Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
Abstract
C43H78CaO22S2, monoclinic, P21 (no. 4), a = 18.2539(3) Å, b = 15.3118(3) Å, c = 18.3718(4) Å, β = 91.2637(17)°, Z = 4, V = 5133.65(18) Å3, Rgt(F) = 0.0544, wRref(F2) = 0.1622, T = 293(2) K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless |
| Size: | 0.22 × 0.16 × 0.13 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 2.48 mm-1 |
| Diffractometer, scan mode: | Xcalibur, ω |
| θmax, completeness: | 70.9°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 25633, 15314, 0.027 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 12706 |
| N(param)refined: | 1271 |
| Programs: | Bruker [1], SHELX [2], [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.1185 (3) | 0.3018 (3) | 0.7137 (3) | 0.0319 (10) |
| H1A | 0.0834 | 0.2837 | 0.6762 | 0.038* |
| H1B | 0.1625 | 0.2671 | 0.7083 | 0.038* |
| C1" | 0.6199 (3) | 0.5511 (3) | 0.2148 (3) | 0.0326 (10) |
| H1"A | 0.5870 | 0.5296 | 0.1768 | 0.039* |
| H1"B | 0.6662 | 0.5206 | 0.2106 | 0.039* |
| C1′ | −0.1129 (2) | 0.2983 (3) | 1.2771 (2) | 0.0309 (10) |
| H1′A | −0.0771 | 0.2789 | 1.3134 | 0.037* |
| H1′B | −0.1571 | 0.2643 | 1.2832 | 0.037* |
| C1A | 0.3838 (3) | 0.5465 (3) | 0.7801 (2) | 0.0312 (10) |
| H1AA | 0.4216 | 0.5291 | 0.8151 | 0.037* |
| H1AB | 0.3407 | 0.5113 | 0.7888 | 0.037* |
| C2 | 0.0864 (3) | 0.2838 (3) | 0.7888 (3) | 0.0396 (11) |
| H2A | 0.0738 | 0.2224 | 0.7926 | 0.048* |
| H2B | 0.1227 | 0.2971 | 0.8265 | 0.048* |
| C2" | 0.5876 (3) | 0.5312 (3) | 0.2889 (3) | 0.0395 (11) |
| H2"A | 0.5795 | 0.4688 | 0.2932 | 0.047* |
| H2"B | 0.6222 | 0.5485 | 0.3271 | 0.047* |
| C2′ | −0.0830 (3) | 0.2809 (3) | 1.2010 (3) | 0.0382 (11) |
| H2′A | −0.0718 | 0.2193 | 1.1961 | 0.046* |
| H2′B | −0.1200 | 0.2959 | 1.1644 | 0.046* |
| C2A | 0.4103 (3) | 0.5281 (4) | 0.7036 (3) | 0.0429 (12) |
| H2AA | 0.4226 | 0.4666 | 0.6993 | 0.051* |
| H2AB | 0.3714 | 0.5410 | 0.6684 | 0.051* |
| C3 | 0.0185 (3) | 0.3389 (4) | 0.8001 (2) | 0.0357 (11) |
| H3 | −0.0167 | 0.3253 | 0.7606 | 0.043* |
| C3" | 0.5160 (3) | 0.5788 (4) | 0.2988 (2) | 0.0361 (11) |
| H3" | 0.4825 | 0.5612 | 0.2592 | 0.043* |
| C3′ | −0.0144 (3) | 0.3341 (4) | 1.1884 (2) | 0.0358 (11) |
| H3′ | 0.0219 | 0.3181 | 1.2261 | 0.043* |
| C3A | 0.4771 (3) | 0.5829 (4) | 0.6870 (2) | 0.0410 (12) |
| H3A | 0.5152 | 0.5686 | 0.7235 | 0.049* |
| C4 | 0.0339 (3) | 0.4375 (3) | 0.7977 (2) | 0.0342 (10) |
| C4" | 0.5241 (3) | 0.6787 (3) | 0.2957 (3) | 0.0363 (11) |
| C4′ | −0.0270 (2) | 0.4330 (3) | 1.1931 (2) | 0.0324 (10) |
| C4A | 0.4630 (3) | 0.6813 (4) | 0.6916 (2) | 0.0356 (11) |
| C5 | 0.0685 (2) | 0.4548 (3) | 0.7212 (2) | 0.0286 (9) |
| H5 | 0.0307 | 0.4357 | 0.6860 | 0.034* |
| C5" | 0.5599 (2) | 0.6993 (3) | 0.2204 (2) | 0.0310 (10) |
| H5" | 0.5244 | 0.6776 | 0.1839 | 0.037* |
| C5′ | −0.0607 (2) | 0.4507 (3) | 1.2700 (2) | 0.0288 (9) |
| H5′ | −0.0226 | 0.4313 | 1.3048 | 0.035* |
| C5A | 0.4329 (2) | 0.6991 (3) | 0.7696 (2) | 0.0298 (9) |
| H5A | 0.4724 | 0.6799 | 0.8028 | 0.036* |
| C6 | 0.0795 (3) | 0.5522 (3) | 0.7042 (3) | 0.0389 (11) |
| H6A | 0.1185 | 0.5754 | 0.7352 | 0.047* |
| H6B | 0.0349 | 0.5840 | 0.7144 | 0.047* |
| C6" | 0.5655 (3) | 0.7978 (3) | 0.2050 (3) | 0.0432 (12) |
| H6"A | 0.6022 | 0.8236 | 0.2373 | 0.052* |
| H6"B | 0.5188 | 0.8255 | 0.2144 | 0.052* |
| C6′ | −0.0705 (3) | 0.5474 (3) | 1.2871 (3) | 0.0387 (11) |
| H6′A | −0.1101 | 0.5710 | 1.2570 | 0.046* |
| H6′B | −0.0260 | 0.5788 | 1.2757 | 0.046* |
| C6A | 0.4237 (3) | 0.7963 (3) | 0.7866 (3) | 0.0406 (11) |
| H6AA | 0.3830 | 0.8198 | 0.7579 | 0.049* |
| H6AB | 0.4677 | 0.8276 | 0.7734 | 0.049* |
| C7 | 0.0989 (3) | 0.5650 (4) | 0.6239 (3) | 0.0432 (12) |
| H7A | 0.0577 | 0.5480 | 0.5928 | 0.052* |
| H7B | 0.1093 | 0.6262 | 0.6149 | 0.052* |
| C7" | 0.5863 (3) | 0.8134 (4) | 0.1251 (3) | 0.0432 (12) |
| H7"A | 0.5470 | 0.7935 | 0.0929 | 0.052* |
| H7"B | 0.5934 | 0.8754 | 0.1169 | 0.052* |
| C7′ | −0.0877 (3) | 0.5606 (4) | 1.3674 (3) | 0.0428 (12) |
| H7′A | −0.0460 | 0.5429 | 1.3975 | 0.051* |
| H7′B | −0.0972 | 0.6219 | 1.3766 | 0.051* |
| C7A | 0.4095 (3) | 0.8096 (4) | 0.8680 (3) | 0.0452 (13) |
| H7AA | 0.4524 | 0.7921 | 0.8964 | 0.054* |
| H7AB | 0.4003 | 0.8709 | 0.8774 | 0.054* |
| C8 | 0.1648 (3) | 0.5107 (3) | 0.6059 (2) | 0.0331 (10) |
| C8" | 0.6555 (3) | 0.7649 (3) | 0.1089 (2) | 0.0344 (10) |
| C8′ | −0.1537 (3) | 0.5073 (3) | 1.3864 (2) | 0.0316 (9) |
| C8A | 0.3448 (3) | 0.7565 (3) | 0.8899 (2) | 0.0334 (10) |
| C9 | 0.1524 (2) | 0.4139 (3) | 0.6189 (2) | 0.0258 (8) |
| H9 | 0.1074 | 0.3980 | 0.5921 | 0.031* |
| C9" | 0.6483 (2) | 0.6672 (3) | 0.1210 (2) | 0.0267 (9) |
| H9" | 0.6041 | 0.6490 | 0.0937 | 0.032* |
| C9′ | −0.1419 (2) | 0.4104 (3) | 1.3739 (2) | 0.0266 (9) |
| H9′ | −0.0958 | 0.3950 | 1.3989 | 0.032* |
| C9A | 0.3551 (2) | 0.6598 (3) | 0.8769 (2) | 0.0246 (8) |
| H9AA | 0.4015 | 0.6434 | 0.9011 | 0.030* |
| C10 | 0.1374 (2) | 0.3989 (3) | 0.7024 (2) | 0.0253 (9) |
| C10" | 0.6325 (2) | 0.6496 (3) | 0.2037 (2) | 0.0257 (9) |
| C10′ | −0.1301 (2) | 0.3953 (3) | 1.2901 (2) | 0.0246 (8) |
| C10A | 0.3648 (2) | 0.6436 (3) | 0.7925 (2) | 0.0257 (9) |
| C11 | 0.2138 (2) | 0.3555 (3) | 0.5897 (2) | 0.0336 (10) |
| H11A | 0.2604 | 0.3746 | 0.6106 | 0.040* |
| H11B | 0.2056 | 0.2959 | 0.6054 | 0.040* |
| C11" | 0.7115 (2) | 0.6126 (3) | 0.0911 (2) | 0.0338 (10) |
| H11E | 0.7049 | 0.5520 | 0.1049 | 0.041* |
| H11F | 0.7573 | 0.6328 | 0.1127 | 0.041* |
| C11′ | −0.2015 (2) | 0.3523 (3) | 1.4063 (2) | 0.0303 (9) |
| H11C | −0.1944 | 0.2925 | 1.3906 | 0.036* |
| H11D | −0.2492 | 0.3714 | 1.3882 | 0.036* |
| C11A | 0.2948 (2) | 0.6020 (3) | 0.9101 (2) | 0.0310 (9) |
| H11G | 0.2473 | 0.6218 | 0.8922 | 0.037* |
| H11H | 0.3014 | 0.5422 | 0.8940 | 0.037* |
| C12 | 0.2179 (2) | 0.3576 (3) | 0.5063 (2) | 0.0320 (10) |
| H12 | 0.2224 | 0.4190 | 0.4919 | 0.038* |
| C12" | 0.7156 (2) | 0.6188 (3) | 0.0076 (2) | 0.0317 (10) |
| H12" | 0.7200 | 0.6806 | −0.0054 | 0.038* |
| C12′ | −0.1999 (2) | 0.3559 (3) | 1.4902 (2) | 0.0299 (9) |
| H12′ | −0.1897 | 0.4165 | 1.5041 | 0.036* |
| C12A | 0.2964 (2) | 0.6045 (3) | 0.9938 (2) | 0.0299 (9) |
| H12A | 0.3078 | 0.6646 | 1.0083 | 0.036* |
| C13 | 0.1501 (3) | 0.3217 (4) | 0.4679 (2) | 0.0386 (11) |
| C13" | 0.6494 (2) | 0.5829 (4) | −0.0319 (2) | 0.0348 (11) |
| C13′ | −0.1414 (3) | 0.3005 (4) | 1.5257 (3) | 0.0415 (12) |
| C13A | 0.3531 (3) | 0.5471 (4) | 1.0289 (3) | 0.0357 (11) |
| C14 | 0.1208 (3) | 0.2437 (5) | 0.4696 (3) | 0.0563 (15) |
| H14 | 0.1396 | 0.1969 | 0.4963 | 0.068* |
| C14" | 0.6164 (3) | 0.5071 (5) | −0.0270 (3) | 0.0503 (14) |
| H14" | 0.6312 | 0.4621 | 0.0040 | 0.060* |
| C14′ | −0.1386 (4) | 0.2157 (5) | 1.5393 (3) | 0.0597 (16) |
| H14′ | −0.1745 | 0.1758 | 1.5250 | 0.072* |
| C14A | 0.3521 (3) | 0.4620 (4) | 1.0451 (3) | 0.0520 (14) |
| H14A | 0.3133 | 0.4244 | 1.0345 | 0.062* |
| C15 | 0.0541 (4) | 0.2406 (6) | 0.4235 (4) | 0.069 (2) |
| H15A | 0.0594 | 0.1995 | 0.3838 | 0.083* |
| H15B | 0.0119 | 0.2242 | 0.4517 | 0.083* |
| C15" | 0.5523 (3) | 0.5030 (6) | −0.0779 (4) | 0.0650 (19) |
| H15E | 0.5577 | 0.4563 | −0.1130 | 0.078* |
| H15F | 0.5073 | 0.4941 | −0.0517 | 0.078* |
| C15′ | −0.0705 (4) | 0.1931 (7) | 1.5804 (5) | 0.095 (3) |
| H15C | −0.0816 | 0.1699 | 1.6280 | 0.114* |
| H15D | −0.0420 | 0.1504 | 1.5542 | 0.114* |
| C15A | 0.4218 (4) | 0.4364 (6) | 1.0822 (4) | 0.077 (2) |
| H15G | 0.4129 | 0.4143 | 1.1306 | 0.092* |
| H15H | 0.4469 | 0.3919 | 1.0546 | 0.092* |
| C16 | 0.1040 (4) | 0.3769 (6) | 0.4219 (4) | 0.073 (2) |
| C16" | 0.6087 (4) | 0.6348 (6) | −0.0861 (4) | 0.070 (2) |
| C16′ | −0.0736 (4) | 0.3380 (6) | 1.5559 (4) | 0.071 (2) |
| C16A | 0.4243 (3) | 0.5808 (5) | 1.0549 (3) | 0.0564 (16) |
| C17 | 0.2271 (3) | 0.5462 (4) | 0.5866 (3) | 0.0444 (13) |
| H17A | 0.2314 | 0.6067 | 0.5837 | 0.053* |
| H17B | 0.2669 | 0.5109 | 0.5759 | 0.053* |
| C17" | 0.7168 (3) | 0.8051 (4) | 0.0924 (3) | 0.0481 (13) |
| H17E | 0.7183 | 0.8658 | 0.0905 | 0.058* |
| H17F | 0.7586 | 0.7728 | 0.0827 | 0.058* |
| C17′ | −0.2156 (3) | 0.5431 (4) | 1.4072 (3) | 0.0458 (13) |
| H17C | −0.2195 | 0.6035 | 1.4104 | 0.055* |
| H17D | −0.2553 | 0.5079 | 1.4185 | 0.055* |
| C17A | 0.2842 (3) | 0.7938 (4) | 0.9125 (3) | 0.0464 (13) |
| H17G | 0.2810 | 0.8543 | 0.9149 | 0.056* |
| H17H | 0.2446 | 0.7595 | 0.9259 | 0.056* |
| C18 | −0.0390 (3) | 0.4863 (5) | 0.8016 (3) | 0.0560 (16) |
| H18A | −0.0667 | 0.4772 | 0.7573 | 0.084* |
| H18B | −0.0297 | 0.5475 | 0.8080 | 0.084* |
| H18C | −0.0662 | 0.4647 | 0.8420 | 0.084* |
| C18" | 0.4472 (3) | 0.7177 (5) | 0.2960 (3) | 0.065 (2) |
| H18G | 0.4203 | 0.6927 | 0.3352 | 0.098* |
| H18H | 0.4225 | 0.7051 | 0.2506 | 0.098* |
| H18I | 0.4505 | 0.7798 | 0.3025 | 0.098* |
| C18′ | 0.0481 (3) | 0.4777 (5) | 1.1904 (3) | 0.0536 (15) |
| H18D | 0.0416 | 0.5386 | 1.1797 | 0.080* |
| H18E | 0.0767 | 0.4511 | 1.1532 | 0.080* |
| H18F | 0.0730 | 0.4715 | 1.2367 | 0.080* |
| C18A | 0.5369 (3) | 0.7292 (5) | 0.6843 (3) | 0.0592 (17) |
| H18J | 0.5621 | 0.7069 | 0.6430 | 0.089* |
| H18K | 0.5663 | 0.7201 | 0.7276 | 0.089* |
| H18L | 0.5281 | 0.7906 | 0.6779 | 0.089* |
| C19 | 0.0853 (3) | 0.4683 (4) | 0.8602 (3) | 0.0408 (12) |
| H19A | 0.1275 | 0.4299 | 0.8633 | 0.049* |
| H19B | 0.1027 | 0.5266 | 0.8495 | 0.049* |
| C19" | 0.5699 (3) | 0.7154 (4) | 0.3598 (3) | 0.0464 (13) |
| H19E | 0.6138 | 0.6802 | 0.3662 | 0.056* |
| H19F | 0.5851 | 0.7744 | 0.3481 | 0.056* |
| C19′ | −0.0761 (3) | 0.4670 (3) | 1.1313 (2) | 0.0365 (10) |
| H19C | −0.1186 | 0.4293 | 1.1261 | 0.044* |
| H19D | −0.0933 | 0.5250 | 1.1437 | 0.044* |
| C19A | 0.4102 (3) | 0.7129 (4) | 0.6309 (2) | 0.0394 (11) |
| H19G | 0.3677 | 0.6748 | 0.6287 | 0.047* |
| H19H | 0.3934 | 0.7713 | 0.6424 | 0.047* |
| C20 | 0.2071 (2) | 0.4226 (3) | 0.7465 (2) | 0.0331 (10) |
| H20A | 0.2491 | 0.3989 | 0.7230 | 0.050* |
| H20B | 0.2042 | 0.3987 | 0.7947 | 0.050* |
| H20C | 0.2116 | 0.4850 | 0.7495 | 0.050* |
| C20" | 0.6996 (3) | 0.6786 (4) | 0.2493 (2) | 0.0362 (11) |
| H20G | 0.6944 | 0.6596 | 0.2987 | 0.054* |
| H20H | 0.7034 | 0.7411 | 0.2481 | 0.054* |
| H20I | 0.7430 | 0.6533 | 0.2296 | 0.054* |
| C20′ | −0.2000 (2) | 0.4209 (3) | 1.2474 (2) | 0.0329 (10) |
| H20D | −0.2026 | 0.4833 | 1.2433 | 0.049* |
| H20E | −0.2421 | 0.3999 | 1.2726 | 0.049* |
| H20F | −0.1991 | 0.3954 | 1.1997 | 0.049* |
| C20A | 0.2934 (2) | 0.6685 (3) | 0.7525 (2) | 0.0318 (10) |
| H20J | 0.2916 | 0.6410 | 0.7055 | 0.048* |
| H20K | 0.2913 | 0.7307 | 0.7466 | 0.048* |
| H20L | 0.2525 | 0.6493 | 0.7803 | 0.048* |
| Ca1 | 0.00043 (6) | 0.34412 (7) | 0.99371 (6) | 0.0317 (2) |
| Ca1A | 0.49034 (5) | 0.58920 (7) | 0.49181 (5) | 0.0345 (2) |
| O1 | −0.0148 (2) | 0.3157 (3) | 0.86785 (19) | 0.0495 (10) |
| H1 | −0.051 (3) | 0.282 (4) | 0.8592 (13) | 0.074* |
| O1" | 0.4842 (2) | 0.5523 (3) | 0.36639 (19) | 0.0494 (10) |
| H1" | 0.450 (3) | 0.515 (4) | 0.3584 (14) | 0.074* |
| O1′ | 0.0151 (2) | 0.3109 (3) | 1.11907 (19) | 0.0498 (10) |
| H1′ | 0.050 (3) | 0.275 (4) | 1.1256 (13) | 0.075* |
| O1A | 0.5044 (2) | 0.5585 (3) | 0.61679 (19) | 0.0596 (13) |
| H1AC | 0.542 (3) | 0.525 (4) | 0.6229 (13) | 0.089* |
| O2 | 0.0489 (2) | 0.4688 (3) | 0.92948 (18) | 0.0449 (9) |
| H2 | 0.065 (3) | 0.514 (2) | 0.9527 (14) | 0.067* |
| O2" | 0.5310 (2) | 0.7165 (3) | 0.42693 (19) | 0.0491 (9) |
| H2" | 0.533 (3) | 0.7685 (12) | 0.444 (2) | 0.074* |
| O2′ | −0.0390 (2) | 0.4708 (2) | 1.06272 (17) | 0.0389 (8) |
| H2′ | −0.050 (3) | 0.5197 (15) | 1.0433 (15) | 0.058* |
| O2A | 0.4443 (2) | 0.7138 (3) | 0.56072 (18) | 0.0442 (9) |
| H2AC | 0.427 (3) | 0.758 (2) | 0.5383 (14) | 0.066* |
| O3 | 0.0469 (3) | 0.3288 (5) | 0.3965 (3) | 0.096 (2) |
| O3" | 0.5527 (3) | 0.5872 (5) | −0.1130 (3) | 0.0843 (17) |
| O3′ | −0.0325 (3) | 0.2736 (6) | 1.5865 (3) | 0.101 (2) |
| O3A | 0.4638 (3) | 0.5151 (5) | 1.0855 (3) | 0.0828 (18) |
| O4 | 0.1095 (4) | 0.4524 (5) | 0.4072 (4) | 0.127 (3) |
| O4" | 0.6209 (4) | 0.7075 (5) | −0.1064 (4) | 0.116 (3) |
| O4′ | −0.0538 (3) | 0.4126 (5) | 1.5550 (4) | 0.110 (2) |
| O4A | 0.4481 (3) | 0.6530 (5) | 1.0514 (3) | 0.093 (2) |
| O5 | 0.2895 (2) | 0.2110 (3) | 0.4973 (2) | 0.0547 (10) |
| O5" | 0.7888 (2) | 0.4726 (3) | −0.0010 (2) | 0.0497 (9) |
| O5′ | −0.3022 (2) | 0.2396 (3) | 1.5038 (2) | 0.0514 (10) |
| O5A | 0.1884 (2) | 0.4931 (3) | 1.0031 (2) | 0.0532 (10) |
| O6 | 0.2979 (2) | 0.3132 (3) | 0.3967 (2) | 0.0514 (10) |
| O6" | 0.7939 (2) | 0.5735 (3) | −0.10293 (19) | 0.0487 (9) |
| O6′ | −0.2805 (2) | 0.3379 (3) | 1.60480 (19) | 0.0498 (10) |
| O6A | 0.2139 (2) | 0.5863 (3) | 1.10723 (19) | 0.0510 (10) |
| O7 | 0.3607 (2) | 0.3415 (3) | 0.5112 (2) | 0.0551 (11) |
| O7" | 0.85846 (19) | 0.6054 (3) | 0.0098 (2) | 0.0519 (10) |
| O7′ | −0.3406 (2) | 0.3889 (3) | 1.4940 (2) | 0.0554 (11) |
| O7A | 0.1583 (2) | 0.6459 (3) | 0.9971 (2) | 0.0583 (12) |
| O8 | 0.1273 (2) | 0.3110 (5) | 1.0020 (3) | 0.088 (2) |
| H8A | 0.1373 | 0.2552 | 1.0085 | 0.132* |
| H8B | 0.1534 | 0.3414 | 1.0407 | 0.132* |
| O8A | 0.3616 (2) | 0.5747 (4) | 0.4684 (3) | 0.0828 (17) |
| H8AA | 0.3758 | 0.5252 | 0.4821 | 0.124* |
| H8AB | 0.3506 | 0.5806 | 0.4212 | 0.124* |
| O9 | −0.0060 (2) | 0.1870 (3) | 0.9931 (3) | 0.0615 (12) |
| H9A | 0.0242 | 0.1531 | 1.0316 | 0.092* |
| H9B | −0.0582 | 0.1580 | 0.9878 | 0.092* |
| O9A | 0.4922 (3) | 0.4324 (3) | 0.4928 (3) | 0.0651 (12) |
| H9AB | 0.5341 | 0.4083 | 0.4717 | 0.098* |
| H9AC | 0.4526 | 0.4073 | 0.4673 | 0.098* |
| O10 | −0.12636 (19) | 0.3260 (3) | 0.9881 (3) | 0.0545 (10) |
| H10A | −0.1449 | 0.3688 | 0.9655 | 0.082* |
| H10B | −0.1399 | 0.2696 | 0.9941 | 0.082* |
| O10A | 0.61798 (18) | 0.5681 (3) | 0.4985 (2) | 0.0475 (9) |
| H10C | 0.6453 | 0.6058 | 0.4674 | 0.071* |
| H10D | 0.6335 | 0.5102 | 0.4853 | 0.071* |
| S1 | 0.29810 (6) | 0.30140 (9) | 0.47466 (7) | 0.0347 (3) |
| S1" | 0.79544 (6) | 0.56290 (10) | −0.02483 (6) | 0.0328 (3) |
| S1′ | −0.28769 (6) | 0.32806 (9) | 1.52647 (6) | 0.0330 (3) |
| S1A | 0.20726 (6) | 0.57929 (10) | 1.02853 (6) | 0.0358 (3) |
| C21 | 0.7993 (5) | 0.6146 (5) | 0.6792 (4) | 0.074 (2) |
| C22 | 0.7951 (6) | 0.5534 (7) | 0.6155 (5) | 0.110 (4) |
| H22A | 0.7720 | 0.4999 | 0.6299 | 0.165* |
| H22B | 0.8436 | 0.5412 | 0.5992 | 0.165* |
| H22C | 0.7669 | 0.5798 | 0.5767 | 0.165* |
| C23 | 0.7288 (5) | 0.6368 (7) | 0.7128 (5) | 0.100 (3) |
| H23A | 0.7212 | 0.6988 | 0.7102 | 0.150* |
| H23B | 0.7299 | 0.6187 | 0.7628 | 0.150* |
| H23C | 0.6896 | 0.6074 | 0.6872 | 0.150* |
| O11 | 0.8569 (4) | 0.6453 (5) | 0.7008 (3) | 0.097 (2) |
| C21A | 0.6926 (4) | 0.3687 (5) | 0.8209 (4) | 0.0679 (18) |
| C22A | 0.7650 (5) | 0.3973 (6) | 0.7937 (5) | 0.088 (2) |
| H22D | 0.7675 | 0.3852 | 0.7425 | 0.131* |
| H22E | 0.8034 | 0.3662 | 0.8192 | 0.131* |
| H22F | 0.7709 | 0.4589 | 0.8017 | 0.131* |
| C23A | 0.6926 (6) | 0.3011 (7) | 0.8801 (5) | 0.105 (3) |
| H23D | 0.6434 | 0.2923 | 0.8961 | 0.157* |
| H23E | 0.7228 | 0.3206 | 0.9203 | 0.157* |
| H23F | 0.7115 | 0.2471 | 0.8617 | 0.157* |
| O11A | 0.6358 (3) | 0.3990 (5) | 0.7973 (3) | 0.096 (2) |
| O14 | 0.2371 (5) | 0.5421 (5) | 0.3628 (4) | 0.133 (3) |
| H14B | 0.1965 | 0.5325 | 0.3832 | 0.199* |
| H14C | 0.2450 | 0.5175 | 0.3192 | 0.199* |
| O19 | 0.6069 (4) | 0.4479 (5) | 0.6579 (3) | 0.117 (3) |
| H19I | 0.6489 | 0.4437 | 0.6392 | 0.175* |
| H19J | 0.6053 | 0.4191 | 0.7037 | 0.175* |
| O15 | 0.2159 (4) | 0.7090 (5) | 0.2986 (3) | 0.113 (2) |
| H15I | 0.2252 | 0.6640 | 0.3271 | 0.169* |
| H15J | 0.2438 | 0.7470 | 0.3335 | 0.169* |
| O16 | 0.2711 (4) | 0.4682 (5) | 0.2127 (4) | 0.125 (3) |
| H16A | 0.2493 | 0.5097 | 0.1904 | 0.187* |
| H16B | 0.2659 | 0.4280 | 0.1792 | 0.187* |
| O12 | 0.8746 (3) | 0.7059 (5) | 0.8383 (3) | 0.0848 (18) |
| H12B | 0.8579 | 0.7120 | 0.7951 | 0.127* |
| H12C | 0.8478 | 0.6664 | 0.8566 | 0.127* |
| O17 | 0.2466 (4) | 0.3092 (5) | 0.1245 (4) | 0.122 (3) |
| H17I | 0.2346 | 0.2942 | 0.1656 | 0.183* |
| H17J | 0.2794 | 0.2718 | 0.1148 | 0.183* |
| O13 | 0.3789 (4) | 0.4407 (5) | 0.3225 (4) | 0.117 (3) |
| H13A | 0.3527 | 0.4550 | 0.2856 | 0.175* |
| H13B | 0.3559 | 0.3986 | 0.3416 | 0.175* |
| O18 | 0.1169 (3) | 0.7024 (4) | 0.1821 (3) | 0.0770 (15) |
| H18M | 0.1336 | 0.7078 | 0.2254 | 0.115* |
| H18N | 0.1502 | 0.6665 | 0.1705 | 0.115* |
Source of material
Andrographolide calcium bisulfite crude product was provided by Jiangsu Jiuxu Pharmaceutical Com. Ltd. To begin with, the raw materials were dissolved in mixture of absolute water and acetone and then slow evaporation at room temperature for 3 days. Finally, we obtained the crystals of the title compound.
Experimental details
All hydrogen atmos were located in calculated positions and refined as riding atoms. The hydrogen atoms of tertiary and secondary carbons for isotropic displacement factors Uiso(H) were set to 1.2 times Ueq(C), 1.5 times Ueq(C) of methyl hydrogen atoms and 1.5 times Ueq(O).
Comment
Andrographolide, a diterpenoid lactone, can be obtained from the extract of traditional herb Andrographis paniculata with extraordinary efficacy for treating various diseases including diarrhea, hepatic protection, rheumatoid arthritis, upperrespiratory tract infection, cancer and so on [4], [5], [6], [7], and the Andrographolide’s contribution of these effects had been proved by pharmacological researches [8]. Furthermore, Andrographolide is a neuroprotection regulator in central nervous system (CNS) [9]. Andrographolide has been identified an anti-inflammatory, anti-bacteria and anti-virus drug, however, which blocks its excellent clinically application prospect for extremely poor water solubility and low bioavailability. Given inadequacies of Andrographolide, researchers improved its solubility by introducing hydrophilic sodium bisulfite group, resulting in Andrographolide sodium bisulfite (ASB). In a recent study, we found Andrographolide calcium bisulfite (ACB) which is a related impurity of ASB.
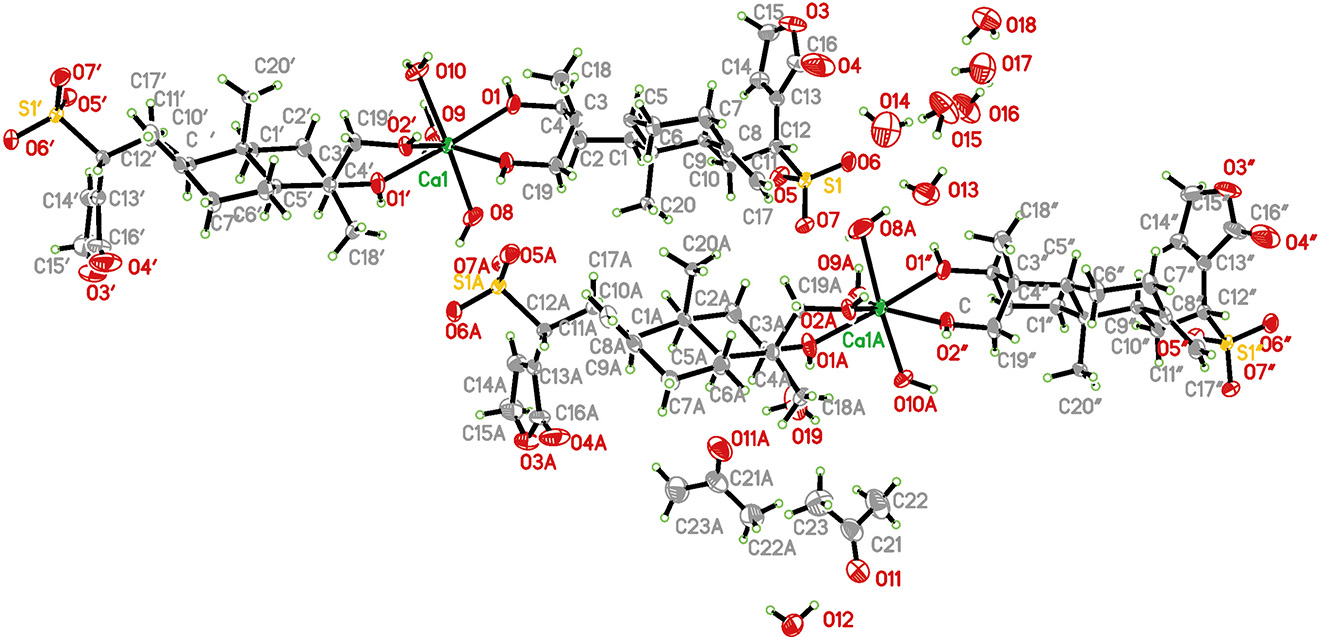
The asymmetric unit contains four andrographolide bisulfite anions, two calcium cations, two molecules of acetone and 14 molecules of water. Ca1 adopts a distorted polyhedron geometry by coordinating to four oxygen atoms of hydroxyl from a pair of organic ligands and four oxygen atoms of four water ligands (see the figure). In contrast Ca1a is sevenfold coordinated by four hydroxy groups of two organic ligands and three water ligands.
The organic ligand andrographolide calcium bisulfite consists of two fused cyclohexanes adopting chair conformations and a nearly planar five-membered lactonic ring. Ca–O distances are in the range of those observed in reported Ca(II) complexes [10], [11]. The O–S distances coincided with the normal ranges. And bond angles around the central Ca (II) ion vary from 71.47 (13)° to 164.85 (19)°. The Flack parameter (absolute configuration) was determined to 0.003(7), thus the configuration at atoms C3, C4, C5, C9, C10 and C12 are in R, R, S, R, S and R, respectively.
Funding source: Science and Technology Bureau of Henan Province for the Cooperation Research Project Fund
Award Identifier / Grant number: 152107000041
Acknowledgments
The Analysis and Testing Center of Zhengzhou University is acknowledged for the single crystal X-ray diffraction facility.
Research funding: This work was supported by the Science and Technology Bureau of Henan Province for the Cooperation Research Project Fund (No. 152107000041 for WL) and Jiangsu Jiuxu Pharmaceutical Com. Ltd.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX3, SAINT–Plus, XPREEP; Bruker AXS Inc.: Madison, Wisconsin, USA, 2017.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122 https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal structure refinement with SHELX. Acta Crystallogr. 2015, C71, 3–8 https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Suriyo, T., Pholphana, N., Rangkadilok, N., Thiantanawat, A., Watcharasit, P., Satayavivad, J. Andrographis paniculata extracts and major constituent diterpenoids inhibit growth of intrahepatic cholangiocarcinoma cells by inducing cell cycle arrest and apoptosis. Planta Med. 2014, 80, 533–543 https://doi.org/10.1055/s-0034-1368399.Search in Google Scholar PubMed
5. Bensch, K., Tiralongo, J., Schmidt, K., Matthias, A., Bone, K. M., Lehmann, R., Tiralongo, E. Investigations into the antiadhesive activity of herbal extracts against Campylobacter jejuni. Phytother. Res. 2011, 25, 1125–1132 https://doi.org/10.1002/ptr.3384.Search in Google Scholar PubMed
6. Dai, Y., Chen, S. R., Chai, L., Zhao, J., Wang, Y., Wang, Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. Nutr. 2019, 59, S17–S29 https://doi.org/10.1080/10408398.2018.1501657.Search in Google Scholar PubMed
7. Saxena, R. C., Singh, R., Kumar, P., Yadav, S. C., Negi, M. P., Saxena, V. S., Joshua, A. J., Vijayabalaji, V., Goudar, K. S., Venkateshwarlu, K., Amit, A. A randomized double blind placebo controlled clinical evaluation of extract of Andrographis paniculata (KalmCold) in patients with uncomplicated upper respiratory tract infection. Phytomedicine 2010, 17, 178–185 https://doi.org/10.1016/j.phymed.2009.12.001.Search in Google Scholar PubMed
8. Tan, W., Liao, W., Zhou, S., Wong, W. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem. Pharmacol. 2017, 139, 71–81 https://doi.org/10.1016/j.bcp.2017.03.024.Search in Google Scholar PubMed
9. Lu, J., Ma, Y., Wu, J., Huang, H., Wang, X., Chen, Z., Chen, J., He, H., Huang, C. A review for the neuroprotective effects of andrographolide in the central nervous system. Biomed. Pharmacother. 2019, 117, 109078 https://doi.org/10.1016/j.biopha.2019.109078.Search in Google Scholar PubMed
10. Ding, Y.-M., Li, R.-P., Chang, Y.-J., Zhao, J., Liu, H.-M., Li, W. Crystal structure of triaqua-bis(2-(6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)-1-(2-oxo-2,5-dihydrofuran-3-yl)ethane-1-sulfonato-κ2O,O′)calcium(II) – ethanol (1/2), C44H76CaO19S2. Z. Kristallogr. NCS 2020, 235, 515–517 https://doi.org/10.1515/ncrs-2019-0709.Search in Google Scholar
11. Tai, X. S., Wang, X. Synthesis, structural characterization and antitumor activity of a Ca(II) coordination polymer based on 4-formyl-1,3-benzenedisulfonate-2-furoic acid hydrazide ligands. Crystallogr. Rep. 2017, 62, 242–245 https://doi.org/10.1134/s1063774517020286.Search in Google Scholar
© 2020 Ying-Jie Chang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3

