Abstract
C22H26N2O, monoclinic, C2/c (no. 15), a = 47.4887(9) Å, b = 5.0220(1) Å, c = 16.4551(3) Å, β = 108.993(1)°, V = 3710.70(12) Å3, Z = 8, Rgt(F) = 0.0345, wRref(F2) = 0.0935, T = 150(2) K.
CCDC Nr.: 2025941
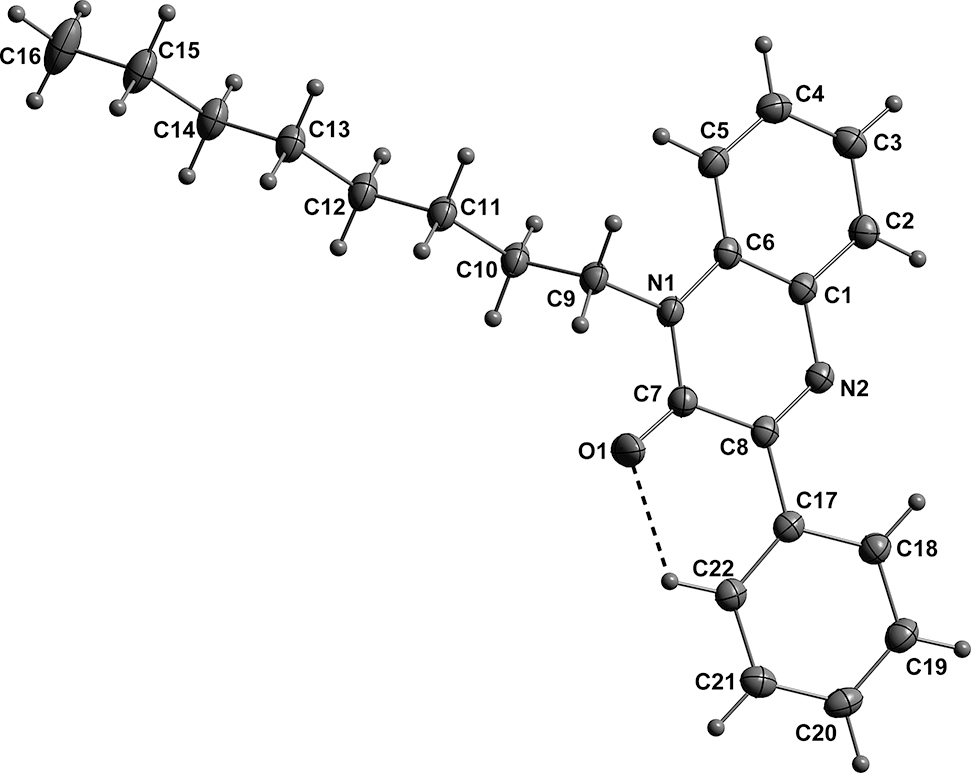
The molecular structure is shown in the Figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.18 × 0.14 × 0.12 mm |
| Wavelength: | Cu Kα radiation (1.54178 Å) |
| μ: | 0.57 mm−1 |
| Diffractometer, scan mode: | D8 VENTURE PHOTON 100, ω |
| θmax, completeness: | 72.5°, >99% |
| N(hkl)meas., N(hkl)unique, Rint: | 14888, 3644, 0.032 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3231 |
| N(param)refined: | 331 |
| Programs: | Bruker [1], SHELX [2], [3], [4], Diamond [5] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| O1 | 0.37437 (2) | 0.16657 (17) | 0.45497 (6) | 0.0368 (2) |
| N1 | 0.38156 (2) | 0.49212 (17) | 0.36879 (5) | 0.0222 (2) |
| N2 | 0.44122 (2) | 0.34815 (17) | 0.41442 (5) | 0.0219 (2) |
| C1 | 0.42983 (2) | 0.5480 (2) | 0.35551 (6) | 0.0216 (2) |
| C2 | 0.44919 (2) | 0.6824 (2) | 0.32061 (6) | 0.0251 (2) |
| H2 | 0.4704 (3) | 0.630 (3) | 0.3419 (8) | 0.028 (3)* |
| C3 | 0.43885 (2) | 0.8820 (2) | 0.26107 (7) | 0.0274 (2) |
| H3 | 0.4528 (3) | 0.975 (3) | 0.2376 (9) | 0.036 (3)* |
| C4 | 0.40877 (2) | 0.9525 (2) | 0.23542 (7) | 0.0280 (2) |
| H4 | 0.4012 (3) | 1.095 (3) | 0.1932 (8) | 0.031 (3)* |
| C5 | 0.38934 (2) | 0.8274 (2) | 0.26973 (7) | 0.0264 (2) |
| H5 | 0.3684 (3) | 0.882 (3) | 0.2512 (8) | 0.036 (3)* |
| C6 | 0.39973 (2) | 0.6243 (2) | 0.33069 (6) | 0.0216 (2) |
| C7 | 0.39167 (2) | 0.2841 (2) | 0.42594 (6) | 0.0238 (2) |
| C8 | 0.42397 (2) | 0.2217 (2) | 0.44838 (6) | 0.0212 (2) |
| C9 | 0.35041 (2) | 0.5759 (2) | 0.35087 (7) | 0.0250 (2) |
| H9A | 0.3451 (3) | 0.519 (3) | 0.4022 (8) | 0.031 (3)* |
| H9B | 0.3501 (3) | 0.775 (3) | 0.3471 (8) | 0.030 (3)* |
| C10 | 0.32942 (2) | 0.4528 (2) | 0.26888 (7) | 0.0281 (2) |
| H10A | 0.3285 (3) | 0.252 (3) | 0.2781 (9) | 0.040 (4)* |
| H10B | 0.3378 (3) | 0.480 (3) | 0.2221 (8) | 0.031 (3)* |
| C11 | 0.29832 (2) | 0.5728 (2) | 0.24530 (7) | 0.0287 (2) |
| H11A | 0.2896 (3) | 0.545 (3) | 0.2937 (9) | 0.042 (4)* |
| H11B | 0.2999 (3) | 0.774 (3) | 0.2375 (9) | 0.040 (4)* |
| C12 | 0.27681 (2) | 0.4609 (2) | 0.16224 (8) | 0.0311 (3) |
| H12A | 0.2751 (3) | 0.263 (3) | 0.1679 (10) | 0.050 (4)* |
| H12B | 0.2852 (3) | 0.488 (3) | 0.1154 (9) | 0.042 (4)* |
| C13 | 0.24592 (2) | 0.5850 (2) | 0.13698 (7) | 0.0305 (3) |
| H13A | 0.2368 (3) | 0.550 (3) | 0.1833 (9) | 0.040 (4)* |
| H13B | 0.2477 (3) | 0.784 (3) | 0.1309 (9) | 0.042 (4)* |
| C14 | 0.22466 (2) | 0.4791 (3) | 0.05239 (8) | 0.0335 (3) |
| H14A | 0.2230 (3) | 0.273 (3) | 0.0567 (10) | 0.049 (4)* |
| H14B | 0.2338 (3) | 0.509 (3) | 0.0055 (9) | 0.044 (4)* |
| C15 | 0.19380 (3) | 0.6022 (3) | 0.02715 (8) | 0.0374 (3) |
| H15A | 0.1846 (3) | 0.570 (3) | 0.0730 (10) | 0.050 (4)* |
| H15B | 0.1960 (4) | 0.812 (4) | 0.0221 (11) | 0.062 (5)* |
| C16 | 0.17279 (3) | 0.4975 (4) | −0.05752 (9) | 0.0515 (4) |
| H16A | 0.1526 (4) | 0.587 (3) | −0.0718 (10) | 0.058 (5)* |
| H16B | 0.1699 (5) | 0.298 (5) | −0.0545 (13) | 0.082 (6)* |
| H16C | 0.1812 (4) | 0.530 (4) | −0.1049 (12) | 0.063 (5)* |
| C17 | 0.43803 (2) | 0.0145 (2) | 0.51375 (6) | 0.0219 (2) |
| C18 | 0.46925 (2) | 0.0058 (2) | 0.54544 (7) | 0.0263 (2) |
| H18 | 0.4807 (3) | 0.139 (3) | 0.5266 (8) | 0.029 (3)* |
| C19 | 0.48400 (2) | −0.1845 (2) | 0.60508 (7) | 0.0298 (2) |
| H19 | 0.5058 (3) | −0.187 (3) | 0.6259 (9) | 0.035 (3)* |
| C20 | 0.46794 (3) | −0.3703 (2) | 0.63474 (7) | 0.0287 (2) |
| H20 | 0.4778 (3) | −0.506 (3) | 0.6765 (9) | 0.035 (3)* |
| C21 | 0.43712 (2) | −0.3632 (2) | 0.60437 (7) | 0.0269 (2) |
| H21 | 0.4252 (3) | −0.490 (3) | 0.6244 (8) | 0.033 (3)* |
| C22 | 0.42213 (2) | −0.1728 (2) | 0.54443 (6) | 0.0237 (2) |
| H22 | 0.4000 (3) | −0.169 (3) | 0.5242 (8) | 0.030 (3)* |
Source of material
3-Phenylquinoxalin-2-one 1 (4.5 mmol), potassium carbonate (5.85 mmol) and tetrakis(n-butyl)ammonium bromide (0.5 mmol) in DMF (20 mL) were added to 1-bromooctane (9 mmol). Stirring was maintained at room temperature for 24 h. The crude residue was filtered and the solvent removed. The residue was extracted with water. The organic compounds were purified by column chromatography using hexane-ethyl acetate (v/v, 9/1). A portion of the product was dissolved in ethanol, the solution was filtered and the filtrate was left undisturbed for 7 days to form colorless block crystals.
Experimental details
Crystal data, data collection and structure refinement details are summarized in Table 1. Hydrogen atoms were added using riding models [4].
Comment
Quinoxaline and its derivatives have received considerable attention due to their pharmacological activity [6], [7] and industrial properties [8], [9], [10]. Our research group has recently reported the synthesis of novel quinoxaline-based compounds [11], [12], [13], [14]. In continuation of our efforts toward the discovery of novel quinoxaline derivatives [15], [16], [17], [18], [19], [20], [21].
The dihydroquinoxaline moiety excepting N1 is planar within 0.0139(9) Å (r.m.s. deviation of the fitted atoms = 0.0072) with N1 0.0458(11) Å out of the above plane. The C17…C22 benzene ring is inclined by only 12.90(4)° from the aforementioned plane, due in part by a weak, intramolecular C22–H22…O1 hydrogen bond (see the Figure). In the crystal, the molecules form oblique stacks extending in the b-axis direction and parallel to the ab plane. The stacks are associated through “intercalation” of the “fully extended” n-octyl groups to form a typical micellar array.
In the crystal, the molecules are linked through C–H…π(ring) interactions and intercalation of the n-octyl groups.
Author contribution: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: The support of NSF-MRI Grant #1228232 for the purchase of the diffractometer and Tulane University for support of the Tulane Crystallography Laboratory are gratefully acknowledged.
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. APEX3 – Diffractometer Control and Data Reduction Software Package; Bruker AXS Inc.: Madison, WI, USA, 2016.Search in Google Scholar
2. Krause, L., Herbst-Irmer, R., Sheldrick, G. M., Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10; https://doi.org/10.1107/s1600576714022985.10.1107/S1600576714022985Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. SHELXT – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.10.1107/S2053229614024218Search in Google Scholar PubMed PubMed Central
5. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System, Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
6. Ramli, Y., Essassi, E. M. Advances in synthetic approaches, functionalization and biological properties of quinoxaline derivatives. In Advances in Chemistry Research; Taylor, J. C., Ed; Nova Science Publishers: New York, Vol. 27, 2015, pp. 109–160.Search in Google Scholar
7. Ramli, Y., Moussaif, A., Karrouchi, K., Essassi, E. M. Pharmacological profile of quinoxalinone. J. Chem. 2014, 2014, Article 563406; https://doi.org/10.1155/2014/563406.10.1155/2014/563406Search in Google Scholar
8. Zarrouk, A., Zarrok, H., Ramli, Y., Bouachrine, M., Hammouti, B., Sahibed-dinee, A., Bentisse, F. Inhibitive properties, adsorption and theoretical study of 3,7-dimethyl-1-(prop-2-yn-1-yl)quinoxalin-2(1H)-one as efficient corrosion inhibitor for carbon steel in hydrochloric acid solution. J. Mol. Liq. 2016, 222, 239–252; https://doi.org/10.1016/j.molliq.2016.07.046.10.1016/j.molliq.2016.07.046Search in Google Scholar
9. Laabaissi, T., Benhiba, F., Missioui, M., Rouifi, Z., Rbaa, M., Ouddaa, H., Ramli, Y., Guenbour, A., Warad, I., Zarrouk, A. Coupling of chemical, electrochemical and theoretical approach to study the corrosion inhibition of mild steel by new quinoxaline compounds in 1 M HCl. Heliyon 2020, 6, e03939; https://doi.org/10.1016/j.heliyon.2020.e03939.10.1016/j.heliyon.2020.e03939Search in Google Scholar PubMed PubMed Central
10. El Ouali, I., Hammouti, B., Aouniti, A., Ramli, Y., Azougagh, M., Essassi, E. M., Bouachrine, M. Thermodynamic characterisation of steel corrosion in HCl in the presence of 2-phenylthieno (3,2-b) quinoxaline. J. Mater. Environ. Sci. 2010, 1, 1–8.Search in Google Scholar
11. Abad, N., Hajji, M., Ramli, Y., Belkhira, M., Elmgirhi, S. M. H., Habib, M. A., Guerfel, T., Mague, J. T., Essassi, E. M. A newly synthesized nitrogen-rich derivative of bicyclic quinoxaline—structural and conceptual DFT reactivity study. J. Phys. Org. Chem. 2020, 33, e4055; https://doi.org/10.1002/poc.4055.10.1002/poc.4055Search in Google Scholar
12. Abad, N., Lgaz, H., Atioglu, Z., Akkurt, M., Mague, J. T., Ali, I. H., Ill-Chung, M., Salghih, R., Essassi, E. M., Ramli, Y. Synthesis, crystal structure, Hirshfeld surface analysis, DFT computations and molecular dynamics study of 2-(benzyloxy)-3-phenylquinoxaline. J. Mol. Struct. 2020, 1221, 128727; https://doi.org/10.1016/j.molstruc.2020.128727.10.1016/j.molstruc.2020.128727Search in Google Scholar
13. Missioui, M., Essassi, E. M., Mague, J. T., Ramli, Y. Synthesis and crystal structure of (E)-1-benzyl-3-(4-methoxystyryl)quinoxalin-2(1H)-one, C24H20N2O2. Z. Kristallogr. NCS 2020, 235; https://doi.org/10.1515/ncrs-2020–0300.10.1515/ncrs-2020-0300Search in Google Scholar
14. Ramli, Y., Essassi, E. M. Condensation study of hydrazonoyl chloride with 3-methylquinoxalin-2-one and 3-styrylquinoxalin-2-one. J. Mar. Chim. Heterocycl. 2019, 18, 39–47.Search in Google Scholar
15. Missioui, M., El Fal, M., Taoufik, J., Essassi, E. M., Mague, J. T., Ramli, Y. 2-(3–Methyl-2-oxo-1,2-dihydroquinoxalin-1-yl)acetic acid dihydrate. IUCrData 2018, 3, x180882; https://doi.org/10.1107/s2414314618008829.10.1107/S2414314618008829Search in Google Scholar
16. Ramli, Y., El Bakri, Y., El Ghayati, L., Essassi, E. M., Mague, J. T. 1–Benzyl-3-methylquinoxalin-2(1H)-one. IUCrData 2018, 3, x180390; https://doi.org/10.1107/s2414314618003905.10.1107/S2414314618003905Search in Google Scholar
17. Missioui, M., Mague, J. T., El Fal, M., Taoufik, J., Essassi, E. M., Ramli, Y. Ethyl 2-[(3-methylquinoxalin-2-yl)sulfanyl]acetate. IUCrData 2017, 2, x171763; https://doi.org/10.1107/s2414314617017631.10.1107/S2414314617017631Search in Google Scholar
18. Ramli, Y., Karrouchi, K., Essassi, E. M., El Ammari, L. N′-Phenyl-N′-[3-(2,4,5-triphenyl-2,5-dihydro-1H-pyrazol-3-yl)quinoxalin-2-yl]benzohydrazide. Acta Crystallogr. 2013, E69, o1320–o1321; https://doi.org/10.1107/s1600536813020035.10.1107/S1600536813020035Search in Google Scholar PubMed PubMed Central
19. Ramli, Y., Slimani, R., Zouihri, H., Lazar, S., Essassi, E. M. 3-Methyl-1-(prop-2-en-1-yl)quinoxalin-2(1H)-one. Acta Crystallogr. 2010, E66, o1767; https://doi.org/10.1107/s1600536810023640.10.1107/S1600536810023640Search in Google Scholar PubMed PubMed Central
20. Benzeid, H., Essassi, E. M., Saffon, N., Garrigues, B., Ng, S. W. 1-Methyl-3-phenylquinoxalin-2(1H)-one. Acta Crystallogr. 2009, E65, o2323; https://doi.org/10.1107/s160053680903414x.10.1107/S160053680903414XSearch in Google Scholar PubMed PubMed Central
21. Benzeid, H., Saffon, N., Garrigues, B., Essassi, E. M., Ng, S. W. 1-Benzyl-3-phenylquinoxalin-2(1H)-one. Acta Crystallogr. 2009, E65, o2685; https://doi.org/10.1107/s1600536809039944.10.1107/S1600536809039944Search in Google Scholar PubMed PubMed Central
© 2020 Nadeem Abad et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- The crystal structure of 3-oxo-urs-12-en-28-oic acid, C30H46O3·1/6H2O
- The crystal structure of (3S,12R,20R,24R)-3,12-diacetyl-20,24-epoxy-dammarane-3,12,25–triol–ethyl acetate (4/1), C34H56O6⋅ 0.25(C4H8O2)
- A new polymorph of tetrakis(dimethylammonium) catena-poly[tetrakis(μ2-sulfato-κ2O:O′)dizinc(II)], Zn2C8H32N4O16S4
- Crystal structure of 10-oxysophoridine, C15H22N2O2
- The crystal structure of (5R,8R,9R,10R,12R,13R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl)tetradecahydro-3H-cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O4
- Synthesis, crystal structure and optical property of 1,6-bis(p-tolylthio)pyrene, C30H22S2
- The crystal structure of hexakis(2-(pyridin-2-ylamino)pyridin-1-ium) decavanadate(V) dihydrate, C60H64N18O30V10
- Preparation and crystal structure of a cationic olefin polymerization precatalyst: (1,7-bis(2,6–dichlorophenyl)-1,7-di-aza-4-oxo-heptan-1,4,7-triyl)dimethyl zirconium(IV), C18H20Cl4N2OZr
- The crystal structure of fac-tricarbonyl(4,4-dimethyl-2,2-dipyridyl-κ2N,N′)- (pyrazole-κN)rhenium(I) nitrate, C18H16O3N4Re
- Synthesis and crystal structure of hexaaquacopper(II) 2,5-dicarboxyterephthalate, C10H16O14Cu
- The crystal structure of (8R,10R,12R,14R)- 12-hydroxy-16-(5-(2-hydroxypropan-2-yl)-2-methyltetrahydrofuran-2-yl)- 4,4,8,10,14-pentamethyltetradecahydro-3H- cyclopenta[a]phenanthrene-3,6(2H)-dione, C30H48O5
- Structure of the mixed crystal (S)-(6-(bromo/chloro)-2-methoxy-2,6-dihydroquinolin-3-yl)(phenyl)methanol, C17H14Br0.5Cl0.5NO2
- The crystal structure of trans-tetraaqua-bis(4-acetylphenoxyacetato-κ1O)manganese(II), C20H26O12Mn
- Crystal structure of (E)-2-(4-fluoro-3-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- Crystal structure of DL-α-(methylaminomethyl)benzyl alcohol, C9H13NO
- The crystal structure of dipentaerthritol hexanitrate, C10H16N6O19
- Crystal structure of N,N-diphenylformamide, C13H11NO
- Crystal structure of (E)-2-(3,5-bis(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen- 1(2H)-one, C20H14F6O2
- Crystal structure of ortho-methoxy benzaldehyde, C8H8O2 – a second polymorph and deposition of 3D coordinates
- Crystal structure of catena-poly[diaqua-bis(μ2-2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O:O')-(2-(4-(2,4,4-trimethylpentan-2-yl)phenoxy)propanoato-κ2O,O')yttrium(III)], C51H79O11Y
- Crystal structure of benzylthiouronium chloride, C8H11ClN2S
- Synthesis and crystal structure of tert-butyl (2′R,3R,3′R,4a′R,9a′S)-1-acetyl-5-chloro-3″-methyl-2,5″,9′-trioxo-1″-phenyl-1″,4a′,5″,9a′-tetrahydro-1′H,3′H,9′H-dispiro[indoline-3,4′-xanthene-2′,4″-pyrazole]-3′-carboxylate, C36H32ClN3O7
- Crystal structure of 2-hydroxy-4-methoxy benzaldehyde, C8H8O3
- Crystal structure of poly[diaqua-(m3-3′,5′-dicarboxy-[1,1′-biphenyl]-3,4-dicarboxylato-K4O,O′:O″:O‴) cadmium(II)], C16H11O10Cd
- Crystal structure of {tetraaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)}-{triaqua-bis(1-(4-hydroxy-2-oxotetrahydrofuran-3-yl)-2-((4aS,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethane-1-sulfonato-k2O,O') calcium(II)} – water – acetone (1/1/8/2)
- Synthesis and crystal structure of bis{2-bromo-6-((E)-((4-((E)-1-(methoxy-imino)ethyl)phenyl)imino)methyl)phenolato- κ2N,O}zinc(II)-methanol(1/2), C65H60Br4N8O9Zn2
- Crystal structure of benzenesulphonic acid
- Crystal structure of N-benzyl-N-nicotinoyl-nicotine amide C19H15N3O2
- Crystal structure of poly[aqua(μ3-2,4-diamino-benzenesulfonato-κ4N:N′,O:O′)silver(I)], C12H18O8N4S2Ag2
- Crystal structure of 1,4-bis(methylpyridinium benzene) bis(1,2-dicyanoethene-1,2-dithiolato-κ2S:S)nickel(II), C26H18N6NiS4
- Crystal structure of the Cu(II) complex chlorido-(6-oxo-2-phenyl-1,6-dihydropyrimidine-4-carboxylato-k2N,O)-(phenanthroline-k2N,N')copper(II), C23H15ClCuN4O3
- Crystal structure of phenarsazine chloride acetic acid solvate, C14H13AsClNO2
- Crystal structure of poly[aqua-(μ2-3,3′,4,5′-biphenyl tetracarboxylate- κ3O,O′:O′′) -(μ2-4,4′-bis(pyrid-4-yl)biphenyl-κ2N:N′)zinc(II)], C27H18NO9Zn
- Crystal structure of catena-poly[(μ2-3-amino-benzenedisulfonato-κ2N:O)-bis (3-methyl-isoquinoline-κN)silver(I)], C26H24N3O3SAg
- Crystal structure of 2-((4-Aminophenyl)thio)acetic acid, C8H9NO2S
- Crystal structure of phenarsazine chloride dimethylsulfoxide solvate, C14H15AsClNOS
- Synthesis and crystal structure of 2-azido-N-phenylacetamide, C8H8N4O
- Crystal structure of chlorido{hydridotris[3-phenyl-5-methylpyrazol-1-yl-κN3]borato}copper(II), C30H28BClCuN6
- Crystal structure of benzanthrone – a redetermination for correct molecular geometry and localization of hydrogen atoms
- Crystal structure of 4-bromobenzaldehyde – complete redetermination at 200 K, C7H5BrO
- Crystal structure and spectroscopic properties of chlorido{hydridotris[3-,5-dimethylpyrazol-1-yl-κN3]borato}(3-,5-dimethylpyrazol-1-yl-κN)copper(II), C20H30BClCuN8
- The crystal structure of 4-((2-hydroxynaphthalen-1-yl)(pyrrolidin-1-yl)methyl)benzonitrile, C22H20N2O
- Crystal structure of 4-ethyl-3-phenylisoquinolin-1(2H)-one, C17H15NO
- Crystal structure of (tricyclohexylphosphane-κP)-[(Z)-N-(3-fluorophenyl)-O-methylthiocarbamato-k1S]gold(I), C26H40AuFNOPS
- Crystal structure of (3S,8R,10R,12R,14R)-12-hydroxy-4,4,8,10,14-pentamethyl-17-((R)-2,6,6-trimethyltetrahydro-2H-pyran-2-yl) hexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate, C32H54O4
- The crystal structure of 2-[(S)-1-(naphthalen-1-yl)ethyl]-2,3,7,7a- tetrahydro-3a,6-epoxyisoindol-1(6H)-one, C19H20NO2
- Crystal structure of {hydridotris[3-(t-butyl)-5-isopropylpyrazol-1-yl-κN3]borato}thallium(I), C30H52BN6Tl
- Synthesis and crystal structure of 1-octyl-3-phenylquinoxalin-2(1H)-one, C22H26N2O
- The crystal structure of 2,6-difluorophenol, C6H4F2O
- 4-(9H-Fluoren-9-yl)-4-methylmorpholin-4-ium bromide, C18H20BrNO
- The crystal structure of 2,4-dimethylimidazole monohydrate, C5H10N2O
- The crystal structure of 1,2-dimethylimidazole, C5H8N2
- The crystal structure of 3-ammonio-4-aminobenzoate, C7H8N2O2 – a second polymorph
- The crystal structure of 4-hydroxy-2,5-bis(1-methyl-1H-imidazol-3-ium-2-ylthio)-3,6-dioxocyclohexa-1,4-dienolate chloride monohydrate, C14H15N4O5S2Cl
- The crystal structure of butyrylferrocene, C14H16FeO
- The crystal structure of bi-1,1′-cyclopentane-1,1′-diol, C10H18O2
- The crystal structure of 2-iso-propylimidazole, C6H10N2
- The crystal structure of aqua-tris (1,3-diphenylpropane-1,3-dionato-κ2O,O′)-lanthanum(III), C45H35LaO7
- Crystal structure of (3E,5E)-3,5-bis-4-methoxy-3-(trifluoromethyl)benzylidene)-1-methylpiperidin-4-one, C24H21F6NO3
- The crystal structure of 3,5-dichloro-6-diazo-2,4-dinitrocyclohexa-2,4-dien-1-one, C6Cl2N4O5
- Crystal structure of carbonyl(2-methylquinolin-8-olato-κ2N,O)(triphenylarsine-κAs)rhodium(I), C29H23AsNO2Rh
- Crystal structure of (1aS,1a1S,2S)-4a-butoxy-1a,1a1,2,4a,5,6-hexahydro-1H-cyclobuta[de]naphthalen-2-yl-4-nitrobenzoate, C22H25NO5
- Crystal structure of carbonyl(2-oxopyridin-1(2H)-olato-k2O,O′)(triphenylarsine-κAs)rhodium(I), C24H19AsNO3Rh
- Crystal structure of catena-poly[triqua-bis(μ2-4-carboxy-2-(1H-tetrazol-1-yl)-1H-imidazole-5-carboxylato-k3N,O:O′)barium(II)] tetrahydrate, C14H14BaN12O15
- Crystal structure of (E)-3′,6′-bis(ethylamino)-2-((quinoxalin-2-ylmethylene)amino)spiro[isoindoline-1,9′-xanthen]-3-one, C35H32N6O2
- Crystal structure of diaqua-bis(μ2-5-chloro-salicylato-κ3O,O′:O′)-bis(5-chloro-salicylato-κ2O,O′)-bis(1,10-phenanthroline-κ2N,N′) dilead(II) – water (1/2), C52H36C14N4O14Pb2·2(H2O)
- Crystal structure of (E)-2-(4-ethoxycarbonyl-3,5-dimethyl-2-(pyrrole-2-ylmethyleneamino)-3′,6′-dihydroxylspiro[isoindoline-1,9′-xanthen]-3-one-methanol (1/1), C31H29N3O7
- The crystal structure of 5H-dibenzo[b,e]azepine-6,11-dione, C14H9NO2
- Crystal structure of (E)-2-(4-fluoro-2-(trifluoromethyl)benzylidene)-7-methoxy-3,4-dihydronaphthalen-1(2H)-one, C19H14F4O2
- The crystal structure of N-(2-methoxy-4,5-bis[phenylselanyl]phenyl)picolinamide, C25H20N2O2Se2
- The crystal structure of (E)-2-(5-bromo-2-hydroxybenzylidene)-N-phenylhydrazine-1- carboxamide monohydrate, C14H14BrN3O3
- Crystal structure of fac-tricarbonyl-(nitrato-k1O)-bis(pyridine-κN)-rhenium, C13H10O6N3Re
- Crystal structure of (E)-2-(((1H-pyrrol-2-yl)methylene)amino)-3′,6′-dihydroxyspiro[isoindoline-1,9′-xanthen]-3-one — methanol (1/2), C27H25N3O6
- The crystal structure of 4-amino-N′-(4-aminobenzoyl)benzohydrazide monohydrate, C14H16N4O3
- Crystal structure of bis(amino(carbamothioylamino)methaniminium) 5-hydroxyisophthalate monohydrate, C12H20N8O6S2
- The crystal structure of 2-(chloromethyl)pyridine, C6H6ClN
- The crystal structure of 1-bromo-4-iodo-benzene, C6H4BrI
- The crystal structure of 2,6-dimethyl-4-nitro-phenol, C8H9NO3
- The crystal structure of 3-chloropropionic acid, C3H5ClO2
- The crystal structure of 2-(2-methoxyphenyl)acetic acid, C9H10O3

