Abstract
Antimicrobial peptide (AMP) self-assembly is an effective way to synthesis antimicrobial biomaterials. In previous studies, we found PAF26 AMP (Ac-RKKWFW-NH2) and its derivative K2–F2 peptide (Ac-KKRKKWFWFF-NH2) could both self-assemble into hydrogels, but they had distinct microscopic structures. Therefore, in this work five PAF26 peptide derivatives with different numbers of aromatic amino acids are designed to better understand the self-assembly mechanism of aromatic AMP. The transmission electron microscopy, infrared spectroscopy, circular dichroism, and fluorescence spectroscopy characterizations are carried out to study the microscope structure, secondary conformation, and molecular interactions. It is found that the five peptide derivatives have different microscopic structures, and the number of aromatic amino acids will affect the peptide hydrogen bonding and aromatic stacking interactions, causing significant differences in the secondary conformation and microscopic structure. This work will enhance the comprehension of aromatic AMP self-assembly.
1 Introduction
Antimicrobial peptides (AMPs), due to their broad-spectrum antibacterial ability and low bacterial resistance, are emerging as promising antibiotic substitutes, and by now several AMPs have been commercially used in clinic (1). However, there are still some challenges that hinder the applications of AMPs, such as weak bioactivity, immature stability, and high toxicity (2). In recent years, several strategies have been developed to deal with these problems, for example combining AMPs with metal materials to enhance the antimicrobial effect (3), self-assembling AMPs to improve the stability and activity (4,5), developing environment responsive AMPs to reduce the toxicity (6), and so on (7,8). Among them, the self-assembling method shows outstanding advantages (9).
Peptide self-assembly is a spontaneous process that mainly depends on the weak non-covalent molecular interactions including hydrogen bonding interaction, electrostatic interaction, aromatic stacking interaction, and hydrophobic interaction (10). Through self-assembly, peptides could change their conformations and aggregate into a variety of nanostructures including micelles (11), fibers (12), ribbons (13), and tubes (14) under mild conditions, which could be widely used for drug delivery and tissue engineering (15,16). In the past decades, a lot of studies have been reported to control the peptides self-assembly (17,18,19,20), and these achievements gave excellent referees for AMP self-assembly.
AMP is a special kind of peptide that has amphiphile structure, which gives it the potential to self-assemble in water. Despite using native AMP, the peptide sequence could be artificially modified to enhance the self-assembly possibility (21,22). Lombardi et al. (5) designed an AMP amphiphile WMR PA by linking native antibacterial WMR peptide with a peptide segment of aliphatic residues (AAAAAAA) containing a lipidic tail (C19H38O2). It was found that the WMR PA peptide could self-assemble into stable nanofibers, inhibit biofilm formation, and eradicate the already formed biofilms. Liu et al. (23) designed and synthesized an amphiphilic peptide CG3R6TAT containing hydrophobic cholesterol (C) and hydrophilic R6TAT peptide sequence. This peptide could self-assemble into core-shell structured nanoparticles, which could strongly enhance its antimicrobial activity. But one question is that with different molecular designs, the nanoarchitectures of AMPs are distinct.
In our previous works, we found that though PAF26 AMP (Ac-RKKWFW-NH2) and its derivative K2–F2 peptide (Ac-KKRKKWFWFF-NH2) both could self-assemble into hydrogels, they had distinct microscopic structures: one tended to self-assemble into nanoparticles, and the other tended to self-assemble into nanofibers (24,25). Therefore, to better understand the self-assembly mechanism of aromatic AMP, five PAF26 AMP derivatives were designed. As shown in Table 1, we divided the five peptides into two groups: In group I, the peptides had the same hydrophilic KRKK peptide sequence and different numbers of aromatic amino acids; In group II, the peptides had the same hydrophilic KKRKK peptide sequence and different numbers of aromatic amino acids. In addition, the chemical structure of each peptide is shown in Scheme 1.
Peptide sequence and molecular weight of each designed peptide
| Group | Abbreviation | Peptide sequence | Molecular weight (g·mol−1) |
|---|---|---|---|
| Group I | KRW6 | Ac-KRKKWFW-NH2 | 1,119.36 |
| KRW6-F | Ac-KRKKWFWF-NH2 | 1266.54 | |
| KRW6-FF | Ac-KRKKWFWFF-NH2 | 1413.71 | |
| Group IIa | KKRW6 | Ac-KKRKKWFW-NH2 | 1247.54 |
| KKRW6-F | Ac-KKRKKWFWF-NH2 | 1394.71 |
- a
The Ac-KKRKKWFWFF-NH2 peptide has already been studied in a previous report (25).

The chemical structures of KRW6, KRW6-F, KRW6-FF, KKRW6, and KKRW6-F peptides.
2 Materials and methods
2.1 Materials
Five PAF26 peptide derivatives: KRW6, KRW6-F, KRW6-FF, KKRW6, and KKRW6-F peptides (purity ≥95%) were purchased from Shanghai GL Biochem Ltd (Shanghai, China). Sodium hydroxide (AR) and hydrochloric acid (37 wt%) were purchased from Sinopharm chemical reagent Co., Ltd (Shanghai, China). Potassium bromide (SP) was purchased from Shanghai Yi En chemical technology Co., Ltd (Shanghai, China). Thioflavin T (ThT) was purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China). All the materials were used as received.
2.2 AMP self-assembly experiments
40 mg KRW6, KRW6-F, KRW6-FF, KKRW6, and KKRW6-F peptide was dissolved in 1 mL of distilled water separately and then the pH value of each peptide solution was adjusted to 3 using diluted hydrochloric acid solution. To trigger peptide self-assembly, 0.5 mol·L−1 diluted sodium hydroxide solution was added into each peptide solution slowly. The addition of sodium hydroxide solution was stopped when the peptide solution was observed to lose flowability, and the solution was kept overnight to let it self-assemble completely.
2.3 Transmission electron microscopy (TEM)
To study the microscopic structures of KRW6, KRW6-F, KRW6-FF, KKRW6, and KKRW6-F peptide self-assemblies, the self-assemblies were diluted ten times and immediately dropped on ultra-thin carbon films, air dried, and observed under a TEM (JEOL JEM 2100Plus, Japan).
2.4 Fourier transform infrared spectroscopy (FTIR)
To study the secondary conformation and hydrogen bonding interactions of KRW6, KRW6-F, KRW6-FF, KKRW6, and KKRW6-F peptide self-assemblies, the self-assemblies were frozen in liquid nitrogen and lyophilized using a freeze-dryer (LGJ-10, China). Then, each sample was mixed with potassium bromide, ground, and repressed into piece. The FTIR spectra were recorded on a spectrophotometer (Nicolet iS50, USA).
2.5 Circular dichroism (CD)
The circular dichroism information of KRW6, KRW6-F, KRW6-FF, KKRW6, and KKRW6-F peptide self-assemblies was studied using a solid spectropolarimeter (JASCO J-815, Japan) with the wavelength ranging from 190 to 280 nm.
2.6 Fluorescence spectroscopy
To evaluate the aromatic stacking interactions during the peptide self-assembly, the fluorescence spectra of KRW6, KRW6-F, KRW6-FF, KKRW6, and KKRW6-F peptide solutions at pH 3, and KRW6, KRW6-F, KRW6-FF, KKRW6, and KKRW6-F peptide self-assemblies were detected on a fluorescence spectrophotometer (F-7100, Japan). λ ex = 310 nm and λ em = 330–600 nm.
2.7 ThT fluorescence assay
The ThT working solution was prepared by dissolving 4 mg ThT into 4 mL of distilled water and filtered through a 0.2 μm syringe filter. In each 0.5 mL of KRW6, KRW6-F, KRW6-FF, KKRW6, or KKRW6-F peptide self-assembly, 0.5 mL of ThT working solution was added and mixed, then each mixed solution was kept in room temperature for 5 min to allow ThT to bind with the peptide assembly. At last, the fluorescence excitation spectra of ThT working solution and KRW6, KRW6-F, KRW6-FF, KKRW6, and KKRW6-F peptide mixed solutions were measured in the range of 300–455 nm with the emission wavelength of 482 nm, and the fluorescence excitation spectra were measured in the range of 465–700 nm with the excitation wavelength of 450 nm.
3 Results and discussion
3.1 Microscopic structure of KRW6, KRW6-F, and KRW6-FF peptide self-assemblies
Peptide self-assembly is a process in which peptide molecules aggregate together under the force of weak molecular interactions, and the microscopic structure of self-assembly is always different with distinct molecular sequence and molecular interactions. As shown in Figure 1, KRW6 peptide was self-assembled into long nanofibers with pronounced twisting structures; KRW6-F peptide was self-assembled into mixed nanofibers and small nanoparticles; KRW6-FF peptide was self-assembled into nanofibers with seldom twisting structures. In addition, it was seen that the microscopic structures of KRW6 and KRW6-FF peptides were more regular than that of KRW6-F peptide.

TEM images of (a1 and a2) KRW6, (b1 and b2) KRW6-F, and (c1 and c2) KRW6-FF peptide self-assemblies. Scale bars: (a1–c1) 200 nm and (a2–c2) 100 nm.
3.2 Self-assembly mechanism of KRW6, KRW6-F, and KRW6-FF peptides
The microscopic structure of peptide self-assembly is always related to peptide secondary conformation. And the FTIR absorptions in the amide I region could give information about the secondary conformation of peptide self-assemblies (26). As shown in Figure 2a, KRW6, KRW6-F, and KRW6-FF peptides showed strong absorptions at around 1,630–1,632 cm−1, which were the characteristic feature of β-sheet conformation (27). And the absorptions at around 1,677–1,681 cm−1 showed that they might adopt antiparallel β-sheet conformation or β-turn conformation (28,29). In addition, as shown in the solid circular dichroism spectra (Figure 2b), KRW6 peptide exhibited a broad negative peak at 215 nm (nπ* transition), suggesting that it adopted β-sheet conformation (30). KRW6-F peptide exhibited a negative peak at 200 nm (ππ* transition), showing that it adopted random coil conformation. And it also showed less pronounced peaks at 213 and 238 nm (nπ* transition), indicating that it also contained β-sheet and β-turn conformations (31). Similar to KRW6-F peptide, KRW6-FF peptide exhibited a negative peak at 200 nm (ππ* transition), and its peak at 236 nm (nπ* transition) was much stronger than that of KRW6-F peptide, demonstrating that it contained larger number of β-turn conformations. ThT is a popular fluorescent dye for detecting protein or peptide aggregate with amyloid fibril structure, and it was reported that β-sheet structure could cause large enhancement in ThT fluorescence (32,33). In Figure 2c, it was shown that new excitation peaks (λ em = 482 nm) at around 450 nm were displayed for KRW6, KRW6-F, and KRW6-FF peptides. And compared with ThT solution, the fluorescence emission intensity (λ ex = 450 nm) of KRW6 peptide at 494 nm, KRW6-F peptide at 504 nm, and KRW6-FF peptide at 501 nm were strongly increased (Figure 2d) and the maximum fluorescence intensity of KRW6-F peptide was much higher than that of KRW6 and KRW6-FF peptides. These results indicated that there were β-sheet structures in KRW6, KRW6-F, and KRW6-FF peptide self-assemblies, and KRW6-F peptide had more β-sheet structures than KRW6 and KRW6-FF peptides (33,34).

(a) FTIR and (b) CD spectra of KRW6, KRW6-F, and KRW6-FF peptide self-assemblies; (c) fluorescence excitation spectra and (d) fluorescence emission of ThT solution (1 mg·mL−1) and KRW6, KRW6-F, and KRW6-FF peptide self-assemblies dyed with ThT with λ em = 482 nm and λ ex = 450 nm, respectively.
The FTIR absorptions in the amide A band region could be applied to distinguish the intermolecular and intramolecular hydrogen bonding interactions in the self-assembly (35,36). As shown in Figure 2a, the amide A band absorption of KRW6 peptide was about 3,300 cm−1, showing that KRW6 peptide had more possibility of adopting intramolecular hydrogen bonding interaction in the self-assembly. For KRW6-F and KRW6-FF peptides, the amide A band absorptions were 3,285 and 3,277 cm−1 (below 3,300 cm−1), showing that the self-assemblies were dominated by intermolecular hydrogen bonding interactions (37). In addition, the absorption of KRW6-FF peptide was lower than that of KRW6-F peptide, probably demonstrating that the intermolecular hydrogen bonding interaction of KRW6-FF peptide was stronger than that of KRW6-F peptide.
For aromatic peptides, the aromatic stacking interactions could be investigated by fluorescence emission spectroscopy. As shown in Figure 3, the emission spectra of peptide solutions and self-assemblies were measured. The emission maximum of KRW6 peptide after self-assembly was red-shifted from 362 to 396 nm; KRW6-F peptide after self-assembly was red-shifted from 366 to 417 nm; KRW6-FF peptide after self-assembly was red-shifted from 356 to 400 nm and 414 nm. The red-shifting of KRW6-F and KRW6-FF peptide was bigger than that of KRW6 peptide, showing that the aromatic stacking interactions in KRW6-F and KRW6-FF peptide self-assemblies were stronger than that of KRW6 peptide self-assembly. In addition, all the three peptide self-assemblies showed excimer emission peak at around 477 nm, which was the extended aggregation of aromatic motifs.
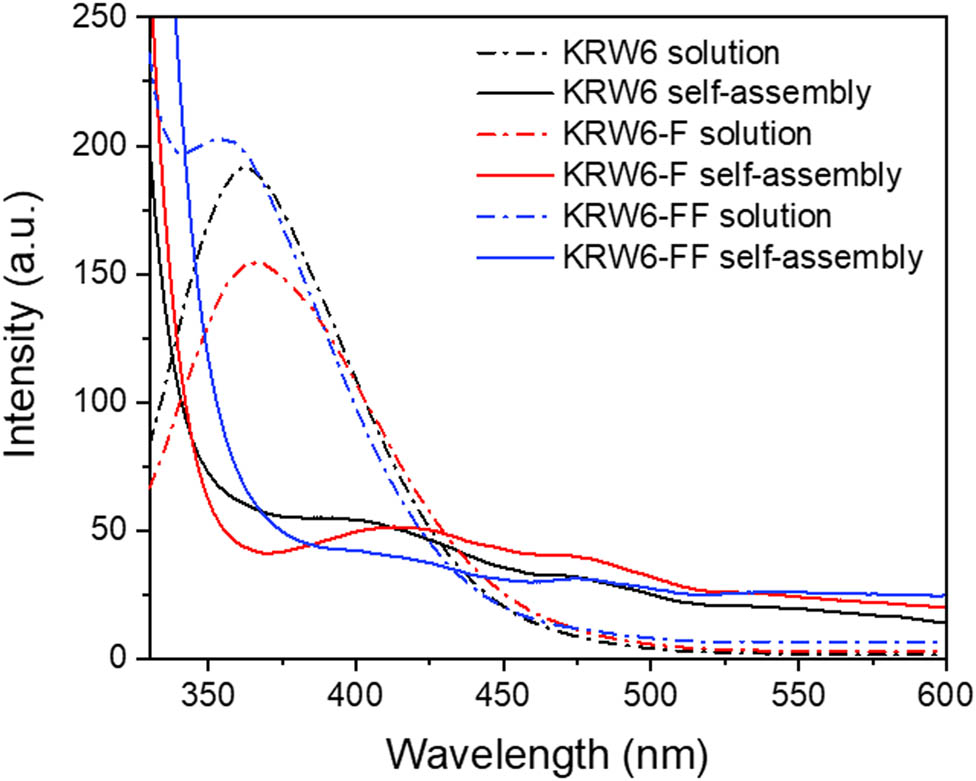
Fluorescence spectra of KRW6, KRW6-F, and KRW6-FF peptide solutions (40 mg·mL−1, pH = 3) and peptide self-assemblies.
3.3 Microscopic structure of KKRW6 and KKRW6-F peptide self-assemblies
The microscopic structures of KKRW6 and KKRW6-F peptide self-assemblies are shown in Figure 4. It was shown that KKRW6 peptide was self-assembled into short nanofibers, mixed with some irregular structures; KKRW6-F peptide was self-assembled into long nanofibers with some twisting structures, and there were also some nanoparticles and irregular structures.

TEM images of (a1 and a2) KKRW6 and (b1 and b2) KKRW6-F peptide self-assemblies. Scale bars: (a1 and b1) 200 nm and (a2 and b2) 100 nm.
3.4 Self-assembly mechanism of KKRW6 and KKRW6-F peptides
As shown in the FTIR spectra (Figure 5a), in the amide I region, KKRW6 and KKRW6-F peptides had strong absorptions at around 1,630–1,631 cm−1, showing that they adopted β-sheet conformation. And the absorptions at 1,682 cm−1 confirmed that they might adopt antiparallel β-sheet conformation or β-turn conformation. In the solid circular dichroism spectra (Figure 5b), KKRW6 peptide exhibited a broad negative peak at 216 nm (nπ* transition), suggesting that it adopted β-sheet conformation. Besides, the negative peak at 200 nm (ππ* transition) showed that it also contained a number of random coil conformation. KKRW6-F peptide showed a negative peak at 200 nm (ππ* transition), demonstrating the existence of random coil conformation. Besides, the positive peak at 208 nm (ππ* transition) and less pronounced peaks at 211 and 255 nm (nπ* transition), indicated that it also contained β-sheet and β-turn conformations (16,31). From the ThT fluorescence excitation spectra (Figure 5c), it was shown that new excitation peaks (λ em = 482 nm) at around 445 nm were displayed for KKRW6 and KKRW6-F peptides. And compared with ThT solution, the fluorescence emission intensity (λ ex = 450 nm) of KKRW6 peptide at 487 nm and KKRW6-F peptide at 503 nm were strongly increased (Figure 5d). These results indicated that there were β-sheet structures in KKRW6 and KKRW6-F peptide self-assemblies. Besides, the maximum fluorescence intensity of KKRW6 peptide was much higher than that of KKRW6-F peptide, showing that KKRW6 peptide had more β-sheet structures than KKRW6-F peptide.

(a) FTIR and (b) CD spectra of KKWR6 and KKWR6-F peptide self-assemblies; (c) fluorescence excitation spectra and (d) fluorescence emission of ThT solution (1 mg·mL−1) and KKWR6 and KKWR6-F peptide self-assemblies dyed with ThT with λ em = 482 nm and λ ex = 450 nm, respectively.
Besides, the amide A band absorption of KKRW6 peptide was about 3,305 cm−1, showing that it had more probability to self-assemble through intramolecular hydrogen bonding interaction. For KKRW6-F peptide, the absorption was 3,288 cm−1 (below 3,300 cm−1), showing that the self-assembly was dominated by intermolecular hydrogen bonding interaction.
The fluorescence emission spectra of KKRW6 and KKRW6-F peptide solutions and self-assemblies are shown in Figure 6. The emission maximum of KKRW6 peptide after self-assembly was red-shifted from 364 to 424 nm, and KKRW6-F peptide after self-assembly was red-shifted from 362 to 412 nm, suggesting that both of them had efficient aromatic overlaps. In addition, it was found that both of them showed excimer emission peak at around 469 nm, which was the extended aggregation of aromatic motifs.

Fluorescence spectra of KKRW6 and KKRW6-F peptide solutions (40 mg·mL−1, pH = 3) and peptide self-assemblies.
Aromatic AMP belongs to a special kind of peptide that is always called aromatic peptide amphiphile, due to its special amphiphile structure and sufficient aromatic motifs. In aromatic peptide amphiphile self-assembly systems, the aromatic motifs played an extremely important role that they were more likely to form nanofibers, nanoribbons, or nanotubes through stacking arrangements (38–43). In this work, comparing KRW6 peptide with KRW6-F and KRW6-FF peptides, we could find that the peptides with larger number of aromatic amino acids had stronger aromatic stacking interaction. In addition, the stronger aromatic stacking interaction was coupled with stronger intermolecular hydrogen bonding interaction, making peptide molecules tend to adopt β-turn conformation. Similar to KRW6, KRW6-F, and KRW6-FF peptides, KKRW6-F peptide with larger number of aromatic amino acids also showed stronger intermolecular hydrogen bonding interaction and stronger tendency to adopt β-turn conformation than KKRW6 peptide, showing the important influences of aromatic motifs.
4 Conclusion
Peptide self-assembly is a spontaneous process forced by weak molecular interactions. In this work, the five aromatic AMPs had similar peptide sequences, but the microscopic structures and secondary conformations were different. It was found that the peptides with larger number of aromatic amino acids tended to have stronger intermolecular hydrogen bonding and aromatic stacking interactions and were more likely to adopt β-turn conformation; the peptides with smaller number of aromatic amino acids tended to have stronger intramolecular hydrogen bonding interaction and were more likely to adopt β-sheet conformation. However, the effects of aromatic amino acids on the microscopic structure of self-assemblies were more complex and still needed to be revealed in the future.
-
Funding information: This work was financially supported by the National Natural Science Foundation of China (No. 51703260, 51603236), and Program for Science and Technology of Henan Province (No. 212102210292).
-
Author contributions: Fengyi Cao: conceptualization, methodology, data curation, writing – original draft, and writing – review and editing; Genxing Zhu: formal analysis and validation; Meng Song: formal analysis and validation; Xiaoli Zhao: methodology and formal analysis; Gangqing Ma and Mengqing Zhang: methodology.
-
Conflict of interest: Authors declare no conflict of interest.
References
(1) Chen CH, Lu TK. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics. 2020;9(1):24. 10.3390/antibiotics9010024.Search in Google Scholar PubMed PubMed Central
(2) Yan Y, Li Y, Zhang Z, Wang X, Niu Y, Zhang S, et al. Advances of peptides for antibacterial applications. Colloids Surf, B. 2021;202:111682. 10.1016/j.colsurfb.2021.111682.Search in Google Scholar PubMed
(3) Casciaro B, Moros M, Rivera-Fernandez S, Bellelli A, de la Fuente JM, Mangoni ML. Gold-nanoparticles coated with the antimicrobial peptide Esculentin-1a(1-21)NH2 as a reliable strategy for antipseudomonal drugs. Acta Biomater. 2017;47:170–81. 10.1016/j.actbio.2016.09.041.Search in Google Scholar PubMed
(4) Tu Z, Hao J, Kharidia R, Meng X, Liang J. Improved stability and selectivity of lytic peptides through self-assembly. Biochem Biophys Res Commun. 2007;361(3):712–7. 10.1016/j.bbrc.2007.06.178.Search in Google Scholar PubMed
(5) Lombardi L, Shi Y, Falanga A, Galdiero E, de Alteriis E, Franci G, et al. Enhancing the potency of antimicrobial peptides through molecular engineering and self-assembly. Biomacromolecules. 2019;20(3):1362–74. 10.1021/acs.biomac.8b01740.Search in Google Scholar PubMed
(6) Wan Y, Liu L, Yuan S, Sun J, Li Z. pH-responsive peptide supramolecular hydrogels with antibacterial activity. Langmuir. 2017;33(13):3234–40. 10.1021/acs.langmuir.6b03986.Search in Google Scholar PubMed
(7) Li L, An H, Peng B, Zheng R, Wang H. Self-assembled nanomaterials: design principle, nanostructural effect, functional mechanism as antimicrobial or detection agent. Mater Horiz. 2019;6(9):1794–811. 10.1039/c8mh01670d.Search in Google Scholar
(8) Alavi M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. e-Polym. 2019;19(1):103–19. 10.1515/epoly-2019-0013.Search in Google Scholar
(9) Tian X, Sun F, Zhou X, Luo S, Chen L. Role of peptide self-assembly in antimicrobial peptides. J Pept Sci. 2015;21(7):530–9. 10.1002/psc.2788.Search in Google Scholar PubMed
(10) Wang J, Liu K, Xing R, Yan X. Peptide self-assembly: thermodynamics and kinetics. Chem Soc Rev. 2016;45(20):5589–604. 10.1039/c6cs00176a.Search in Google Scholar PubMed
(11) Dai Y, Jiang Z, Li J, Wang M, Liu C, Qi W, et al. Co-assembly of curcumin and a cystine bridged peptide to construct tumor-responsive nano-micelles for efficient chemotherapy. J Mater Chem B. 2020;8(9):1944–51. 10.1039/c9tb02625h.Search in Google Scholar PubMed
(12) Wychowaniec JK, Smith AM, Ligorio C, Mykhaylyk OO, Miller AF, Saiani A. Role of sheet-edge interactions in β-sheet self-assembling peptide hydrogels. Biomacromolecules. 2020;21(6):2285–97. 10.1021/acs.biomac.0c00229.Search in Google Scholar PubMed PubMed Central
(13) Rüter A, Kuczera S, Stenhammar J, Zinn T, Narayanan T, Olsson U. Tube to ribbon transition in a self-assembling model peptide system. Phys Chem Chem Phys. 2020;22(33):18320–7. 10.1039/d0cp03204b.Search in Google Scholar PubMed
(14) Gokula RP, Mahato J, Tripathi A, Singh HB, Chowdhury A. Self-assembly of nicotinic acid-conjugated selenopeptides into mesotubes. ACS Appl Bio Mater. 2021;4(2):1912–9. 10.1021/acsabm.0c01551.Search in Google Scholar PubMed
(15) Hu X, Liao M, Gong H, Zhang L, Cox H, Waigh TA, et al. Recent advances in short peptide self-assembly: from rational design to novel applications. Curr Opin Colloid Interface Sci. 2020;45:1–13. 10.1016/j.cocis.2019.08.003.Search in Google Scholar
(16) Dou X, Li P, Zhang D, Feng C. RGD anchored C2-benzene based PEG-like hydrogels as scaffolds for two and three dimensional cell cultures. J Mater Chem B. 2013;1(29):3562–8. 10.1039/c3tb20155d.Search in Google Scholar PubMed
(17) Firipis K, Boyd-Moss M, Long B, Dekiwadia C, Hoskin W, Pirogova E, et al. Tuneable hybrid hydrogels via complementary self-assembly of a bioactive peptide with a robust polysaccharide. ACS Biomater Sci Eng. 2021;7(7):3340–50. 10.1021/acsbiomaterials.1c00675.Search in Google Scholar PubMed
(18) Dai K, Fores JR, Wanzke C, Winkeljann B, Bergmann AM, Lieleg O, et al. Regulating chemically fueled peptide assemblies by molecular design. J Am Chem Soc. 2020;142(33):14142–9. 10.1021/jacs.0c04203.Search in Google Scholar PubMed
(19) Cui H, Cheetham AG, Pashuck ET, Stupp SI. Amino acid sequence in constitutionally isomeric tetrapeptide amphiphiles dictates architecture of one-dimensional nanostructures. J Am Chem Soc. 2014;136(35):12461–8. 10.1021/ja507051w.Search in Google Scholar PubMed PubMed Central
(20) Zagorodko O, Melnyk T, Rogier O, Nebot VJ, Vicent MJ. Higher-order interfiber interactions in the self-assembly of benzene-1,3,5-tricarboxamide-based peptides in water. Polym Chem. 2021;12(23):3478–87. 10.1039/d1py00304f.Search in Google Scholar PubMed PubMed Central
(21) Tan P, Fu H, Ma X. Design, optimization, and nanotechnology of antimicrobial peptides: From exploration to applications. Nano Today. 2021;39:101229. 10.1016/j.nantod.2021.101229.Search in Google Scholar
(22) Cao F, Ma G, Mei L, Zhu G, Song M, Qin Q, et al. Development of disulfide bond crosslinked antimicrobial peptide hydrogel. Colloids Surf A. 2021;626:127026. 10.1016/j.colsurfa.2021.127026.Search in Google Scholar
(23) Liu L, Xu K, Wang H, Tan PK, Fan W, Venkatraman SS, et al. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nat Nanotechnol. 2009;4(7):457–63. 10.1038/nnano.2009.153.Search in Google Scholar PubMed
(24) Cao F, Mei L, Zhu G, Song M, Zhang X. An injectable molecular hydrogel assembled by antimicrobial peptide PAF26 for antimicrobial application. RSC Adv. 2019;9(53):30803–8. 10.1039/c9ra06130d.Search in Google Scholar PubMed PubMed Central
(25) Cao F, Ma G, Song M, Zhu G, Mei L, Qin Q. Evaluating the effects of hydrophobic and cationic residues on antimicrobial peptide self-assembly. Soft Matter. 2021;17(16):4445–51. 10.1039/d1sm00096a.Search in Google Scholar PubMed
(26) Lee NR, Bowerman CJ, Nilsson BL. Effects of varied sequence pattern on the self-assembly of amphipathic peptides. Biomacromolecules. 2013;14(9):3267–77. 10.1021/bm400876s.Search in Google Scholar PubMed
(27) Zhou X, Dai Q, Huang X, Qin Z. Preparation and characterizations of antibacterial-antioxidant film from soy protein isolate incorporated with mangosteen peel extract. e-Polym. 2021;21(1):575–89. 10.1515/epoly-2021-0058.Search in Google Scholar
(28) Han S, Cao S, Wang Y, Wang J, Xia D, Xu H, et al. Self-assembly of short peptide amphiphiles: the cooperative effect of hydrophobic interaction and hydrogen bonding. Chem - Eur J. 2011;17(46):13095–102. 10.1002/chem.201101970.Search in Google Scholar PubMed
(29) Qin SY, Jiang HF, Liu XJ, Pei Y, Cheng H, Sun YX, et al. High length–diameter ratio nanotubes self-assembled from a facial cyclopeptide. Soft Matter. 2014;10(7):947–51. 10.1039/c3sm52730a.Search in Google Scholar PubMed
(30) Pashuck ET, Stupp SI. Direct observation of morphological tranformation from twisted ribbons into helical ribbons. J Am Chem Soc. 2010;132(26):8819–21. 10.1021/ja100613w.Search in Google Scholar PubMed PubMed Central
(31) Ma M, Kuang Y, Gao Y, Zhang Y, Gao P, Xu B. Aromatic-aromatic interactions induce the self-assembly of pentapeptidic derivatives in water to form nanofibers and supramolecular hydrogels. J Am Chem Soc. 2010;132(8):2719–28. 10.1021/ja9088764.Search in Google Scholar PubMed
(32) Namioka S, Yoshida N, Konno H, Makabe K. Residue-specific binding mechanisms of thioflavin T to a surface of flat β-sheets within a peptide self-assembly mimic. Biochemistry. 2020;59(30):2782–7. 10.1021/acs.biochem.0c00280.Search in Google Scholar PubMed
(33) Erickson DP, Ozturk OK, Selling G, Chen F, Campanella OH, Hamaker BR. Corn zein undergoes conformational changes to higher β-sheet content during its self-assembly in an increasingly hydrophilic solvent. Int J Biol Macromol. 2020;157:232–9. 10.1016/j.ijbiomac.2020.04.169.Search in Google Scholar PubMed
(34) Qin S, Pei Y, Liu X, Zhuo R, Zhang X. Hierarchical self-assembly of a β-amyloid peptide derivative. J Mater Chem B. 2013;1(5):668–75. 10.1039/c2tb00105e.Search in Google Scholar PubMed
(35) Toniolo C, Palumbo M. Solid-state infrared absorption spectra and chain arrangement in some synthetic homooligopeptides in the intermolecularly hydrogen-bonded pleated-sheet β-conformation. Biopolymers. 1977;16(1):219–24. 10.1002/bip.1977.360160116.Search in Google Scholar PubMed
(36) Yurong G, Dapeng L. Preparation and characterization of corn starch/PVA/glycerol composite films incorporated with ε-polylysine as a novel antimicrobial packaging material. e-Polym. 2020;20(1):154–61. 10.1515/epoly-2020-0019.Search in Google Scholar
(37) Muraoka T, Cui H, Stupp SI. Quadruple helix formation of a photoresponsive peptide amphiphile and its light-triggered dissociation into single fibers. J Am Chem Soc. 2008;130(10):2946–7. 10.1021/ja711213s.Search in Google Scholar PubMed
(38) Fleming S, Ulijn RV. Design of nanostructures based on aromatic peptide amphiphiles. Chem Soc Rev. 2014;43(23):8150–77. 10.1039/c4cs00247d.Search in Google Scholar PubMed
(39) Zhou P, Deng L, Wang Y, Lu JR, Xu H. Different nanostructures caused by competition of intra-and inter-β-sheet interactions in hierarchical self-assembly of short peptides. J Colloid Interface Sci. 2016;464:219–28. 10.1016/j.jcis.2015.11.030.Search in Google Scholar PubMed
(40) Ryan DM, Anderson SB, Nilsson BL. The influence of side-chain halogenation on the self-assembly and hydrogelation of Fmoc-phenylalanine derivatives. Soft Matter. 2010;6(14):3220–31. 10.1039/c0sm00018c.Search in Google Scholar
(41) Yang L, Gan S, Guo Q, Zhang H, Chen Q, Li H, et al. Stimuli-controlled peptide self-assembly with secondary structure transitions and its application in drug release. Mater Chem Front. 2021;5(12):4664–71. 10.1039/d1qm00430a.Search in Google Scholar
(42) Li Q, Zhang J, Wang Y, Zhang G, Qi W, You S, et al. Self-assembly of peptide hierarchical helical arrays with sequence-encoded circularly polarized luminescence. Nano Lett. 2021;21(15):6406–15. 10.1021/acs.nanolett.1c00697.Search in Google Scholar PubMed
(43) Ghosh G, Kartha KK, Fernández G. Tuning the mechanistic pathways of peptide self-assembly by aromatic interactions. Chem Commun. 2021;57(13):1603–6. 10.1039/d0cc07199d.Search in Google Scholar PubMed
© 2022 Fengyi Cao et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

