than those of protons [10, 11, 22]. Since a metal ion may affect the two relaxation rates (R1 and R2) individually, there are two corresponding relaxivities, denoted by r1 and r2. By definition:
Since R1 and R2 are given in s−1 and C is measured in mg/l, r1 and r2 have units of l/mg s. constant1 and constant2 are the relaxation rates of clean water.The relaxivity depends on the temperature, field strength, and the substance in which the metal ion is dissolved.
Online measurements of metal ions were carried out using a TD-NMR system [25], which has been modified for flowing samples [15, 17, 18].AMATLAB software script written by the authors was used for controlling the pump, conducting the TD-NMR measurements, fitting the magnetization decays and calculating the relaxation rates.
The measurement procedure was as follows (Figure 3):
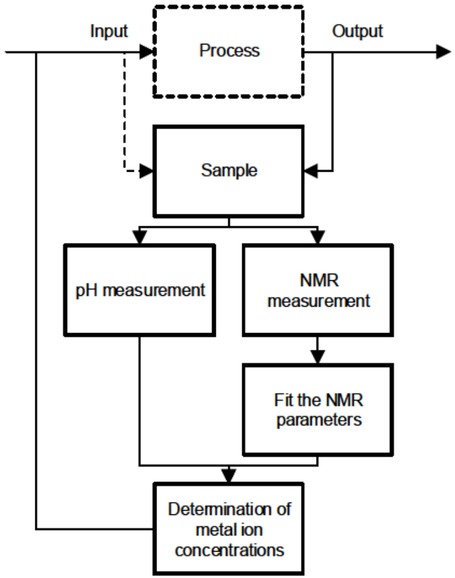
Flow chart of the measurement procedure.
An input or output sample of a water treatment process was delivered to a container, where it was continuously mixed.
The pH of the sample was measured.
The sample was automatically pumped through the magnet system. The pump was stopped. The transverse magnetization decay was measured.
The decay was fitted, and the NMR parameter was solved.
A model was applied to determine metals present in the sample.
The R2 and r2 values were used for determination of the metal ion concentrations via Equation 2.
A new sample was pumped in.
2.3 Calculation of chemical speciations
The concentrations of the metal ions as a function of pH were estimated by means of ChemEQL software [26],which is a computer program for calculating chemical speciation equilibria. The experimental concentrations of the metal ions at pH 4.62 (see Table 1) were used as the initial ion concentrations. Then, the concentration at each pH was estimated by the ChemEQL software; finally, the total relaxation rate R2 results were calculated as a function of pH using the following equation:
where n is the number of ions, the r2i values are from Table 2 and the concentration C is calculated by the software.
Relaxivity r2 results for metal ions obtained from a water-based solution by TD-NMR. Numbers in parentheses show the errors of the relaxivities.
| Metal ion | Relaxivity r2 (l/mg s) |
|---|---|
| Mn2+ | 0.81765(226) |
| Fe3+ | 0.28799(365) |
| Fe2+ | 0.01194(1) |
| Cu2+ (CuSO4) | 0.01493(10) |
| Cu2+ (CuCl2) | 0.01443(14) |
| Ni2+ | 0.01239(9) |
| Zn2+ | 0.00069(1) |
3 Results
3.1 Relaxivities of metals
In this paper, we focus on the relaxation rate R2 and relaxivity r2 results for several metal ions. The relaxivity measurements were conducted for the following ions: Mn2+, Fe3+, Fe2+, Cu2+, Zn2+ and Ni2+. In addition, the Cu2+ relaxivities were determined using two different chemicals. As an example, the correlation between the chemical concentration of Fe3+ and its relaxation rate R2 is presented in Figure 4, showing a very high correlation. The results of all the ions are collected in Table 2. In summary, the measurements for the concentrations of single metals are very accurate.
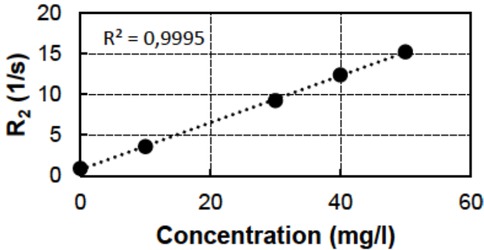
Relaxation rate R2 as a function of Fe3+ concentration.
3.2 Relaxation rates of metal mixtures
The participation of simple mixtures of metal ions was studied by TD-NMR in the laboratory. The relaxation rates of two mixtures of Mn2+ and Fe3+ ions in different concentrations
(Fe3+ 20 mg/l + Mn2+ 13 mg/l and Fe3+ 40 mg/l + Mn2+ 20 mg/l) as a function of pH are shown in Figure 5.
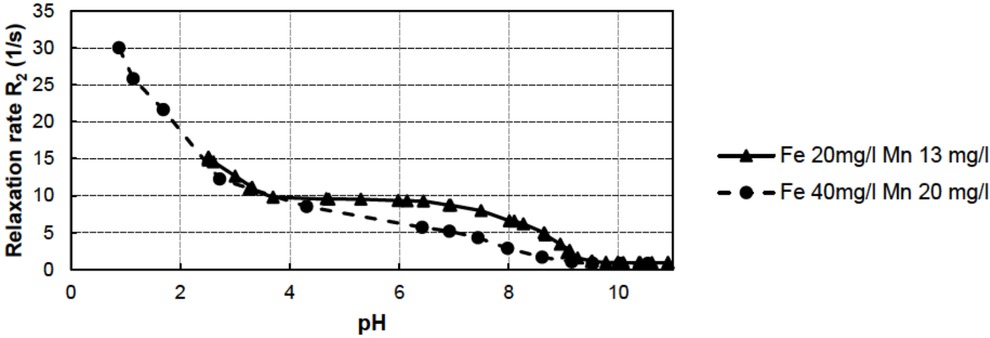
Relaxation rate R2 results for two different mixtures of Mn2+ and Fe3+ as a function of pH.
Remarkably, the relaxation rates of these two mixtures behave quite differently at pH values of 4–10. This is probably due to a change in the redox value of the mixtures. In the case of the latter mixture (Fe3+ 40 mg/l + Mn2+ 20 mg/l), the redox potential eventually increases so much that the precipitation of Mn2+ begins at a lower pH. The main conclusion to be drawn here is that the behaviour of mixtures of metal ions is, in general, observable by the TD-NMR method, even if the behaviour is controlled by changes in the redox potential of the solution.
3.3 Mining water measurements
In real-world conditions, metals in water are typically bound within mixtures of several metal components. Therefore, the relaxation rates of a real mining water sample were also measured, and the results were plotted as a function of pH. The experimental relaxation rates of the water are shown in Figure 6. (“Experimental”). The Mn, Fe, Cu and Zn concentrations of the samples were also measured by the XRF method. Using the concentration values in Table 1 and the relaxivities presented in Table 2, the corresponding relaxation rates were calculated using Equation 3 (Figure 6; “Calculated”). The error between the experimental and calculated R2 values was 2.9%, which indicates the good accuracy of the NMR method. It also confirms that the NMR effects of Al and Mg ions are small compared to those of other paramagnetic ions.
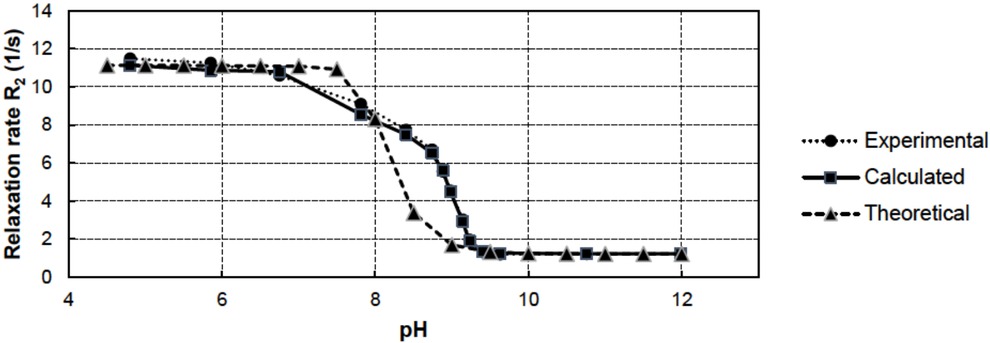
Total relaxation rate R2 results for mixtures of Mn2+, Cu2+, Fe3+, Fe2+ and Zn2+ in mining water as a function of pH.
In addition, the concentrations of metal ions were estimated by means of ChemEQL software [26]. Again, using these r2 values and Equation 3, the theoretical relaxation rates were calculated (Figure 6; “Theoretical”). All three determinations are in relativity good agreement. The results show that among the measured, calculated, and theoretical results, there is a more dramatic decrease in the relaxation rate at lower pH values for the NMR measurements, whereas the decrease in the relaxation rate of the theoretical calculations starts later. The results indicate that the difference between the experimental and theoretical values may arise from the redox reactions of the experimental samples.
3.4 Online measurements
The approach was tested in a real process: the estimation of metal ion contents in mining water during continuous purification. In the test shown in Figure 7, the change in pH of the output of this process ranges between 7 and 10. According to the data from Table 1, the concentration of only Mn2+ can be detected. All the other metals are minor concentrations at these pH levels, so they could be neglected. The Mn2+ concentration was calculated using Equation 2, and the relaxivity of Mn2+ is presented in Table 2. R2 = 1.27 s−1 at pH = 11.05 was used as constant2 in Equation 2.
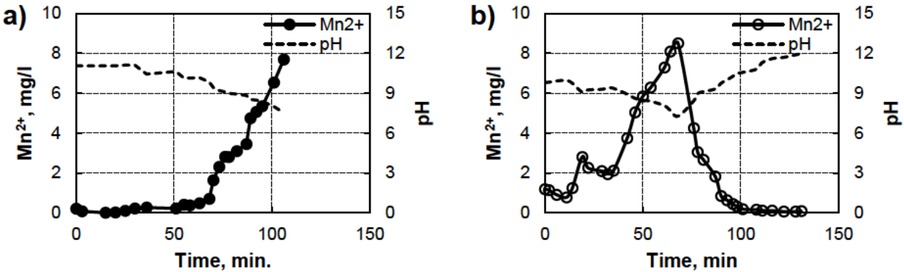
Concentration of Mn2+ in the output purified mining water as a function of pH, measured by TD-NMR, on day 1 (a) and day 2 (b).
4 Discussion
In this paper, TD-NMR technology was applied for monitoring the concentrations of Mn2+, Cu2+, Fe3+, Fe2+, Zn2+ and Ni2+ ions in water-based solutions. These paramagnetic ions are often the principal metal components in mining waters. XRF measurements and chemical speciation equilibria calculations were compared to the TD-NMR results. The results show the potential of TD-NMR for monitoring metal ion concentrations in process and mining water treatment.
Determination of the concentration of single paramagnetic ions in aqueous solution by TD-NMR relaxation is a robust approach, as previously demonstrated [9, 10, 11].Moreover, TD-NMR was applied to the online monitoring of the precipitation of metals in real mines [14], and several investigations were performed in situ by measurements of samples placed in standard NMR tubes or by single-sided NMR [9, 12]. However, TD-NMR technology has rarely been applied to the online determination of chemical concentrations in industrial environments.
The measurement of metals in liquid is a new field of research and business because reliable online determination methods for different kinds of metal ions in process and mining waters do not exist. The results of this work and earlier papers [14, 16, 17, 18] indicate that the online TD-NMR method is not sensitive to fouling. Specifically, TD-NMR was successfully applied for the determination of the content of solids in black liquor at a pulp mill in a longterm test [18]. This property may provide a cost benefit due to lower maintenance costs. When considering the potential for producing commercial products, the results of this approach may offer a starting point for achieving cost savings by reducing both energy consumption and chemical consumption.
Online measurement of metal concentrations will be the first step towards more efficient and optimized control of water-intensive processes. Better measurement and control enable the optimization of processes, making it possible to achieve optimal purification, energy savings via the optimization of mixing processes, and chemical savings via the optimization of chemical dosage.
In the long term, better management of metals in liquid makes it possible to reduce the environmental impacts of water-intensive processes; thus, the well-being of the people, plants and other point sources of release in a mine’s sphere of influence may improve.
5 Conclusion
The measuring system presented in this paper is applicable for continuous real-time monitoring of the concentrations of a number of paramagnetic ions in water and controlling the water purification processes. TD-NMR is a non-fouling technique and might be beneficial for use in harsh industrial environments, such as mines, and municipal or industrial wastewater treatment processes. The performance of the system in various water-intensive processes will be further tested.
Acknowledgement
This research is a part of the QualityMeas projects, which are funded by the Centre for Economic Development, Transport and the Environment and four companies.
References
[1] Fu F, Wang Q. Removal of heavy metal ions from wastewaters: a review. J Environ Manage. 2011 Mar;92(3):407–18.10.1016/j.jenvman.2010.11.011Suche in Google Scholar PubMed
[2] Nasiri EF, Kebria DY, Qaderi F. An Experimental Study on the Simultaneous Phenol and Chromium Removal From Water Using Titanium Dioxide Photocatalyst, Civil. Eng J (NY). 2018 Mar;4(3):585–93.10.28991/cej-0309117Suche in Google Scholar
[3] Beidokhti MZ, Naeeni ST. AbdiGhahroudi M.S., Biosorption of Nickel (II) from Aqueous Solutions onto Pistachio Hull Waste as a Low-Cost Biosorbent, Civil. Eng J (NY). 2019 Feb;5(2):447–57.10.28991/cej-2019-03091259Suche in Google Scholar
[4] Nkansah MA, Shamsu–Deen M, Opoku F. Phytocompounds, Heavy Metal and Mineral Contents in honey Samples from Selected Markets in the Kumasi Metropolis. Emerging Science Journal. 2018 Oct;2(5):287–94.10.28991/esj-2018-01152Suche in Google Scholar
[5] Gomes B.F., da Silva Burato J.S., Silva Lobo C.M, and Colnago L.A., Use of the Relaxometry Technique for Quantification of Paramagnetic Ions in Aqueous Solutions and a Comparison with Other Analytical Methods, Hindawi Publishing Corporation International Journal of Analytical Chemistry Volume 2016, Article ID 8256437, 5 pages, DOI: https://doi.org/10.1155/2016/825643710.1155/2016/8256437Suche in Google Scholar PubMed PubMed Central
[6] Dalitz F, Cudaj M, Maiwald M, Guthausen G. Process and reaction monitoring by low-field NMR spectroscopy. Prog Nucl Magn Reson Spectrosc. 2012 Jan;60:52–70.10.1016/j.pnmrs.2011.11.003Suche in Google Scholar PubMed
[7] Mitchell J, Gladden LF, Chandrasekera TC, Fordham EJ. Low-field permanent magnets for industrial process and quality control. Prog Nucl Magn Reson Spectrosc. 2014 Jan;76:1–60.10.1016/j.pnmrs.2013.09.001Suche in Google Scholar PubMed
[8] Blümich B, Casanova F, Appelt S. NMR at low magnetic fields. Chem Phys Lett. 2009;477(4-6):231–40.10.1016/j.cplett.2009.06.096Suche in Google Scholar
[9] Mitreiter I, Oswald SE, Stallmach F. Investigation of Iron(III)-Release in the Pore Water of Natural Sands by NMR Relaxometry. Open Magn Reson J. 2010;3(1):46–51.10.2174/1874769801003010046Suche in Google Scholar
[10] Kock FV,Machado MP, Athayde GP, Colnago LA, Barbosa LL. Quantification of paramagnetic ions in solution using time domain NMR. PROS and CONS to optical emission spectrometry method. Microchem J. 2018;137:204–7.10.1016/j.microc.2017.10.013Suche in Google Scholar
[11] Cobra PF, Gomes BF, Mitre CI, Barbosa LL,Marconcini LV, Colnago LA. Measuring the solubility product constant of paramagnetic cations using time-domain nuclear magnetic resonance relaxometry. Microchem J. 2015;121:14–7.10.1016/j.microc.2015.02.002Suche in Google Scholar
[12] Gomes BF, Nunes LM, Lobo CM, Carvalho AS, Cabeça LF, Colnago LA. In situ analysis of copper electrodeposition reaction using unilateral NMR sensor. J Magn Reson. 2015 Dec;261:83–6.10.1016/j.jmr.2015.09.018Suche in Google Scholar PubMed
[13] Colnago LA, Andrade FD, Souza AA, Azeredo RB, Lima AA, Cerioni LM, et al. Why is Inline NMR Rarely Used as Industrial Sensor? Challenges and Opportunities. Chem Eng Technol. 2014;37(2):191–203.10.1002/ceat.201300380Suche in Google Scholar
[14] Nikolskaya E, Liukkonen M, Kankkunen J, Hiltunen Y. A non-fouling online method for monitoring precipitation of metal ions in mine waters, IFAC Proceedings Volumes, 2015, 48-17, 98–101, DOI:https://doi.org/10.1016/j.ifacol.2015.10.08510.1016/j.ifacol.2015.10.085Suche in Google Scholar
[15] Nikolskaya E, Hiltunen Y. Determination of Carbon Chain Lengths of Fatty Acid Mixtures by Time Domain NMR. Appl Magn Reson. 2018;49(2):185–93.10.1007/s00723-017-0953-2Suche in Google Scholar PubMed PubMed Central
[16] Nikolskaya E, Hiltunen Y. Molecular Properties of Fatty Acid Mixtures Estimated by Online Time-Domain NMR. Appl Magn Reson. 2019;50(1-3):159–70.10.1007/s00723-018-1046-6Suche in Google Scholar
[17] Raunio J, Nikolskaya E, Hiltunen Y. On-line monitoring of cationic starch gelatinization and retrogradation by 1H NMR-relaxometry. Nord Pulp Paper Res J. 2018;33(4):625–31.10.1515/npprj-2018-0010Suche in Google Scholar
[18] Nikolskaya E, Janhunen P, Haapalainen M, Hiltunen Y. Solids content of black liquor measured by online Time-Domain NMR. Appl Sci (Basel). 2019;9(10):2169.10.3390/app9102169Suche in Google Scholar
[19] Maiwald M, Gräßer P, Wander L, Zientek N, Guhl S, Meyer K, et al. Strangers in the Night—Smart Process Sensors in Our Current Automation Landscape. Proceedings. 2017;1(4):628.10.3390/proceedings1040628Suche in Google Scholar
[20] Eisen K, Eifert T, Herwig C,Maiwald M. Current and future requirements to industrial analytical infrastructure-part 1: process analytical laboratories. Anal Bioanal Chem. 2020 Apr;412(9):2027– 35.10.1007/s00216-020-02420-2Suche in Google Scholar PubMed PubMed Central
[21] Eifert T, Eisen K, Maiwald M, Herwig C. Current and future requirements to industrial analytical infrastructure-part 2: smart sensors. Anal Bioanal Chem. 2020 Apr;412(9):2037–45.10.1007/s00216-020-02421-1Suche in Google Scholar PubMed PubMed Central
[22] Bloembergen N, Purcell E, Pound R. Relaxation Effects in Nuclear Magnetic Resonance Absorption. Phys Rev. 1948;73(7):679–712.10.1142/9789814540223_0039Suche in Google Scholar
[23] Carr H, Purcell E. Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys Rev. 1954;94(3):630–8.10.1103/PhysRev.94.630Suche in Google Scholar
[24] Meiboom S, Gill D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev Sci Instrum. 1958;29(8):688–91.10.1063/1.1716296Suche in Google Scholar
[25] Web pages of Resonance Systems, http://www.nmr-design.com accessed 2018, March 28.Suche in Google Scholar
[26] Web pages of Eawag - Swiss Federal Institute of Aquatic Science and Technology, https://www.eawag.ch/en/department/surf/projects/chemeql/ accessed 2019, March 28.Suche in Google Scholar
© 2020 E. Nikolskaya et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Fabrication of aluminium covetic casts under different voltages and amperages of direct current
- Inhibition effect of the synergistic properties of 4-methyl-norvalin and 2-methoxy-4-formylphenol on the electrochemical deterioration of P4 low carbon mold steel
- Logistic regression in modeling and assessment of transport services
- Design and development of ultra-light front and rear axle of experimental vehicle
- Enhancement of cured cement using environmental waste: particleboards incorporating nano slag
- Evaluating ERP System Merging Success In Chemical Companies: System Quality, Information Quality, And Service Quality
- Accuracy of boundary layer treatments at different Reynolds scales
- Evaluation of stabiliser material using a waste additive mixture
- Optimisation of stress distribution in a highly loaded radial-axial gas microturbine using FEM
- Analysis of modern approaches for the prediction of electric energy consumption
- Surface Hardening of Aluminium Alloy with Addition of Zinc Particles by Friction Stir Processing
- Development and refinement of the Variational Method based on Polynomial Solutions of Schrödinger Equation
- Comparison of two methods for determining Q95 reference flow in the mouth of the surface catchment basin of the Meia Ponte river, state of Goiás, Brazil
- Applying Intelligent Portfolio Management to the Evaluation of Stalled Construction Projects
- Disjoint Sum of Products by Orthogonalizing Difference-Building ⴱ
- The Development of Information System with Strategic Planning for Integrated System in the Indonesian Pharmaceutical Company
- Simulation for Design and Material Selection of a Deep Placement Fertilizer Applicator for Soybean Cultivation
- Modeling transportation routes of the pick-up system using location problem: a case study
- Pinless friction stir spot welding of aluminium alloy with copper interlayer
- Roof Geometry in Building Design
- Review Articles
- Silicon-Germanium Dioxide and Aluminum Indium Gallium Arsenide-Based Acoustic Optic Modulators
- RZ Line Coding Scheme With Direct Laser Modulation for Upgrading Optical Transmission Systems
- LOGI Conference 2019
- Autonomous vans - the planning process of transport tasks
- Drivers ’reaction time research in the conditions in the real traffic
- Design and evaluation of a new intersection model to minimize congestions using VISSIM software
- Mathematical approaches for improving the efficiency of railway transport
- An experimental analysis of the driver’s attention during train driving
- Risks associated with Logistics 4.0 and their minimization using Blockchain
- Service quality of the urban public transport companies and sustainable city logistics
- Charging electric cars as a way to increase the use of energy produced from RES
- The impact of the truck loads on the braking efficiency assessment
- Application of virtual and augmented reality in automotive
- Dispatching policy evaluation for transport of ready mixed concrete
- Use of mathematical models and computer software for analysis of traffic noise
- New developments on EDR (Event Data Recorder) for automated vehicles
- General Application of Multiple Criteria Decision Making Methods for Finding the Optimal Solution in City Logistics
- The influence of the cargo weight and its position on the braking characteristics of light commercial vehicles
- Modeling the Delivery Routes Carried out by Automated Guided Vehicles when Using the Specific Mathematical Optimization Method
- Modelling of the system “driver - automation - autonomous vehicle - road”
- Limitations of the effectiveness of Weigh in Motion systems
- Long-term urban traffic monitoring based on wireless multi-sensor network
- The issue of addressing the lack of parking spaces for road freight transport in cities - a case study
- Simulation of the Use of the Material Handling Equipment in the Operation Process
- The use of simulation modelling for determining the capacity of railway lines in the Czech conditions
- Proposals for Using the NFC Technology in Regional Passenger Transport in the Slovak Republic
- Optimisation of Transport Capacity of a Railway Siding Through Construction-Reconstruction Measures
- Proposal of Methodology to Calculate Necessary Number of Autonomous Trucks for Trolleys and Efficiency Evaluation
- Special Issue: Automation in Finland
- 5G Based Machine Remote Operation Development Utilizing Digital Twin
- On-line moisture content estimation of saw dust via machine vision
- Data analysis of a paste thickener
- Programming and control for skill-based robots
- Using Digital Twin Technology in Engineering Education – Course Concept to Explore Benefits and Barriers
- Intelligent methods for root cause analysis behind the center line deviation of the steel strip
- Engaging Building Automation Data Visualisation Using Building Information Modelling and Progressive Web Application
- Real-time measurement system for determining metal concentrations in water-intensive processes
- A tool for finding inclusion clusters in steel SEM specimens
- An overview of current safety requirements for autonomous machines – review of standards
- Expertise and Uncertainty Processing with Nonlinear Scaling and Fuzzy Systems for Automation
- Towards online adaptation of digital twins
- Special Issue: ICE-SEAM 2019
- Fatigue Strength Analysis of S34MnV Steel by Accelerated Staircase Test
- The Effect of Discharge Current and Pulse-On Time on Biocompatible Zr-based BMG Sinking-EDM
- Dynamic characteristic of partially debonded sandwich of ferry ro-ro’s car deck: a numerical modeling
- Vibration-based damage identification for ship sandwich plate using finite element method
- Investigation of post-weld heat treatment (T6) and welding orientation on the strength of TIG-welded AL6061
- The effect of nozzle hole diameter of 3D printing on porosity and tensile strength parts using polylactic acid material
- Investigation of Meshing Strategy on Mechanical Behaviour of Hip Stem Implant Design Using FEA
- The effect of multi-stage modification on the performance of Savonius water turbines under the horizontal axis condition
- Special Issue: Recent Advances in Civil Engineering
- The effects of various parameters on the strengths of adhesives layer in a lightweight floor system
- Analysis of reliability of compressed masonry structures
- Estimation of Sport Facilities by Means of Technical-Economic Indicator
- Integral bridge and culvert design, Designer’s experience
- A FEM analysis of the settlement of a tall building situated on loess subsoil
- Behaviour of steel sheeting connections with self-drilling screws under variable loading
- Resistance of plug & play N type RHS truss connections
- Comparison of strength and stiffness parameters of purlins with different cross-sections of profiles
- Bearing capacity of floating geosynthetic encased columns (GEC) determined on the basis of CPTU penetration tests
- The effect of the stress distribution of anchorage and stress in the textured layer on the durability of new anchorages
- Analysis of tender procedure phases parameters for railroad construction works
- Special Issue: Terotechnology 2019
- The Use of Statistical Functions for the Selection of Laser Texturing Parameters
- Properties of Laser Additive Deposited Metallic Powder of Inconel 625
- Numerical Simulation of Laser Welding Dissimilar Low Carbon and Austenitic Steel Joint
- Assessment of Mechanical and Tribological Properties of Diamond-Like Carbon Coatings on the Ti13Nb13Zr Alloy
- Characteristics of selected measures of stress triaxiality near the crack tip for 145Cr6 steel - 3D issues for stationary cracks
- Assessment of technical risk in maintenance and improvement of a manufacturing process
- Experimental studies on the possibility of using a pulsed laser for spot welding of thin metallic foils
- Angular position control system of pneumatic artificial muscles
- The properties of lubricated friction pairs with diamond-like carbon coatings
- Effect of laser beam trajectory on pocket geometry in laser micromachining
- Special Issue: Annual Engineering and Vocational Education Conference
- The Employability Skills Needed To Face the Demands of Work in the Future: Systematic Literature Reviews
- Enhancing Higher-Order Thinking Skills in Vocational Education through Scaffolding-Problem Based Learning
- Technology-Integrated Project-Based Learning for Pre-Service Teacher Education: A Systematic Literature Review
- A Study on Water Absorption and Mechanical Properties in Epoxy-Bamboo Laminate Composite with Varying Immersion Temperatures
- Enhancing Students’ Ability in Learning Process of Programming Language using Adaptive Learning Systems: A Literature Review
- Topical Issue on Mathematical Modelling in Applied Sciences, III
- An innovative learning approach for solar power forecasting using genetic algorithm and artificial neural network
- Hands-on Learning In STEM: Revisiting Educational Robotics as a Learning Style Precursor
Artikel in diesem Heft
- Regular Articles
- Fabrication of aluminium covetic casts under different voltages and amperages of direct current
- Inhibition effect of the synergistic properties of 4-methyl-norvalin and 2-methoxy-4-formylphenol on the electrochemical deterioration of P4 low carbon mold steel
- Logistic regression in modeling and assessment of transport services
- Design and development of ultra-light front and rear axle of experimental vehicle
- Enhancement of cured cement using environmental waste: particleboards incorporating nano slag
- Evaluating ERP System Merging Success In Chemical Companies: System Quality, Information Quality, And Service Quality
- Accuracy of boundary layer treatments at different Reynolds scales
- Evaluation of stabiliser material using a waste additive mixture
- Optimisation of stress distribution in a highly loaded radial-axial gas microturbine using FEM
- Analysis of modern approaches for the prediction of electric energy consumption
- Surface Hardening of Aluminium Alloy with Addition of Zinc Particles by Friction Stir Processing
- Development and refinement of the Variational Method based on Polynomial Solutions of Schrödinger Equation
- Comparison of two methods for determining Q95 reference flow in the mouth of the surface catchment basin of the Meia Ponte river, state of Goiás, Brazil
- Applying Intelligent Portfolio Management to the Evaluation of Stalled Construction Projects
- Disjoint Sum of Products by Orthogonalizing Difference-Building ⴱ
- The Development of Information System with Strategic Planning for Integrated System in the Indonesian Pharmaceutical Company
- Simulation for Design and Material Selection of a Deep Placement Fertilizer Applicator for Soybean Cultivation
- Modeling transportation routes of the pick-up system using location problem: a case study
- Pinless friction stir spot welding of aluminium alloy with copper interlayer
- Roof Geometry in Building Design
- Review Articles
- Silicon-Germanium Dioxide and Aluminum Indium Gallium Arsenide-Based Acoustic Optic Modulators
- RZ Line Coding Scheme With Direct Laser Modulation for Upgrading Optical Transmission Systems
- LOGI Conference 2019
- Autonomous vans - the planning process of transport tasks
- Drivers ’reaction time research in the conditions in the real traffic
- Design and evaluation of a new intersection model to minimize congestions using VISSIM software
- Mathematical approaches for improving the efficiency of railway transport
- An experimental analysis of the driver’s attention during train driving
- Risks associated with Logistics 4.0 and their minimization using Blockchain
- Service quality of the urban public transport companies and sustainable city logistics
- Charging electric cars as a way to increase the use of energy produced from RES
- The impact of the truck loads on the braking efficiency assessment
- Application of virtual and augmented reality in automotive
- Dispatching policy evaluation for transport of ready mixed concrete
- Use of mathematical models and computer software for analysis of traffic noise
- New developments on EDR (Event Data Recorder) for automated vehicles
- General Application of Multiple Criteria Decision Making Methods for Finding the Optimal Solution in City Logistics
- The influence of the cargo weight and its position on the braking characteristics of light commercial vehicles
- Modeling the Delivery Routes Carried out by Automated Guided Vehicles when Using the Specific Mathematical Optimization Method
- Modelling of the system “driver - automation - autonomous vehicle - road”
- Limitations of the effectiveness of Weigh in Motion systems
- Long-term urban traffic monitoring based on wireless multi-sensor network
- The issue of addressing the lack of parking spaces for road freight transport in cities - a case study
- Simulation of the Use of the Material Handling Equipment in the Operation Process
- The use of simulation modelling for determining the capacity of railway lines in the Czech conditions
- Proposals for Using the NFC Technology in Regional Passenger Transport in the Slovak Republic
- Optimisation of Transport Capacity of a Railway Siding Through Construction-Reconstruction Measures
- Proposal of Methodology to Calculate Necessary Number of Autonomous Trucks for Trolleys and Efficiency Evaluation
- Special Issue: Automation in Finland
- 5G Based Machine Remote Operation Development Utilizing Digital Twin
- On-line moisture content estimation of saw dust via machine vision
- Data analysis of a paste thickener
- Programming and control for skill-based robots
- Using Digital Twin Technology in Engineering Education – Course Concept to Explore Benefits and Barriers
- Intelligent methods for root cause analysis behind the center line deviation of the steel strip
- Engaging Building Automation Data Visualisation Using Building Information Modelling and Progressive Web Application
- Real-time measurement system for determining metal concentrations in water-intensive processes
- A tool for finding inclusion clusters in steel SEM specimens
- An overview of current safety requirements for autonomous machines – review of standards
- Expertise and Uncertainty Processing with Nonlinear Scaling and Fuzzy Systems for Automation
- Towards online adaptation of digital twins
- Special Issue: ICE-SEAM 2019
- Fatigue Strength Analysis of S34MnV Steel by Accelerated Staircase Test
- The Effect of Discharge Current and Pulse-On Time on Biocompatible Zr-based BMG Sinking-EDM
- Dynamic characteristic of partially debonded sandwich of ferry ro-ro’s car deck: a numerical modeling
- Vibration-based damage identification for ship sandwich plate using finite element method
- Investigation of post-weld heat treatment (T6) and welding orientation on the strength of TIG-welded AL6061
- The effect of nozzle hole diameter of 3D printing on porosity and tensile strength parts using polylactic acid material
- Investigation of Meshing Strategy on Mechanical Behaviour of Hip Stem Implant Design Using FEA
- The effect of multi-stage modification on the performance of Savonius water turbines under the horizontal axis condition
- Special Issue: Recent Advances in Civil Engineering
- The effects of various parameters on the strengths of adhesives layer in a lightweight floor system
- Analysis of reliability of compressed masonry structures
- Estimation of Sport Facilities by Means of Technical-Economic Indicator
- Integral bridge and culvert design, Designer’s experience
- A FEM analysis of the settlement of a tall building situated on loess subsoil
- Behaviour of steel sheeting connections with self-drilling screws under variable loading
- Resistance of plug & play N type RHS truss connections
- Comparison of strength and stiffness parameters of purlins with different cross-sections of profiles
- Bearing capacity of floating geosynthetic encased columns (GEC) determined on the basis of CPTU penetration tests
- The effect of the stress distribution of anchorage and stress in the textured layer on the durability of new anchorages
- Analysis of tender procedure phases parameters for railroad construction works
- Special Issue: Terotechnology 2019
- The Use of Statistical Functions for the Selection of Laser Texturing Parameters
- Properties of Laser Additive Deposited Metallic Powder of Inconel 625
- Numerical Simulation of Laser Welding Dissimilar Low Carbon and Austenitic Steel Joint
- Assessment of Mechanical and Tribological Properties of Diamond-Like Carbon Coatings on the Ti13Nb13Zr Alloy
- Characteristics of selected measures of stress triaxiality near the crack tip for 145Cr6 steel - 3D issues for stationary cracks
- Assessment of technical risk in maintenance and improvement of a manufacturing process
- Experimental studies on the possibility of using a pulsed laser for spot welding of thin metallic foils
- Angular position control system of pneumatic artificial muscles
- The properties of lubricated friction pairs with diamond-like carbon coatings
- Effect of laser beam trajectory on pocket geometry in laser micromachining
- Special Issue: Annual Engineering and Vocational Education Conference
- The Employability Skills Needed To Face the Demands of Work in the Future: Systematic Literature Reviews
- Enhancing Higher-Order Thinking Skills in Vocational Education through Scaffolding-Problem Based Learning
- Technology-Integrated Project-Based Learning for Pre-Service Teacher Education: A Systematic Literature Review
- A Study on Water Absorption and Mechanical Properties in Epoxy-Bamboo Laminate Composite with Varying Immersion Temperatures
- Enhancing Students’ Ability in Learning Process of Programming Language using Adaptive Learning Systems: A Literature Review
- Topical Issue on Mathematical Modelling in Applied Sciences, III
- An innovative learning approach for solar power forecasting using genetic algorithm and artificial neural network
- Hands-on Learning In STEM: Revisiting Educational Robotics as a Learning Style Precursor

