Abstract
Chitosan is an amino-polysaccharide, traditionally obtained by the partial deacetylation of chitin from exoskeletons of crustaceans. Properties such as biocompatibility, hemostasis, and the ability to absorb physiological fluids are attributed to this biopolymer. Chitosan’s biological properties are regulated by its origin, polymerization degree, and molecular weight. In addition, it possesses antibacterial and antifungal activities. It also has been used to prepare films, hydrogels, coatings, nanofibers, and absorbent sponges, all utilized for the healing of skin wounds. In in vivo studies with second-degree burns, healing has been achieved in at least 80% of the cases between the ninth and twelfth day of treatment with chitosan coatings. The crucial steps in the treatment of severe burns are the early excision of damaged tissue and adequate coverage to minimize the risk of infection. So far, partial-thickness autografting is considered the gold standard for the treatment of full-thickness burns. However, the limitations of donor sites have led to the development of skin substitutes. Therefore, the need for an appropriate dermal equivalent that functions as a regeneration template for the growth and deposition of new skin tissue has been recognized. This review describes the properties of chitosan that validate its potential in the treatment of skin burns.
1 Introduction
The increase of the world population has reached the generation of waste in an excessive way. Specifically, in the marine-food industry, by-products and wastes have been generated over the years. Head, scales, shell, bones that increase excessively and affect not only the environment and biodiversity, but also humans. Marine wastes are regularly burned, thrown into landfills or back to the ocean, negatively affecting coastal areas with an unpleasant odor and bacterial contamination during decomposition (1).
Various studies on the elaboration of biological materials for their application in different areas have directed their attention to renewable resources. Chitin is an abundant biopolymer mainly found in marine ecosystems. It is a natural amino-polysaccharide consisting of a linear chain of repeated monomer units of d-glucosamine and N-acetyl-glucosamine, whose content and sequence are variable (2).
Chitosan is obtained through partial deacetylation of chitin by thermo-alkaline hydrolysis of chitin (3), biological-enzymatic (4), and physical methods (5). Chitosan extracted from shellfish residues of crustaceans has gained powerful attention for its potential applications in the mining industry, water treatment and soil leachate (6), the food industry (7), agriculture (8), the cosmetic industry (9), and the textile industry (10). The amino groups allow specific chemical reactions and give important functional properties to chitosan (11), which are responsible for all of its different applications.
In recent years, biodegradable polymers have been recommended for the preparation of coatings for wounds based on their biological properties such as biocompatibility, non irritant and antimicrobial properties, among others (12). Biodegradable polymers, in general, are attractive for tissue engineering. Natural gum polysaccharides (13), bovine type I collagen (14), tilapia skin collagen for the pediatric treatment of burns (15) have reported favorable results for the biomedical sector. Spider silk is also a biomaterial recognized for its outstanding mechanical and biocompatibility properties for potential medical applications (16).
In the medical field, tissue engineering has been achieved with the production of advanced bandages for the treatment of acute and chronic wounds, which act in a timely and cost-effective manner. Among these materials are hydrogels, hydrocolloids, gauzes, foams, and fibrous dressings such as alginates and occlusive synthetic materials (17). Coatings for wounds provide a permeable barrier for moisture and oxygen and prevent colonization and proliferation of microorganisms in the wound (18).
Wounds are classified as acute and chronic as a result of a thermal, physical, or chemical injury. Wound healing takes place in five stages: hemostasis, inflammation, migration, proliferation, remodeling, and maturation. The healing periods for acute and chronic wounds are 8–12 days, respectively (19).
The main objective of burn treatment is to improve the healing process including proliferation, granulation, epithelialization, and collagenation (20); from this perspective, the modern concept of wound healing is based on establishing an optimal environment with adequate humidity around the wound, effective circulation of oxygen to favor cell division and tissue regeneration, and a low bacterial density that allows normal movement of epithelial cells, thus accelerating the rehabilitation phase and wound healing (21).
The proper choice of a topical coating to restore the balance of moisture in a wound depends on factors such as the amount of exudate, the anatomic location of the wound, the condition of the surrounding skin, whether a full recovery is expected or not, also should form a rapid film, show a broad antibacterial spectrum and strong antibacterial activity, and be cost effective (22). This study presents a detailed literary review of chitosan as a biopolymer with regenerative capacity in burn injuries, including clinical cases that confirm its therapeutic value.
2 Chitosan
Chitosan is a promising biopolymer in many different areas of science. Structurally, it is an amino-polysaccharide consisting of a linear chain of two repeating monomers of d-glucosamine (deacetylated units) and N-acetyl-d-glucosamine (acetylated units) linked by β-(1,4) glycosidic bonds (3). Chitosan (Figure 1) is composed of less than 20% of β-(1,4)-2-acetamido-d-glucopyranose and more than 80% of β-(1,4)-2-amino-d-glucopyranose (23). It is obtained from the alkaline hydrolysis of chitin, one of the most abundant natural amino-polysaccharides extracted from the exoskeleton of crustaceans and insects (24).
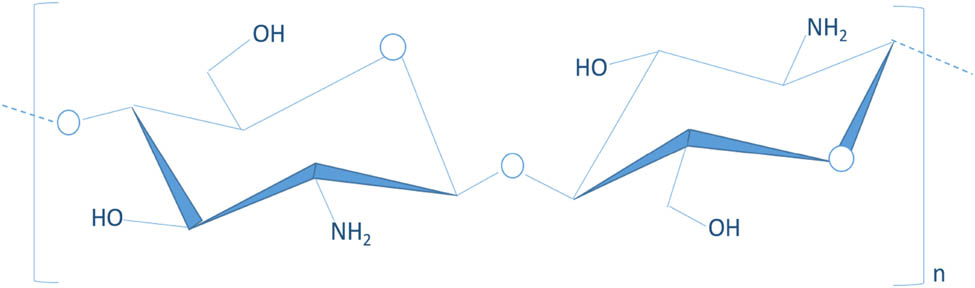
Chemical structure of chitosan.
2.1 Functional properties
The deacetylated form of chitosan in acidic solutions can be easily processed as powder, paste, gel, membranes, sponges, nanofibers, beads, microparticles, nanoparticles, and nanofibers (25).
The properties of chitosan depend on the range of deacetylation and the distribution of the acetyl groups along its main chain (26). In addition, this biopolymer only shows antimicrobial activity in acid media and, in this condition, achieves its highest solubility (2).
Chitosan offers distinct advantages that provide safe degradation products with no inflammatory effects; therefore, its exceptional properties, such as polycationic, nontoxic biocompatibility, biodegradability, bioadhesivity, antigenic capacity, antitumor, bioresorbable, and hemostasis (27,28), have led to its broad spectrum of biomedical applications in the pharmaceutical industry, especially in the elaboration of controlled release of drugs/enzymes/vaccine, tissue engineering, wound healing, blood anticoagulant, bone regeneration, as well as biosensing/biomembrane manufacturing among others (29). Since the 1980s, chitosan and its derivatives have been used in Japanese products for the management of skin wounds (30).
Chemical properties of chitosan can be highly variable, depending on the source of obtaining, the method of deacetylation of chitin, molecular weight, viscosity, among others, and in turn impact on the functional properties of chitosan so that they can be manipulated according to its purpose. However, in the management of burn wounds, chitosan has been shown to have little or poor mechanical and handling properties. This is an important factor because the healing material that is used to counteract the loss of moisture by burning must be able to handle, provide moisture, absorb exudates, and promote healing.
For this reason, research has focused on finding coadjuvants that are compatible with chitosan to support its weaknesses and that can maximize or enhance the skin restoration effect (31,32). New formulations have been reported to adapt these biocomposites to the needs of the injury. Some studies like Alizadeh et al. (33) studied the interaction of gelatin–chitosan for the formulation of sponges as hemostatic agents. Wang et al. (34) studied hydrogel sheets based on chitosan with honey and gelatin as a cover for burn wounds. Zamora-Mora et al. (35) reported the preparation of chitosan–agarose hydrogels for their application in tissue engineering. Kang et al. (36) developed chitosan biocomposites treated with sodium hydroxide and sodium tripolyphosphate for hemostatic use. Ong et al. (37) prepared biocomposites based on chitosan incorporating polyphosphate as a precoagulant and silver as antimicrobial, both to accelerate blood coagulation, platelet adhesion, and absorb significantly more blood than pure chitosan composites. Nanomaterials based on chitosan show physical and chemical properties superior to those of pure chitosan. These new materials have a large surface area, have porosity, and have better resistance to deformation (38). Another advantage of chitosan in the treatment of burns is its ability to permeate oxygen for all tissues (39).
The molecular weight of chitosan is probably the most important property. Researchers have determined that using low molecular weight chitosan, even chitosan oligomers and monomers accelerate wound healing (40). Chitosan materials have been used to evaluate the acceleration of wound healing, and have been applied in different shapes such as powders, sponges, and granules. However, at the same time, cotton or polyester composites are used, and therefore the contact area between chitosan and injury site is too low to be able to estimate the effect of chitosan in accelerating wound healing.
2.2 Healing ability
Wound healing is a specific biological process related to the phenomenon of tissue growth and regeneration. Healing depends on several factors, including the person’s medical condition, and the process progresses through a series of interdependent and overlapping stages, which involve biochemical complex and cellular processes. These stages include hemostasis, inflammation, proliferation, and remodeling (41).
In several studies, it has been reported that the use of chitosan accelerates the healing process and benefits each stage involved in the healing process. The hemostatic properties of chitosan are directly due to the electrostatic interactions between the negative charges of the erythrocyte cell membrane and the positively charged chitosan (42). Clinically, when the chitosan materials are in contact with a wound, they adhere to cover the site of the injury by attracting red blood cells, forming a seal that prevents future hemorrhages (2).
In addition, chitosan is a promoter of tissue granulation by increasing the expression of collagen, among other components of the extracellular matrix, and accelerating wound healing (32). It also acts to improve the functions of proliferation and migration of inflammatory cells such as polymorphonuclear leukocytes, macrophages, and fibroblasts at the site of injury (Figure 2) (43).
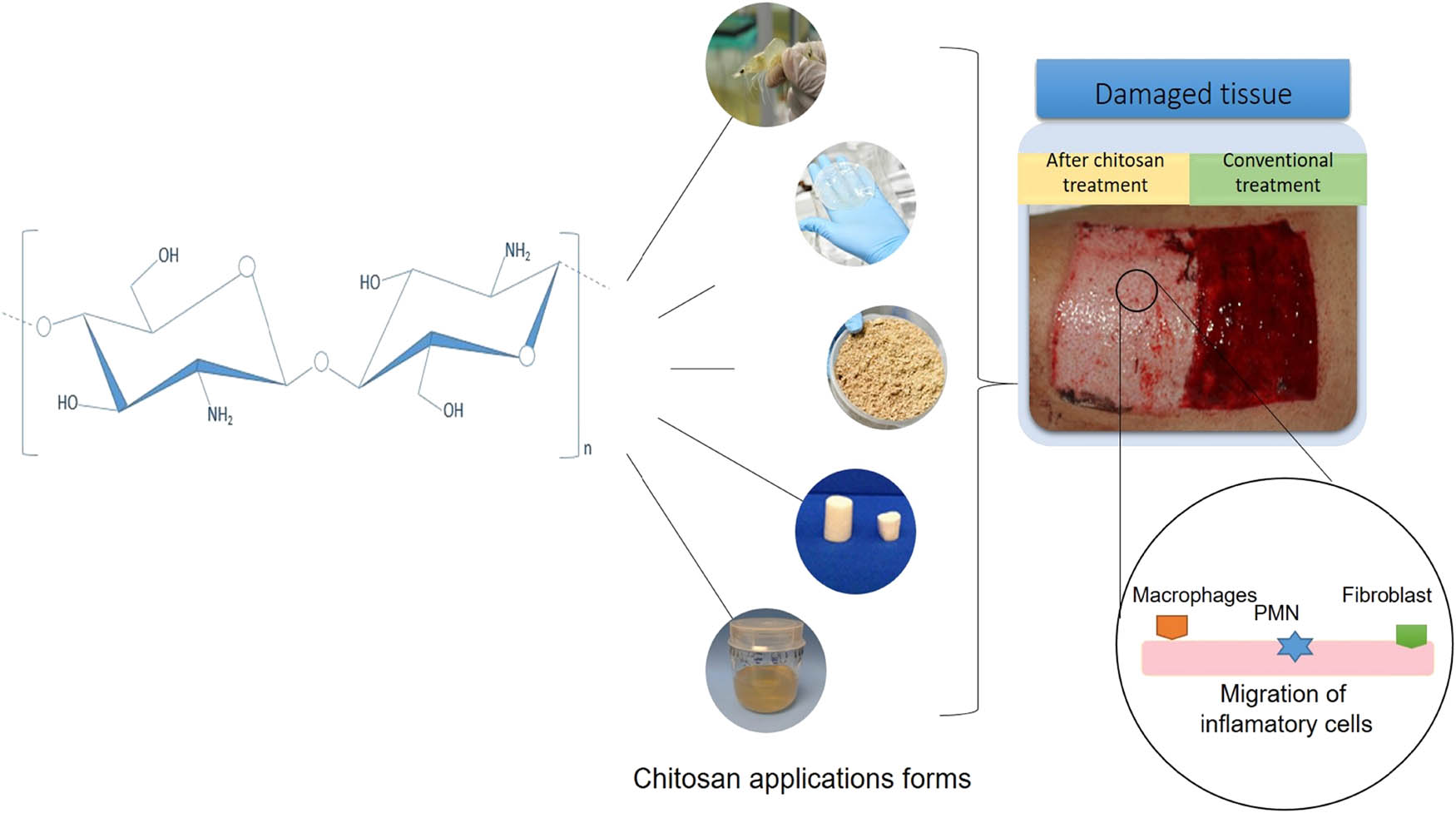
Graphic representation of the application of chitosan in wound healing.
Chitosan’s biodegradable effect is attributed to lysozyme, an enzyme that is found in the human body and in various plants (25,38). It is known as a glycoside hydrolase that possesses the ability to slowly hydrolyze β-(1–4) bonds between N-acetylmuramic acid and N-acetyl-d-glucosamine residues or between N-acetyl-d-glucosamine residues of chitosan membranes (2). The degradation by lysozyme allows the reabsorption of the coating and the release of N-acetyl glucosamine among other oligomers, which activate the macrophages and are incorporated into the extracellular matrix for the reconstruction of normal physiological tissues (44).
2.3 Antimicrobial activity
An antimicrobial is an agent that eliminates microorganisms or inhibits their growth (25). Despite great progress in the management of burn scars, infections are the greatest challenge causing morbidity and mortality. Candida spp. it is one of the microorganisms that often colonize burn scars causing persistent infections, loss of skin graft, and delaying the healing process (45).
For an antimicrobial polymer to be ideal, it must be economically synthesized, stable in the long term, soluble in water or neutral medium, must not be decomposed into toxic products or emit them, and must have bactericidal activity against pathogens in short contact times (46). Chitosan has been shown to have advantages over other antimicrobials because it has a high antimicrobial activity, a broad spectrum of activity, and a higher mortality rate (47).
Chitosan is a potent antimicrobial agent of cationic nature at a pH lower than 6.3 (48). Hypotheses indicate that chitosan could interact with anionic groups on the cell surface of microorganisms increasing the permeability of membranes, facilitating the leakage of proteins and other intracellular constituents of microorganisms (43). Another mechanism involves the formation of chitosan chelates with trace elements or nutrients resulting in the inhibition of enzymatic activity (49) due to the chitosan–DNA interaction that alters the synthesis of messenger RNA (50). The antimicrobial effects are regulated by intrinsic factors that include the type of chitosan, the degree of polymerization of the chitosan, the source, and the chemical composition of the substrates.
Malinowska-Panczyk et al. (51) studied the antimicrobial properties of chitosan films of different degrees of deacetylation (DD) and molecular weight against Gram-positive and Gram-negative bacteria. With this, they demonstrated that the stage of inactivation of the bacteria increases with the increase in the DD of the biopolymer; however, the mechanism of bacteriostatic and bactericidal actions is not fully known.
Some studies on animals have been carried out using chitosan, proving that this product is superior to others in the elimination of infection and in the promotion of new tissue. However, few clinical cases have been used chitosan in the treatment of skin lesions such as ulcers (52).
The chitosan–gelatin composites prepared by Pereda et al. (53) have a inhibitory activity against Gram-positive and Gram-negative microorganisms. Ahmed and Ikram (25) reported that the biocomposites with more than 0.025% chitosan inhibit the growth of Escherichia coli, Fusarium, Alternaria, and Helminthosporium. Wang et al. (34) evaluated the antimicrobial activity of chitosan, honey, and gelatin hydrogels as possible coatings for the healing of burn wounds and reported that the hydrogels have an action against Staphylococcus aureus and E. coli. Liu et al. (48) added polyvinyl alcohol (PVA) to chitosan solutions for the production of nanofibers with multiple applications and reported bacteriostatic activity against E. coli. According to research conducted by Anisha et al. (54), the application of chitosan sponges for the treatment of diabetic foot ulcers prevents polymicrobial infection and decreases the risk of amputation.
3 In vitro studies
The safety and effectiveness of medical devices made of resorbable biomaterials depend to a large extent on their complete biocompatibility (55). That is, the tissues and the human body fully accept the implantable material and do not cause a massive immune response as a result of a threatening material.
Hemocompatibility studies have established that the lower the value of the hemolysis ratio, the better the compatibility of the biomaterial with the blood. Some authors have reported that a value of up to 5% hemolysis is permissible for biomaterials. In vitro studies with nanomaterials based on chitosan, hemolysis values of 1.14% have been reported, this after 60 min of contact of the material with the blood (56).
Chen et al. (57) investigated the hemostatic mechanism of biomaterials, through the reaction with erythrocytes, comparing conventional medical gauzes, polyurethane sponges, absorbent dressings of chitosan, and the aqueous solution of chitosan. In these experimental studies, changes were observed in the shape of the erythrocytes on contact with conventional gauze and polyurethane sponges, attributed to the morphological changes of the red blood cells by external stimuli and no aggregation was observed. While in the porous sponges of chitosan, the erythrocytes showed deformation and their incorporation on the surface of the sponges, as a result of a hemostatic reaction. The agglutination of blood cells by the action of chitosan can be the result of the interaction of the positive charges of the polymer with the receptors on the cell surface of erythrocytes.
Alizadeh et al. (33) investigated the in vitro biodegradation of chitosan–gelatin dressings with lysozyme and observed that the destruction of the structure was 28% at day 8 at 37°C. Additionally, Arpornmaeklong et al. (58) evaluated enzyme activity in lyophilized hydrogels of chitosan–collagen. These dressings exhibited a dynamic degradation from day 14. This was attributed to the hydrophobicity of the sponges that causes the biodegradation and excision of the bonds after the swelling of the matrix.
The epidermal growth factor (EGF) is a mitogenic peptide, involved in the regulation of physiological processes such as growth, cell proliferation, regeneration, and wound repair. It also stimulates messenger RNA, DNA, and protein synthesis in many cell types and has been shown to stimulate keratinocyte division in vitro and epidermal regeneration in vivo (59).
Ceren et al. (60) determined the in vitro release of EGF from the chitosan gel formulation to be 97.3% at a pH of 5.8 in a period of 24 h. However, the release kinetics was of first order. This result indicates that the rate of EGF release from the gel is variable with time.
Yenilmez et al. (59) evaluated the stability of chitosan by changes in pH for 6 months. It was determined that the chitosan solution of lower concentration remained unchanged in its pH. Later, egg yolk oil and EGF were added to the stable formulation for future in vivo studies.
Because of low molecular weight chitosan, even chitosan oligomers and monomers accelerate wound healing and have better antimicrobial properties. Recent research studies new formulations mixing chitosan with curcumin nanoparticles and using microwave technology to reduce molecular weight and increase the DD. Curcumin is a drug with powerful antioxidant and antibacterial attributes that promote tissue regeneration. Hafiz et al. (61) determined that chitosan of low molecular weight, low viscosity, smaller particle size, and higher surface charge helps to produce smaller nanoparticles with a more efficient penetration at the site of the injury and therefore releases the drug more effectively.
Other investigations on the incorporation of antibiotic nanoparticles, such as gentamicin to chitosan, showed that the release of the drug is controlled by the diffusion and degradation of the polymeric matrix (62).
4 Studies in animal models
Burns can be classified according to their degree of severity as wounds with loss of tissue and wounds without loss of tissue. For this cause, few studies have reported the effectiveness of chitosan alone, as an antimicrobial and healing agent in its different forms. Bayat et al. (20) performed the application of 2% (w/v) chitosan nanofibers on second-degree burns in rats. The nanofibers were changed every 2 days, and biopsies were taken within 21 days. On day 7, granulation tissue formation was observed, including fibroblast proliferation and angiogenesis. The results showed almost complete regeneration of skin and hair at 21 days.
Nacer Khodja et al. (63) evaluated the healing activity of chitosan coatings in second-degree burns in rats. The coatings were replaced every 2 days in the inflammatory phase, every 3–4 days in the proliferative phase, and every 4–7 days during the maturation phase. The authors observed that 90% of the healing of the burn was achieved between days 9 and 12. Similar results were reported by Sung et al. (64) when applying chitosan dressings loaded with antibiotics, observing that the size of the induced wound decreases approximately 90% at day 15.
To assess the healing efficiency in female pigs, chitosan hydrogel was applied as a coating in third-degree burns, and the dermal–epidermal reconstruction and re-epithelialization of the affected area were observed without irritations or harmful effects. After 10 months of skin involvement, it was observed that the quality of healing, in terms of thickness, was better with chitosan hydrogels than with commercial gauze (65).
Honardar et al. (66) studied the speed and effectiveness of the healing of second-degree burns in rabbits by applying chitosan gel. They confirmed the acceleration of healing microscopically on the 20th day, as a consequence of the proliferation of epidermal cells with complete epithelialization of the affected area.
Histological observations of the effect of local application of chitosan and heparin on partial depth burns in adult rats indicate that burns are less severe than control wounds (67). This is because the mixture of chitosan and heparin inhibits the inflammatory reaction.
Pereira et al. (68) developed a gel of microparticles based on chitosan added with Aloe vera and vitamin E for the treatment of burns. This material has mucoadhesive properties influenced by chitosan, which favored re-epithelialization after 14 days of treatment in mice.
Dantas et al. (31) proposed to combine sodium alginate with chitosan for the production of films with the aim of improving cutaneous healing by burns in rats. They concluded that the combination of low-level laser therapy with chitosan and alginate-based films improves healing, specifically with respect to epithelialization and vascularization.
Wang et al. (34) used chitosan hydrogels with gelatin and honey as coatings for second-degree burns in rabbits. The mixture of chitosan and honey showed a positive synergism in terms of antibacterial activity. This fulfills the primary objective of the treatment of burns, which implies preventing infection and acting as an effective promoter of healing.
Bano et al. (12) analyzed second-degree burns on rabbits, using chitosan-PVA membranes, and observed initial phases of peripheral inflammation in the first 3 days of experimentation; chitosan membranes showed a significant healing effect over a period of 15 days.
Ceren et al. (60) determined that the efficient release of growth factors that accelerate burn healing should be considered. Chitosan was proposed as a polymer for controlled release. The results were evaluated immunohistochemically on the 7th day of therapy; the maximum cell proliferation was found in the application of the gel formulation with EGF.
Yenilmez et al. (59) measured burn wound healing contraction over a 21 day period. The researchers used the mixture of chitosan-egg yolk oil and EGF. The formulation showed significant shrinkage compared to commercial Silverdin® products and control groups (no treatment). The most dramatic changes were evidenced on day 21, epithelialization was mostly completed, and even the formation of a new vein and collagen fibers between layers was detected. Table 1 summarizes the main results reported in the in vivo studies.
Summary of in vivo studies and main findings
| Year | Characteristics of chitosan | Chitosan blend | Animal model | Main results | References |
|---|---|---|---|---|---|
| 2021 | ND | Carboxymethyl chitosan, hyaluronic acid, and silver | Burns on rats | Significative wound contraction, granulation tissue formation, inflammatory infiltration, and collagen fibers deposit | (69) |
| 2019 | DD 98%, MW 15 kDa | Glycerol-plasticized chitosan and PVA membranes | Burns on rabbits | Phase of peripheral inflammation in 3 days. Significant healing effect over a period of 15 days | (12) |
| 2019 | Low molecular weight chitosan | Chitosan and bromelain nanofibers | Burns on rats | Granulation tissue on day 7. Complete regeneration of skin and hair on day 21 | (20) |
| 2016 | ND | Chitosan-based topical gel | Burns on rabbits | Acceleration of healing microscopically on day 20 | (66) |
| 2015 | Highly viscous | Chitosan, egg yolk oil, and EGF | Burns on rats | Dramatic changes in wound contraction on day 21 day | (59) |
| 2014 | Low molecular weight chitosan | Aloe vera, chitosan, and vitamin E | Burns on rats | The material has mucoadhesive properties influenced by chitosan. Re-epithelialization after 14 days | (68) |
| 2013 | DD 70%, MW 471 kDa | Chitosan and PVA | Burns on rats | 90% of the healing was achieved between days 9 and 12 | (63) |
| 2011 | ND | Sodium alginate and chitosan films | Burns on rats | Low level laser therapy with chitosan and alginate improves epithelialization and vascularization | (31) |
| 2010 | DD >75%, viscosity 800–2,000 cps | Minocycline-loaded dressings with PVA and chitosan | Burns on rats | Wound decreases approximately 90% on day 15 | (64) |
| 2007 | DA 2.6%, MW 540,000 g·mol−1 | Chitosan hydrogels | Wounds on female pigs backs | Quality of healing in terms of thickness was better with chitosan hydrogels than with commercial gauze | (65) |
| 2007 | DD >90%, MW 500,000 g·mol−1 | Chitosan and heparin | Burns on rats | Mixture of chitosan and heparin inhibit the inflammatory reaction | (67) |
| 2006 | MW 650,000 g·mol−1, viscosity 7,903 mPa at 25°C | Chitosan with EGF | Burns on rats | Treatment with EGF and chitosan gel decreased the wound healing period | (60) |
DA, degree of acetylation; DD, degree of deacetylation; MW, molecular weight; and ND, not determined.
5 Clinical cases
The data on the toxicity of chitosan in human studies are very limited. Hamedi et al. (70) summarize trials conducted in humans in recent years. It was reported that quantities above 4.5 g of chitosan taken daily did not cause toxic effects, and other clinical trials performed for more than 12 weeks, showed no significant clinical symptoms and no allergic response.
Escárcega-Galaz et al. (52) reported a clinical case where eight patients received treatment in wounds and ulcerative lesions associated with diabetes mellitus located in the lower limbs, reported that at the end of the treatment period only one of the patients experienced complete recovery, while the other patients experienced significant progress in their injuries.
Carles et al. (71) presented four cases of the use of a commercial product of chitosan (Celox®) to stop potentially fatal obstetric hemorrhage for the patient: two cases applied gauzes with chitosan and two applied the chitosan powder product, and the results indicated that the chitosan quickly stopped the bleeding, constituting a new economic alternative and that may be available in developing countries.
Some chitosan products available on the market are used for hemostatic use, among which are HemCon Dental Dressing (HemCon Medical Technologies, Inc, Beaverton, OR), ChitoSAM™ 100 is a 100% chitosan dressing, Celox™ (72) sells chitosan products (OMNI-STAT™) for topical application in hospital procedures and in the emergency room, the clinical trials performed showed significant time reduction to achieve hemostasis. Axiostat®,- brand has products based on clinically validated patents of Axio clotting technology, recommended to stop arterial hemorrhages and during vascular procedures.
Those individuals who have suffered from severe skin loss from burns are at higher risk of dying from fluid loss, as well as massive infections. The application of chitosan biomaterials for the healing of various burns has been reported in recent clinical studies. Table 2 describes the clinical trials where chitosan-based dressings were applied as a coating for wounds. In all cases, uniform adherence to the wound is reported, which is a requirement for a successful biomaterial. Another requirement is the promotion of hemostasis, healing, and rapid re-epithelialization of the affected area, transforming it into new healthy and aesthetically acceptable tissue.
Summary of clinical trials using chitosan in the treatment of chronic wounds
| Year | Coatings | No. of patients | Wound type | Main results | References |
|---|---|---|---|---|---|
| 2020 | Topical gel containing chitosan | 36 | Incision for lower third molar tooth extraction | • 2 days after surgical removal, patients evaluated wound healing on a scale of good, acceptable, and bad | (73) |
| • Chitosan gel was considered good in 41.7 and 0% on the placebo treatment | |||||
| 2020 | Chitosan-based chewing gum | 36 | Reduction of salivary Streptococcus mutans counts | • Chitosan chewing gum has a positive effect on the reduction of numbers of salivary S. mutans colonies | (74) |
| 2019 | Chitosan dressings | 10 | Cutaneous lesions | • Patients showed significant (30%) or complete (70%) improvement after 8 weeks of therapy. At 16 weeks posttreatment, all cases were completely cured | (75) |
| • It was well tolerated, and there were no product-related adverse events such as allergic reaction or infection | |||||
| 2019 | Chitosan brush | 11 | Dental implants with peri-implant mucositis | • Implants treated with the chitosan brush had a better improvement in bleeding on probing at 2 and 4 weeks compared with titanium curettes | (76) |
| 2018 | Chitosan dressings | 277 | Lower third molar extraction | • Better radiographic findings at second week. Improved bone formation | (43) |
| 2018 | Chitosan films | 244 | Superficial wounds | • Showed less exudate and less odor | (77) |
| • Ease removal, no edema, or localized warmth was observed | |||||
| 2017 | Chitosan dressings with silver | 10 | Partial thickness pediatric burns | • No infection | (42) |
| • Full healing in 8.3 days | |||||
| • The coating forms a gel to moisten with saline maintaining its original size | |||||
| • The lack of contraction of the coating provides a high degree of patient comfort | |||||
| 2008 | Chitosan films with oleic acid | 2 | Tissue donor area burn type AB/B | • Complete epithelialization on day 12 | (39) |
| • Complete epithelialization at day 15, with good appearance and functionality of the scar | |||||
| • Uniform adhesion of the film to the wound | |||||
| • Healthy, clean, and bright pink area on the 10th day | |||||
| 2004 | Chitosan films | 20 | Tissue donor area | • Pain assessment less than 2 | (30) |
| • Abundant formation of inflammatory cells, fibroblasts, and keratinocytes | |||||
| • Re-epithelialization faster than the control areas | |||||
| 2000 | Chitosan dressings | 20 | Tissue donor area | • Healing in 10.4 days | (78) |
| • Biopsies showed small nerve fibers and flexible connective tissue in the papillary dermis | |||||
| 1991 | Carboxymethyl chitosan dressings | 4 | Tissue donor area | • Better histological order and vascularization | (79) |
| • Absence of inflammatory cells |
6 Conclusions
Burn trauma is one of the most difficult challenges that patients can present; as a consequence, it is common to observe infections, wounds, scars, and psychological damage. In this review, it has been highlighted recent research that shows the biological and antimicrobial properties of chitosan favor the restoration of cutaneous tissues and benefit human health. In addition, clinical trials are shown regarding the treatment of burns and donor areas with films or coatings based on chitosan. Although there are few clinical cases reported to date on the application of chitosan in burns, the results have indicated that the therapeutic application of chitosan is effective for skin regeneration, constituting an effective alternative treatment especially in developing countries.
Acknowledgements
The corresponding author is grateful to CONACYT (408169).
-
Funding information: This research was financed under Project No. 248160 from CONACYT-PN2014 and by Project PROFAPI No. 2020-0105 from Instituto Tecnológico de Sonora.
-
Author contributions: Dalia I. Sánchez-Machado: supervision – oversight and leadership responsibility for the research activity, funding acquisition – acquisition of the financial support for the project leading to this publication, validation – verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs; Diana M. Martínez-Ibarra: writing – original draft, writing – review and editing, preparation, creation, and/or presentation of the published work by those from the original research group, specifically critical review, commentary or revision – including prepublication or postpublication stages; Jaime López-Cervantes: supervision – oversight and leadership responsibility for the research activity; Ana A. Escárcega-Galaz: investigation – conducting a research and investigation process; and Claudia A. Vega-Cázarez: investigation – conducting a research and investigation process.
-
Conflict of interest: Authors state no conflict of interest.
References
(1) Balitaan JN, Yeh JM, Santiago KS. Marine waste to a functional biomaterial: green facile synthesis of modified-β-chitin from Uroteuthis duvauceli pens (gladius). Int J Biol Macromol. 2019;154:1565–75. 10.1016/j.ijbiomac.2019.11.041.Suche in Google Scholar
(2) Martínez-Ibarra DM, López-Cervantes J, Sánchez-Machado DI, Sanches-Silva A. Chitosan and xyloglucan-based hydrogels: an overview of synthetic and functional utility. In: Dongre R, editor. Chitin-Chitosan – Myriad Functionalities in Science and Technology. London: Rajendra Dongre, IntechOpen; 2018a. p. 183–218. 10.5772/intechopen.74646.Suche in Google Scholar
(3) Martínez-Ibarra DM, Sánchez-Machado DI, López CJ, Campas-Baypoli ON, Sanches-Silva A, Madera-Santana TJ. Hydrogel wound dressings based on chitosan and xyloglucan: development and characterization. J Appl Polym Sci. 2018b;136:47342. 10.1002/app.47342.Suche in Google Scholar
(4) Aguila E, Gomes L, Andrade C, Silva J, Paschoalin V. Biocatalytic production of chitosan polymers from shrimp shells, using a recombinant enzyme produced by Pichia pastoris. Am J Mol Biol. 2012;2:341–50. 10.4236/ajmb.2012.24035.Suche in Google Scholar
(5) Duarte M, Ferreira M, Marvão M, Rocha J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int J Biol Macromolecules. 2002;31:1–8. 10.1016/S0141-8130(02)00039-9.Suche in Google Scholar
(6) Hwang JH, Pathank P, Wang X, Rodriguez KL, Park J, Cho HJ, et al. A novel Fe-chitosan-coated carbon electrode sensor for in situ As(iii) detection in mining wastewater and soil leachate. Sens Actuators B Chem. 2019;294:89–97. 10.1016/j.snb.2019.05.044.Suche in Google Scholar
(7) Yuan G, Chen X, Li D. Chitosan films and coatings containing essential oils: the antioxidant and antimicrobial activity, and application in food systems. Food Res Int. 2016;89:117–28. 10.1016/j.foodres.2016.10.004.Suche in Google Scholar PubMed
(8) Malerba M, Cerana R. Chitosan Effects on Plant Systems. Int J Mol Sci. 2016;17:996. 10.3390/ijms17070996.Suche in Google Scholar PubMed PubMed Central
(9) Sakulwech S, Lourith N, Ruktannochai U, Kanlayavattanakul M. Preparation and characterization of nanoparticles from quaternized cyclodextrin-grafted chitosan associated with hyaluronic acid for cosmetics. Asian J Pharm Sci. 2018;13:498–504. 10.1016/j.ajps.2018.05.006.Suche in Google Scholar PubMed PubMed Central
(10) Islam SU, Butola BS. Recent advances in chitosan polysaccharide and its derivatives in antimicrobial modification of textile materials. Int J Biol Macromol. 2019;121:905–12. 10.1016/j.ijbiomac.2018.10.102.Suche in Google Scholar PubMed
(11) Guan Y, Qi MX, Chen GG, Peng F, Sun CR. Facile approach to prepare drug-loading film from hemicelluloses and chitosan. Carbohyd polym. 2016;153:542–8. 10.1016/j.carbpol.2016.08.008.Suche in Google Scholar PubMed
(12) Bano I, Muhammad A, Yasin A, Afzal MG. Preparation, characterization and evaluation of glycerol plasticized chitosan/PVA blends for burn wounds. Biomac. 2019;124:155–62. 10.1016/j.ijbiomac.2018.11.073.Suche in Google Scholar PubMed
(13) Aruna K, Prabhakar O. Natural gum polysaccharides as efficient tissue engineering and drug delivery biopolymers. J Drug Deliv Sci Technol. 2021;63:102431. 10.1016/j.jddst.2021.102431.Suche in Google Scholar
(14) Brum I, Elias C, Carvalho J, Pires J, Pereira M, Biasi R. Properties of a bovine collagen type I membrane for guided bone regeneration applications. E-Polymers. 2021;21:210–21. 10.1515/epoly-2021-0021.Suche in Google Scholar
(15) Lima Júnior EM, Moraes Filho MO, Forte AJ, Costa BA, Fechine FV, Alves A, et al. Pediatric Burn treatment using tilapia skin as a xenograft for superficial-partial thickness wounds: a pilot study. J Burn Care Res. 2020;19:241–7. 10.1093/jbcr/irz149.Suche in Google Scholar PubMed
(16) Yunqing G, Lingzhi Y, Jiegang M, Denghao W, Peijian Z, Maosen X. Mechanical propierties and application analysis of spider silk bionic material. E-polymers. 2020;20:443–57. 10.1515/epoly-2020-0049.Suche in Google Scholar
(17) Mo X, Cen J, Gibson E, Wang R, Percival S. An open multicenter comparative randomized clinical study on chitosan. Wound Rep Reg. 2015;23:518–24. 10.1111/wrr.12298.Suche in Google Scholar PubMed
(18) Mogoşanu GD, Grumezescu AM. Natural and synthetic polymers for wounds and burns dressing. Int J Pharm. 2014;463:127–36. 10.1016/j.ijpharm.2013.12.015.Suche in Google Scholar PubMed
(19) Alavi M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. E-Polymers. 2019;19:103–19. 10.1515/epoly-2019-0013.Suche in Google Scholar
(20) Bayat S, Amiri N, Pishavar E, Kalalinia F, Movaffagh J, Hahsemi M. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019;229:57–66. 10.1016/j.lfs.2019.05.028.Suche in Google Scholar PubMed
(21) Bagheri MK, Alipoor E, Vaghardoost R, Saberi IM, Yaseri M, Djafarian K, et al. The effect of a hydrolyzed collagen-based supplement on wound healing in patients with burn: a randomized double-blind pilot clinical trial. Burns. 2019;46(1):156–63. 10.1016/j.burns.2019.02.015.Suche in Google Scholar PubMed
(22) Cheng Y, Hu Z, Zhao Y, Zou Z, Lu S, Zhang B, et al. Sponges of carboxymethyl chitosan grafted with collagen peptides for wound healing. Int J Mol Sci. 2019;20:3890. 10.3390/ijms20163890.Suche in Google Scholar PubMed PubMed Central
(23) Lopes PP, Tanabe EH, Bertuol DA. Chitosan as biomaterial in drug delivery and tissue engineering. In Gopi S, Thomas S, Pius A, editors. Handbook of chitin and chitosan. Santa Maria, RS, Brazil: Elsevier; 2020. p. 407–31. 10.1016/B978-0-12-817966-6.00013-3.Suche in Google Scholar
(24) Pella CG, Lima-Tenório MK, Tenorio-Neto ET, Guilherme MR, Muniz EC, Rubira AF. Chitosan-based hydrogel: from preparation to Biomedical applications. Carbohyd Polym. 2018;196:233–45. 10.1016/j.carbpol.2018.05.033.Suche in Google Scholar PubMed
(25) Ahmed S, Ikram S. Chitosan based scaffolds and their applications in wound healing. Achiev Life Sci. 2016;10:27–37. 10.1016/j.als.2016.04.001.Suche in Google Scholar
(26) Rinaudo M. Chitin and chitosan: properties and applications. Prog Polym Sci. 2006;31:603–32. 10.1016/j.progpolymsci.2006.06.001.Suche in Google Scholar
(27) Feng Y, Kopplin G, Kimihiko S, Draget KI, Vårum KM. Alginate gels with a combination of calcium and chitosan oligomer mixtures as crosslinkers. Carbohydr Polym. 2016;156:490–7. 10.1016/j.carbpol.2016.09.006.Suche in Google Scholar PubMed
(28) Pinheiro AC, Bourbon AI, Cerqueira MA, Maricato E, Nunes C, Coimbra MA, et al. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr Polym. 2015;115:1–9. 10.1016/j.carbpol.2014.07.016.Suche in Google Scholar PubMed
(29) Shariatinia Z, Jalali AM. Chitosan-based hydrogels: preparation, properties and applications. Int J Biol Macromol. 2018;115:194–220. 10.1016/j.ijbiomac.2018.04.034.Suche in Google Scholar PubMed
(30) Azad KA, Sermsintham N, Chandrkrachang S, Stevens FW. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res. 2004;69B(2):216–22. 10.1002/jbm.b.30000.Suche in Google Scholar PubMed
(31) Dantas MD, Cavalcante DR, Araújo FE, Barretto SR, Aciole GT, Pinheiro AL, et al. Improvement of dermal burn healing by combining sodium alginate/chitosan-based films and low level laser therapy. J Photochem Photobiol B. 2011;105:51–9. 10.1016/j.jphotobiol.2011.06.009 Suche in Google Scholar PubMed
(32) Sohrabi S, Haeri A, Mahboubi A, Mortazavi A, Dadashzadeh S. Chitosan gel-embedded moxifloxacin niosomes: an efficient antimicrobial hybrid system for burn infection. Int J Biol Macromol. 2016;85:625–33. 10.1016/j.ijbiomac.2016.01.013.Suche in Google Scholar PubMed
(33) Alizadeh M, Abbasi F, Khoshfetrat AB, Ghaleh H. Microstructure and characteristic properties of gelatin/chitosan scaffold prepared by a combined freeze-drying/leaching method. Mater Sci Eng C. 2013;33:3958–67. 10.1016/j.msec.2013.05.039.Suche in Google Scholar PubMed
(34) Wang T, Zhu XK, Xue XT, Wu DY. Hydrogel sheets of chitosan, honey and gelatin as burn wound dressings. Carbohydr Polym. 2012;88:75–83. 10.1016/j.carbpol.2011.11.069.Suche in Google Scholar
(35) Zamora-Mora VZ, Velasco D, Hernández R, Mijangos C, Kumacheva E. Chitosan/agarose hydrogels: cooperative properties and microfluidic preparation. Carbohydr Polym. 2014;111:348–55. 10.1016/j.carbpol.2014.04.087.Suche in Google Scholar PubMed
(36) Kang PL, Chang SJ, Manousakas I, Lee CW, Yao CH, Lin FH, et al. Development and assessment of hemostasis chitosan dressings. Carbohydr Polym. 2011;85:565–70. 10.1016/j.carbpol.2011.03.015.Suche in Google Scholar
(37) Ong SY, Wu J, Moochhala SM, Tan MH, Lu J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29:4323–32. 10.1016/j.biomaterials.2008.07.034.Suche in Google Scholar PubMed
(38) Baouche-Mati N, Elchinger PH, Baynast H, Pierre G, Delattre C, Michaud P. Chitosan as an adhesive. Eur Polym J. 2014;60:198–212. 10.1016/j.eurpolymj.2014.09.008.Suche in Google Scholar
(39) Cárdenas G, Anaya P, von Plessing C, Rojas C, Sepúlveda J. Chitosan composite films. Biomedical applications. J Mater Sci: Mater Med. 2008;19:2397–405. 10.1007/s10856-007-3275-3.Suche in Google Scholar PubMed
(40) Ibrahim A. Chitosan topical gel formulation in management of burn wounds. Int J Biol Macromol. 2009;45:16–21. 10.1016/j.ijbiomac.2009.03.010.Suche in Google Scholar PubMed
(41) Mohandas A, Deepthi S, Biswas R, Jayakumar R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact Mater. 2017;3:267–77. 10.1016/j.bioactmat.2017.11.003.Suche in Google Scholar PubMed PubMed Central
(42) Massad S, Cheema F, Brown S, Davis WJ, Burkey B, Grat PM. The use of a chitosan dressing with silver in the management of paediatric burn wound: a pilot study. J Wound Care. 2017;26(4):S26–30. 10.12968/jowc.2017.26.Sup4.S26.Suche in Google Scholar PubMed
(43) Gupta A, Rattan V, Rai S. Efficacy of Chitosan in promoting wound healing in extraction socket: a prospective study. J Oral Biol Craniofac Res. 2019;9:91–5. 10.1016/j.jobcr.2018.11.001.Suche in Google Scholar PubMed PubMed Central
(44) Adekogbe I, Ghanem A. Fabrication and characterization of DTBP-crosslinked chitosan scaffolds for skin tissue engineering. Biomaterials. 2005;26:7241–50. 10.1016/j.biomaterials.2005.05.043.Suche in Google Scholar PubMed
(45) Farjah MH, Farahpour MR. Efficacy of topical platelet-rich plasma and chitosan co-administration on Candida albicans-infected partial thickness burn wound healing. Burns. 2020;46:S0305417920303855. 10.1016/j.burns.2020.05.019.Suche in Google Scholar PubMed
(46) Badawy ME, Rabea EI. A biopolymer chitosan and its derivatives as promising antimicrobial agents against plant pathogens and their applications in crop protection. Int J Carbohyd Chem. 2011;2011:29. 10.1155/2011/460381. Article ID: 460381.Suche in Google Scholar
(47) Younes I, Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs. 2015;13(3):1133–74. 10.3390/md13031133.Suche in Google Scholar PubMed PubMed Central
(48) Liu R, Xu X, Zhuang X, Cheng B. Solution blowing of chitosan/PVA hydrogel nanofiber mats. Carbohydr Polym. 2014;101:1116–21. 10.1016/j.carbpol.2013.10.056.Suche in Google Scholar PubMed
(49) Abdelgawad AM, Hudson SM, Rojas OJ. Antimicrobial wound dressing nanofiber mats from multicomponent (Chitosan/silver-NPs/polyvinyl alcohol). Carbohyd Polym. 2014;100:166–79. 10.1016/j.carbpol.2012.12.043.Suche in Google Scholar PubMed
(50) Hafdani NF, Sadeghinia N. A review on application of chitosan as a natural antimicrobial. World Acad Sci Eng Technol. 2011;50:252–6. 10.5281/zenodo.1062688.Suche in Google Scholar
(51) Malinowska-Panczyk MW, Staroszczyk H, Gottfried K, Kolodziejska I, Pajak WA. Antimicrobial properties of chitosan solutions, chitosan films and gelatin-chitosan films. Polimery. 2015;60:735–41. 10.14314/polimery.2015.735.Suche in Google Scholar
(52) Escárcega-Galaz AA, De la Cruz-Mercado JL, López-Cervantes J, Sánchez-Machado DI, Brito-Zurita OR, Ornelas-Aguirre JM. Chitosan treatment for skin ulcers associated with diabetes. Saudi J Biol Sci. 2018;25:130–5. 10.1016/j.sjbs.2017.03.017.Suche in Google Scholar PubMed PubMed Central
(53) Pereda M, Ponce AG, Marcovich NE, Ruseckaite RA, Martucci JF. Chitosan-gelatin composites and bi-layer films with potential Antimicrobial activity. Food Hydrocoll. 2011;25:1371–81. 10.1016/j.foodhyd.2011.01.001.Suche in Google Scholar
(54) Anisha BS, Biswas R, Chennazhi KP, Jayakumar R. Chitosan-hyaluronic acid/nanosilver composites sponges for drug resistant bacterial infected diabetic wounds. Int J Biol Macromol. 2013;62:310–20. 10.1016/j.ijbiomac.2013.09.011.Suche in Google Scholar PubMed
(55) Villa T, Brianza S. Form and function of resorbable materials–based medical devices. In Perale G, Hilborn J, editors. Bioresorbable polymers for biomedical applications. Politecnico di Milano, Milano, Italy: Woodhead publishing; 2017. p. 95–100. 10.1016/B978-0-08-100262-9.00005-7.Suche in Google Scholar
(56) Archana D, Dutta J, Dutta PK. Evaluation of chitosan nano dressing for wound healing: characterization, in vitro and in vivo studies. Int J Biol Macromol. 2013;57:193–203. 10.1016/j.ijbiomac.2013.03.002.Suche in Google Scholar PubMed
(57) Chen C, Liu L, Huang T, Wang Q, Fang Y. Bubble template fabrication of chitosan/poly(vinyl alcohol) sponges for wound dressing applications. Int J Biol Macromol. 2013;62:188–93. 10.1016/j.ijbiomac.2013.08.042.Suche in Google Scholar PubMed
(58) Arpornmaeklong P, Pripatnanont P, Suwatwirote N. Properties of chitosan-collagen sponges and osteogenic differentiation of rat-bone-marrow stromal cells. Int J Oral Maxillofac Surg. 2008;37:357–66. 10.1016/j.ijom.2007.11.014.Suche in Google Scholar PubMed
(59) Yenilmez E, Başaran E, Arslan R, Berkman M, Güven U, Yazan Y. Chitosan gel formulations containing egg yolk oil and epidrmal growth factor for dermal burn treatment. Pharmazie. 2015;70:67–73. 10.1691/ph.2015.4126.Suche in Google Scholar
(60) Ceren A, Zelihagül D, Nevin C, Fatih Z, Serdar Ö, Deniz E. An investigation on burn wound healing in rats with chitosan gel formation containing epidermal growth factor. Burns. 2006;32:319–27. 10.1016/j.burns.2005.10.015.Suche in Google Scholar PubMed
(61) Hafiz M, Mohd-Cairul I, Shiow-Fern N, Haliza K, Shefaat U, Nauman R. Formulation and Evaluation of microwave-modified chitosan-curcumin nanoparticles-A promising nanomaterials plataform for skin tissue regeneration applications following burn wounds. Polymers (Basel). 2020;12:2608. 10.3390/polym12112608.Suche in Google Scholar PubMed PubMed Central
(62) Asgarirad H, Ebrahimnejad P, Mahjoub MA, Jalalian M, Morad H, Ataee R, et al. A promising technology for wound healing; in-vitro and in-vivo evaluation of chitosan nano-biocomoposite films containing gentamicin. J Microencapsul. 2021;38:100–7. 10.1080/02652048.2020.1851789.Suche in Google Scholar PubMed
(63) Nacer Khodja A, Mahlous M, Tahtat D, Benamer S, Larbi Youcef S, Chader H, et al. Evaluation of healing activity if PVA/Chitosan hydrogels on deep second degree burn: pharmacological and toxicological test. Burns. 2013;39:98–104. 10.1016/j.burns.2012.05.021.Suche in Google Scholar PubMed
(64) Sung HJ, Hwang M-R, Kim OJ, Lee HJ, Kim IY, Kim HJ, et al. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int J Pharm. 2010;392:232–40. 10.1016/j.ijpharm.2010.03.024.Suche in Google Scholar PubMed
(65) Boucard N, Viton C, Agay D, Mari E, Roger T, Cancerelle Y, et al. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials. 2007;28:3478–88. 10.1016/j.biomaterials.2007.04.021.Suche in Google Scholar PubMed
(66) Honardar S, Kordestani SS, Daliri M, NayebHabib F. The effect of chitosan-based gel on second degree burn wounds. J Wound care. 2016;25(8):488–94. 10.12968/jowc.2016.25.8.488.Suche in Google Scholar PubMed
(67) Jin Y, Ling XP, He YL, Zhang MT. Effects of chitosan and heparin on early extension of burns. Burns. 2007;33:1027–31. 10.1016/j.burns.2006.12.002.Suche in Google Scholar PubMed
(68) Pereira GG, Santos-Oliveira R, Albernaz MS, Canema D, Weismüller G, Barros EB, et al. Microparticles of Aloe vera/vitamin E/chitosan: microscopic a nuclear imaging and an in vivo test analysis for burn treatment. Eur J Pharm Biopharm. 2014;86:292–300. 10.1016/j.ejpb.2013.10.011.Suche in Google Scholar PubMed
(69) Gonçalves R, Signini R, Martins L, Pereira Y, Clare M, Junior R. Carboxymethyl chitosan hydrogel formulations enhance the healing process in experimental partial-thickness (second-degree) burn wound healing. Acta Cir Bras. 2021;36:e360303. 10.1590/ACB360303.Suche in Google Scholar PubMed PubMed Central
(70) Hamedi H, Moradi S, Hudson MS, Tonelli AE. Chitosan based hydrogels and their applications for drug delivery in wound dressings: a review. Carbohyd Polym. 2018;199:445–60. 10.1016/j.carbpol.2018.06.114.Suche in Google Scholar PubMed
(71) Carles G, Dabiri C, Mchirgui A, Saoudi EO, Hcini N, Pouget K, et al. Uses of chitosan for treating different forms of serious obstetrics hemorrhages. J Gynecol Obstet Hum Reprod. 2017;46:693–5. 10.1016/j.jogoh.2017.08.003.Suche in Google Scholar
(72) Celox™, UK, A powerful topical hemostatic agent. http://www.celoxmedical.com/cx-product/omnistat/2018. (accessed 16 August 2018).Suche in Google Scholar
(73) Sáez-Alcaide L, Molinero-Mourelle P, González-Serrano J, Rubio-Alonso M, López-Quiles J. Efficacy of a topical gel containing chitosan, chlorhexidine, allantoin and dexpanthenol for pain and inflammation control after third molar surgery: a randomized and placebo-controlled clinical trial. Med Oral Patol Oral Cir Bucal. 2020;1(25(5)):e644–51. 10.4317/medoral.23661.Suche in Google Scholar
(74) Khamverdi Z, Farhadian F, Khazaei S, Adabi M. Efficacy of chitosan-based chewing gum on reducing salivary S. mutans counts and salivary pH: a randomised clinical trial. Acta Odontol Scand. 2020;3:1–7. 10.1080/00016357.2020.1836392.Suche in Google Scholar
(75) Fahimeh A, Hamideh M, Sahar D, Hamid M, Mehdi M, Hamid M. Chitosan-based biocompatible dressing for treatment of recalcitrant lesions of cutaneous leishmaniasis: a pilot clinical study. Indian J Dermatol Venereol Leprol. 2019;85(6):609–14. 10.4103/ijdvl.IJDVL_189_18.Suche in Google Scholar
(76) Wohlfahrt JC, Aass AM, Koldsland OC. Treatment of peri-implant mucositis with a chitosan brush-A pilot randomized clinical trial. Int J Dent Hyg. 2019;17(2):170–6. 10.1111/idh.12381.Suche in Google Scholar
(77) Halim AS, Nor FM, Saad-Mat AZ, Norsa’adah NB, Ujang Z. Efficacy of chitosan derivative films versus hydrocolloid dressing on superficial wounds. J Taibah Univ Med Sci. 2018;13(6):512–20. 10.1016/j.jtumed.2018.10.004.Suche in Google Scholar
(78) Stone CA, Clarke WT, Powell R, Devaraj VS. Healing at skin graft donor sites dressed with chitosan. Br J Plast Surg. 2000;53:601–6. 10.1054/bjps.2000.3412.Suche in Google Scholar
(79) Biagini G, Bertani A, Muzzarelli R, Damadei A, Dibenedetto G, Belligolli A, et al. Wound management with N-carboxybutyl chitosan. Biomaterials. 1991;2:281–6. 10.1016/0142-9612(91)90035-9.Suche in Google Scholar
© 2022 Dalia I. Sánchez-Machado et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Artikel in diesem Heft
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

