Abstract
C14H13AsClNO2, triclinic, P
CCDC no.: 2031779
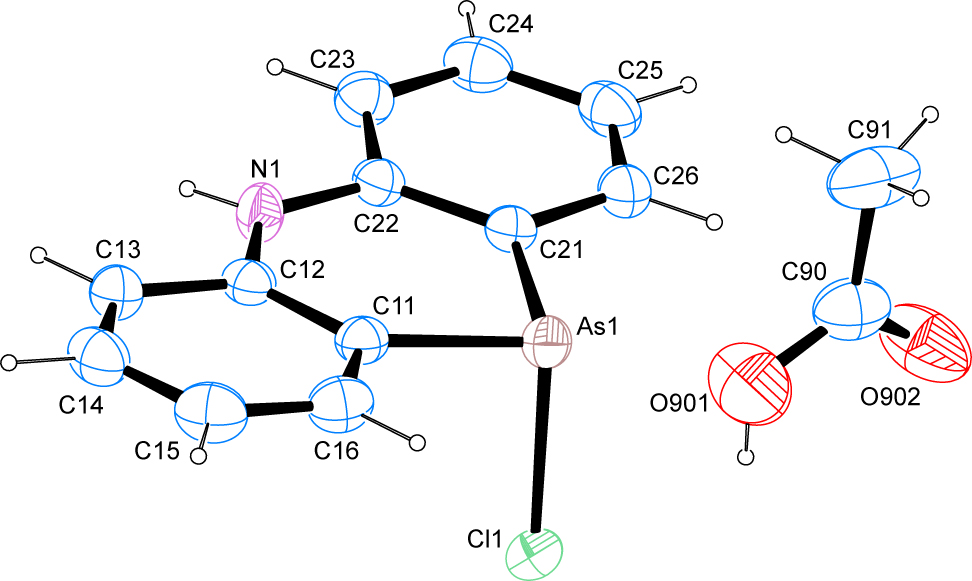
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Green needles |
| Size: | 0.57 × 0.11 × 0.07 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.65 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 28.4°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 12,346, 3435, 0.020 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3118 |
| N(param)refined: | 178 |
| Programs: | Bruker [1], SHELX [2], WinGX/ORTEP [3], Mercury [4], PLATON [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| As1 | 0.00496 (2) | 0.312269 (18) | 0.293392 (12) | 0.02873 (6) |
| Cl1 | −0.17051 (5) | 0.14354 (5) | 0.26643 (3) | 0.03300 (9) |
| N1 | 0.14376 (19) | 0.11283 (16) | 0.50831 (11) | 0.0293 (3) |
| H71 | 0.169 (3) | 0.056 (2) | 0.5632 (17) | 0.040 (5)* |
| C11 | 0.2286 (2) | 0.13103 (18) | 0.29625 (12) | 0.0267 (3) |
| C12 | 0.2633 (2) | 0.05636 (17) | 0.39836 (12) | 0.0259 (3) |
| C13 | 0.4281 (2) | −0.08185 (19) | 0.38971 (14) | 0.0312 (3) |
| H13 | 0.4516 | −0.1350 | 0.4584 | 0.037* |
| C14 | 0.5557 (2) | −0.1408 (2) | 0.28286 (15) | 0.0363 (3) |
| H14 | 0.6669 | −0.2342 | 0.2783 | 0.044* |
| C15 | 0.5238 (2) | −0.0654 (2) | 0.18137 (15) | 0.0395 (4) |
| H15 | 0.6130 | −0.1061 | 0.1077 | 0.047* |
| C16 | 0.3622 (2) | 0.0682 (2) | 0.18865 (13) | 0.0349 (3) |
| H16 | 0.3403 | 0.1195 | 0.1192 | 0.042* |
| C21 | −0.0989 (2) | 0.35162 (17) | 0.45842 (12) | 0.0264 (3) |
| C22 | −0.0190 (2) | 0.25111 (17) | 0.53955 (12) | 0.0262 (3) |
| C23 | −0.1072 (2) | 0.2915 (2) | 0.65904 (13) | 0.0324 (3) |
| H23 | −0.0545 | 0.2241 | 0.7149 | 0.039* |
| C24 | −0.2697 (2) | 0.4280 (2) | 0.69596 (14) | 0.0360 (3) |
| H24 | −0.3283 | 0.4534 | 0.7772 | 0.043* |
| C25 | −0.3492 (2) | 0.5292 (2) | 0.61610 (16) | 0.0374 (4) |
| H25 | −0.4608 | 0.6238 | 0.6421 | 0.045* |
| C26 | −0.2637 (2) | 0.49007 (19) | 0.49924 (15) | 0.0333 (3) |
| H26 | −0.3177 | 0.5588 | 0.4444 | 0.040* |
| O901 | 0.2404 (2) | 0.5009 (2) | −0.02779 (18) | 0.0679 (4) |
| H901 | 0.1690 | 0.4427 | −0.0303 | 0.102* |
| O902 | −0.0334 (2) | 0.70098 (19) | 0.03518 (16) | 0.0653 (4) |
| C90 | 0.1390 (3) | 0.6554 (3) | 0.00838 (15) | 0.0456 (4) |
| C91 | 0.2483 (4) | 0.7744 (3) | 0.01386 (18) | 0.0602 (6) |
| H91A | 0.2979 | 0.8108 | −0.0648 | 0.090* |
| H91B | 0.3543 | 0.7159 | 0.0454 | 0.090* |
| H91C | 0.1649 | 0.8748 | 0.0646 | 0.090* |
Source of material
The title compound was synthesized upon the recrystallization of phenarsazine chloride from hot glacial acetic acid. Crystals suitable for the diffraction studies were obtained upon cooling of the solution to room temperature.
Experimental details
Carbon-bound H atoms were placed in calculated positions (C–H 0.95 Å for aromatic carbon atoms) and were included in the refinement in the riding model approximation, with U(H) set to 1.2 Ueq(C).
The H atoms of the methyl group were allowed to rotate with a fixed angle around the C–C bond to best fit the experimental electron density (HFIX 137 in the SHELX program suite [3]), with U(H) set to 1.5 Ueq(C).
The H atoms of the hydroxyl groups were allowed to rotate with a fixed angle around the C–O bond to best fit the experimental electron density (HFIX 147 in the SHELX program suite [3]), with U(H) set to 1.5 Ueq(O).
The nitrogen-bound H atom was located on a difference Fourier map and refined freely.
Comment
The effect of size and steric hindrance of large ions on chemical and spectroscopic properties of compounds have been a focus of research for many decades. Among the many effects associated with the spatial requirements of counterions have been the glass transition temperature in ionomers [6], surfactant modifying properties [7], the charge transfer in radical ions [8] and polymer-modified electrodes [9], as well as the structural and vibrational spectroscopic behaviour of DNA building blocks [10]. Gaining crystallographic information about a vast variety of large anions has seen significant growth upon the introduction of bulky cations that have simplified the crystallization of these compounds. [11], as for instance showed for chlorido coordination compounds of gallium that incorporate cations derived from the phenarsazine scaffold [12], [13].
At the onset of a research project aimed at the characterization of large anionic compounds, we set out to create a novel set of phenarsazine-inspired cations via phenarsazine chloride as the starting material. To confirm the successful synthesis of the latter, a diffraction study of the compound recrystallized from acetic acid was conducted. Very recently, we have determined the molecular and crystal structures of the arsenic-based compound as the DMSO solvate [14]. Furthermore, the molecular and crystal structures of solvent-free phenarsazine chloride and bromide [15] as well as the para-xylene solvate of phenarsazine chloride [16] are available in the literature.
The crystal structure shows the presence of the desired phenarsazine chloride product as well as one molecule of acetic acid in the asymmetric unit. The As–Cl bond length of 2.3022(4) Å is slightly longer than the most commonly reported arsenic–chlorine bond lengths deposited with the Cambridge Structural Database [17]. The angles around the arsenic atom were measured at 97.18(4)° and 97.96(4)° towards the chlorine atom as well as 96.76(6)° for the intracyclic angle thus ruling out classical hybridization of the heavier pnicogen atom. The least-squares planes as defined by the respective atoms of the two aromatic moieties on the one hand as well as the central six-membered heterocyclic ring on the other hand enclose angles of 3.13(7)° and 3.48(7)°, while the two planes of the outer aromatic moieties intersect at an angle of 6.18(7)°.
In the crystal, classical hydrogen bonds of the O–H…O type and the N–H…Cl type are observed next to C–H…O and C–H…Cl contacts. The O–H…O hydrogen bonds give rise to the familiar pattern of dimers among the carboxylic acid groups of the acetic acid solvate molecule, whose descriptor in terms of graph-set analysis [18], [19] requires a
Acknowledgments
The authors thank Mr Friedhelm Averdunk for logistical support.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX2, SAINT and SADABS; Brucker AXS Inc.: Madison, Wisconsin, USA, 2012.Search in Google Scholar
2. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
3. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
4. Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J., Wood, P. A. Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470; https://doi.org/10.1107/s0021889807067908.Search in Google Scholar
5. Spek, A. L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155; https://doi.org/10.1107/s090744490804362x.Search in Google Scholar
6. Enokida, J. S., Hu, W., Fang, H., Morgan, B. F., Beyer, F. L., Winter, H. H., Coughlin, E. B. Modifying the structure and dynamics of ionomers through counterion sterics. Macromolecules 2020, 53, 1767–1776; https://doi.org/10.1021/acs.macromol.9b02116.Search in Google Scholar
7. Oh, S. G., Shah, D. O. Effect of counterions on the interfacial tension and emulsion droplet size in the oil/water/dodecyl sulfate system. J. Phys. Chem. 1993, 97, 284–286; https://doi.org/10.1021/j100104a003.Search in Google Scholar
8. Piotrowiak, P., Miller, J. R. Counterion effects in intramolecular charge transfer in radical anions. J. Phys. Chem. 1993, 97, 13052–13060; https://doi.org/10.1021/j100152a004.Search in Google Scholar
9. Mathias, M. F., Haas, O. Effect of counterion type on charge transport at redox polymer-modified electrodes. J. Phys. Chem. 1993, 97, 9217–9225; https://doi.org/10.1021/j100138a025.Search in Google Scholar
10. Minguirbara, A., Vamhindi, B. S. D. R., Koyambo-Konzapa, S. J. b, Nsangou, M. Effects of counterions and solvents on the geometrical and vibrational features of dinucleoside-monophosphate (dNMP): case of 3′,5′-dideoxycytidine-monophosphate (dDCMP). J. Mol. Model. 2020, 26, Article no. 99; https://doi.org/10.1007/s00894-020-04369-6.Search in Google Scholar
11. Roof, L. C., Kolis, J. W. New developments in the coordination chemistry of inorganic selenide and telluride ligands. Chem. Rev. 1993, 93, 1037–1080; https://doi.org/10.1021/cr00019a010.Search in Google Scholar
12. Burford, N., Ragogna, P. J., Sharp, K., McDonald, R., Ferguson, M. J. Arsinophosphonium cations from arsenium-phosphine and -bisphosphine coordination chemistry. Inorg. Chem. 2005, 44, 9453–9460; https://doi.org/10.1021/ic050989w.Search in Google Scholar
13. Conrad, E., Burford, N., Werner-Zwanziger, U., McDonald, R., Ferguson, M. J. Phosphinopnictinophosphonium frameworks. Chem. Commun. 2010, 46, 2465–2467; https://doi.org/10.1039/b924918d.Search in Google Scholar
14. Averdunk, A., Hosten, E. C., Betz, R. The molecular and crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS. Z. Kristallogr. NCS. accepted.Search in Google Scholar
15. Fukuyo, M., Nakatsu, K., Shimada, A. The crystal and molecular structure of 10-halo-5, 10-dihydrophenarsazine. Bull. Chem. Soc. Jpn. 1966, 39, 1614–1615; https://doi.org/10.1246/bcsj.39.1614.Search in Google Scholar
16. Camerman, A., Trotter, J. 115. Stereochemistry of arsenic. Part XIII. 10-chloro-5,10-dihydrophenarsazine. J. Chem. Soc. 1965, 730–738; https://doi.org/10.1039/jr9650000730.Search in Google Scholar
17. Allen, F. H. The Cambridge Structural Database: a quarter of a million crystal structures and rising. Acta Crystallogr. 2002, B58, 380–388; https://doi.org/10.1107/s0108768102003890.Search in Google Scholar
18. Bernstein, J., Davis, R. E., Shimoni, L., Chang, N.-L. Patterns in hydrogen bonding: functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. Engl. 1995, 34, 1555–1573; https://doi.org/10.1002/anie.199515551.Search in Google Scholar
19. Etter, M. C., MacDonald, J. C., Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. 1990, B46, 256–262; https://doi.org/10.1107/s0108768189012929.Search in Google Scholar
© 2020 Arthur Averdunk et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3


