Abstract
Polylactic acid (PLA) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate) (P(3HB-co-4HB)) nanofibers were prepared by melt extrusion of immiscible blends of PLA/polyvinyl alcohol (PVA) and P(3HB-co-4HB)/PVA via in situ formation of microfibrils during the melt extrusion process. The morphology of the blends and nanofibers after removal of PVA with water was studied using scanning electron microscopy. The intermolecular interactions in the blends were studied by Fourier-transform infrared spectroscopy. The compatibility of the components of the PVA/PLA blends was better than that of the PVA/P(3HB-co-4HB) blends. By varying the process conditions, the average diameter of the PLA nanofibers could be controlled in the range of 78–150 nm and that of the P(3HB-co-4HB) nanofibers could be controlled in the range of 274–424 nm.
1 Introduction
Polylactic acid (PLA) can be extracted from corn, cassava, and other crops via microbial fermentation, followed by refining, dehydration, oligomerization, and high-temperature pyrolysis polymerization (1–4). Polyhydroxy fatty acid (PHA) ester is an intracellular polyester that can be synthesized by numerous bacteria and serves primarily as a carbon and energy storage substance in organisms. Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) P(3HB-co-4HB) is a fourth-generation PHA industrial product (5,6). PLA and PHA are biodegradable, biocompatible, optically active, and present piezoelectric properties and gas separation ability. The properties of PLA and PHA are generally superior to those of synthetic plastics. Nanofibers have large surface areas and length-to-diameter ratios and can strongly interpenetrate with other substances. Moreover, nanofibers fabricated using PLA and PHA present high porosity, good flexibility, and strong adsorbability. Owing to the continuous development of nanofiber materials for various fields, it is anticipated that completely biodegradable PLA and PHA nanofibers will be increasingly used in the fields of information, medicine, and energy, as well as environmental and human protection, and healthcare (1,7,8).
Common methods of fabricating fibers include melt spraying, electrospinning, and melt extrusion (9–12). In melt spraying, liquid polymers are extruded and subsequently stretched through a hot air injector to produce non-woven fibers (13). Because melt spraying is a simple and efficient process, it is favored in industrial settings. Li et al. (14) fabricated PLA fibers via melt spraying. The average and smallest diameters of the fabricated fibers were 5 and 1 μm, respectively. Electrospinning is a distinct fiber manufacturing process that uses strong electric fields to jet-spin polymer solutions or melts (5,9,10,15,16). However, toxic solvents, such as chloroform, dichloromethane, and N,N-dimethyl formamide, which are typically used to fabricate nanofibers, volatilize during solution electrospinning and can cause environmental pollution. Melt electrospinning does not involve the use of solvents. Morikawa et al. (17) fabricated polyethylene fibers with diameters of 10 ± 4 μm via melt electrospinning. However, owing to the high viscosity of the molten polymers, the diameter of the fabricated nanofibers was typically >500 nm (18). The process parameters and environmental factors have a strong impact on electrospinning, leading to complex and expensive fabrication procedures with poor repeatability. To achieve electrostatic-assisted melt spraying, an electrostatic field is applied directly near the melt nozzle to combine the melt spray and electric field effects. Pu et al. (19) used electrostatic-assisted melt spraying to prepare polypropylene microfibers that were thinner than the conventional melt-sprayed fibers (average diameter of 0.96 μm). However, the diameters of the fabricated fibers did not meet the size criteria for nanofibers.
In this study, a melt extrusion method, which is a new manufacturing process, is used to fabricate high-yield and environmentally friendly thermoplastic nanofibers (20–22). The melt extrusion method is derived from an in-situ polymer fiber fabrication method (23). Two incompatible polymers are melted and extruded using a twin-screw extruder. Upon extrusion, the blended polymer melts are elongated and deformed under the action of shear forces, and a composite force-field is induced in the spray head, resulting in the formation of nanofiber bundles. Thereafter, the matrix material is removed, and thermoplastic nanofibers with desired dimensions are obtained. Unlike other methods used to fabricate nanofibers, the melt extrusion method is environmentally friendly, controllable, and affords high yields. Wang et al. used mixtures of cellulose butyrate acetate (CAB) with various thermoplastic polymers to fabricate polypropylene, polyethylene, polyethylene terephthalate, and polytrimethylene terephthalate polymer nanofibers and evaluated their morphological and thermoplastic properties (23,24,25). However, because the removal of the CAB matrix after melting and extrusion through the twin-screw extruder required the use of acetone, the method was not environmentally friendly.
Herein, we use PVA, which is a non-toxic polymer, as the matrix. PVA, which is a water-soluble polymer that biodegrades into water and carbon dioxide, is environmentally friendly, inexpensive, and readily available (26,27). To explore the green fabrication of biodegradable nanofibers, water-soluble PVA is plasticized and mixed with PLA or P(3HB-co-4HB) in a twin-screw extruder. Biodegradable PLA or P(3HB-co-4HB) nanofibers are obtained by dissolving the PVA matrices in water. The effect of the compatibility of the component polymers in the blends on the morphology of the as-fabricated nanofibers is evaluated.
2 Materials and methods
2.1 Materials
Industrial grade PVA (1788, Shanxi 3D Group Co., Ltd) was used as a matrix phase. PLA (REVODE190) was procured from Zhejiang Haizheng Biomaterials Co., Ltd. P(3HB-co-4HB) (EM10050) was purchased Shandong Yikeman Technology Co., Ltd. Analytical grade glycerine and polyethylene glycol (PEG 200; Tianjin Kemio Chemical Reagent Co., Ltd.) were used as the complex plasticizer.
2.2 Preparation of nanofibers
The fabrication of the nanofibers is illustrated in Figure 1. First, PVA, glycerine, and ethylene glycol (70/20/10 wt%) were plasticized at 70°C in a vacuum oven for 4 h until a homogeneous mixture was obtained. The mixture was then extruded through a twin-screw extruder (D = 16 mm; L/D = 40) to obtain plasticized PVA particles. Subsequently, the plasticized PVA, PLA, or P(3HB-co-4HB) particles were dried at 80°C for 6 h. To characterize the morphology and properties of the dispersed phase, prior to extrusion, PLA and P(3HB-co-4HB) were mixed with PVA in a series of blend ratios using a solid mixer. The blending temperature and screw speed were 180°C and 150 rpm, respectively. After melt mixing and extruding, the PVA/P(3HB-co-4HB) and PVA/PLA composite fibers were obtained. Finally, PLA and P(3HB-co-4HB) nanofibers were obtained by immersion in deionized water for 24 h; the solvent was changed every 4 h to ensure complete removal of the PVA matrix.
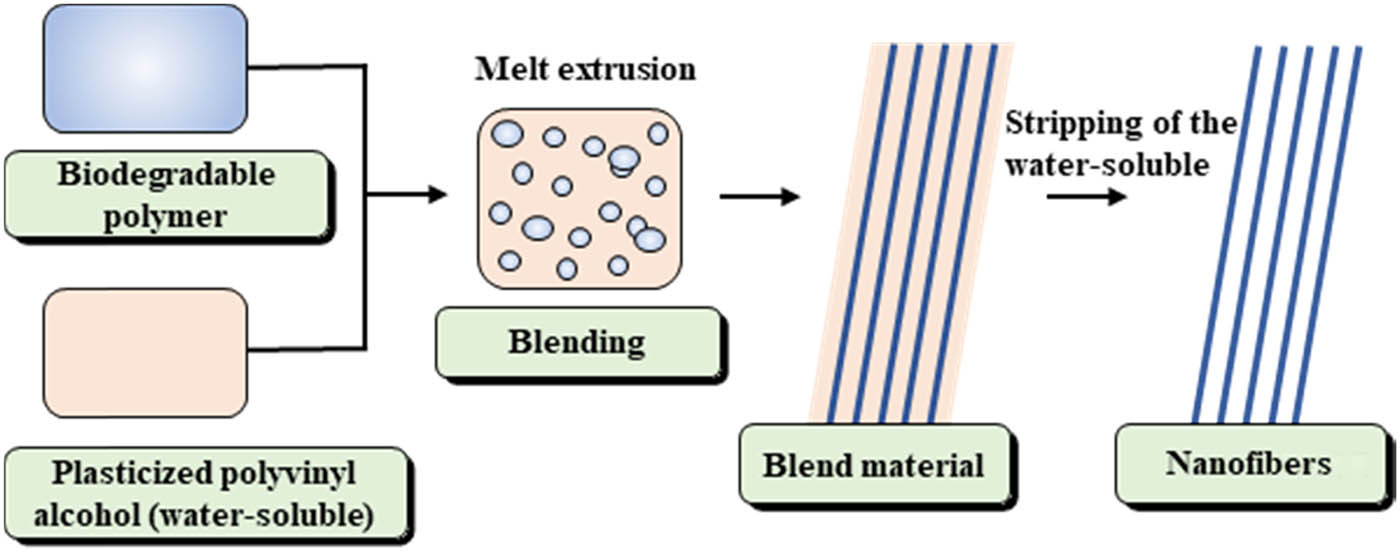
Schematic illustration of fabrication of biodegradable nanofibers.
2.3 Analysis and characterization
2.3.1 Scanning electron microscopy (SEM)
The samples were observed using a field-emission scanning electron microscope (FESEM, JSM-7500). The composite fibers were fractured in liquid nitrogen and coated with gold to improve the image quality for observing the cross-sectional morphology of the composite fibers. The morphology and structure of the nanofibers were analyzed directly after gold spray treatment.
2.3.2 Fourier-transform infrared (FTIR) spectroscopy
The chemical composition of pure PVA, plasticized PVA, pure P(3HB-co-4HB), and the blend samples with different mass ratios were qualitatively investigated using Fourier-transform infrared spectroscopy (FTIR, Nicolet210-IR, Shanghai Jinghong Experiment Equipment Co., Ltd.) in the wavenumber range of 4,000 to 400 cm−1.
3 Results
3.1 SEM imaging of PVA/P(3HB-co-4HB) and PVA/PLA blends
The compatibility of the blend components directly affects the morphology of polymer blends, and the morphology of the dispersed phase determines whether nanofibers can be obtained. To evaluate the compatibility between the components and the phase structure of the PVA/P(3HB-co-4HB) and PVA/PLA blends, splines were extruded from the twin-screw extruder and frozen using liquid nitrogen to eliminate the effects of temperature and stretching on the particle morphology.
Figure 2 shows the cross-sectional SEM images of the PVA/P(3HB-co-4HB) blends with different blend ratios. “Sea–island” formations were present in the samples fabricated via melting extrusion, in which the PVA matrices and P(3HB-co-4HB) dispersed phases represented the sea and islands, respectively. The distinct PVA–P(3HB-co-4HB) interfaces indicate poor compatibility between PVA and P(3HB-co-4HB). Several spherical and ellipsoidal cavities were present in the PVA matrices. The surfaces of the cavity walls were smooth, and the edges of the cavities were distinct. This is attributed to recoil of the P(3HB-co-4HB) particles from the PVA matrix, indicating the weak bonding forces at the PVA–P(3HB-co-4HB) interface. Furthermore, for the PVA/P(3HB-co-4HB) blends with low P(3HB-co-4HB) contents, the dispersed phase was present in smaller amounts and was more uniformly distributed, whereas for the PVA/P(3HB-co-4HB) blends with higher P(3HB-co-4HB) contents, the amount of dispersed phase increased, the distribution widened, and the heterogeneity increased (Figure 2).
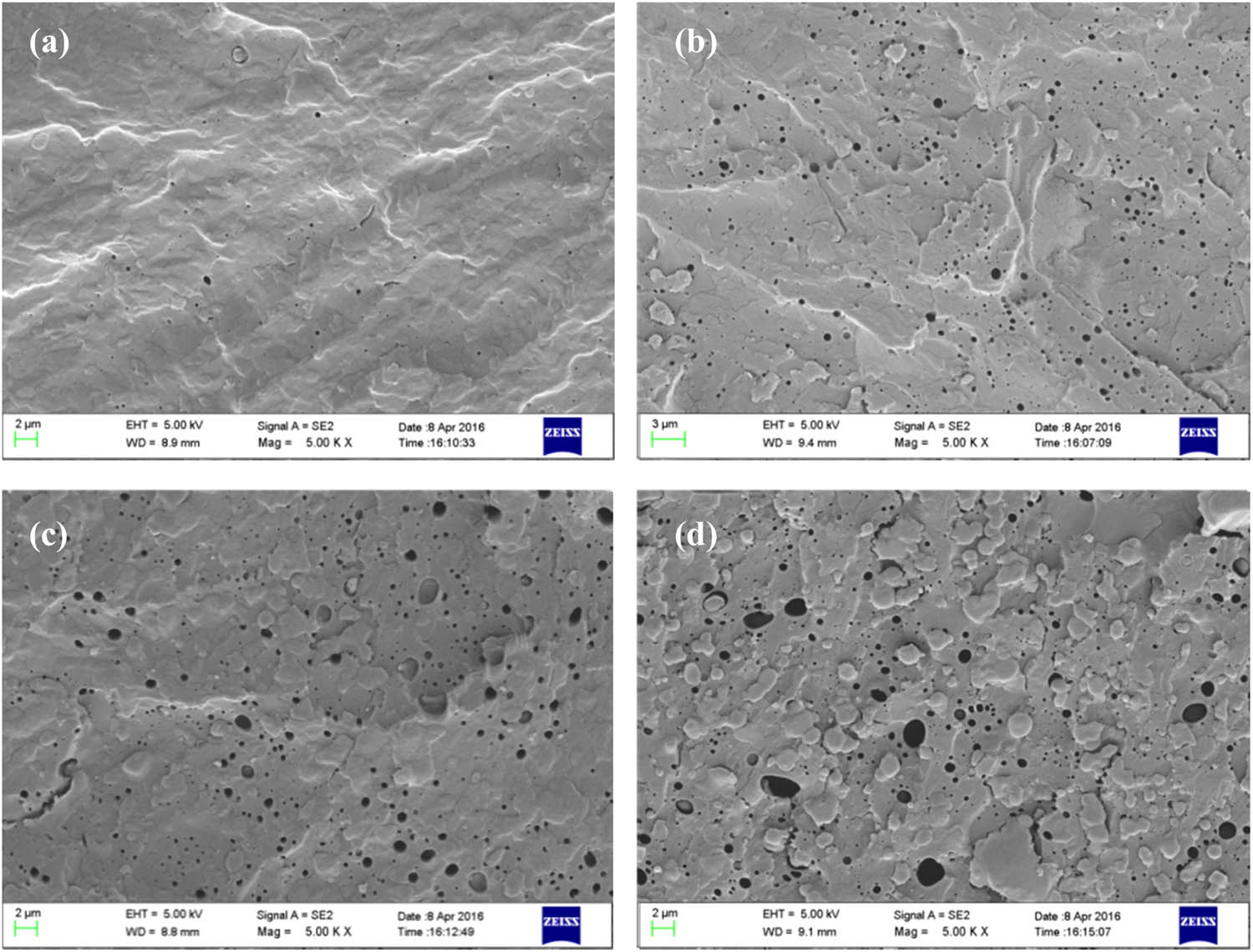
Fracture surfaces of (a) PVA/P(3HB-co-4HB) (PVA 95 wt%), (b) PVA/P(3HB-co-4HB) (PVA 90 wt%), (c) PVA/P(3HB-co-4HB) (PVA 80 wt%), and (d) PVA/P(3HB-co-4HB) (PVA 70 wt%).
Figure 3 shows the cross-sectional SEM images of the PVA/PLA blends with different blending ratios. This blend also adopted a sea–island morphology after melt extrusion, in which the PVA matrix formed the “sea,” and PLA formed the “islands.” The PLA particles dispersed in the PVA matrices of the PVA/PLA blends were more uniform in size but were significantly smaller than the P(3HB-co-4HB) particles dispersed in the PVA matrices of the PVA/P(3HB-co-4HB) blends. Moreover, the PVA–PLA interface was distorted, and the morphology of the PLA islands was fibrous. These findings indicate that PLA was well dispersed within the PVA matrix, PLA and PVA are highly compatible, and the bonding forces at the PLA–PVA interface are strong.
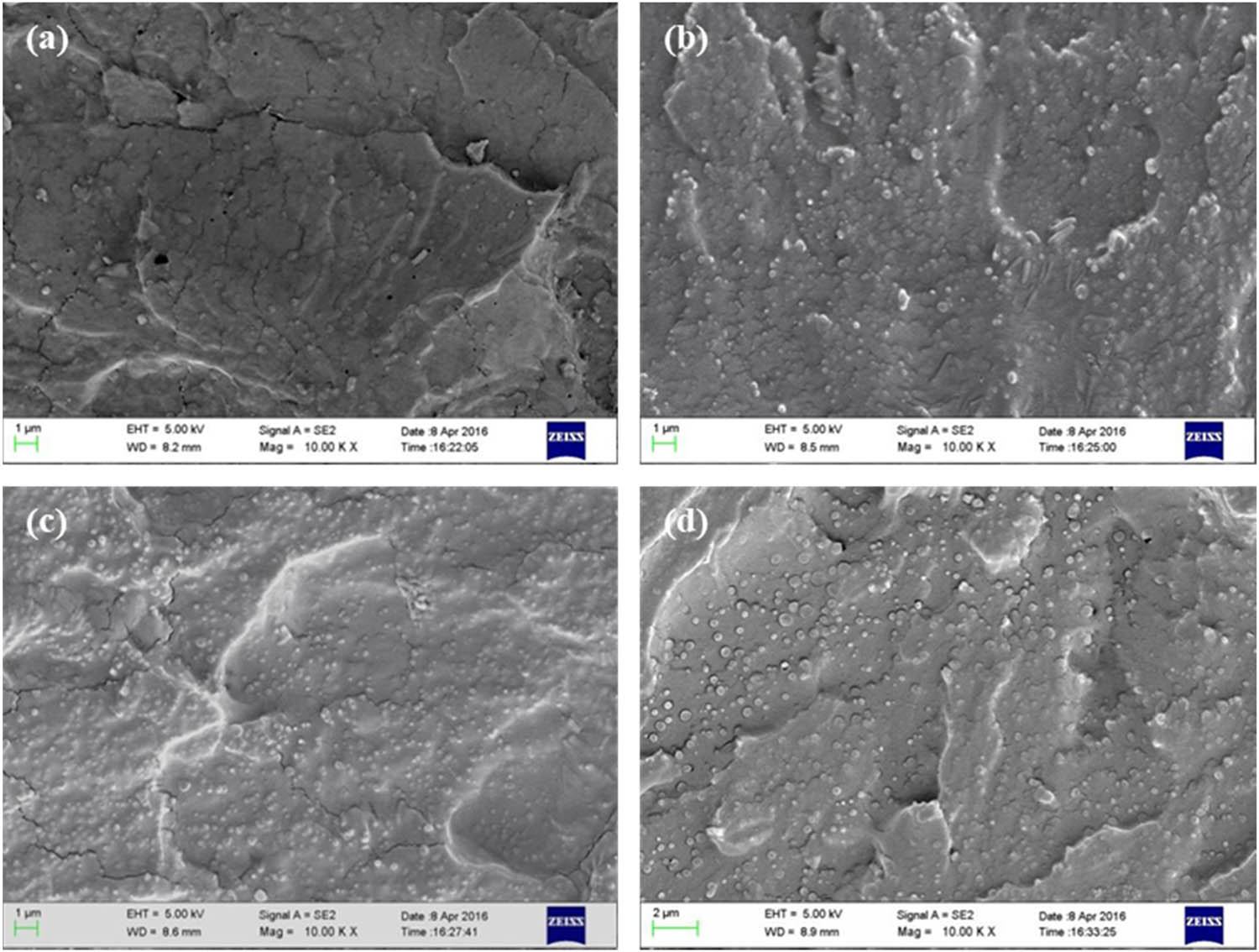
Fracture surfaces of (a) PVA/PLA (PVA 95 wt%), (b) PVA/PLA (PVA 90 wt%), (c) PVA/PLA (PVA 80 wt%), and (d) PVA/PLA (PVA 70 wt%).
The SEM images of the PVA/P(3HB-co-4HB) and PVA/PLA blends (Figures 2 and 3, respectively) suggest that the components of the PVA/PLA blends were significantly more compatible than those of the PVA/P(3HB-co-4HB) blends. The compatibility of the continuous and dispersed phases determines the success or failure of in situ fiber formation. Mixtures of incompatible matrices and dispersed phases favor the formation of island fibers. Due to the low entanglement between the PVA and P(3HB-co-4HB) molecules, the PVA–P(3HB-co-4HB) interfaces were weak. This promoted the slip and orientation of the P(3HB-co-4HB) dispersed phase in the melt. However, the morphological structure arising from the poor compatibility between PVA and P(3HB-co-4HB) was not conducive to generating a strong interface, and the weak PVA–P(3HB-co-4HB) interface bonds rendered the blends unsuitable for effective stress transfer. Therefore, the PVA/PLA blends are more suitable for preparing biodegradable nanofibers than the PVA/P(3HB-co-4HB) blends. The interactions between the PLA islands in the PVA/PLA blends were stronger than those between the P(3HB-co-4HB) islands in the PVA/P(3HB-co-4HB) blends, thereby promoting uniform distribution of the PLA nanofibers in the PVA matrices.
3.2 FTIR analysis of PVA/P(3HB-co-4HB) and PVA/PLA blends
The FTIR spectra of blends can be used to determine the presence of specific interactions between molecules. Therefore, the FTIR spectra of the PVA/P(3HB-co-4HB) and PVA/PLA blends were acquired to determine the reasons for the differences in the compatibility between the blend components. The –OH groups of PVA are strong proton donors, whereas the carbonyl groups of PLA are typical proton acceptors. Therefore, hydrogen bonds can be formed between PVA and PLA in PVA/PLA blends. Moreover, P(3HB-co-4HB) contains numerous carbonyl groups. Therefore, hydrogen bonds can also be formed between the –OH groups of PVA and carbonyl groups of P(3HB-co-4HB).
Figure 4 shows the FTIR spectra of pure PVA, pure PLA, PVA/PLA (PVA 90 wt%), and PVA/PLA (PVA 70 wt%). The absorption peaks at 3,373, 1,336, and 1,094 cm−1 in the FTIR spectrum of pure PVA are the characteristic peaks of the O–H telescopic, CH–OH bending, and C–O telescopic vibrations, respectively. The absorption peaks ascribed to the expansion and bending vibrations of the C–H bonds emerged at 2,943 and 1,431 cm−1, respectively. The absorption peak of polyvinyl acetate appearing at 1,737 cm−1 in the FTIR spectra of the samples is characteristic of incompletely hydrolyzed PVA (28).
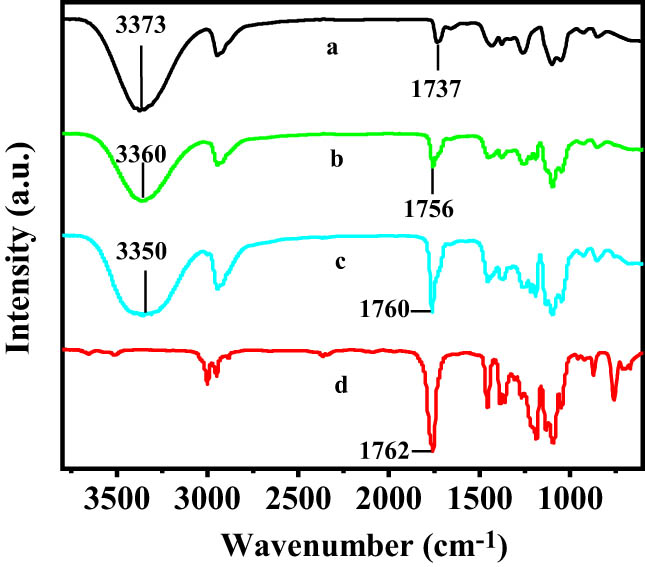
FTIR of (a) PVA, (b) PVA/PLA (PVA 90 wt%), (c) PVA/PLA (PVA 70 wt%), and (d) PLA.
The absorption peak of the −OH groups of PVA shifted from 3,373 cm−1 (for pure PVA) to lower frequency (3,360 cm−1 for PVA/PLA (PVA 90 wt%) and 3,350 cm−1 for PVA/PLA (PVA 70 wt%)) as the PLA content increased (Figure 4). Moreover, peak broadening was observed with increasing PLA content. The formation of hydrogen bonds often causes the bond length to increase and the bond energy to decrease. For telescopic vibrations, the stronger the hydrogen bonds, the wider the spectral bands, the greater the absorption intensity, and the greater the downshift. Consequently, the downshift and width of the absorption peak at 3,373 cm−1 indicate that the −OH groups of PVA formed hydrogen bonds with the carbonyl groups of PLA.
The absorption peak of the carbonyl groups shifted from 1,762 cm−1 (for pure PVA) to lower frequency (1,760 cm−1 for PVA/PLA (PVA 70 wt%) and 1,576 cm−1 for PVA/PLA (PVA 90 wt%)) when the PLA content was increased (Figure 4). These results suggest that hydrogen bonds were formed between the carbonyl groups of PVA and the −OH groups of PLA (Figure 5).
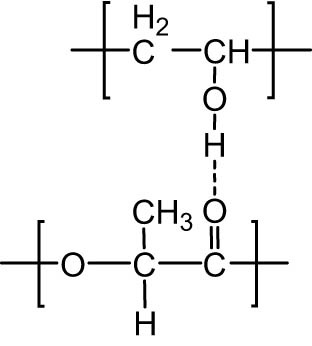
Schematic of the hydrogen bonding between PVA and PLA.
Figure 6 shows the FTIR spectra of pure PVA, pure P(3HB-co-4HB), PVA/P(3HB-co-4HB) (PVA 90 wt%), and PVA/P(3HB-co-4HB) (PVA 70 wt%). The characteristic hydroxyl peaks in the FTIR spectrum of PVA at 3,373 cm−1 were unchanged in the FTIR spectra of the PVA/P(3HB-co-4HB) blends. The carbonyl peak of P(3HB-co-4HB), which was observed at 1,737 cm−1, did not shift in the FTIR spectra of the PVA/P(3HB-co-4HB) blends. These results indicate that no hydrogen bonds were formed between PVA and P(3HB-co-4HB). The formation of intermolecular hydrogen bonds can decrease the degree of freedom of the polymer chains in polymer blends and control the aggregate structure of polymers at the molecular level. Therefore, inducing intermolecular hydrogen bonds between the polymers in blends can improve the compatibility of the polymers.
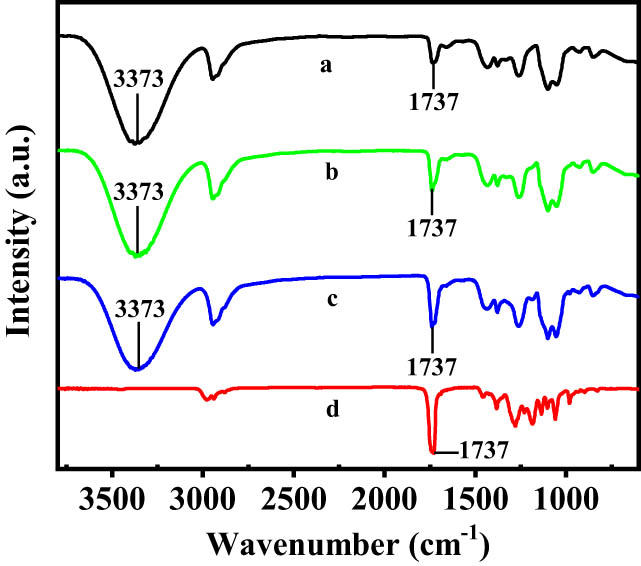
FTIR spectra of (a) PVA, (b) PVA/(P(3HB-co-4HB) (PVA 90 wt%), (c) PVA/P(3HB-co-4HB) (PVA 70wt%), and (d) P(3HB-co-4HB).
The FTIR analysis indicated that the PVA/PLA blends were more compatible than the PVA/P(3HB-co-4HB) blends because of the formation of hydrogen bonds between the PVA and PLA chains, which is also the underlying cause for the differences between the cross-sectional morphology of the two types of blends.
3.3 Morphologies of the P(3HB-co-4HB) and PLA nanofibers
The relative content of the dispersed and continuous phases in polymer blends is a critical factor influencing the phase morphology of the blends. The relative content of each phase determines which polymers form the dispersed and continuous phases and also affects the morphology, size, and size distribution of the dispersed phase (29). High or low contents of the dispersed phase are not conducive to the formation of nanofibers using polymer blends. An optimal composition ratio of the blended system is thus required to achieve the desired properties. The phase structures obtained using polymer mixtures with different blend ratios were different. Good fiber morphology and size could be achieved by changing the composition of the blends to regulate the morphology of the dispersed phase. Herein, PVA/PLA and PVA/P(3HB-co-4HB) blends with various compositions were fabricated under the same extrusion conditions, and the effect of the blend composition on the morphology of the as-fabricated PLA and P(3HB-co-4HB) nanofibers was analyzed.
The extruded PVA/PLA and PVA/P(3HB-co-4HB) blends were stretched five times and then added to water to dissolve the PVA matrices and obtain PLA and P(3HB-co-4HB) nanofibers, respectively. The SEM images of the P(3HB-co-4HB) nanofibers are presented in Figure 7. The diameter distributions of the P(3HB-co-4HB) nanofibers obtained using the blends with different compositions intuitively reflect the changes in the morphology of the P(3HB-co-4HB) nanofibers. Upon increasing the PVA content of the PVA/P(3HB-co-4HB) blends, the diameter of the P(3HB-co-4HB) nanofibers decreased, and the diameter distribution narrowed gradually. The average diameters of the PVA/P(3HB-co-4HB) (PVA 70 wt%) and PVA/P(3HB-co-4HB) (PVA 90 wt%) nanofibers were 424 and 274 nm, respectively.
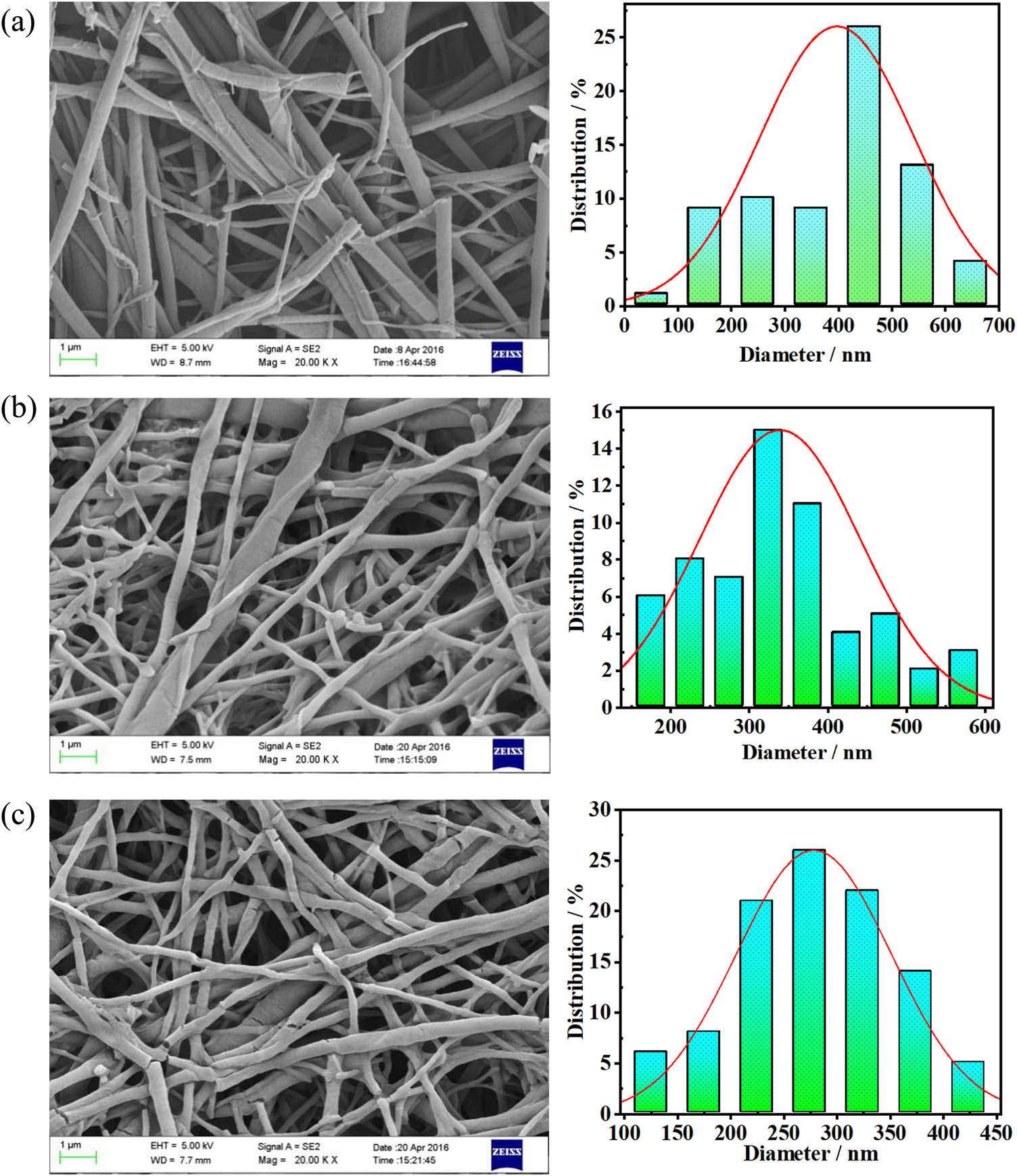
Morphology of the P(3HB-co-4HB) nanofibers and diameter distribution with different blend ratios: (a) PVA/P(3HB-co-4HB) (PVA 70 wt%), (b) PVA/P(3HB-co-4HB) (PVA 80 wt%), and (c) PVA/P(3HB-co-4HB) (PVA 90 wt%).
The SEM images and diameter distribution of the PLA nanofibers fabricated using the PVA/PLA blends with different PVA:PLA ratios (Figure 8) were similar to those of the P(3HB-co-4HB) nanofibers. The average diameters of the PVA/PLA (PVA 70 wt%) and PVA/PLA (PVA 90 wt%) nanofibers fabricated using the PVA/PLA blends were 150 and 78 nm, respectively. Therefore, the diameters of the PLA nanofibers were significantly lower than those of the P(3HB-co-4HB) nanofibers. These results are consistent with the compatibility analysis and IR spectroscopy data.
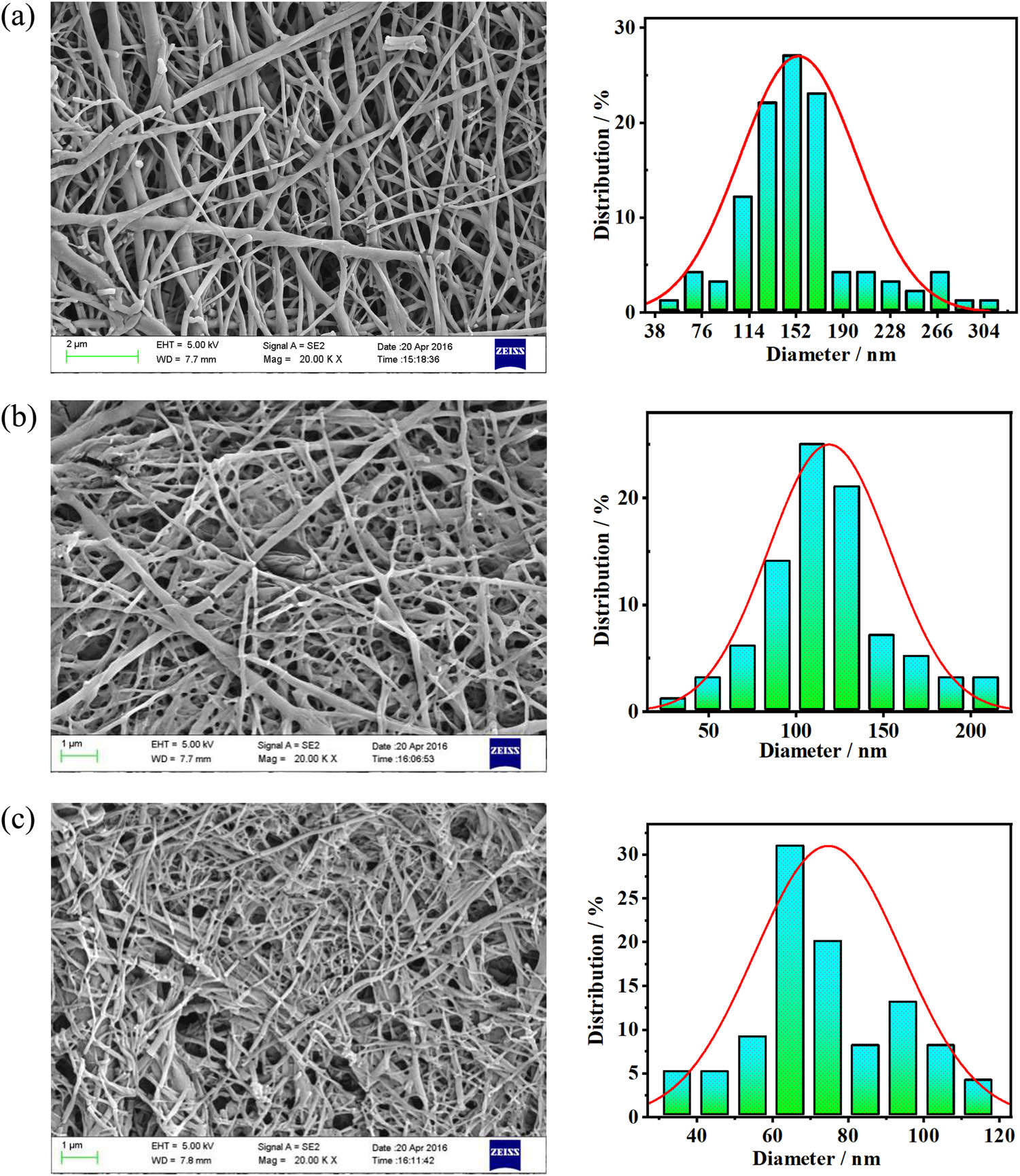
Morphology of the PLA nanofibers and diameter distribution of the nanofibers fabricated using PVA/PLA blends with different blend ratios: (a) PVA/PLA (PVA 70 wt%), (b) PVA/PLA (PVA 80 wt%), and (c) PVA/PLA (PVA 90 wt%).
4 Conclusions
The effect of the compatibility of the components of PVA/PLA and PVA/P(3HB-co-4HB) blends on the morphology of nanofibers fabricated from the melt-blended extrusions was studied experimentally. P(3HB-co-4HB) and PLA nanofibers were prepared by dissolving the PVA matrices of the PVA/PLA and PVA/P(3HB-co-4HB) blends, respectively. The compatibility of the components of the PVA/PLA blends was significantly better than that of the PVA/P(3HB-co-4HB) blends. The −OH groups of PVA formed intermolecular hydrogen bonds with the carbonyl groups of PLA in the PVA/PLA blends. In contrast, no hydrogen bonds were observed between PVA and P(3HB-co-4HB) in the PVA/P(3HB-co-4HB) blends. By varying the PVA content in the blends from 70 wt% to 90 wt%, the average diameter of the resulting P(3HB-co-4HB) nanofibers could be controlled in the range of 424–274 nm and that of the PLA nanofibers could be tailored from 150 to 78 nm.
-
Funding information: The authors appreciate the financial support received from the Key Research Program of Higher Education of Henan Province (21A540006).
-
Author contributions: Zheng Guo: investigation, conceptualization, methodology, writing – original draft, writing – review and editing, supervision; Zebo Wang: writing – original draft, writing – review and editing; Yajie Qin: writing – review and editing, formal analysis; Jintao Zhang: formal analysis; Yu Qi: formal analysis; Binguo Liu: resources, formal analysis; Wei Pan: writing – original draft, writing – review and editing, supervision, formal analysis.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sets generated and/or analyzed during this study can be obtained from the corresponding authors upon reasonable request.
References
(1) Boey JY, Mohamad L, Khok YS, Tay GS, Baidurah S. A review of the applications and biodegradation of polyhydroxyalkanoates and poly(lactic acid) and its composites. Polym (Basel). 2021;13(10):1544. 10.3390/polym13101544.Search in Google Scholar PubMed PubMed Central
(2) Li G, Zhao M, Xu F, Yang B, Li X, Meng X, et al. Synthesis and biological application of polylactic acid. Molecules. 2020;25(21):5023. 10.3390/molecules25215023.Search in Google Scholar PubMed PubMed Central
(3) Madhavan Nampoothiri K, Nair NR, John RP. An overview of the recent developments in polylactide (PLA) research. Bioresour Technol. 2010;101(22):8493–501. 10.1016/j.biortech.2010.05.092.Search in Google Scholar PubMed
(4) Singhvi MS, Zinjarde SS, Gokhale DV. Polylactic acid: synthesis and biomedical applications. J Appl Microbiol. 2019;127(6):1612–26. 10.1111/jam.14290.Search in Google Scholar PubMed
(5) Chai JM, Amelia TSM, Mouriya GK, Bhubalan K, Amirul AA, Vigneswari S, et al. Surface-modified highly biocompatible bacterial-poly(3-hydroxybutyrate-co-4-hydroxybutyrate): a review on the promising next-generation biomaterial. Polym (Basel). 2020;13(1):51. 10.3390/polym13010051.Search in Google Scholar PubMed PubMed Central
(6) Al-Kaddo KB, Mohamad F, Murugan P, Tan JS, Sudesh K, Samian MR. Production of P(3HB-co-4HB) copolymer with high 4HB molar fraction by Burkholderia contaminans Kad1 PHA synthase. Biochem Eng J. 2020;153:107394. 10.1016/j.bej.2019.107394.Search in Google Scholar
(7) Faisalina AF, Sonvico F, Colombo P, Amirul AA, Wahab HA, Majid MIA. Docetaxel-loaded poly(3HB-co-4HB) biodegradable nanoparticles: impact of copolymer composition. Nanomaterials (Basel). 2020;10(11):2123. 10.3390/nano10112123.Search in Google Scholar PubMed PubMed Central
(8) Li X, Liu K, Liu Z, Lu X, Li Y, Wang H, et al. Poly (ethylene-butylacrylate-glycidyl methacrylate) reaction compatibilized poly (lactic acid)/poly (3-hydroxybutyrate-4-hydroxybutyrate) blends with enhanced mechanical property, biodegradability and thermal stability. Polym Test. 2022;111:107610. 10.1016/j.polymertesting.2022.107610.Search in Google Scholar
(9) Ramier J, Boubaker MB, Guerrouache M, Langlois V, Grande D, Renard E. Novel routes to epoxy functionalization of PHA-based electrospun scaffolds as ways to improve cell adhesion. J Polym Sci Part A Polym Chem. 2014;52(6):816–24. 10.1002/pola.27063.Search in Google Scholar
(10) Li Y, Lim CT, Kotaki M. Study on structural and mechanical properties of porous PLA nanofibers electrospun by channel-based electrospinning system. Polymer. 2015;56:572–80. 10.1016/j.polymer.2014.10.073.Search in Google Scholar
(11) Drabek J, Zatloukal M. Meltblown technology for production of polymeric microfibers/nanofibers: a review. Phys Fluids. 2019;31(9):091301. 10.1063/1.5116336.Search in Google Scholar
(12) Wang D, Wang K, Xu W. Novel fabrication of magnetic thermoplastic nanofibers via melt extrusion of immiscible blends. Polym Adv Technol. 2013;24(1):70–4. 10.1002/pat.3051.Search in Google Scholar
(13) Jafari M, Shim E, Joijode A. Fabrication of poly(lactic acid) filter media via the meltblowing process and their filtration performances: a comparative study with polypropylene meltblown. Sep Purif Technol. 2021;260:118185. 10.1016/j.seppur.2020.118185.Search in Google Scholar
(14) Li H, Zhang H, Hu J-J, Wang G-F, Cui J-Q, Zhang Y-F, et al. Facile preparation of hydrophobic PLA/PBE micro-nanofiber fabrics via the melt-blown process for high-efficacy oil/water separation. Polymers. 2022;14(9):1667. 10.3390/polym14091667.Search in Google Scholar PubMed PubMed Central
(15) Wang Y, Li X, Dai X, Zhan Y, Ding X, Wang M, et al. Hybrid electrospun porous fibers of poly(lactic acid) and nano ZIF-8@C600 as effective degradable oil sorbents. J Chem Technol Biotechnol. 2019;95(3):730–8. 10.1002/jctb.6256.Search in Google Scholar
(16) Shibata T, Yoshimura N, Kobayashi A, Ito T, Hara K, Tahara K. Emulsion-electrospun polyvinyl alcohol nanofibers as a solid dispersion system to improve solubility and control the release of probucol, a poorly water-soluble drug. J Drug Delivery Sci Technol. 2022;67:102953. 10.1016/j.jddst.2021.102953.Search in Google Scholar
(17) Morikawa K, Vashisth A, Bansala T, Verma P, Green MJ, Naraghi M. Melt electrospinning polyethylene fibers in inert atmosphere. Macromol Mater Eng. 2020;305(12):2000106. 10.1002/mame.202000106.Search in Google Scholar
(18) Li X, Zheng Y, Mu X, Xin B, Lin L. The effects of electric field on jet behavior and fiber properties in melt electrospinning. Fibers Polym. 2020;21(5):984–92. 10.1007/s12221-020-9849-0.Search in Google Scholar
(19) Pu Y, Zheng J, Chen F, Long Y, Wu H, Li Q, et al. Preparation of polypropylene micro and nanofibers by electrostatic-assisted melt blown and their application. Polym (Basel). 2018;10(9):959. 10.3390/polym10090959.Search in Google Scholar PubMed PubMed Central
(20) Xue CH, Wang D, Xiang B, Chiou B-S, Sun G. Controlled and high throughput fabrication of poly(trimethylene terephthalate) nanofibers via melt extrusion of immiscible blends. Mater Chem Phys. 2010;124(1):48–51. 10.1016/j.matchemphys.2010.07.039.Search in Google Scholar
(21) Aumnate C, Soatthiyanon N, Makmoon T, Potiyaraj P. Polylactic acid/kenaf cellulose biocomposite filaments for melt extrusion based-3D printing. Cellulose. 2021;28(13):8509–25. 10.1007/s10570-021-04069-1.Search in Google Scholar
(22) Andrade-Guel M, Cabello-Alvarado C, Romero-Huitzil RL, Rodríguez-Fernández OS, Ávila-Orta CA, Cadenas-Pliego G, et al. Nanocomposite PLA/C20A nanoclay by ultrasound-assisted melt extrusion for adsorption of uremic toxins and methylene blue dye. Nanomaterials (Basel). 2021;11(10):2477. 10.3390/nano11102477.Search in Google Scholar PubMed PubMed Central
(23) Dong J, Qi Y, Sun J, Wei L, Qin S. In situ fibrillation of poly(trimethylene terephthalate) in polyolefin elastomer through multistage stretching extrusion. J Appl Polym Sci. 2016;133(33):43797(1–9). 10.1002/app.43797.Search in Google Scholar
(24) Wang D, Sun G. Formation and morphology of cellulose acetate butyrate (CAB)/polyolefin and CAB/polyester in situ microfibrillar and lamellar hybrid blends. Eur Polym J. 2007;43(8):3587–96. 10.1016/j.eurpolymj.2007.05.018.Search in Google Scholar
(25) Wang D, Sun G, Yu L-W. Recyclability of cellulose acetate butyrate (CAB) matrix for controllable and productive fabrication of thermoplastic nanofibers [J]. Carbohydrate Polymers. 2011;83(1):95–100. 10.1016/j.carbpol.2010.08.082.Search in Google Scholar
(26) Lu Y, Jing R, Kong Q-M, Zhu P-X. Solid state grafting copolymerization of acrylamide onto poly(vinyl alcohol) initiated by redox system. J Appl Polym Sci. 2014;131(4):39938(1–7). 10.1002/app.39938.Search in Google Scholar
(27) Husain MSB, Gupta A, Alashwal BY, Sharma S. Synthesis of PVA/PVP based hydrogel for biomedical applications: a review. Energy Sources Part A Recovery Util Environ Eff. 2018;40(20):2388–93. 10.1080/15567036.2018.1495786.Search in Google Scholar
(28) Domene-Lopez D, Guillen MM, Martin-Gullon I, Garcia-Quesada JC, Montalban MG. Study of the behavior of biodegradable starch/polyvinyl alcohol/rosin blends. Carbohydr Polym. 2018;202:299–305. 10.1016/j.carbpol.2018.08.137.Search in Google Scholar PubMed
(29) Yi X, Xu L, Wang Y-L, Zhong G-J, Ji X, Li Z-M. Morphology and properties of isotactic polypropylene/poly(ethylene terephthalate) in situ microfibrillar reinforced blends: Influence of viscosity ratio. Eur Polym J. 2010;46(4):719–30. 10.1016/j.eurpolymj.2009.12.027.Search in Google Scholar
© 2022 Zheng Guo et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

