Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
Abstract
C32H54O4, monoclinic, P21 (no. 4), a = 7.8727(1) Å, b = 19.9173(2) Å, c = 19.1801(2) Å, β = 94.539(1)°, V = 2998.06(6) Å3, Z = 4, Rgt(F) = 0.0370, wRref(F2) = 0.0983, T = 293 K.
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.13 × 0.10 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.55 mm−1 |
| Diffractometer, scan mode | Xcalibur |
| θmax, completeness: | 66.5°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 43,442, 10,562, 0.029 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 9800 |
| N(param)refined: | 669 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3], SUPERFLIP [4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.1680 (3) | 0.06139 (12) | 1.00958 (12) | 0.0476 (5) |
| H1A | 0.113929 | 0.104685 | 1.001042 | 0.057* |
| H1B | 0.128413 | 0.031685 | 0.971645 | 0.057* |
| C2 | 0.1130 (3) | 0.03301 (14) | 1.07813 (13) | 0.0548 (6) |
| H2A | 0.144863 | 0.063853 | 1.116069 | 0.066* |
| H2B | −0.009825 | 0.027675 | 1.075041 | 0.066* |
| C3 | 0.1977 (3) | −0.03412 (14) | 1.09272 (12) | 0.0527 (6) |
| H3 | 0.164856 | −0.063883 | 1.053250 | 0.063* |
| C4 | 0.3925 (3) | −0.03125 (14) | 1.10138 (13) | 0.0528 (5) |
| C5 | 0.4468 (3) | 0.00180 (12) | 1.03268 (12) | 0.0450 (5) |
| H5 | 0.407261 | −0.029844 | 0.995772 | 0.054* |
| C6 | 0.6404 (3) | 0.00520 (14) | 1.02797 (14) | 0.0562 (6) |
| H6A | 0.687306 | 0.041741 | 1.056864 | 0.067* |
| H6B | 0.692225 | −0.036298 | 1.045489 | 0.067* |
| C7 | 0.6818 (3) | 0.01612 (14) | 0.95263 (14) | 0.0561 (6) |
| H7A | 0.644159 | −0.022801 | 0.925199 | 0.067* |
| H7B | 0.804491 | 0.019410 | 0.951400 | 0.067* |
| C8 | 0.5995 (3) | 0.07922 (12) | 0.91843 (12) | 0.0446 (5) |
| C9 | 0.4061 (2) | 0.08137 (11) | 0.93185 (11) | 0.0405 (4) |
| H9 | 0.356250 | 0.042679 | 0.906148 | 0.049* |
| C10 | 0.3620 (2) | 0.06991 (11) | 1.00917 (11) | 0.0406 (4) |
| C11 | 0.3197 (3) | 0.14259 (13) | 0.89570 (13) | 0.0493 (5) |
| H11A | 0.199058 | 0.141260 | 0.902691 | 0.059* |
| H11B | 0.366360 | 0.182958 | 0.918098 | 0.059* |
| C12 | 0.3404 (3) | 0.14725 (13) | 0.81729 (13) | 0.0523 (6) |
| H12 | 0.276463 | 0.110686 | 0.793188 | 0.063* |
| C13 | 0.5277 (3) | 0.14166 (12) | 0.80294 (12) | 0.0471 (5) |
| H13 | 0.586437 | 0.179902 | 0.826215 | 0.057* |
| C14 | 0.6088 (3) | 0.07720 (12) | 0.83671 (12) | 0.0477 (5) |
| C15 | 0.7886 (4) | 0.08003 (18) | 0.81006 (16) | 0.0702 (7) |
| H15A | 0.863448 | 0.107885 | 0.840479 | 0.084* |
| H15B | 0.837235 | 0.035353 | 0.808528 | 0.084* |
| C16 | 0.7659 (4) | 0.10999 (16) | 0.73718 (16) | 0.0668 (7) |
| H16A | 0.780626 | 0.075611 | 0.702340 | 0.080* |
| H16B | 0.849756 | 0.144960 | 0.732001 | 0.080* |
| C17 | 0.5829 (3) | 0.13972 (13) | 0.72739 (13) | 0.0536 (6) |
| H17 | 0.511239 | 0.106227 | 0.701817 | 0.064* |
| C18 | 0.5734 (3) | 0.20449 (13) | 0.68316 (13) | 0.0554 (6) |
| C19 | 0.6497 (4) | 0.19132 (18) | 0.61326 (16) | 0.0734 (8) |
| H19A | 0.772965 | 0.190965 | 0.620806 | 0.088* |
| H19B | 0.613637 | 0.147368 | 0.596053 | 0.088* |
| C20 | 0.5960 (5) | 0.2440 (2) | 0.55813 (17) | 0.0897 (11) |
| H20A | 0.639933 | 0.287572 | 0.573234 | 0.108* |
| H20B | 0.643467 | 0.232778 | 0.514453 | 0.108* |
| C21 | 0.4042 (5) | 0.2470 (3) | 0.54697 (17) | 0.0940 (11) |
| H21A | 0.361828 | 0.204545 | 0.527856 | 0.113* |
| H21B | 0.371914 | 0.281861 | 0.513175 | 0.113* |
| C22 | 0.3219 (4) | 0.2613 (2) | 0.61478 (15) | 0.0772 (9) |
| C23 | 0.0015 (3) | −0.10468 (13) | 1.14579 (13) | 0.0539 (6) |
| C24 | −0.0582 (4) | −0.12987 (18) | 1.21271 (16) | 0.0732 (8) |
| H24A | −0.135615 | −0.097974 | 1.230349 | 0.110* |
| H24B | 0.037755 | −0.135802 | 1.246235 | 0.110* |
| H24C | −0.115384 | −0.172073 | 1.204715 | 0.110* |
| C25 | 0.4579 (4) | 0.00528 (18) | 1.16905 (14) | 0.0695 (8) |
| H25A | 0.405104 | 0.048641 | 1.170555 | 0.104* |
| H25B | 0.579280 | 0.010579 | 1.169955 | 0.104* |
| H25C | 0.430186 | −0.020628 | 1.208800 | 0.104* |
| C26 | 0.4579 (4) | −0.10434 (17) | 1.10499 (19) | 0.0763 (8) |
| H26A | 0.405298 | −0.127976 | 1.141222 | 0.114* |
| H26B | 0.579307 | −0.104382 | 1.115081 | 0.114* |
| H26C | 0.429813 | −0.126097 | 1.060926 | 0.114* |
| C27 | 0.4149 (3) | 0.12945 (13) | 1.05750 (13) | 0.0546 (6) |
| H27A | 0.399706 | 0.170695 | 1.031857 | 0.082* |
| H27B | 0.532460 | 0.124732 | 1.074271 | 0.082* |
| H27C | 0.345493 | 0.129998 | 1.096447 | 0.082* |
| C28 | 0.6985 (3) | 0.14067 (14) | 0.94981 (14) | 0.0581 (6) |
| H28A | 0.725268 | 0.133750 | 0.998981 | 0.087* |
| H28B | 0.629617 | 0.180239 | 0.942787 | 0.087* |
| H28C | 0.802005 | 0.146155 | 0.927119 | 0.087* |
| C29 | 0.5223 (4) | 0.01358 (13) | 0.80307 (14) | 0.0618 (6) |
| H29A | 0.529415 | 0.014796 | 0.753357 | 0.093* |
| H29B | 0.404757 | 0.012455 | 0.813127 | 0.093* |
| H29C | 0.579145 | −0.025808 | 0.821970 | 0.093* |
| C30 | 0.6610 (4) | 0.26291 (16) | 0.72245 (17) | 0.0732 (8) |
| H30A | 0.678400 | 0.298778 | 0.690339 | 0.110* |
| H30B | 0.769108 | 0.248359 | 0.743899 | 0.110* |
| H30C | 0.590957 | 0.278441 | 0.757886 | 0.110* |
| C31 | 0.3453 (6) | 0.3348 (2) | 0.6367 (2) | 0.0997 (12) |
| H31A | 0.308354 | 0.340797 | 0.682791 | 0.149* |
| H31B | 0.278810 | 0.362943 | 0.604337 | 0.149* |
| H31C | 0.463405 | 0.346781 | 0.636673 | 0.149* |
| C32 | 0.1343 (5) | 0.2444 (3) | 0.6084 (2) | 0.1093 (15) |
| H32A | 0.119854 | 0.197573 | 0.597584 | 0.164* |
| H32B | 0.077394 | 0.270803 | 0.571793 | 0.164* |
| H32C | 0.086520 | 0.253981 | 0.651848 | 0.164* |
| C33 | 0.3862 (3) | 0.02020 (12) | 0.43299 (13) | 0.0492 (5) |
| H33A | 0.432325 | 0.014825 | 0.387902 | 0.059* |
| H33B | 0.422059 | 0.063706 | 0.451509 | 0.059* |
| C34 | 0.4589 (3) | −0.03468 (13) | 0.48254 (14) | 0.0549 (6) |
| H34A | 0.430258 | −0.078358 | 0.462562 | 0.066* |
| H34B | 0.582097 | −0.030949 | 0.487785 | 0.066* |
| C35 | 0.3899 (3) | −0.02922 (12) | 0.55297 (14) | 0.0510 (5) |
| H35 | 0.428608 | 0.013445 | 0.574150 | 0.061* |
| C36 | 0.1955 (3) | −0.03220 (13) | 0.55253 (14) | 0.0530 (6) |
| C37 | 0.1224 (3) | 0.02019 (12) | 0.49728 (12) | 0.0447 (5) |
| H37 | 0.160048 | 0.063750 | 0.516571 | 0.054* |
| C38 | −0.0716 (3) | 0.02421 (16) | 0.49193 (14) | 0.0603 (7) |
| H38A | −0.118791 | −0.013725 | 0.465222 | 0.072* |
| H38B | −0.111895 | 0.021989 | 0.538380 | 0.072* |
| C39 | −0.1325 (3) | 0.08930 (15) | 0.45654 (14) | 0.0575 (6) |
| H39A | −0.093194 | 0.126796 | 0.485796 | 0.069* |
| H39B | −0.256126 | 0.089981 | 0.452874 | 0.069* |
| C40 | −0.0701 (3) | 0.09896 (11) | 0.38303 (12) | 0.0437 (5) |
| C41 | 0.1267 (2) | 0.08521 (10) | 0.38582 (11) | 0.0377 (4) |
| H41 | 0.177704 | 0.120985 | 0.415664 | 0.045* |
| C42 | 0.1901 (3) | 0.01832 (11) | 0.42290 (12) | 0.0416 (4) |
| C43 | 0.1949 (3) | 0.09742 (11) | 0.31429 (12) | 0.0464 (5) |
| H43A | 0.317338 | 0.090669 | 0.318395 | 0.056* |
| H43B | 0.145523 | 0.064394 | 0.281495 | 0.056* |
| C44 | 0.1566 (3) | 0.16761 (12) | 0.28471 (12) | 0.0456 (5) |
| H44 | 0.220993 | 0.200695 | 0.313951 | 0.055* |
| C45 | −0.0326 (3) | 0.18342 (10) | 0.28441 (11) | 0.0397 (4) |
| H45 | −0.093108 | 0.150762 | 0.253326 | 0.048* |
| C46 | −0.0968 (3) | 0.17357 (12) | 0.35823 (12) | 0.0434 (5) |
| C47 | −0.2832 (3) | 0.19727 (16) | 0.34474 (15) | 0.0638 (7) |
| H47A | −0.353530 | 0.162176 | 0.322440 | 0.077* |
| H47B | −0.329890 | 0.209931 | 0.388157 | 0.077* |
| C48 | −0.2737 (3) | 0.25804 (14) | 0.29629 (14) | 0.0565 (6) |
| H48A | −0.280046 | 0.299321 | 0.322780 | 0.068* |
| H48B | −0.367796 | 0.257209 | 0.260471 | 0.068* |
| C49 | −0.1011 (3) | 0.25420 (11) | 0.26218 (11) | 0.0440 (5) |
| H49 | −0.024715 | 0.287304 | 0.285936 | 0.053* |
| C50 | −0.1189 (3) | 0.27284 (11) | 0.18404 (12) | 0.0463 (5) |
| C51 | −0.2034 (4) | 0.34220 (13) | 0.17510 (15) | 0.0596 (6) |
| H51A | −0.324739 | 0.337512 | 0.179356 | 0.072* |
| H51B | −0.157751 | 0.371388 | 0.212506 | 0.072* |
| C52 | −0.1761 (4) | 0.37490 (15) | 0.10539 (16) | 0.0697 (8) |
| H52A | −0.233127 | 0.348803 | 0.067785 | 0.084* |
| H52B | −0.225017 | 0.419620 | 0.103946 | 0.084* |
| C53 | 0.0128 (4) | 0.37919 (13) | 0.09525 (16) | 0.0646 (7) |
| H53A | 0.067141 | 0.408966 | 0.130290 | 0.078* |
| H53B | 0.027831 | 0.398270 | 0.049612 | 0.078* |
| C54 | 0.0993 (4) | 0.31055 (12) | 0.10086 (13) | 0.0549 (6) |
| C55 | 0.6127 (3) | −0.07488 (15) | 0.63096 (13) | 0.0574 (6) |
| C56 | 0.6691 (5) | −0.1358 (2) | 0.6713 (2) | 0.0979 (12) |
| H56A | 0.781282 | −0.128563 | 0.693396 | 0.147* |
| H56B | 0.671024 | −0.173503 | 0.640143 | 0.147* |
| H56C | 0.591403 | −0.144699 | 0.706292 | 0.147* |
| C57 | 0.1288 (4) | −0.10390 (15) | 0.53810 (19) | 0.0766 (9) |
| H57A | 0.170814 | −0.132992 | 0.575481 | 0.115* |
| H57B | 0.167566 | −0.119740 | 0.494819 | 0.115* |
| H57C | 0.006524 | −0.103698 | 0.534985 | 0.115* |
| C58 | 0.1476 (4) | −0.0111 (2) | 0.62530 (16) | 0.0775 (9) |
| H58A | 0.205933 | −0.039268 | 0.660058 | 0.116* |
| H58B | 0.026754 | −0.015685 | 0.627675 | 0.116* |
| H58C | 0.179944 | 0.034818 | 0.633746 | 0.116* |
| C59 | 0.1394 (4) | −0.04407 (12) | 0.37884 (15) | 0.0614 (7) |
| H59A | 0.148762 | −0.034323 | 0.330275 | 0.092* |
| H59B | 0.024004 | −0.056183 | 0.385883 | 0.092* |
| H59C | 0.213848 | −0.080654 | 0.392859 | 0.092* |
| C60 | −0.1722 (3) | 0.05077 (14) | 0.33225 (16) | 0.0620 (7) |
| H60A | −0.179008 | 0.007381 | 0.353645 | 0.093* |
| H60B | −0.115999 | 0.046807 | 0.289816 | 0.093* |
| H60C | −0.285023 | 0.068218 | 0.321782 | 0.093* |
| C61 | −0.0090 (4) | 0.22401 (13) | 0.41058 (13) | 0.0560 (6) |
| H61A | 0.109468 | 0.212728 | 0.418791 | 0.084* |
| H61B | −0.062172 | 0.222102 | 0.453878 | 0.084* |
| H61C | −0.019668 | 0.268548 | 0.391605 | 0.084* |
| C62 | −0.2182 (4) | 0.21914 (14) | 0.14080 (14) | 0.0622 (7) |
| H62A | −0.258495 | 0.237575 | 0.096285 | 0.093* |
| H62B | −0.313476 | 0.204524 | 0.165184 | 0.093* |
| H62C | −0.144944 | 0.181607 | 0.133796 | 0.093* |
| C63 | 0.0535 (5) | 0.26695 (15) | 0.03698 (15) | 0.0712 (8) |
| H63A | 0.090975 | 0.221759 | 0.046436 | 0.107* |
| H63B | 0.108367 | 0.284225 | −0.002214 | 0.107* |
| H63C | −0.067747 | 0.267357 | 0.026428 | 0.107* |
| C64 | 0.2907 (4) | 0.31845 (16) | 0.11139 (17) | 0.0711 (8) |
| H64A | 0.319344 | 0.344771 | 0.152577 | 0.107* |
| H64B | 0.331418 | 0.340600 | 0.071500 | 0.107* |
| H64C | 0.342810 | 0.274987 | 0.116774 | 0.107* |
| O1 | 0.1343 (2) | −0.06352 (10) | 1.15541 (9) | 0.0622 (5) |
| O2 | 0.2658 (3) | 0.20991 (12) | 0.79560 (11) | 0.0815 (7) |
| H2 | 0.286174 | 0.217622 | 0.755102 | 0.122* |
| O3 | 0.3913 (2) | 0.21650 (10) | 0.66925 (9) | 0.0604 (4) |
| O4 | −0.0686 (3) | −0.11719 (12) | 1.08954 (10) | 0.0750 (6) |
| O5 | 0.4589 (2) | −0.08384 (9) | 0.59764 (10) | 0.0630 (5) |
| O6 | 0.2184 (3) | 0.16755 (11) | 0.21699 (10) | 0.0745 (6) |
| H6 | 0.184532 | 0.201280 | 0.195675 | 0.112* |
| O7 | 0.0558 (2) | 0.27785 (8) | 0.16543 (8) | 0.0471 (3) |
| O8 | 0.6930 (3) | −0.02449 (13) | 0.62787 (11) | 0.0752 (6) |
Source of material
A solution of 20(R)-panoxadiol and acetic anhydride in pyridine was stirred for 11 h at room temperature. After evaporation under reduced pressure, the residue was dissolved in ethyl acetate and washed successively by water and brine, and dried over anhydrous sodium sulfate. After filtration, the ethyl acetate was removed in vacuo to yield a white solid, which was purified by silica-gel column chromatography (petroleum ether:ethyl acetate = 1:2 v/v). Suitable crystals were obtained by slow evaporation in ethyl acetate.
Experimental details
All H atoms were included in calculated positions and refined as riding atoms, with C–H = 0.96–0.98 Å and with O–C = 1.190–1.448 Å. The absolute configuration was derived from the synthesis and the configuration of the educts.
Comment
20(R)–Panoxadiol which was achieved by acid degradation from leaves and stems of ginseng can inhibit the release of inflammatory cytokine interleukin-6 and increase the release of anti-inflammatory mediator interleukin-10 [5], [6]. It has been reported to possess anti-inflammatory effect [7], [8]. It is a potent approach to modify the structures of natural products and preparing a variety of derivatives for the search of new lead compound. Therefore 20(R)-panoxadiol and its derivatives have attracted much attention.
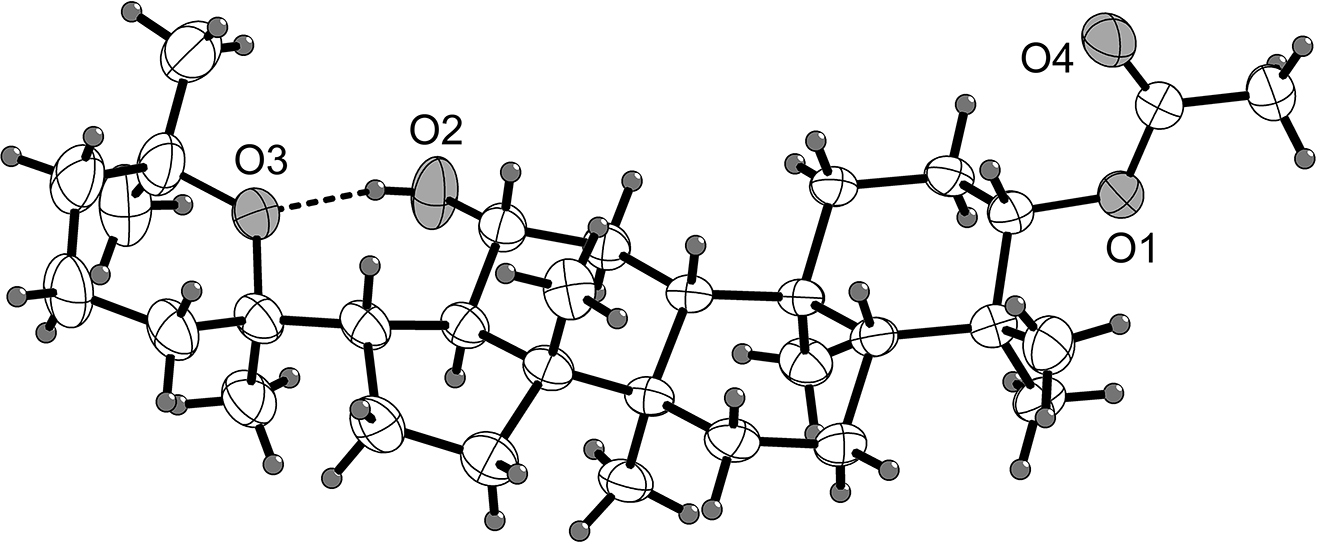
There are two molecules in the asymmetric unit of the title structure. One of the two molecules is shown in the figure. Bond lengths and angles are very similar to those given in the literature for protopanaxadiol [9], [10], [11], [12], [13].
As shown in the figure, the structure of the title compound and panoxadiol are equivalent except for the substituents at O(1). The title compound contains a classical intramolecular O–H⋯O hydrogen bond. All these bond lengths and angles are in the expected ranges. And it would be more interesting to compare the two crystallographically independent molecules in this structure.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 81473104, 81773563
Acknowledgments
X-ray data were collected at Institute of Medical Biotechnology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100050, Peoples Republic of China.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Funding information: This work was supported by the National Natural Science Foundation of China (No. 81473104, 81773563).
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Rigaku Oxford Diffracion. CrysAlisPRO software system, version 1.171.39.32a; Rigaku Corporation: Oxford, UK, 2017.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Palatinus, L., Chapuis, G. SUPERFLIP: a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790; https://doi.org/10.1107/s0021889807029238.Search in Google Scholar
5. Liu, J., Xu, Y. R., Yang, J. J., Wang, W. Z., Zhang, J. Q., Zhang, R. M., Meng, Q. G. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J. Ginseng Res. 2017, 41, 373–378; https://doi.org/10.1016/j.jgr.2017.01.001.Search in Google Scholar
6. Zhang, J. Q., Zhang, Q., Xu, Y. R., Li, H. X., Zhao, F. L., Wang, C. M., Liu, Z., Liu, P., Liu, Y. N., Meng, Q. G., Zhao, F. Synthesis and in vitro anti-inflammatory activity of C20 epimeric ocotillol-type triterpenes and protopanaxadiol. Planta Med. 2018, 23, 292–301; https://doi.org/10.1055/a-0770-0994.Search in Google Scholar
7. Zhang, S. N., Zhao, Y. Q. Crystallization separation of anti-tumor constituent 20(R) 25-OCH3-PPD. Chin. Tradit. Herbal Drugs 2014, 45, 770–773.Search in Google Scholar
8. Yang, Y. Y., Lee, J. S., Rhee, M. H., Yu, T., Baek, K. S., Sung, N. Y., Kim, Y., Yoon, K., Kim, J. H., Kwak, Y. S., Hong, S., Kim, J. H., Cho, J. Y. Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J. Ginseng Res. 2015, 39, 61–68; https://doi.org/10.1016/j.jgr.2014.06.002.Search in Google Scholar
9. Puff, H., Friedrichs, E., Habscheid, M., Quante, G. Panaxadiol and panaxatriol-monohydrat. Acta Crystallogr. 1986, C42, 576–579; https://doi.org/10.1107/s0108270186095343.Search in Google Scholar
10. Liu, Z., Xu, Y. R., An, X. S., Yang, J. J., Meng, Q. G., Hou, G. G. Synthesis and crystal structure of ocotillol-type metabolites derived from (20R)-protopanaxadiol. J. Chem. Res. 2017, 41, 216–220; https://doi.org/10.3184/174751917x14894997017612.Search in Google Scholar
11. Xu, Y. R., Yang, J. J., Liu, J., Hou, G. G., Meng, Q. G. Synthesis and crystal structures of C24-epimeric 20(R)-ocotillol-type saponins. Acta Crystallogr. 2016, C72, 498–503; https://doi.org/10.1107/s2053229616007270.Search in Google Scholar
12. Liu, J., Wang, W. Z., Wang, J. Z., Hou, G. G., Meng, Q. G. Synthesis and crystal structures of 3,6-diacetylated C24 epimeric 20(R)-ocotillol-type saponins. J. Chem. Soc. Pak. 2019, 41, 452–457; https://doi.org/10.3184/174751917x14894997017612.Search in Google Scholar
13. Deng, J.-Q., Mu, X.-D., Zhao, R.-L., Liu, Z., Tang, H.-J., He, M., Meng, Q.-G. Crystal structure of (20R)-20,25-epoxy-dammaran-3,12-dione, C30H48O3. Z. Kristallogr. NCS 2019, 234, 145–147.10.1515/ncrs-2018-0237Search in Google Scholar
© 2020 Ying Ma et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3

