Abstract
C20H30BClCuN8, monoclinic, P21/c (no. 14), a = 17.1345(7) Å, b = 7.8207(2) Å, c = 19.0213(8) Å, β = 108.268(1)°, V = 2420.46(16) Å3, Z = 4, Rgt(F) = 0.0322, wRref(F2) = 0.0876, T = 184 K.
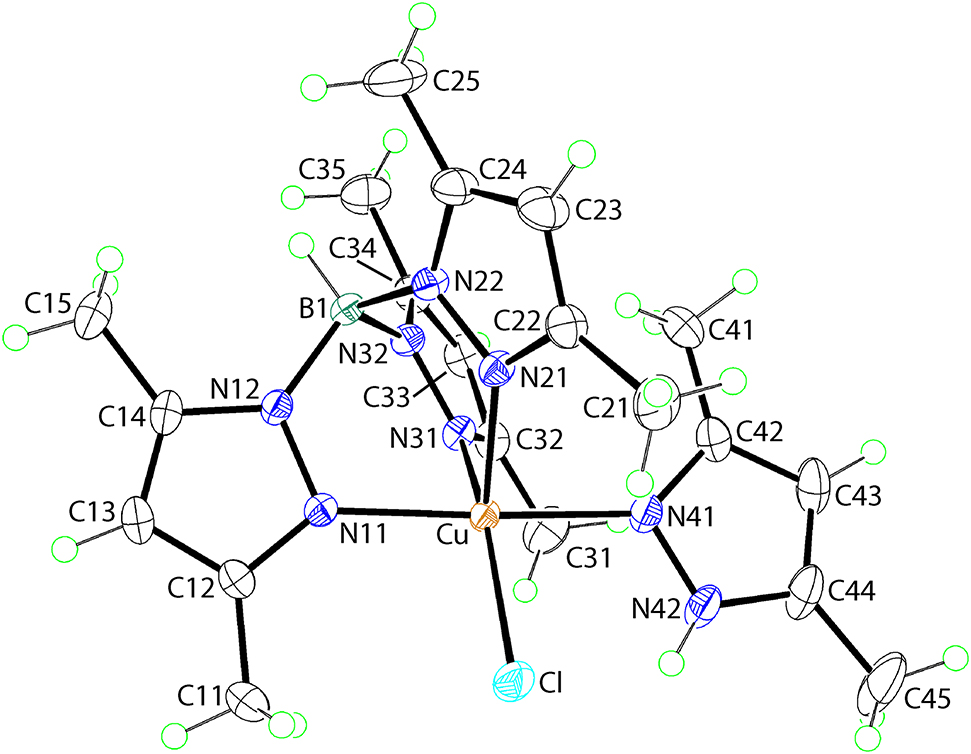
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Green needle |
|---|---|
| Size: | 0.25 × 0.05 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.04 mm-1 |
| Diffractometer, scan mode: | Rigaku Mercury70, ω |
| θmax, completeness: | 27.5°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 18647, 5501, 0.022 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 5223 |
| N(param)refined: | 291 |
| Programs: | REQAB [1], CrystalClear [2], SIR2014 [3], SHELX [4], WinGX/ORTEP [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Cu | 0.26204 (2) | 0.12884 (2) | 0.19167 (2) | 0.01650 (7) |
| Cl | 0.23805 (3) | −0.01354 (6) | 0.28935 (3) | 0.03204 (12) |
| N11 | 0.37327 (8) | 0.02157 (17) | 0.20311 (8) | 0.0185 (3) |
| N12 | 0.39609 (8) | 0.02229 (17) | 0.13957 (8) | 0.0185 (3) |
| N21 | 0.21502 (8) | −0.00725 (18) | 0.08711 (8) | 0.0194 (3) |
| N22 | 0.25958 (9) | 0.01586 (17) | 0.03908 (8) | 0.0193 (3) |
| N31 | 0.30330 (9) | 0.33060 (18) | 0.14730 (8) | 0.0191 (3) |
| N32 | 0.32879 (8) | 0.29815 (18) | 0.08695 (8) | 0.0188 (3) |
| N41 | 0.15275 (9) | 0.25600 (18) | 0.17602 (8) | 0.0221 (3) |
| N42 | 0.12066 (10) | 0.2654 (2) | 0.23280 (9) | 0.0273 (3) |
| H42N | 0.1445 (13) | 0.203 (3) | 0.2717 (9) | 0.033* |
| C11 | 0.43654 (12) | −0.0759 (3) | 0.33436 (11) | 0.0339 (4) |
| H11A | 0.423730 | 0.033881 | 0.353069 | 0.051* |
| H11B | 0.491504 | −0.113169 | 0.364326 | 0.051* |
| H11C | 0.395926 | −0.161461 | 0.337332 | 0.051* |
| C12 | 0.43421 (10) | −0.0558 (2) | 0.25567 (10) | 0.0222 (3) |
| C13 | 0.49568 (11) | −0.1068 (2) | 0.22598 (11) | 0.0267 (4) |
| H13 | 0.545249 | −0.165018 | 0.251111 | 0.032* |
| C14 | 0.46997 (10) | −0.0560 (2) | 0.15341 (10) | 0.0240 (3) |
| C15 | 0.51228 (13) | −0.0791 (3) | 0.09596 (12) | 0.0361 (5) |
| H15A | 0.477457 | −0.147612 | 0.054939 | 0.054* |
| H15B | 0.564828 | −0.137743 | 0.118032 | 0.054* |
| H15C | 0.522216 | 0.033035 | 0.077374 | 0.054* |
| C21 | 0.08398 (13) | −0.1345 (3) | 0.08674 (13) | 0.0352 (5) |
| H21A | 0.107531 | −0.116502 | 0.140223 | 0.053* |
| H21B | 0.067897 | −0.254652 | 0.077043 | 0.053* |
| H21C | 0.035546 | −0.061417 | 0.067228 | 0.053* |
| C22 | 0.14649 (11) | −0.0904 (2) | 0.04968 (10) | 0.0246 (3) |
| C23 | 0.14640 (13) | −0.1214 (3) | −0.02276 (11) | 0.0325 (4) |
| H23 | 0.104976 | −0.178056 | −0.060836 | 0.039* |
| C24 | 0.21882 (12) | −0.0527 (2) | −0.02769 (10) | 0.0278 (4) |
| C25 | 0.25174 (16) | −0.0501 (4) | −0.09226 (12) | 0.0451 (6) |
| H25A | 0.255806 | 0.068475 | −0.107451 | 0.068* |
| H25B | 0.214560 | −0.113889 | −0.133648 | 0.068* |
| H25C | 0.306310 | −0.103057 | −0.077757 | 0.068* |
| C31 | 0.27966 (14) | 0.5805 (3) | 0.21757 (13) | 0.0364 (5) |
| H31A | 0.220371 | 0.602436 | 0.201885 | 0.055* |
| H31B | 0.309425 | 0.688642 | 0.231413 | 0.055* |
| H31C | 0.294377 | 0.503118 | 0.260244 | 0.055* |
| C32 | 0.30219 (11) | 0.5004 (2) | 0.15545 (10) | 0.0232 (3) |
| C33 | 0.32633 (12) | 0.5789 (2) | 0.09957 (11) | 0.0282 (4) |
| H33 | 0.330712 | 0.698151 | 0.092141 | 0.034* |
| C34 | 0.34252 (11) | 0.4483 (2) | 0.05736 (10) | 0.0241 (3) |
| C35 | 0.37099 (14) | 0.4584 (3) | −0.00928 (12) | 0.0358 (4) |
| H35A | 0.423883 | 0.399517 | 0.000987 | 0.054* |
| H35B | 0.377389 | 0.578533 | −0.021043 | 0.054* |
| H35C | 0.330362 | 0.403687 | −0.051417 | 0.054* |
| C41 | 0.11463 (13) | 0.3682 (3) | 0.04744 (11) | 0.0332 (4) |
| H41A | 0.147908 | 0.470950 | 0.049066 | 0.050* |
| H41B | 0.061519 | 0.381148 | 0.008724 | 0.050* |
| H41C | 0.143411 | 0.268385 | 0.036519 | 0.050* |
| C42 | 0.10100 (11) | 0.3442 (2) | 0.12042 (11) | 0.0259 (4) |
| C43 | 0.03564 (12) | 0.4065 (3) | 0.14299 (14) | 0.0356 (5) |
| H43 | −0.009678 | 0.472329 | 0.114116 | 0.043* |
| C44 | 0.04986 (13) | 0.3539 (3) | 0.21465 (14) | 0.0357 (5) |
| C45 | 0.00349 (18) | 0.3768 (3) | 0.26903 (19) | 0.0577 (8) |
| H45A | 0.011081 | 0.275640 | 0.300867 | 0.087* |
| H45B | −0.055094 | 0.391742 | 0.242239 | 0.087* |
| H45C | 0.024191 | 0.478073 | 0.299539 | 0.087* |
| B1 | 0.34288 (12) | 0.1108 (2) | 0.06774 (10) | 0.0187 (3) |
| H1 | 0.371706 | 0.107742 | 0.029464 | 0.022* |
Source of material
A solution of Na{HB(3,5-Me2pz)3} (100.5 mg, 0.313 mmol) [6] in dichloromethane (13 mL) was added slowly to a solution of CuCl2⋅2H2O (61.0 mg, 0.358 mmol) in acetone (13 mL). In addition, one equivalent of 3,5-Me2pzH (30.1 mg, 0.313 mmol) in dichloromethane (13 mL) was dropped carefully into this solution. After the mixture was stirred overnight, the solvent was evaporated under vacuum. The resulting solid was extracted with dichloromethane (20 mL). The filtrate was evaporated under vacuum, and a green powder was obtained. Green crystals were obtained by slow evaporation of a saturated dichloromethane/n-octane solution at room temperature. Yield: 62% (95.8 mg, 0.195 mmol). Anal. Calcd. for C20H30BClCuN8. C, 48.79; H, 6.14; N, 22.76%. Found: C; 48.48, H; 6.02, N; 22.54%. IR (JASCO FT/IR-550 spectrophotometer, KBr; cm−1): 3211 (m) ν(N–H), 2927 (m) ν(C–H), 2511 (m) ν(B–H), 1567 (m) ν(C=N), 1542 (s) ν(C=N). UV–Vis (JASCO V-570 at 298 K in dichloromethane); λmax, nm (ε, mol−1 cm−1) 341 (1100), 430 (shoulder, 230), 730 (100). ESR Bruker EMX-T, 5 mm φ quartz tube, dichloromethane: 1,2-dichloroethane = 1:1, 124 K: g‖, 2.32 (A‖, 159 G); g(⊥), 2.07.
Experimental details
The C- and B-bound H atoms were geometrically placed (C–H = 0.95–0.98 Å & B–H = 1.00 Å) and refined as riding with Uiso(H) = 1.2–1.5 Ueq(C) and 1.2 Ueq(B). The N-bound H atom was refined with N–H = 0.88 ± 0.01 Å, and with Uiso(H) = 1.2 Ueq(N).
Comment
Boron-substituted poly(1-pyrazolyl)borates occupy a prominent position in current coordination chemistry [6]. Among this class of ligand, the methyl substituted hydridotris(3,5-dimethyl-pyrazolyl-1-yl]borato-κN3 anion is very common. It has been designated as “Tp*” [7–10] and also named “homoscorpionates-first generation” [7, 10]. The Tp* ligand is relatively sterically unhindered so it can readily form coordinatively saturated six-coordinate, bis-chelate complexes, formulated as [M(Tp*)2]. For copper(II), the structure of [Cu(Tp*)2] has been reported [11]. To avoid the formation of this bis-chelate complex, the synthetic methodology needs to be carefully modified: the solution containing the Tp* ligand needs to be added slowly to a solution containing a small amount of excess metal salt [12]. This work reports the crystal structure and some properties of a five-coordinate copper(II) complex with the first generation Tp* ligand, also containing a coordinated 3,5-dimethylpyrazol-1-yl molecule, i.e. [Cu(Cl)(3,5-Me2pzH){HB(3,5-Me2pz)3}], (I).
It is well-known that four-coordinate copper(II) geometries are not so common in coordination chemistry [13]. In particular, the tetrahedral geometry is very unstable for less sterically hindered ligands such as Tp*. Indeed, the slow reaction of a copper(II) salt, such as CuCl2, with Tp* yielded a mixture of four- (red) and five-coordinate (green) complexes. To ensure the formation of a five-coordinate complex, one equivalent of pyrazole was also added to the reaction mixture. In the IR spectrum of (I), the ν (C=N) signals were split, with absorptions at 1567 and 1542 cm−1, reflecting the presence of two kinds of pyrazole ligands. The IR also provides evidence for hydrogen bonding by the pzH–N–H residue with a very sharp absorption noted at 3211 cm−1. The ground state of (I) is dx2−y2, as confirmed by ESR [14]. This ground state is also supported by UV–Vis spectroscopy. For (I), the d–d transition occurs at 730 nm (100 M−1 cm−1) which is shifted by approximately 200 nm to higher energy compared with that of 996 nm (150 M−1 cm−1) for [Cu(Cl){HB(3,5-iPr2pz)3}] [15] and 906 nm (180 M−1 cm−1) for [Cu(Cl){HB(3-Ph-5-Mepz)3}] [13].
The molecular structure determined by X-ray diffraction of (I) is shown in figure (50% probably displacement ellipsoids). The Cu atom is coordinated by a Cl, three pyrazolyl-N11, N21 and N31 atoms of the tripodal ligand and a pyrazolyl-N41 atom derived from a neutral 3,5-dimethylpyrazol-1-yl molecule. The Cu–Cl bond length in (I) of 2.3107(5) Å is slightly longer than the equivalent Cu–Cl bonds in other five-coordinated chlorido copper(II) complexes, viz. 2.260(2) Å in [Cu(Cl)(dmf){HB(3,5-iPr2pz)3}] [15] and 2.2833(8) Å in [Cu(Cl)(3-Ph-5-MepzH){HB(3-Ph-5-Mepz)3}] [16]. These bond lengths are approximately 0.1 Å longer compared with the Cu–Cl bond lengths in four-coordinate, chlorido copper(II) complexes [13].
Each of the four Cu–N bond lengths in (I) is experimentally distinct from the others. The Cu–N11 [2.0305(14) Å] and Cu–N31 [2.0189(14) Å] bond lengths are different and significantly shorter than the Cu–N21 [2.1756(14) Å] separation. The Cu–N41 bond length [2.0579(14) Å] is intermediate between the extreme Cu–N values formed by the tripodal ligand. The ClN4 donor set defines, to a first approximation a distorted square-pyramidal geometry, with the less tightly bound pyrazolyl-N21 atom occupying the axial position with the N11 and N31 atoms approximately trans to the N41 [N11–Cu–N41 = 174.93(6)°] and Cl [N31–Cu–Cl = 152.45(4)°] atoms. The distortion in the coordination geometry is quantified by the value of τ = 0.37 which compares with values of 0.0 and 1.0 for ideal square-pyramidal and trigonal-bipyramidal geometries, respectively [17]. The orientation of the 3-phenyl-5-methylpyrazol-1-yl ligand is to place the amine-N42–H group in close proximity with the Cl atom which enables the formation of an intramolecular N–H···Cl [N42–H42n···Cl: H42n···Cl = 2.28(2) Å, N42···Cl = 2.9335(17) Å with angle at H42n = 131.3(17)°] hydrogen bond.
In the molecular packing, the only directional contact is a long pyrazolyl-C–H···π(pyrazolyl) [C33–H33···Cg(N21, N22, C22–C24)i: H33···Cg(N21, N22, C22–C24)i = 2.98 Å with angle at H33 = 129° for symmetry operation (i): x, 1 + y, z] contact. The result is the formation of a linear, supramolecular chain parallel to the b-axis. To investigate the molecular packing further, with the aid of Crystal Explorer 17 [18] and following literature methods [19], the Hirshfeld surface along with the full and delineated two-dimensional fingerprint plots were calculated. Consistent with the lack of evident directional interactions in the crystal of (I), the major contribution to the surface comes from H···H contacts, at 73.9%. After this are H···C/C···H [10.9%], H···N/N···H [8.0%] and H···C/C···H [6.8%] contacts.
Funding source: Osaka City University
Funding source: Sunway University
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: KF is grateful for support from the joint usage/research programme “Artificial Photosynthesis” based at Osaka City University. Sunway University Sdn Bhd is thanked for financial support of this work through Grant No. STR-RCTR-RCCM-001-2019.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. REQAB; Rigaku Corporation: Tokyo, Japan, 1998.Search in Google Scholar
2. CrystalClear–SM Expert; Rigaku Corporation: Tokyo, Japan, 2019.Search in Google Scholar
3. Burla, M. C., Caliandro, R., Carrozzini, B., Cascarano, G. L., Cuocci, C., Giacovazzo, C., Mallamo, M., Mazzone, A., Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Cryst. 2015, 48, 306–309; https://doi.org/10.1107/s1600576715001132.Search in Google Scholar
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Cryst. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
6. Trofimenko, S. Boron-pyrazole chemistry. IV. Carbon- and boron-substituted poly(1-pyrazolyl)borates. J. Am. Chem. Soc. 1967, 89, 6288–6294; https://doi.org/10.1021/ja01000a053.Search in Google Scholar
7. Trofimenko, S. In Scorpionates: The Coordination Chemistry of Polypyrazolyborate Ligands; Imperial College Press: London, 1999.10.1142/p148Search in Google Scholar
8. Trofimenko, S. Polypyrazolylborates: scorpionates. J. Chem. Educ. 2005, 82, 1715–1720; https://doi.org/10.1021/ed082p1715.Search in Google Scholar
9. Trofimenko, S. Scorpionates: genesis, milestones, prognosis. Polyhedron 2004, 23, 197–203; https://doi.org/10.1016/j.poly.2003.11.013.Search in Google Scholar
10. Pettinari, C. In Scorpionates II: Chelating Borate Ligands; Imperial College Press: London, 2008.10.1142/p527Search in Google Scholar
11. Marsh, R. E. Bis[hydrotris(3,5-dimethyl-1-pyrazolyl)borato]copper(II). Corrigendum. Acta Crystallogr. 1989, C45, 1269–1270; https://doi.org/10.1107/s010827018900288x.Search in Google Scholar
12. Kitajima, N., Koda, T., Hashimoto, S., Kitagawa, T., Moro-oka, Y. Synthesis and characterization of the dinuclear copper(II) complexes [Cu(HB(3,5-Me2pz)3)]2X (X = O2‐, (OH)22‐, CO32‐, O22‐). J. Am. Chem. Soc. 1991, 113, 5664–5671; https://doi.org/10.1021/ja00015a021.Search in Google Scholar
13. Fujisawa, K., Shimizu, M., Tiekink, E. R. T. Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6. Z. Kristallogr. NCS 2020, 235. NCRS-2020-0410.10.1515/ncrs-2020-0372Search in Google Scholar
14. Solomon, E. I., Heppner, D. E., Johnston, E. M., Ginsbach, J. W., Cirera, J., Qayyum, M., Kieber-Emmons, M. T., Kjaergaard, C. H., Hadt, R. G., Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853; https://doi.org/10.1021/cr400327t.Search in Google Scholar
15. Kitajima, N., Fujisawa, K., Moro-oka, Y. Tetrahedral copper(II) complexes supported by a hindered pyrazolylborate. Formation of the thiolato complex, which closely mimics the spectroscopic characteristics of blue copper proteins. J. Am. Chem. Soc. 1990, 112, 3210–3212; https://doi.org/10.1021/ja00164a052.Search in Google Scholar
16. Lupidi, G., Marchetti, F., Masciocchi, N., Reger, D. L., Tabassum, S., Astolfi, P., Damiani, E., Pettinari, C. Synthesis, structural and spectroscopic characterization and biomimetic properties of new copper, manganese, zinc complexes: identification of possible superoxide-dismutase mimics bearing hydroxyl radical generating/scavenging abilities. J. Inorg. Biochem. 2010, 104, 820–830; https://doi.org/10.1016/j.jinorgbio.2010.03.013.Search in Google Scholar
17. Addison, A. W., Rao, T. N., Reedijk, J., van Rijn, J., Verschoor, G. C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356.10.1039/DT9840001349Search in Google Scholar
18. Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Spackman, P. R., Jayatilaka, D., Spackman, M. A. Crystal Explorer v17; The University of Western Australia: Australia, 2017.Search in Google Scholar
19. Tan, S. L., Jotani, M. M., Tiekink, E. R. T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. 2019, E75, 308–318; https://doi.org/10.1107/s2056989019001129.Search in Google Scholar
© 2020 Kiyoshi Fujisawa and Edward R. T. Tiekink, published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3

