Abstract
C24H19AsNO3Rh, triclinic, P
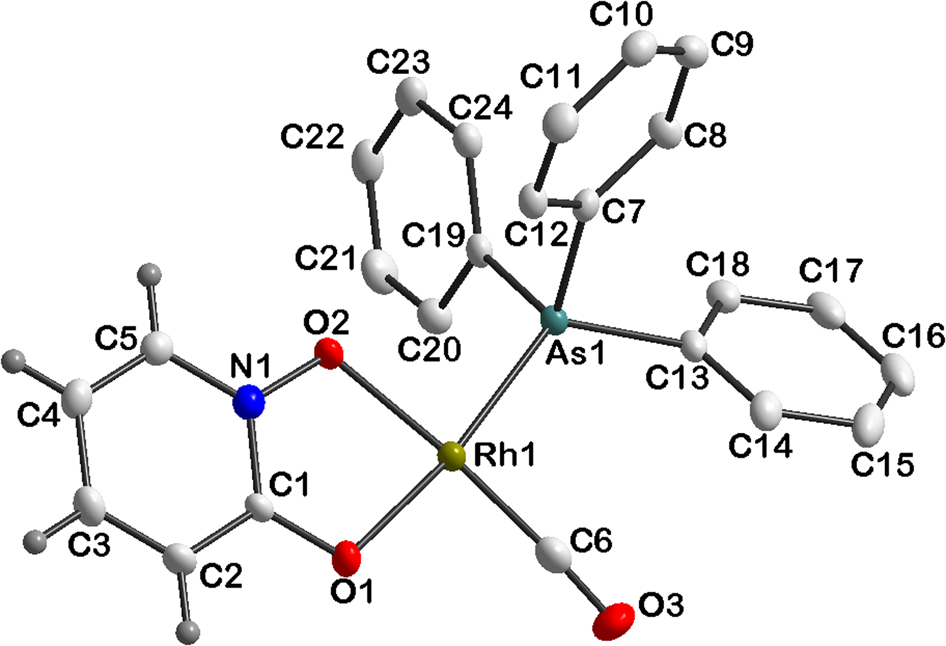
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Yellow plate |
| Size | 0.25 × 0.18 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 2.44 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 28.3°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 13050, 5046, 0.052 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 4283 |
| N(param)refined: | 271 |
| Programs: | SHELX [1], Bruker [2], Diamond [3], OLEX2 [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| As1 | 0.34390 (3) | 0.29682 (3) | 0.68910 (2) | 0.01458 (9) |
| C1 | −0.0073 (3) | 0.2082 (3) | 0.9250 (2) | 0.0159 (6) |
| C2 | −0.0990 (4) | 0.1868 (4) | 0.9968 (3) | 0.0216 (7) |
| H2 | −0.096246 | 0.106811 | 1.038937 | 0.026* |
| C3 | −0.1927 (4) | 0.2794 (4) | 1.0074 (3) | 0.0228 (7) |
| H3 | −0.254851 | 0.264685 | 1.057015 | 0.027* |
| C4 | −0.1972 (4) | 0.3968 (4) | 0.9444 (3) | 0.0215 (6) |
| H4 | −0.264618 | 0.460778 | 0.949425 | 0.026* |
| C5 | −0.1043 (4) | 0.4182 (3) | 0.8763 (3) | 0.0197 (6) |
| H5 | −0.106210 | 0.497969 | 0.833899 | 0.024* |
| C6 | 0.3112 (4) | 0.0396 (4) | 0.8152 (3) | 0.0245 (7) |
| C7 | 0.4641 (3) | 0.4813 (3) | 0.7527 (2) | 0.0158 (6) |
| C8 | 0.5769 (4) | 0.5467 (3) | 0.7066 (3) | 0.0195 (6) |
| H8 | 0.591718 | 0.503615 | 0.641396 | 0.023* |
| C9 | 0.6679 (4) | 0.6757 (4) | 0.7567 (3) | 0.0221 (7) |
| H9 | 0.743827 | 0.721223 | 0.724948 | 0.027* |
| C10 | 0.6476 (4) | 0.7374 (3) | 0.8527 (3) | 0.0225 (7) |
| H10 | 0.710298 | 0.824772 | 0.887129 | 0.027* |
| C11 | 0.5357 (4) | 0.6713 (4) | 0.8985 (3) | 0.0234 (7) |
| H11 | 0.522575 | 0.713399 | 0.964706 | 0.028* |
| C12 | 0.4426 (4) | 0.5441 (3) | 0.8482 (3) | 0.0187 (6) |
| H12 | 0.364669 | 0.500332 | 0.878926 | 0.022* |
| C13 | 0.4939 (3) | 0.2123 (3) | 0.6375 (3) | 0.0168 (6) |
| C14 | 0.6020 (4) | 0.1499 (3) | 0.7128 (3) | 0.0224 (7) |
| H14 | 0.598476 | 0.144413 | 0.788066 | 0.027* |
| C15 | 0.7154 (4) | 0.0955 (4) | 0.6785 (3) | 0.0287 (8) |
| H15 | 0.788727 | 0.052146 | 0.729902 | 0.034* |
| C16 | 0.7208 (4) | 0.1049 (4) | 0.5689 (3) | 0.0311 (8) |
| H16 | 0.799203 | 0.069223 | 0.545710 | 0.037* |
| C17 | 0.6133 (4) | 0.1656 (4) | 0.4931 (3) | 0.0274 (8) |
| H17 | 0.617064 | 0.170712 | 0.417814 | 0.033* |
| C18 | 0.4992 (4) | 0.2195 (3) | 0.5275 (3) | 0.0201 (6) |
| H18 | 0.424950 | 0.261156 | 0.475562 | 0.024* |
| C19 | 0.2027 (3) | 0.3333 (3) | 0.5494 (2) | 0.0161 (6) |
| C20 | 0.0878 (4) | 0.2215 (4) | 0.4912 (3) | 0.0223 (7) |
| H20 | 0.081445 | 0.132005 | 0.520383 | 0.027* |
| C21 | −0.0172 (4) | 0.2407 (4) | 0.3907 (3) | 0.0269 (7) |
| H21 | −0.094072 | 0.163763 | 0.350405 | 0.032* |
| C22 | −0.0102 (4) | 0.3715 (4) | 0.3490 (3) | 0.0242 (7) |
| H22 | −0.083031 | 0.384688 | 0.280597 | 0.029* |
| C23 | 0.1031 (4) | 0.4835 (4) | 0.4071 (3) | 0.0218 (6) |
| H23 | 0.107648 | 0.573354 | 0.378367 | 0.026* |
| C24 | 0.2102 (4) | 0.4645 (3) | 0.5073 (3) | 0.0187 (6) |
| H24 | 0.288163 | 0.541137 | 0.546844 | 0.022* |
| N1 | −0.0074 (3) | 0.3254 (3) | 0.8681 (2) | 0.0211 (6) |
| O1 | 0.0812 (3) | 0.1168 (2) | 0.91090 (19) | 0.0209 (5) |
| O2 | 0.0869 (3) | 0.3463 (2) | 0.80385 (18) | 0.0194 (5) |
| O3 | 0.3684 (4) | −0.0581 (3) | 0.8221 (3) | 0.0394 (7) |
| Rh1 | 0.21129 (3) | 0.18715 (2) | 0.80716 (2) | 0.01565 (8) |
Source of material
[Rh(hopo)(CO)2] (hopo=2-oxopyridineN-oxide) was synthesized according to the method described previously [5]. [Rh(opo)(CO)(AsPh3)] was synthesized by dissolving [Rh(hopo)(CO)2] (0.0210 g, 0.0780 mmol) in 5 cm3 of acetone. Triphenylarsine (0.0239 g, 0.0780 mmol) was added to the aforementioned solution with stirring. Some ice water was added dropwise to precipitate the product. Yellow plate crystals were obtained from recrystallization in acetone and a few drops of water.
IR: νCO 1954 cm−1. 1H NMR (400 MHz, CD2Cl2) δ 8.11 (d, J = 6.2 Hz, 1H), 7.77 (s, 1H), 7.67 (d, J = 6.7 Hz, 15H), 7.56 (d, J = 7.5 Hz, 1H), 7.52 – 7.43 (m, 15H), 7.18 (t, J = 7.5 Hz, 1H), 7.00 (d, J = 8.9 Hz, 1H), 6.76 (d, J = 8.7 Hz, 1H), 6.60 (s, 1H), 6.49 (t, J = 6.3 Hz, 1H).
Experimental details
All H-atoms were positioned on geometrically idealized positions and refined using the riding model with fixed C–H distances for aromatic C–H of 0.93 Å (C–H) [Uiso (H)=1.2 Ueq]. The graphics were obtained using the DIAMOND [3] program with 50% probability ellipsoids.
Comment
The carbonylation of methanol using a rhodium complex as catalyst is an industrial relevant reaction to produce acetic acid. One of the carbonyl ligands in the precursor [Rh(BID)(CO)2] complexes (BID = different monocharged bidentate ligands such as cupferrate and 2-oxopyridine N-oxide, etc.) is substituted by tertiary phosphine ligands (PX3) to form [Rh(BID)(CO)(PX3)] complexes. These complexes have been studied intensively for a potential application in catalytic hydroformylation, hydrogenation, carbonylation, and decarbonylation [6], [7], [8], [9], [10], [11], [12], [13].
The coordination mode in the title complex is similar to the complexes reported previously [14], [15]. In this structure, the ligands are coordinated to rhodium in a distorted square planar geometry. This distortion of the O1-Rh-O2 bond angle from 90° is indicated by the small bite angle of 79.87(2)° of the five membered ring as well as the As1-Rh1-C6, As1-Rh1-O2 and C6-Rh1-O1, angles of 92.37(2), 91.89(2) and 95.89(3)°, respectively. The Rh1-O1, Rh1-O2, Rh1-As1 and Rh1-C6 bond distances are 2.0532(1), 2.0442(1), 2.3377(1) and 1.8010(1) Å, respectively. The lengthening of Rh-O1 compared to Rh-O2 is due to the strong trans influence of the As atom. Phenyl hydrogen atoms in the figure are omitted for clarity (see the figure).
Funding source: University of the Free State
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: The authors would like to thank the University of the Free State for financial support.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar
2. Bruker. SAINT–Plus (Version 7.12) and SADABS (Version 2004/1); Bruker AXS Inc.: Madison, Wisconsin, USA, 2004.Search in Google Scholar
3. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Version 3.0c; Crystal Impact: Bonn, Germany, 2005.Search in Google Scholar
4. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Elmakki, M. A., Koen, R., Venter, J. A., Drost, R. Crystal structure of dicarbonyl(pyridine-2-olate-1-oxido-k2O,O′)rhodium(I), C7H4NO4Rh. Z. Kristallogr. NCS 2016, 231, 703–705; https://doi.org/10.1515/ncrs-2015-0240.Search in Google Scholar
6. Venter, J. A., Leipoldt, J. G., Eldik, R. V. Solvent, temperature, and pressure dependence of oxidative addition of iodomethane to complexes of the type RhI(β-diketone)(CO)(PPh3). Inorg. Chem. 1991, 30, 2207–2209; https://doi.org/10.1021/ic00009a046.Search in Google Scholar
7. Hallinan, N., Hinnenkamp, J. Catalysis of organic reactions. J. Chem. Ind. 2001, 82, 545.Search in Google Scholar
8. Basson, S. S., Leipoldt, J. G., Roodt, A., Venter, J. A. Mechanism for the oxidative addition of iodomethane to carbonyl(N-hydroxy-N-nitrosobenzenaminato-O,O′)- triarylphosphinerhodium(I) complexes and crystal structure of [Rh(cupf)(CO)(CH3)(I)(PPh3)]. Inorg. Chim. Acta 1987, 128, 31–37; https://doi.org/10.1016/s0020-1693(00)84691-5.Search in Google Scholar
9. Brink, A., Roodt, A., Steyl, G., Visser, H. G. Steric vs. electronic anomaly observed from iodomethane oxidative addition to tertiary phosphine modified rhodium(I) acetylacetonato complexes following progressive phenyl replacement by cyclohexyl [PR3= PPh3, PPh2, Cy, PPhCy2, PCy3]. Dalton Trans. 2010, 39, 5572–5578; https://doi.org/10.1039/b922083f.Search in Google Scholar
10. Roodt, A., Visser, H. G., Brink, A. Structure/reactivity relationships and mechanism from X-ray data and spectroscopic kinetic analysis. Crystallogr. Rev. 2011, 17, 241–280; https://doi.org/10.1080/0889311x.2011.593032.Search in Google Scholar
11. Roodt, A., Otto, S., Steyl, G. Structure & solution behaviour of rhodium(I) Vaska-type complexes for correlation of steric & electronic properties of tertiary phosphine ligands. Coord. Chem. Rev. 2003, 245, 121–137; https://doi.org/10.1016/s0010-8545(03)00069-9.Search in Google Scholar
12. Warsink, S., Kotze, P. D. R., Janse van Rensburg, J. M., Venter, J. A., Otto, S., Botha, E., Roodt, A. Kinetic-mechanistic and solid-state study of the oxidative addition and migratory insertion of iodomethane to [rhodium(S,O–BdiPT or N,O-ox)(CO)(PR1R2R3)] complexes. Eur. J. Inorg. Chem. 2018, 32, 3615–3625; https://doi.org/10.1002/ejic.201800293.Search in Google Scholar
13. Elmakki, M. A., Alexander, O. T., Roodt, A. The crystal structure of trans-carbonyl-(diphenylcyclohexyl-phosphine P)iodidomethyl-(2-oxopyridin-1(2H)-olato-k2O,O′)rhodium(III), C25H28INO3PRh. Z. Kristallogr. NCS 2020, 235, 279–281; https://doi.org/10.1515/ncrs-2019-0602.Search in Google Scholar
14. Elmakki, M. A., Alexander, O. T., Venter, G. J. S., Venter, J. A. Crystal structure of carbonyl(2-oxopyridin-1(2H-olato-k2O,Ok2) (diphenylcyclohexylphosphine-P)rhodium(I), C24H25NO3PRh. Z. Kristallogr. NCS 2017, 232, 831–833; https://doi.org/10.1515/ncrs-2017-0066.Search in Google Scholar
15. Elmakki, M. A., Koen, R., Drost, R. M., Alexander, O. T., Venter, G. J. S., Venter, J. A. Crystal structure of carbonyl(2-oxopyridin-1(2H-olato-k2O,O′) (triphenylphosphine-P)rhodium(I), C24H19NO3PRh. Z. Kristallogr. NCS 2016, 231, 781–783; https://doi.org/10.1515/ncrs-2015-0266.Search in Google Scholar
© 2020 Mohammed A. Elmakki et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3


