Abstract
The Cr(vi) ion-imprinted composite membranes (Cr(vi)-IICMs) were prepared by using the surface imprinting method. The template ion was Cr(vi), the functional monomer was 4-vinylpyridine (4-VP), and the nylon filter membrane (nylon-6) was the support membrane. Non-imprinted composite membranes (NICMs) were prepared under the same conditions as the corresponding Cr(vi)-IICM. The adsorption effect of the imprinted membrane can reach 2.4 times that of the corresponding non-imprinted membrane. Meanwhile, the adsorption quantity of Cr(vi)-IICM was 34.60 μmol·g−1. The physical characteristics of membranes were confirmed by Brunauer–Emmett–Teller and scanning electron microscopy. Inductively coupled plasma emission spectrometry was used to analyze their adsorption properties and permeation selectivity. Cr(vi)-IICM and NICM were both mesoporous materials from the structural characterization and performance test results. Their adsorption behavior conformed to the Langmuir isotherm adsorption model. The permeation recognition mechanism of Cr(vi)-IICM was the Piletsky’s gate model. The IICM still has excellent permeability selectivity to Cr(vi) in the presence of competitive ions. The results provided a reference for the isolation and enrichment of Cr(vi).
1 Introduction
Chromium(vi) is a strong oxidizer that can directly act on the surface of the skin and cause skin diseases and even cancer (1,2). If it enters the respiratory system or blood, it can circulate and cause organ damage (3,4,5). The common methods to treat heavy metals in water include chemical precipitation (6,7), biological (8), ion-exchange (9,10), electrolysis (11), adsorption (12,13,14,15,16), and membrane separation (17,18,19) methods. However, these often require complex processes, high costs, and poor selectivity, or they easily cause secondary water pollution (20,21,22). This can aggravate clean water shortages, which directly pose health threats. Therefore, it is urgent to find highly selective adsorption and separation materials to remove Cr(vi) from wastewater.
Ion-imprinted composite membranes (IICMs) (23,24,25) have high fluxes and high selectivities because the support layer can be used for ultrafiltration or microfiltration. The template ions were bound to the functional monomers using chelation or electrostatic interaction. Due to the use of ion imprinting techniques in the preparation process, the material was provided with specifically identified cavities. IICM combine the advantages of membrane separation with the selectivity of ion-imprinting methods because they contain recognition sites on their surfaces, which improve the selection and permeation of the IICM (26,27). At the same time, since the action site was primarily on the surface of the basement membrane, the problem of too-deep encapsulation of the recognition site of the conventionally imprinted polymer was avoided. Compared with traditional particle polymers, IICM has unique advantages such as no abrasion and small diffusion resistance (28,29,30).
In 2005, Araki et al. (31) first applied the surface ion–imprinted technique for the preparation of membrane materials with high selectivity for ions. The polytetrafluoroethylene membrane (PTFE) membrane was used as a support membrane. The results showed that the ion-imprinted membrane had good permeation selectivity for Zn(ii). Vatanpour et al. (32) prepared Ni(ii) ion-imprinted membrane using dithizone as a functional monomer. In the permeation experiments with Co(ii) as the competing ion, the imprinted membrane still showed good selective recognition of Ni(ii). The separation coefficient of the imprinted membrane for Ni(ii) and Co(ii) was 2.6. The work also showed that in membrane permeation experiments, the adsorption of metal ions was the rate-control step of the process. Wang et al. (33) prepared a novel copper ion surface-imprinted membrane using layer-by-layer technology. The binding sites were distributed in a multilayer structure on the membrane surface. Amino and carboxyl groups were detected on the membrane surface by fourier transform infrared spectroscopy, and their effective adsorption was carried out by interaction with Cu(ii). Meanwhile Cu(ii) ion-imprinted membranes showed good reusability in six adsorption–desorption cycles.
The Cr(vi) exists as the
2 Materials and methods
2.1 Materials
α-Methacrylic acid (MAA), acrylamide (AM), 4-vinylpyridine (4-VP), ethylene glycol dimethacrylate (EGDMA), and 2,2-azobisisobutyronitrile (AIBN) were bought from Aladdin, Shanghai. Potassium dichromate (K2Cr2O7), nickel chloride hexahydrate (NiCl2·6H2O), copper chloride (CuCl2), cadmium chloride (CdCl2), phosphoric acid, methanol, ethanol, acetonitrile, isopropanol, and glacial acetic acid were purchased from Tianjin Sailboat Chemical Reagent Technology Co., Ltd. Nylon membrane (nylon-6), PTFE, and polyvinylidene fluoride membrane (PVDF) were procured from Shanghai Xingya Purification Equipment Factory. The purity of all reagents was analytical grade. The water used in the experiment was deionized water.
2.2 Apparatus
The ultraviolet–visible spectrophotometer (UV-2500; Shimadzu, Japan) and inductively coupled plasma optical emission spectrometer (ICP-OES) (Prodigy; Leeman, USA) were used to detect ion concentrations. The surface morphology of the samples was detected with scanning electron microscopy (SEM) (MIRA LMS; TESCAN, the Czech Republic). The Brunauer–Emmett–Teller (BET) surface area analyzer (NOVA2000e, Quantachrome, America) was used to detect surface areas.
2.3 Preparation of membranes
The preparation of Cr(vi)-IICM is outlined in Table 1. About 0.10 mmol of K2Cr2O7 was added to the Erlenmeyer flask and then dissolved with the corresponding pore-forming solvent. A certain amount of functional monomer was added, which was vibrated in a gas bath constant-temperature shaker for 3 h at room temperature. Then, EGDMA and 10.00 mg AIBN were added to the mixed solution, which was sonicated and deaerated for 5 min to form a pre-polymer solution. The support membrane was immersed in the pre-polymer solution for a period of time, followed by reacting at 60°C for 24 h and then eluting with methanol:glacial acetic acid (V:V = 9:1) to remove the template ion. Finally, IICM was eluted with pure methanol to neutral pH, and then membrane was dried for 12 h and obtained. The corresponding NICM was prepared under the same methods but without template ion.
Preparation of membranes
| Membranes | Functional monomer | Support membrane | Dosage of the functional monomer (mmol) | Crosslinking agent (mmol) | Porogen solvent (V:V) | Soaking time (s) |
|---|---|---|---|---|---|---|
| 1 | 4-VP | PTFE | 0.4 | 2 | 1:1 Isopropyl alcohol:water | 180 |
| 2 | MAA | — | — | — | — | — |
| 3 | AM | — | — | — | — | — |
| 4 | 4-VP | — | — | — | 1:1 Methanol:water | — |
| 5 | — | — | — | — | 1:1 Ethanol:water | — |
| 6 | — | — | — | — | 1:1 Acetonitrile:water | — |
| 7 | — | PVDF | — | — | 1:1 Isopropyl alcohol:water | — |
| 8 | — | Nylon-6 | — | — | — | — |
| 9 | — | — | 0.2 | — | — | — |
| 10 | — | — | 0.6 | — | — | — |
| 11 | — | — | 0.8 | — | — | — |
| 12 | — | — | 0.6 | 1 | — | — |
| 13 | — | — | — | 3 | — | — |
| 14 | — | — | — | 4 | — | — |
| 15 | — | — | — | 5 | — | — |
| 16 | — | — | — | 2 | — | 30 |
| 17 | — | — | — | — | — | 60 |
| 18 | — | — | — | — | — | 120 |
| 19 | — | — | — | — | — | 1,800 |
| 20 | — | — | — | — | — | 3,600 |
| 21 | — | — | — | — | 1:2 Isopropyl alcohol:water | 180 |
| 22 | — | — | — | — | 2:1 Isopropyl alcohol:water | — |
| 23 | — | — | — | — | 1:3 Isopropyl alcohol:water | — |
| 24 | — | — | — | — | 7:3 Isopropyl alcohol:water | — |
2.4 Adsorption experiments
ICM or NICM (20.00 mg) was dispersed in 10.00 mL of Cr(vi) solution with 10.00–75.00 mg·L−1. After shaking and adsorbing at 25°C for 12 h, they were filtered to a constant volume. The solutions before and after adsorption were analyzed using UV spectrophotometer. No chromogenic agent was added during the analysis. The maximum absorption wavelength of Cr(vi) was found to be about 350 nm by the UV spectrophotometer, and the subsequent adsorption experiments were performed at this wavelength.
The equilibrium adsorption amount Q and the imprinting factor α were obtained from the changes in the concentration before and after adsorption. The equations are shown in Eqs. 1 and 2, and the adsorption isotherm was drawn as follows:
where Q (μmol·g−1) is the equilibrium adsorption quantity; C e (mmol·L−1) is the concentration at adsorption equilibrium; C 0 (mmol·L−1) is the initial concentration; m (g) represents the mass of Cr(vi)-IICM or NICM; and V (L) represents the volume of the adsorption solution.
2.5 Permeability experiments
The permeation device shown in Figure 1 was used, where the effective membrane permeation area was 1.54 cm2, and the membrane thickness was 0.015 cm.
where ΔC i/Δt is the change in the concentration for each ion in the receiving tank; A (cm2) is the efficacious membrane area; V (mL) is the volume of the tank solution; d (cm) is the thickness of the membrane; C Fi is the concentration for each ion in the supply tank; and C Ri is the concentration for each ion in the receiving tank.
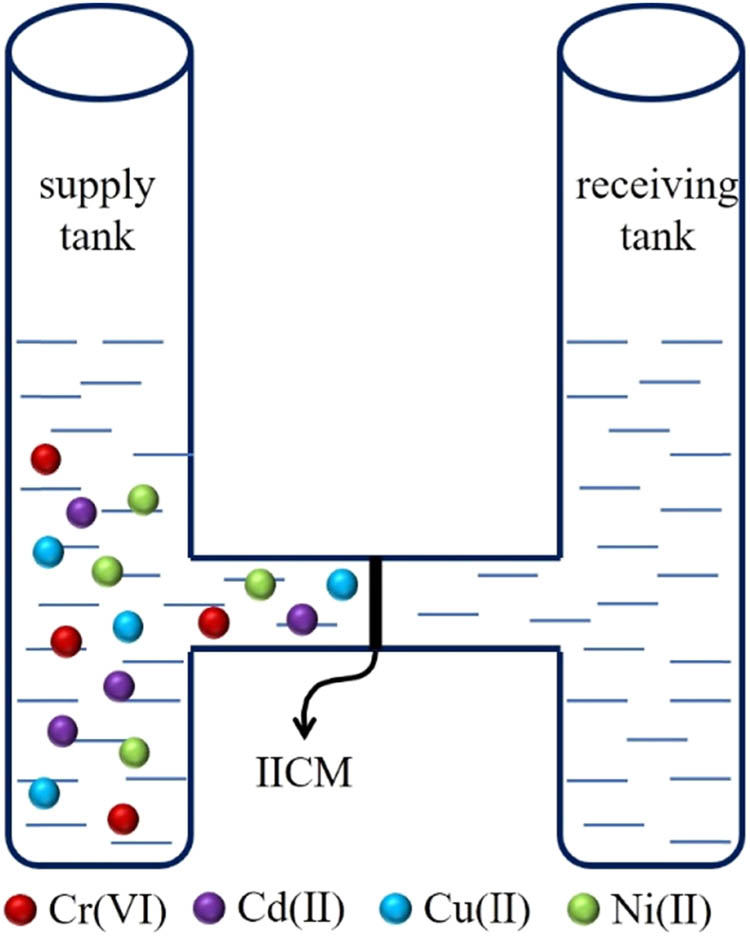
Permeability device.
3 Results
3.1 Optimization of preparation conditions
In this study, the experimental conditions such as the functional monomer, pore-forming solvent, type of base membrane, dosage ratio of imprinted ions to the functional monomer, and crosslinking agent were optimized. Table 2 shows the experimental results. The functional monomer was 4-VP; the support membrane was nylon-6; the pore-forming solvent was a mixture of isopropanol and water with a volume ratio of 1:1; the molar ratio of the imprinted ion, functional monomer, and crosslinker was 1:6:20; and the soaking time of the base membrane in the pre-polymer solution was 180 s. The adsorption effect of the imprinted membrane can reach 2.4 times that of the corresponding non-imprinted membrane. The adsorption quantity of Cr(vi)-IICM was 34.60 μmol·g−1.
Adsorption properties of membranes
| Membranes | Q IICM (mg·g−1) | Q NICM (mg·g−1) | α (Q IICM/Q NICM) |
|---|---|---|---|
| 1 | 23.19 | 15.53 | 1.49 |
| 2 | 25.56 | 18.14 | 1.41 |
| 3 | 23.24 | 23.10 | 1.01 |
| 4 | 11.13 | 9.14 | 1.22 |
| 5 | 8.72 | 7.42 | 1.18 |
| 6 | 18.41 | 17.46 | 1.05 |
| 7 | 21.55 | 13.78 | 1.56 |
| 8 | 25.82 | 14.5 | 1.78 |
| 9 | 21.63 | 13.86 | 1.56 |
| 10 | 34.60 | 14.42 | 2.40 |
| 11 | 19.47 | 15.53 | 1.25 |
| 12 | 19.23 | 16.87 | 1.14 |
| 13 | 28.30 | 16.00 | 1.77 |
| 14 | 27.83 | 13.00 | 2.14 |
| 15 | 19.15 | 17.43 | 1.10 |
| 16 | 32.55 | 26.72 | 1.22 |
| 17 | 30.16 | 28.85 | 1.05 |
| 18 | 33.43 | 27.17 | 1.23 |
| 19 | 31.57 | 30.97 | 1.02 |
| 20 | 31.29 | 30.18 | 1.37 |
| 21 | 11.47 | 5.43 | 2.11 |
| 22 | 36.42 | 26.38 | 1.38 |
| 23 | 30.29 | 26.64 | 1.14 |
| 24 | 34.83 | 30.41 | 1.15 |
3.2 SEM analysis
Cr(vi)-IICM, NICM, and support membrane were characterized using SEM. Figure 2 shows the results. Compared with the support membrane, the surface of composite membranes was rougher. This indicated that cross-linking occurred on the surface of the composite membrane, which also led to changes in the membrane pore structure. In addition, Cr(vi)-IICM contained more pores and a more uniform structure, while NICM had fewer pores and an uneven structure. Compared with NICM, Cr(vi)-IICM may have a templating effect due to the addition of imprinted Cr(vi) ions during preparation. Imprinted holes matching Cr(vi) remained after elution, while NICM had a smooth surface without imprinted holes. These results suggest that there may be imprinted pores produced by interactions with Cr(vi) in Cr(vi)-IICM.
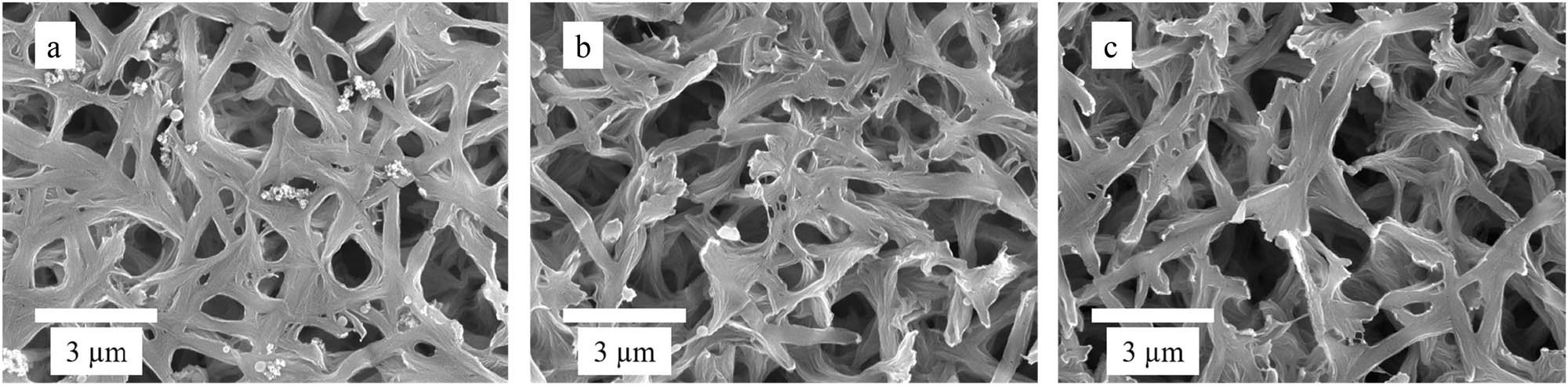
SEM images of IICM (a), NICM (b), and nylon-6 membrane (c).
3.3 BET analysis
Figure 3 shows the BET isotherms, wherein Cr(vi)-IICM and NICM had similar shapes except for the slope. They are both class IV isotherms in the international union of pure and applied chemistry (IUPAC) classification, indicating that they are typical mesoporous materials (37).
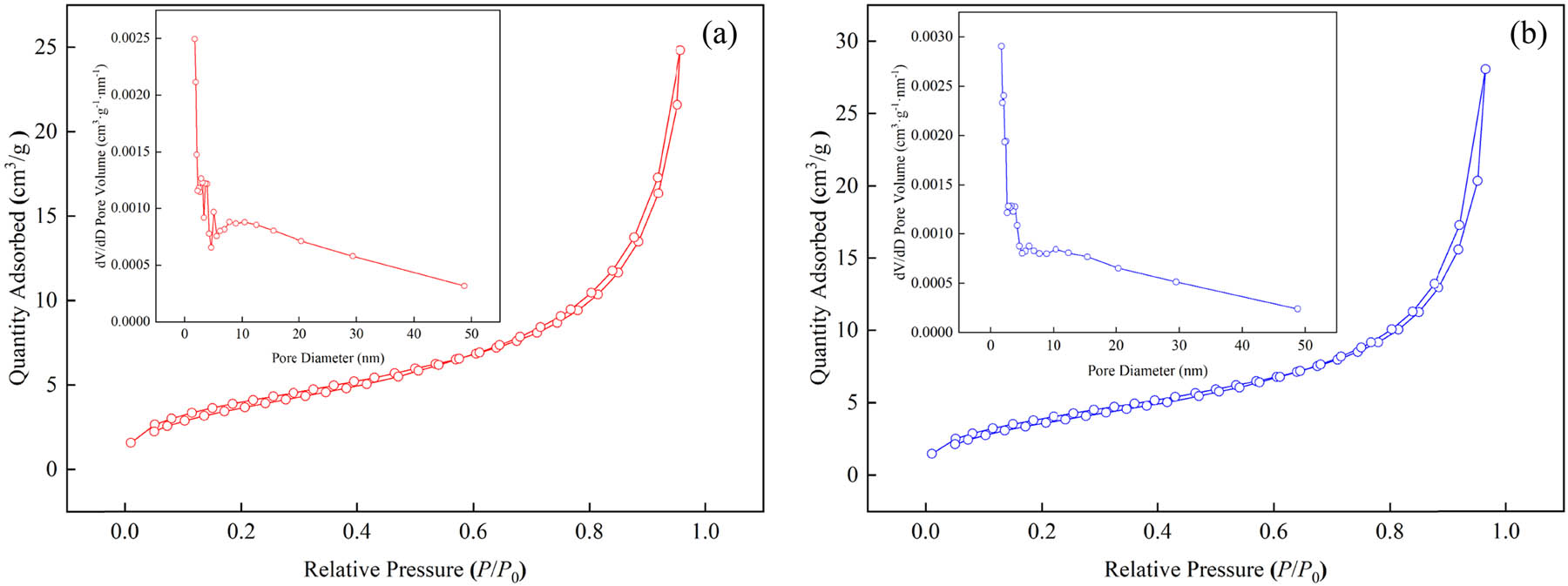
BET isotherms of IICM (a) and NICM (b).
Table 3 shows the BET parameters. The parameters of NICM were smaller than the ones of Cr(vi)-IICM, including specific surface area, pore volume, and pore size. Cr(vi)-IICM and NICM have pore size between 2 to 50 nm. According to IUPAC, they are both mesoporous materials.
BET parameters
| Specific surface area (m2·g−1) | Pore volume (cm3·g−1) | Pore size (nm) | |
|---|---|---|---|
| Cr(vi)-IICM | 9.911 | 0.034 | 11.760 |
| NICM | 8.758 | 0.030 | 11.525 |
3.4 Adsorption isotherm
The isothermal adsorption of Cr(vi) by imprinted composite membranes is usually analyzed by the Freundlich isothermal adsorption, Langmuir isothermal adsorption, and Scatchard models. Their respective fitting equations are shown in the following equations:
where C e is the equilibrium concentration (mg·L−1); Q e is the adsorption quantity of the membrane (μmol·g−1); K f and n are Freundlich constants; Q max is the maximum adsorption quantity (μmol·g−1); b is the Langmuir constant; and K d is the dissociation constant.
Figure 4 shows the adsorption isotherm, Freundlich fitting curves, Langmuir fitting curves, and Scatchard fitting curves of Cr(vi)-IICM. The Scatchard fitting curve in Figure 4d shows that Cr(vi)-IICM had specific adsorption and non-specific adsorption.
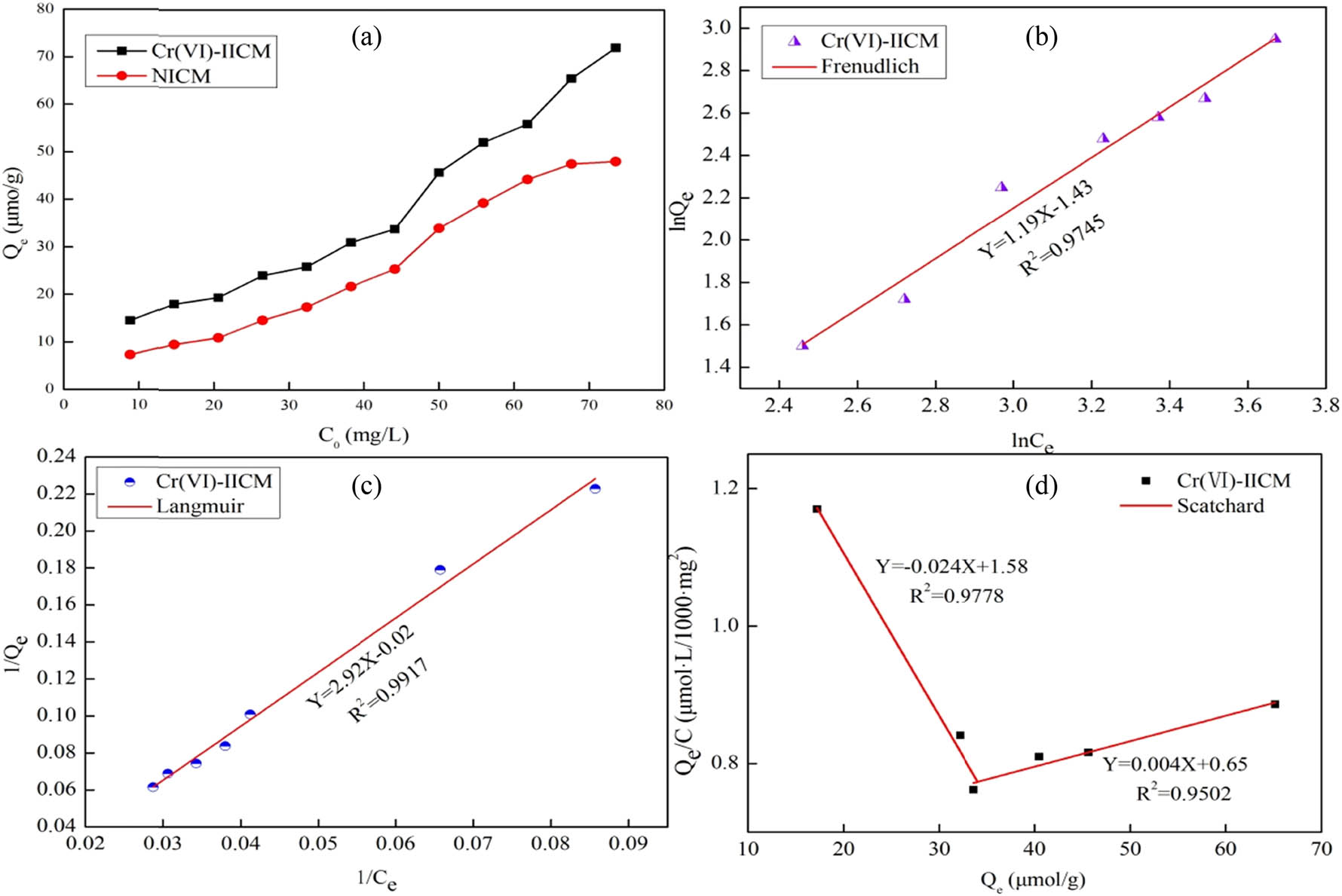
Adsorption isotherms of IICM (a), Freundlich fitting curve (b), Langmuir fitting curve (c), Scatchard fitting curve (d).
Table 4 shows the parameters obtained from the Cr(vi)-IICM fitting curves. The linear correlation coefficient of the Freundlich isothermal adsorption model was 0.9745, while that of the Langmuir isothermal adsorption model was 0.9917. The Langmuir isothermal adsorption model was better suited to represent the adsorption behavior of Cr(vi)-IICM. The adsorption was monomolecular adsorption. The Scatchard fitting curve showed that the sum of the maximum specific and non-specific adsorption capacities was 228.32 μmol·g−1.
Fitting parameters of the Langmuir, Freundlich, and Scatchard adsorption models
| Langmuir | Freundlich | Scatchard | |||
|---|---|---|---|---|---|
| Q max (μmol·g−1) | 50.00 | K f | 0.24 | Q max (μmol·g−1) | 65.82 |
| 162.50 | |||||
| b | 6.84 × 10−3 | 1/n | 1.19 | K d | 41.66 |
| 250.00 | |||||
| R 2 | 0.9917 | R 2 | 0.9745 | R 2 | 0.9778 |
| 0.9502 | |||||
3.5 Adsorption kinetics analysis
Figure 5a shows the change in the adsorption quantity of Cr(vi)-IICM over time. The data were also fitted using Lagergren’s first- and second-order dynamics models. The fitting equations are Eqs. 9 and 10, and the results are shown in Figure 5b.
where Q e represents the equilibrium adsorption quantity (μmol·g−1); Q t represents the adsorption quantity at time t (μmol·g−1); t represents the adsorption time (min); k 1 and k 2 represent the first- and second-order dynamics constants, respectively.
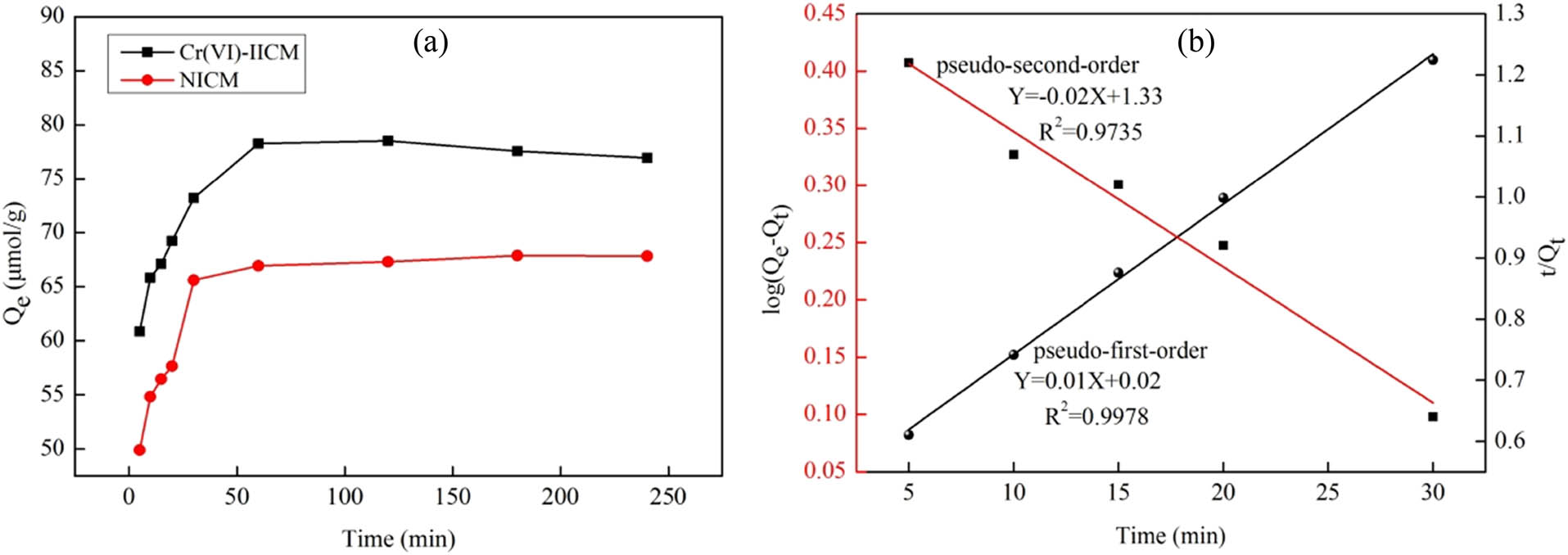
Adsorption quantity of composite membranes over time (a); kinetic fitting curve (b).
The kinetic fitting parameters of Cr(vi)-IICM are shown in Table 5. The equilibrium adsorption quantity fitted by the first-order kinetics model was 21.38 μmol·g−1, which was significantly lower than the experimental value of 78.28 μmol·g−1. The linear correlation coefficient was 0.9735. The equilibrium adsorption quantity Q e fitted by the second-order kinetics model was 100.00 μmol·g−1. This result was similar to the value obtained in the experiment. The linear correlation coefficient was 0.9978. The experimental results suggested that the kinetic adsorption of Cr(vi)-IICM was more in line with the second-order kinetics model. Chemisorption was dominant in this process.
Kinetic fitting parameters of Cr(vi)-IICM
| First-order dynamic model | Second-order dynamic model | ||||
|---|---|---|---|---|---|
| K 1 | Q e (μmol·g−1) | R 2 | K 2 | Q e (μmol·g−1) | R 2 |
| 0.05 | 21.38 | 0.9735 | 0.005 | 100.00 | 0.9978 |
3.6 Adsorption thermodynamic analysis
The temperature was a factor that had a strong influence on the amount of Cr(vi)-IICM adsorption. The temperature range selected for this study was 298–328 K. Figure 6a displays the outcomes. The thermodynamic adsorption of Cr(vi)-IICM was analyzed by the following thermodynamic equations:
where C e denotes the concentration after adsorption equilibrium (mmol·L−1); Q e is the adsorption quantity of composite membrane (μmol·g−1); ΔS is the entropy variation in the adsorption process (kJ·K−1·mol−1); R is the molar gas constant; ΔH represents the enthalpy change during the adsorption process (kJ·mol−1); K represents the Langmuir adsorption constant; and ΔG represents the change of the Gibbs free energy (kJ·mol−1).
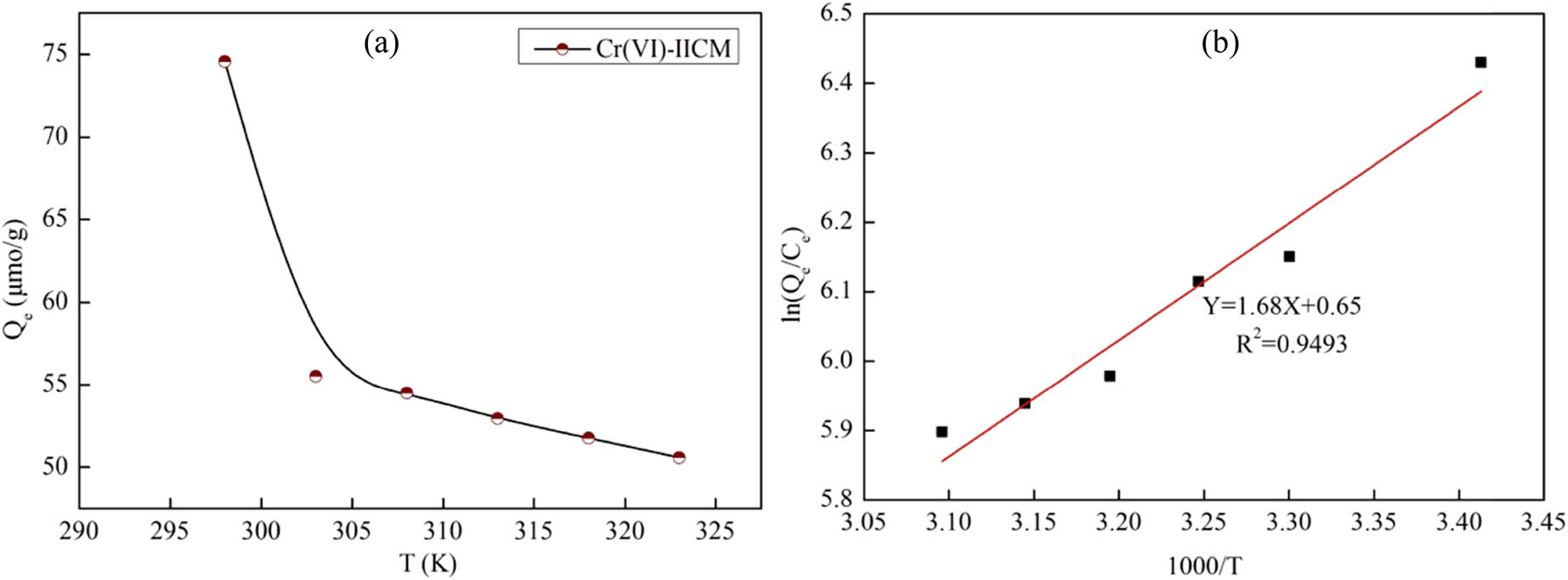
Temperature dependence of the adsorption quantity of Cr(vi)-IICM (a), thermodynamic fitting curve (b).
Using Eq. 11, the change in the adsorption quantity Q e of Cr(vi) with temperature T was fitted. Figure 6b shows the fitting curve. The corresponding ∆H and ∆S values were obtained as the intercept and slope of the fitting curve. According to Eq. 12, ∆G at different temperatures was obtained, and the calculated results are shown in Table 6. The ∆H was negative. This indicates that the adsorption process is an exothermic reaction. The negative value of ∆G and the positive value of ∆S indicate that adsorption was a spontaneous process with an increase in entropy.
Thermodynamic fitting parameters of Cr(VI)-IICM
| T (K) | ∆H | ∆S | ∆G | R 2 |
|---|---|---|---|---|
| 293 | −13.97 | 5.40 | −15.56 | 0.949 |
| 298 | −15.59 | |||
| 303 | −15.61 | |||
| 308 | −15.64 | |||
| 313 | −15.66 | |||
| 318 | −15.69 | |||
| 323 | −15.72 |
3.7 Effect of pH on adsorption
The pH affected the form of Cr(vi) present in the aqueous solution and the recognition site of Cr(vi)-IICM. Therefore, the adsorption performance of Cr(vi)-IICM was investigated in the pH range of 1.5–8 in this experiment. The variation of Cr(vi)-IICM adsorption quantity on Cr(vi) with pH is shown in Figure 7. Cr(vi)-IICM has a high adsorption quantity when the pH was less than 2.5. As the pH exceeded 2.5, the adsorption quantity gradually decreased with the increased pH. This suggested that acidic conditions favored the adsorption of Cr(vi)-IICM. The acidic conditions caused the nitrogen atoms in the pyridine ring to be protonated and adsorbed Cr(vi).
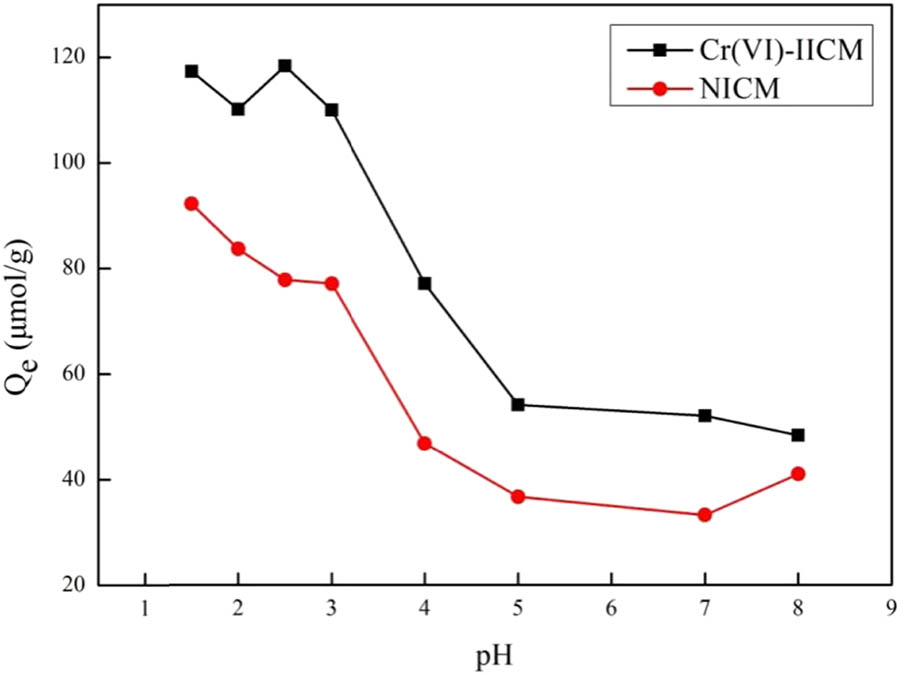
Effect of pH on adsorption.
3.8 Permeation selectivity
3.8.1 Permeation selectivity of metal ions
The permeability of Cr(vi)-IICM to various metal ions is shown in Table 7. The permeability of Cr(vi)-IICM to
Permeability parameters of Cr(vi)-IICM to cations
| Ion | J i (mg·h−1·cm−2) | P i × 10−6 (cm2·h−1) | β | |
|---|---|---|---|---|
| Cr(vi)-IICM | Cr(vi) | 0.42 | 6.80 | — |
| Cd(ii) | 0.19 | 2.99 | 2.27 | |
| Cu(ii) | 0.14 | 2.17 | 3.13 | |
| Ni(ii) | 0.12 | 1.83 | 3.72 |
3.8.2 Permeation selectivity of anionic
Cr(vi) mainly exists as
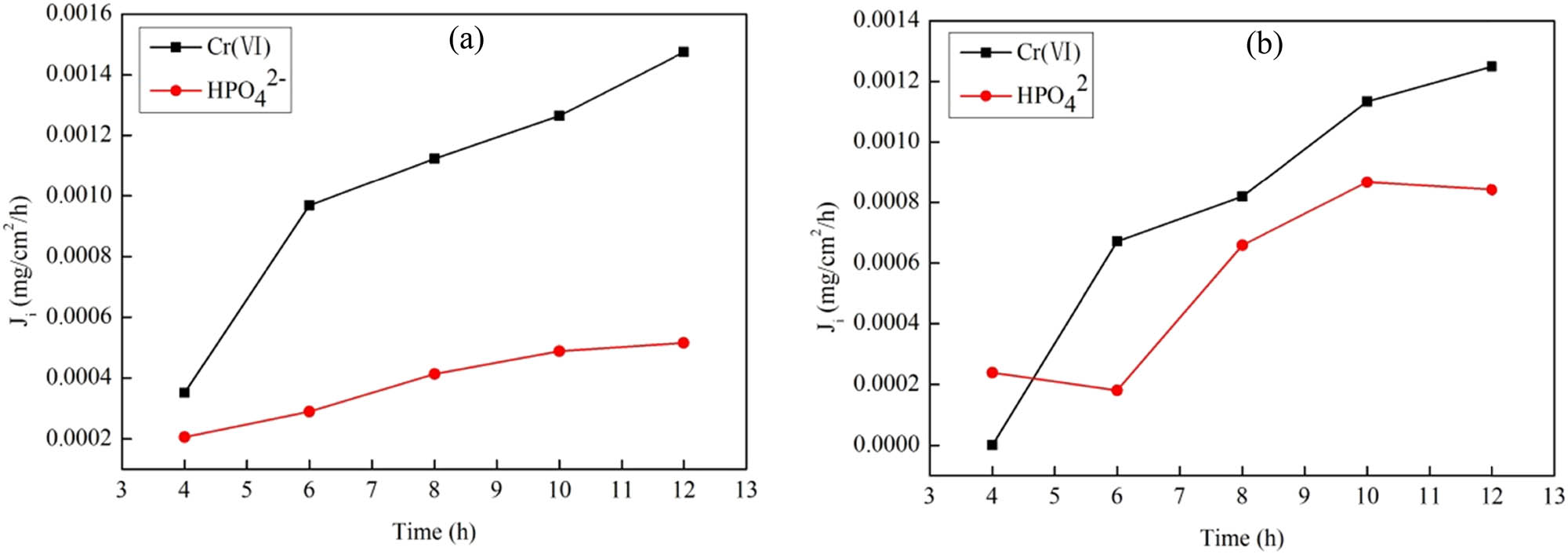
Permeation flux changes of Cr(vi) and
Permeability parameters of Cr(vi)-IICM to anions
| Ion | J i × 10−3 (mg·h−1·cm−2) | P i × 10−6 (cm2·h−1) | β | |
|---|---|---|---|---|
| Cr(vi)-IICM | Cr(vi) | 1.48 | 9.93 | — |
|
|
0.52 | 3.22 | 3.08 |
3.9 Comparison with other methods
Table 9 shows the works (39,40,41,42,43) with good effects in the adsorption of Cr(vi). Many different types of materials were included in these jobs. These materials could be applied to different environments and had reference values for the treatment of Cr(vi). The adsorption quantity of the Cr(vi)-IICM in this work was not the best. However, the Cr(vi)-IICM was a membrane material with better selectivity, which was more advantageous for the adsorption and recovery of single ions in mixed samples. It was easier to separate from the solution after adsorption than conventional adsorbents and had the same advantages for sample recovery and processing.
Comparison of adsorption quantity by different materials
| Adsorbents | Q (mg·g−1) | Ref. |
|---|---|---|
| Ion imprinting polymer@graphene oxide-Fe3O4 | 8.50 | (39) |
| Olive stones | 4.11 | (40) |
| Artemisia monosperma plant powder | 36.90 | (41) |
| Magnetite–humic acid | 3.17–25.06 | (42) |
| Attapulgite–Fe3O4 | 10.49 | (43) |
| Cr(vi)-IICM | 4.07 | This work |
4 Conclusions
Cr(vi)-IICM was one useful material for removing the Cr(vi) from the wastewater. Structural characterization and performance test results showed that both ion-imprinted membranes and non-imprinted membranes were mesoporous materials, and imprinted pores were generated after ion-imprinted membranes interacted with the imprinted ion. The adsorption behavior of Cr(vi)-IICM was in accordance with the Langmuir isothermal adsorption model. This suggested that the whole process was monomolecular adsorption. Kinetic and thermodynamic studies showed that the adsorption behavior was exothermic chemical adsorption that included both specific and non-specific adsorption. Meanwhile, the adsorption of Cr(vi) is more favorable in an acidic environment. The osmotic separation mechanism of Cr(vi)-IICM followed Piletsky’s gate model, which had excellent osmotic selectivity. As a material with high adsorption quantity and high selectivity, Cr(vi)-IICM has a promising research potential for the separation and enrichment of Cr(vi). By comparing with other adsorbents, the imprinted membrane in this work has advantages such as better selectivity and easy recovery. Of course, improving the adsorption quantity while maintaining high selectivity is also the focus of our next research.
Acknowledgment
The authors acknowledge the financial support provided by the National Natural Science Foundation of China.
-
Funding information: This work was supported by the National Natural Science Foundation of China (No. 21764008).
-
Author contributions: Xin Wang: methodology, writing – original draft, data curation; Peng Li: software; Guifang Wang: investigation, data analysis; Li Zhao: investigation; Huiling Cheng: supervision, project administration, funding acquisition, writing – review and editing.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
(1) Kong Z, Du Y, Wei J, Zhang H, Fan L. Synthesis of a new ion-imprinted polymer for selective Cr(vi) adsorption from aqueous solutions effectively and rapidly. J Colloid Interface Sci. 2021;588:749–60. 10.1016/j.jcis.2020.11.107.Search in Google Scholar PubMed
(2) Yang D, Liu J, Wang Q, Hong H, Zhao W, Chen S, et al. Geochemical and probabilistic human health risk of chromium in mangrove sediments: A case study in Fujian, China. Chemosphere. 2019;233:503–11. 10.1016/j.chemosphere.2019.05.245.Search in Google Scholar PubMed
(3) Shooto ND. Removal of toxic hexavalent chromium (Cr(vi)) and divalent lead (Pb(II)) ions from aqueous solution by modified rhizomes of Acorus calamus. Surf Interfaces. 2020;20:100624. 10.1016/j.surfin.2020.100624.Search in Google Scholar
(4) Toranzo R, Ferraro G, Beligni MV, Perez GL, Castiglioni D, Pasquevich D, et al. Natural and acquired mechanisms of tolerance to chromium in a Scenedesmus dimorphus strain. Algal Res. 2020;52:102100. 10.1016/j.algal.2020.102100.Search in Google Scholar
(5) Ambi AA, Isa MT, Ibrahim AB, Bashir M, Ekwuribe S, Sallau AB. Hexavalent chromium bioremediation using Hibiscus sabdariffa calyces extract: Process parameters, kinetics and thermodynamics. Sci Afr. 2020;10:e00642. 10.1016/j.sciaf.2020.e00642.Search in Google Scholar
(6) Chen Q, Yao Y, Li X, Lu J, Zhou J, Huang Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J Water Process Eng. 2018;26:289–300. 10.1016/j.jwpe.2018.11.003.Search in Google Scholar
(7) Fu F, Xie L, Tang B, Wang Q, Jiang S. Application of a novel strategy – Advanced Fenton-chemical precipitation to the treatment of strong stability chelated heavy metal containing wastewater. Chem Eng J. 2012;189–190:283–7. 10.1016/j.cej.2012.02.073.Search in Google Scholar
(8) Li R, Li L, Zhang Z, Chen G, Tang Y. Limiting factors of heavy metals removal during anaerobic biological pretreatment of municipal solid waste landfill leachate. J Hazard Mater. 2021;416:126081. 10.1016/j.jhazmat.2021.126081.Search in Google Scholar PubMed
(9) Kodispathi T, Jacinth Mispa K. Fabrication, characterization, ion-exchange studies and binary separation of polyaniline/Ti(IV) iodotungstate composite ion-exchanger for the treatment of water pollutants. Environ Nanotechnol Monit Manage. 2021;16:100555. 10.1016/j.enmm.2021.100555.Search in Google Scholar
(10) Hosseini SM, Alibakhshi H, Jashni E, Parvizian F, Shen JN, Taheri M, et al. A novel layer-by-layer heterogeneous cation exchange membrane for heavy metal ions removal from water. J Hazard Mater. 2020;381:120884. 10.1016/j.jhazmat.2019.120884.Search in Google Scholar PubMed
(11) Ren L, Dong J, Chi Z, Huang H. Reduced graphene oxide-nano zero value iron (rGO-nZVI) micro-electrolysis accelerating Cr(vi) removal in aquifer. J Env Sci. 2018;73:96–106. 10.1016/j.jes.2018.01.018.Search in Google Scholar PubMed
(12) Rouhaninezhad AA, Hojati S, Masir MN. Adsorption of Cr(vi) onto micro- and nanoparticles of palygorskite in aqueous solutions: Effects of pH and humic acid. Ecotoxicol Env Saf. 2020;206:111247. 10.1016/j.ecoenv.2020.111247.Search in Google Scholar PubMed
(13) Wang Y, Peng C, Padilla-Ortega E, Robledo-Cabrera A, López-Valdivieso A. Cr(vi) adsorption on activated carbon: Mechanisms, modeling and limitations in water treatment. J Environ Chem Eng. 2020;8(4):104031. 10.1016/j.jece.2020.104031.Search in Google Scholar
(14) Huang T, Song D, Wang G, Li G, Geng C, Yao C, et al. High adsorption performance of synthesized hexametaphosphate green rust towards Cr(vi) removal and its mechanism explorations. J Env Manage. 2019;252:109642. 10.1016/j.jenvman.2019.109642.Search in Google Scholar PubMed
(15) Demarchi CA, Michel BS, Nedelko N, lawska-Waniewska A, Dłużewski P, Kaleta A, et al. Preparation, characterization, and application of magnetic activated carbon from termite feces for the adsorption of Cr(vi) from aqueous solutions. Powder Technol. 2019;354:432–41. 10.1016/j.powtec.2019.06.020.Search in Google Scholar
(16) Aborode Abdullahi T, Benedicta D, Abigail Owusuwaa G, Opoku E. Isotherms, kinetics, equilibrium, and thermodynamic studies on the uptake of hexavalent chromium ions from aqueous solution using synthetic hydroxyapatite. Adv J Chem-Sec B Nat Prod Med Chemis. 2020;2(4):214–25. 10.22034/ajcb.2020.113974.Search in Google Scholar
(17) Lu W, Duan C, Zhang Y, Gao K, Dai L, Shen M, et al. Cellulose-based electrospun nanofiber membrane with core-sheath structure and robust photocatalytic activity for simultaneous and efficient oil emulsions separation, dye degradation and Cr(vi) reduction. Carbohydr Polym. 2021;258:117676. 10.1016/j.carbpol.2021.117676.Search in Google Scholar PubMed
(18) Zhang Q, Gao J, Qiu Y-R. Removal of Ni(II) and Cr(III) by complexation-ultrafiltration using rotating disk membrane and the selective separation by shear induced dissociation. Chem Eng Process – Process Intensif. 2019;135:236–44. 10.1016/j.cep.2018.12.005.Search in Google Scholar
(19) Roy Choudhury P, Majumdar S, Sahoo GC, Saha S, Mondal P. High pressure ultrafiltration CuO/hydroxyethyl cellulose composite ceramic membrane for separation of Cr(vi) and Pb(II) from contaminated water. Chem Eng J. 2018;336:570–8. 10.1016/j.cej.2017.12.062.Search in Google Scholar
(20) Vareda JP, Valente AJM, Duraes L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J Env Manage. 2019;246:101–18. 10.1016/j.jenvman.2019.05.126.Search in Google Scholar PubMed
(21) GracePavithra K, Jaikumar V, Kumar PS, SundarRajan P. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J Clean Prod. 2019;228:580–93. 10.1016/j.jclepro.2019.04.117.Search in Google Scholar
(22) Hao S, Jia Z, Wen J, Li S, Peng W, Huang R, et al. Progress in adsorptive membranes for separation – A review. Sep Purif Technol. 2021;255:117772. 10.1016/j.seppur.2020.117772.Search in Google Scholar
(23) Lu J, Qin Y, Wu Y, Meng M, Yan Y, Li C. Recent advances in ion-imprinted membranes: separation and detection via ion-selective recognition. Environ Sci Water Res Technol. 2019;5(10):1626–53. 10.1039/c9ew00465c.Search in Google Scholar
(24) Lian H, Hu Y, Li G. Novel metal ion-mediated complex imprinted membrane for selective recognition and direct determination of naproxen in pharmaceuticals by solid surface fluorescence. Talanta. 2013;116:460–7. 10.1016/j.talanta.2013.07.022.Search in Google Scholar PubMed
(25) Yu C, Lu J, Dai J, Dong Z, Lin X, Xing W, et al. Bio-inspired fabrication of Ester-functionalized imprinted composite membrane for rapid and high-efficient recovery of lithium ion from seawater. J Colloid Interface Sci. 2020;572:340–53. 10.1016/j.jcis.2020.03.091.Search in Google Scholar PubMed
(26) Lu J, Wu Y, Lin X, Gao J, Dong H, Chen L, et al. Anti-fouling and thermosensitive ion-imprinted nanocomposite membranes based on grapheme oxide and silicon dioxide for selectively separating europium ions. J Hazard Mater. 2018;353:244–53. 10.1016/j.jhazmat.2018.04.014.Search in Google Scholar PubMed
(27) Xi Y, Shi H, Liu R, Yin X, Yang L, Huang M, et al. Insights into ion imprinted membrane with a delayed permeation mechanism for enhancing Cd(2+) selective separation. J Hazard Mater. 2021;416:125772. 10.1016/j.jhazmat.2021.125772.Search in Google Scholar PubMed
(28) Liu Y, Hu D, Hu X, Chen S, Zhao L, Chen Y, et al. Preparation and characterization of chromium(vi) ion-imprinted composite membranes with a specifically designed functional monomer. Anal Lett. 2019;53(7):1113–39. 10.1080/00032719.2019.1698589.Search in Google Scholar
(29) Li Z, He G, Zhao G, Niu J, Li L, Bi J, et al. Preparation of a novel ion-imprinted membrane using sodium periodate-oxidized polydopamine as the interface adhesion layer for the direction separation of Li+ from spent lithium-ion battery leaching solution. Sep Purif Technol. 2021;277:119519. 10.1016/j.seppur.2021.119519.Search in Google Scholar
(30) Cui J, Xie A, Liu Y, Xue C, Pan J. Fabrication of multi-functional imprinted composite membrane for selective tetracycline and oil-in-water emulsion separation. Compos Commun. 2021;28:100985. 10.1016/j.coco.2021.100985.Search in Google Scholar
(31) Araki K, Maruyama T, Kamiya N, Goto M. Metal ion-selective membrane prepared by surface molecular imprinting. J Chromatogr B Anal Technol Biomed Life Sci. 2005;818(2):141–5. 10.1016/j.jchromb.2004.12.030.Search in Google Scholar PubMed
(32) Vatanpour V, Madaeni SS, Zinadini S, Rajabi HR. Development of ion imprinted technique for designing nickel ion selective membrane. J Membr Sci. 2011;373(1–2):36–42. 10.1016/j.memsci.2011.02.030.Search in Google Scholar
(33) Wang Z, Kong D, Qiao N, Wang N, Wang Q, Liu H, et al. Facile preparation of novel layer-by-layer surface ion-imprinted composite membrane for separation of Cu2+ from aqueous solution. Appl Surf Sci. 2018;457:981–90. 10.1016/j.apsusc.2018.07.031.Search in Google Scholar
(34) Zeng J, Dong Z, Zhang Z, Liu Y. Preparation of a surface-grafted imprinted ceramic membrane for selective separation of molybdate anion from water solutions. J Hazard Mater. 2017;333:128–36. 10.1016/j.jhazmat.2017.03.016.Search in Google Scholar PubMed
(35) Zhang J, Wang R, Ou X, Zhang X, Liu P, Chen Z, et al. Bio-inspired synthesis of thermo-responsive imprinted composite membranes for selective recognition and separation of ReO4−. Sep Purif Technol. 2021;259:118165. 10.1016/j.seppur.2020.118165.Search in Google Scholar
(36) Xi Y, Huang M, Luo X. Enhanced phosphate adsorption performance by innovative anion imprinted polymers with dual interaction. Appl Surf Sci. 2019;467–468:135–42. 10.1016/j.apsusc.2018.10.095.Search in Google Scholar
(37) Muttakin M, Mitra S, Thu K, Ito K, Saha BB. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int J Heat Mass Transf. 2018;122:795–805. 10.1016/j.ijheatmasstransfer.2018.01.107.Search in Google Scholar
(38) Piletsky SA, Panasyuk TL, Piletskaya EV, Nicholls IA, Ulbricht M. Receptor and transport properties of imprinted polymer membranes – a review. J Membr Sci. 1999;157(2):263–78. 10.1016/S0376-7388(99)00007-1.Search in Google Scholar
(39) Neolaka YAB, Lawa Y, Naat J, Riwu AAP, Lindu YE, Darmokoesoemo H, et al. Evaluation of magnetic material IIP@GO-Fe3O4 based on Kesambi wood (Schleichera oleosa) as a potential adsorbent for the removal of Cr(vi) from aqueous solutions. Reactive Funct Polym. 2021;166:105000. 10.1016/j.reactfunctpolym.2021.105000.Search in Google Scholar
(40) Vilardi G, Ochando-Pulido JM, Verdone N, Stoller M, Di Palma L. On the removal of hexavalent chromium by olive stones coated by iron-based nanoparticles: Equilibrium study and chromium recovery. J Clean Prod. 2018;190:200–10. 10.1016/j.jclepro.2018.04.151.Search in Google Scholar
(41) Ali HM, Essawy AA, Elnasr TAS, Aldawsari AM, Alsohaimi I, Hassan HMA, et al. Selective and efficient sequestration of Cr(vi) in ground water using trimethyloctadecylammonium bromide impregnated on Artemisia monosperma plant powder. J Taiwan Inst Chem Eng. 2021;125:122–31. 10.1016/j.jtice.2021.05.051.Search in Google Scholar
(42) Chang J, Zhang J, Wang H, Bai Y, Liu Y, Bi Y, et al. Cr(vi) adsorption and reduction by magnetite-humic acid adsorption complexes under mildly acidic conditions: Synergistic/antagonistic mechanism and multi-step reaction model. Chem Eng J. 2023;451:138648. 10.1016/j.cej.2022.138648.Search in Google Scholar
(43) Chen L, Wu X, Liu C, Zhao Y, Huang Q. Study on removal of Microcystis aeruginosa and Cr(vi) using attapulgite-Fe3O4 magnetic composite material (MCM). Algal Res. 2021;60:102501. 10.1016/j.algal.2021.102501.Search in Google Scholar
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

