Abstract
C22H25NO5, monoclinic, P21/c (no. 14), a = 15.7241(13) Å, b = 7.0223(5) Å, c = 18.4613(14) Å, β = 100.329(4)°, V = 2005.4(3) Å3, Z = 4, Rgt(F) = 0.0495, wRref(F2) = 0.1564, T = 296(2) K.
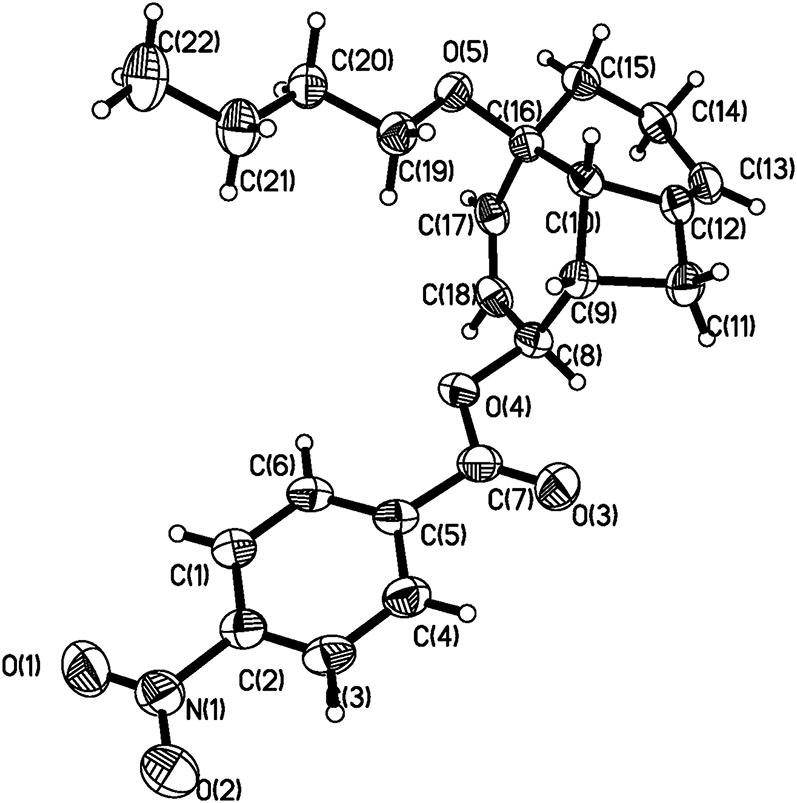
The asymmetric unit of the molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.17 × 0.14 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 28.3°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 21,787, 4985, 0.022 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3644 |
| N(param)refined: | 254 |
| Programs: | Bruker [1], Olex2 [2] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.53113 (10) | 0.7800 (3) | 0.58806 (9) | 0.0644 (4) |
| H1 | 0.5182 | 0.9093 | 0.5844 | 0.077* |
| C2 | 0.60067 (10) | 0.7077 (3) | 0.56136 (8) | 0.0619 (4) |
| C3 | 0.62117 (11) | 0.5176 (3) | 0.56502 (10) | 0.0708 (5) |
| H3 | 0.6681 | 0.4721 | 0.5459 | 0.085* |
| C4 | 0.57076 (10) | 0.3958 (3) | 0.59766 (10) | 0.0694 (4) |
| H4 | 0.5836 | 0.2664 | 0.6005 | 0.083* |
| C5 | 0.50123 (9) | 0.4643 (2) | 0.62621 (9) | 0.0587 (4) |
| C6 | 0.48103 (9) | 0.6559 (2) | 0.62037 (9) | 0.0621 (4) |
| H6 | 0.4333 | 0.7016 | 0.6384 | 0.074* |
| C7 | 0.45188 (10) | 0.3257 (3) | 0.66396 (10) | 0.0663 (4) |
| C8 | 0.33374 (9) | 0.2845 (2) | 0.72671 (8) | 0.0553 (3) |
| H8 | 0.3748 | 0.2123 | 0.7627 | 0.066* |
| C9 | 0.27728 (9) | 0.1439 (2) | 0.67672 (8) | 0.0527 (3) |
| H9 | 0.2860 | 0.1484 | 0.6255 | 0.063* |
| C10 | 0.18052 (9) | 0.14880 (19) | 0.68438 (8) | 0.0507 (3) |
| H10 | 0.1439 | 0.1012 | 0.6394 | 0.061* |
| C11 | 0.28185 (11) | −0.0622 (2) | 0.70937 (10) | 0.0667 (4) |
| H11A | 0.2727 | −0.1624 | 0.6726 | 0.080* |
| H11B | 0.3327 | −0.0867 | 0.7465 | 0.080* |
| C12 | 0.20238 (10) | −0.0100 (2) | 0.74007 (9) | 0.0575 (4) |
| C13 | 0.17531 (11) | −0.0233 (2) | 0.80304 (10) | 0.0642 (4) |
| H13 | 0.1977 | −0.1173 | 0.8366 | 0.077* |
| C14 | 0.10874 (11) | 0.1113 (3) | 0.82123 (10) | 0.0668 (4) |
| H14A | 0.1346 | 0.1897 | 0.8626 | 0.080* |
| H14B | 0.0623 | 0.0387 | 0.8361 | 0.080* |
| C15 | 0.07105 (10) | 0.2403 (2) | 0.75681 (10) | 0.0645 (4) |
| H15A | 0.0419 | 0.3466 | 0.7754 | 0.077* |
| H15B | 0.0281 | 0.1693 | 0.7231 | 0.077* |
| C16 | 0.13886 (9) | 0.3179 (2) | 0.71436 (8) | 0.0522 (3) |
| C17 | 0.20441 (11) | 0.4376 (2) | 0.76263 (9) | 0.0576 (4) |
| H17 | 0.1840 | 0.5288 | 0.7918 | 0.069* |
| C18 | 0.28846 (11) | 0.4244 (2) | 0.76721 (9) | 0.0596 (4) |
| H18 | 0.3226 | 0.5095 | 0.7983 | 0.072* |
| C19 | 0.13871 (11) | 0.4979 (3) | 0.60296 (10) | 0.0674 (4) |
| H19A | 0.1429 | 0.3940 | 0.5691 | 0.081* |
| H19B | 0.1968 | 0.5343 | 0.6259 | 0.081* |
| C20 | 0.09433 (11) | 0.6627 (2) | 0.56185 (9) | 0.0655 (4) |
| H20A | 0.0912 | 0.7666 | 0.5959 | 0.079* |
| H20B | 0.0357 | 0.6264 | 0.5405 | 0.079* |
| C21 | 0.14050 (16) | 0.7310 (3) | 0.50107 (11) | 0.0887 (6) |
| H21A | 0.1981 | 0.7732 | 0.5232 | 0.106* |
| H21B | 0.1468 | 0.6242 | 0.4692 | 0.106* |
| C22 | 0.0959 (2) | 0.8894 (4) | 0.45509 (14) | 0.1190 (9) |
| H22A | 0.0404 | 0.8464 | 0.4300 | 0.178* |
| H22B | 0.1302 | 0.9279 | 0.4196 | 0.178* |
| H22C | 0.0883 | 0.9954 | 0.4862 | 0.178* |
| N1 | 0.65651 (10) | 0.8387 (3) | 0.52871 (9) | 0.0807 (5) |
| O1 | 0.63772 (13) | 1.0053 (3) | 0.52389 (13) | 0.1229 (7) |
| O2 | 0.71968 (11) | 0.7727 (3) | 0.50842 (10) | 0.1181 (6) |
| O3 | 0.47294 (9) | 0.1625 (2) | 0.67558 (10) | 0.0951 (5) |
| O4 | 0.38273 (6) | 0.40487 (16) | 0.68382 (7) | 0.0630 (3) |
| O5 | 0.09169 (7) | 0.43701 (16) | 0.65788 (7) | 0.0653 (3) |
Source of material
All of these reactions were executed in argon atmosphere with dry solvents, unless otherwise noted. All the commercially chemicals were purchased and used without any further purification.
The 4-(but-3-yn-1-yl)-4-butoxycyclohexa-2,5-dien-1-one was synthesized as following: To a well-stirred mixture of 4-(but-3-yn-1-yl)phenol (10 mmol) in butan-1-ol (15 mL) was added the phenyliodine(III) diacetate (15 mmol) at 0 °C in several portions. Then the mixture was warmed to room temperature and stirred overnight followed by quenching with the saturated aqueous NaHCO3 (40 mL) and the saturated aqueous Na2SO3 (20 mL) and extracted with ethyl acetate (30 mL) three times. The combined organic mixture was washed with brine (30 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatography using petroleum ether/ethyl acetate eluent to afford the 4-(but-3-yn-1-yl)-4-butoxycyclohexa-2,5-dien-1-one.
The 4-butoxy-4-(penta-3,4-dien-1-yl)cyclohexa-2,5-dien-1-one was synthesized: To a well-stirred solution of 4-(but-3-yn-1-yl)-4-butoxycyclohexa-2,5-dien-1-one (2 mmol) in dioxane (10 mL) was sequentially added paraformaldehyde (300 mg, 10.0 mmol) CuBr (114.8 mg, 0.8 mmol) and diisopropylamine (0.56 mL, 4.0 mmol) under argon atmosphere. The resulting mixture was stirred at 110 °C for about 1 h (traced by TLC). After cooling to room temperature, the reaction mixture was filtered through a plug of celite and followed by washing with DCM (10 mL × 3). The organic phase was concentrated under reduced pressure and the residue purified by flash column chromatography using PE/ethyl acetate eluent to afford 4-butoxy-4-(penta-3,4-dien-1-yl)cyclohexa-2,5-dien-1-one.
The (R)-4a-butoxy-1,4a,5,6-tetrahydro-2H-cyclobuta[de]naphthalen-2-one was synthesized: To a 25 mL Schlenk tube equipped with magnetic stirring bar was added the 4-butoxy-4-(penta-3,4-dien-1-yl) cyclohexa-2,5-dien-1-one (100.2 mg) and TFE (2.0 mL), The tube was sealed and the resulting mixture was stirred for 4 h at 100 °C, before being cooled down to rt, and the mixture was transferred to a round-bottom flask and followed by concentration under reduced pressure, and then the resulting sticky oil was purified by column chromatography on silica gel using the petroleum ether/ethyl acetate as the eluent to afford the (R)-4a-butoxy-1,4a,5,6-tetrahydro-2H-cyclobuta[de]naphthalen-2-one.
The (1aS,1a1S,2R,4aR)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-ol was synthesized: To a solution of (R)-4a-butoxy-1,4a,5,6-tetrahydro-2H-cyclobuta[de]naphthalen-2-one (151.8 mg, 0.474 mmol) in dry MeOH (5 mL) was added NaBH4 (36.05 mg, 2.0 equiv). The reaction mixture was stirred for another 1 h at room temperature, then concentrated under reduced pressure. The resulting residue was purified by flash column chromatography using PE/EA eluent to afford the (1aS,1a1S,2R,4aR)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-ol.
The (1aS,1a1S,2S,4aR)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta [de]naphthalen-2-yl 4-nitrobenzoate was synthesized: To a stirred solution of alcohol (1aS,1a1S,2R,4aR)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-ol (54 mg, 0.17 mmol), p-nitrobenzoic acid (142.05 mg, 0.85 mmol), and PPh3 (248.2 mg, 0.85 mmol) in THF (5.0 mL) was added DEAD (148.02 mg, 0.13 mL, 0.85 mmol) at 0 °C. The resulting mixture was stirred at room temperature overnight before it was quenched with saturated aq. NaHCO3 solution (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic phases were washed with brine (20 mL) and dried over anhydrous Na2SO4. After filtration and evaporation of the solvent, the residue so obtained was purified by flash column chromatography with petroleum ether/EtOAc (15:1 to 10:1) to give the (1aS,1a1S,2S,4aR)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl 4-nitrobenzoate.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms.
Comment
The cyclobutane and the cyclobutene derivatives containing polycyclic compounds are very common in natural products and bioactive compounds [3], and there are numerous methods for the construction of the cyclobutane and the cyclobutene derivatives via the photochemistry initiation [4] and the transition metal catalyzed [5] and the Lewis acid promoted [6] and the thermal promoted fashion [7]. Recently, we disclosed a powerful, distinct, and atom-economical method for the synthesis of clobutane-fused oxygen containing tricyclic framework via a thermal promoted intramolecular [2+2] cycloaddition of cyclohexadienone-tethered allenes [8]. However, the method for the dicyclobutanes containing ring system remains rather rare reported to date. As our continuing efforts on the efficient synthetic methods for cyclic compounds synthesis associate with allenes [9], we herein report a useful method and the crystal structure of the titled compound.
There is one molecule in the asymmetric unit of the title structure (see the Figure). The single crystal structure verifies that all bond lengths are in normal ranges. Furthermore, the crystal packing doesn’t exhibit strong intramolecular or intermolecular hydrogen bond.
Funding source: Henan Postdoctoral Foundation
Funding source: Anyang Institute of Technology
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: This work was supported by Henan Postdoctoral Foundation and the Foundation of Postdoctoral innovation base of Anyang Institute of Technology.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. BRUKER. SAINT. APEX2 and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2009.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Lou, H., Zheng, S., Li, T., Zhang, J., Fei, Y., Hao, X., Liang, G., Pan, W. Vulgarisin A, a new diterpenoid with a rare 5/6/4/5 ring skeleton from the Chinese medicinal plant Prunella vulgaris. Org. Lett. 2014, 16, 2696–2699; https://doi.org/10.1021/ol5009763.Search in Google Scholar
4. Shepard, M. S., Carreira, E. M. The dendritic effect in molecular recognition: ferrocene dendrimers and their use as supramolecular redox sensors for the recognition of small inorganic anions. J. Am. Chem. Soc. 1997, 119, 2588–2605.10.1021/ja964127tSearch in Google Scholar
5. Gulías, M., Collado, A., Trillo, B., Lopez, F., Onate, E., Esteruelas, M. A., Mascarenas, J. L. Ruthenium-catalyzed (2 + 2) intramolecular cycloaddition of allenenes. J. Am. Chem. Soc. 2011, 133, 7660–7663; https://doi.org/10.1021/ja200784n.Search in Google Scholar
6. Zhao, J.-F., Loh, T.-P. Acid-catalyzed intramolecular [2+2] cycloaddition of ene-allenones: facile access to bicyclo[n.2.0] frameworks. Angew. Chem. Int. Ed. 2009, 48, 7232–7235; https://doi.org/10.1002/anie.200902471.Search in Google Scholar
7. Kimura, M., Horino, Y., Wakamiya, Y., Okajima, T., Tamaru, Y. Pronounced chemo-, regio-, and stereoselective [2 + 2] cycloaddition reaction of allenes toward alkenes and alkynes. J. Am. Chem. Soc. 1997, 119, 10869–10870; https://doi.org/10.1021/ja972614i.Search in Google Scholar
8. Zhai, S., Qiu, S., Chen, L., Niu, Y., Yu, Y., Yang, B., Zhang, B., Han, C., Yang, L., Zhai, H. Synthesis of cyclobutane-fused oxygen-containing tricyclic framework via thermally promoted intramolecular cycloaddition of cyclohexadienone-tethered allenes. Chem. Commun. 2020, 56, 3405–3408; https://doi.org/10.1039/d0cc00061b.Search in Google Scholar
9. Zhai, S., Qiu, S., Chen, X., Tao, C., Li, Y., Cheng, B., Wang, H., Zhai, H. Trifunctionalization of allenes via cobalt-catalyzed MHP-assisted C-H bond functionalization and molecular oxygen activation. ACS Catal. 2018, 8, 6645–6649; https://doi.org/10.1021/acscatal.8b01720.Search in Google Scholar
© 2020 Jiahuan Luo et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3


