Abstract
A semi-crystalline designated nylon 66 polymer is a crucial, high-performance engineering material that is used in wide variety of industrial applications including sensors, electrical insulators, electronic devices, and automotive sector. Using modules based on density functional theory and finite-field approaches, this work explores the optoelectronic and spectroscopic characteristics of this polymer. Absorption, dielectric function, refractive index, and optical conductivity are the principle topics of this study. The effects indicated that nylon 66 is a first-rate insulator and the degree of crystallinity estimated is 46.44%. The simulated bandgap vs. the Tauc relation value is greater than 7.0 eV and has a proportional inaccuracy of 2.36%. Absorption coefficient value, however, suggests that while the refractive index and dielectric function remain stable, the optical conductivity is elevated. In order to determine the advantages appropriate for many applications, this research develops a strong basis and perception of the linear and nonlinear optical properties of nylon 66.
1 Introduction
Research organizations have recently become interested in polymers with different optical properties because of how frequently they are used in optical devices, sensors, and light-emitting diodes. Nylon 66 is a crucial semi-crystalline thermoplastic polymer with strong heat stability and mechanical strength (1,2,3,4). Over 80% of all plastics used worldwide are thermoplastics, making them the material of choice (5). Consequently, accurate molecular structure characterization is necessary to comprehend the macroscopic characteristics of polymeric materials (6,7). One of the most important engineering thermoplastics, nylon 66, is frequently used as an engineering resin in demanding environments for high-performance applications that need superior mechanical, chemical, and thermal properties (8,9,10,11). It serves as a catch-all word for the group of synthetic polymers known as polyamides (PA). Due to the characteristics of the chemical repeat unit, as seen in Figure 1, the polymer can be utilized in a number of industrial applications. The first engineering plastic, polyamide 66 (PA66), is utilized in a variety of products due to its exceptional qualities (12,13,14). For example, nylon fibers are used in carpets, clothing, tire cord, and bearings and gears due to its strong creep modulus and abrasion resistance (15).
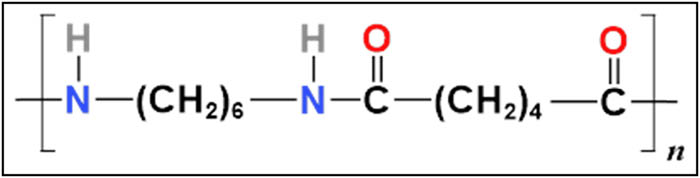
Molecular structure of nylon 66.
During the synthetic process, hexamethylenediamine and adipic acid are mixed to create a polymer. With its long polymer chains that are spaced apart, PA66 crystallization is more stable and forms sheets of hydrogen bonds without the need for molecular deformation. The polymer’s distinct molecular structure allows for modification to enhance performance (16,17,18).
High temperatures cause the semi-crystalline material to melt, nylon 66 has excellent properties but is vulnerable to moisture buildup, which must also be managed during melt processing (19). Polyamide thermoplastics have a high moisture absorption capacity and are sensitive to humidity. Scanning electron microscopy (SEM), X-ray diffraction (XRD), and differential scanning calorimetry (DSC) were used to investigate the effects of humidity/water absorption on microstructure, crystallinity, and glass transition temperatures (20,21).
For a variety of reasons, nylon 66 is employed as a potential material in several fields, including enhancing the properties of cement (22) and lithium-ion batteries (23). There are not many studies on nylon 66’s optoelectronic characteristics in the literature. Similar to this, a number of research on the PA66 polymer have been released. Elzein et al. calculated the degree of crystallinity using infrared spectroscopy (24). Data on the regulation of PA66 hydrogen bonding, extensibility, and crystallinity were reported by Vasanthan et al. in 2004 (25). Because of this, improving the thermophysical properties of polymers, while maintaining their properties, as mentioned above, represents an alluring solution for such particular applications, and over the past 20 years, this research topic has become extremely active at both the industrial and academic levels (26,27,28). Thus, using modules based on density functional theory (DFT) in Material Simulation Software, this work analyzes the optoelectronic and spectroscopic properties and presents the results of the investigation. It is necessary to ascertain the optical properties of polymers, such as absorbance, transmittance, and reflectance. Utilizing optical properties like energy gaps, dielectric loss, dielectric constants, and extinction coefficients, other crucial aspects are computed.
This research explains that in order to specify the kind of electron transition and accurately estimate the optical band gap, Tauc’s model and the optical properties should be investigated. The dielectric loss parameter may be used in recent research to precisely determine the different types of electron transitions. The dielectric loss parameter effectively describes the kinds of electron transitions from Tauc’s model on the basis of theoretical underpinnings and practical techniques. As a result, the results of the current band gap study will address a number of issues that were left unresolved in earlier attempts.
2 Materials and methods
2.1 Nylon 66 structural model
Nylon 66 is a polyamide polymer whose stoichiometry is [(C12H24 N2O2)] n (29,30), and its valence electron configurations are (H: 1s1, C: 2s2 2p2, N: 2s2 2p3, O: 2s2 2p4). Its structure model was placed within an orthorhombic unit cell with the dimensions a = 17.2559 Å, b = 8.639 Å, c = 5.3019 Å, and α = β = γ = 90°. The nylon 66 structural model adopted in the simulation for this research is depicted in Figure 2. The Corey–Pauling–Koltun color scheme was used by Subrata Pal in 2019 to represent atoms. The carbon atom is shown as a gray sphere, the oxygen atom as a red sphere, the hydrogen atom as a white sphere, and the nitrogen atom as a blue sphere (31).
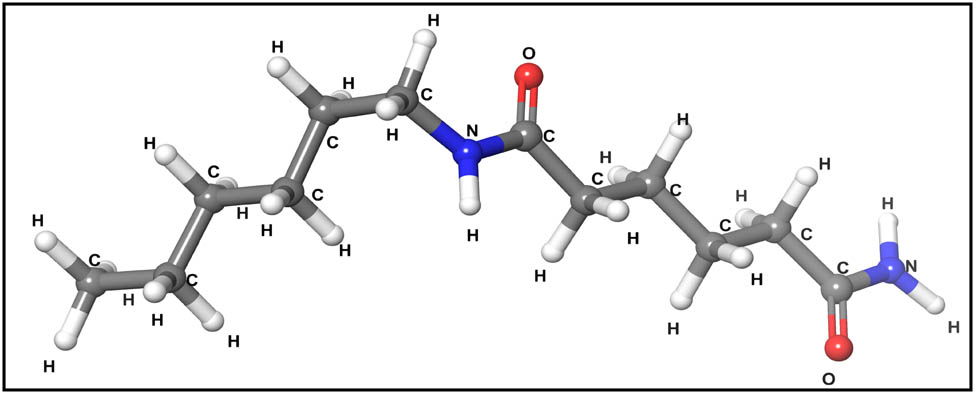
Structure of nylon 66.
Guo et al. selected Materials Studio 2017 software to enhance the model and simulation (32). Software such as Material Studio 2020 and Maestro were used in this study to examine the simulation. Numerous techniques are employed to ascertain the outcomes of optical and electrical qualities, such as CASTEP Modules based on the DFT (33). The nylon 66 Brillouin zone’s K-point was sampled using a 6 × 6 × 1 Monkhorst-Pack grid. The generalized gradient Approximation-Perdew, Burke, and Ernzerhof (GGA-PBE) and hybrid B3LYP/6-31 (Becke, Lee, Yang, and Parr47) functionals were used to estimate the exchange-correlation energy, and all simulations used a plane wave energy cut-off of 600 eV.
2.2 XRD
Figure 3 displays the nylon 66 XRD pattern spectra. X-rays are dispersed in specific directions and high-intensity peaks can be seen when there is a periodic arrangement of atoms. When differentiating between crystalline, semi-crystalline, and amorphous polymers, the type of peak displayed in the XRD patterns tells us the amount of crystallinity in the material.
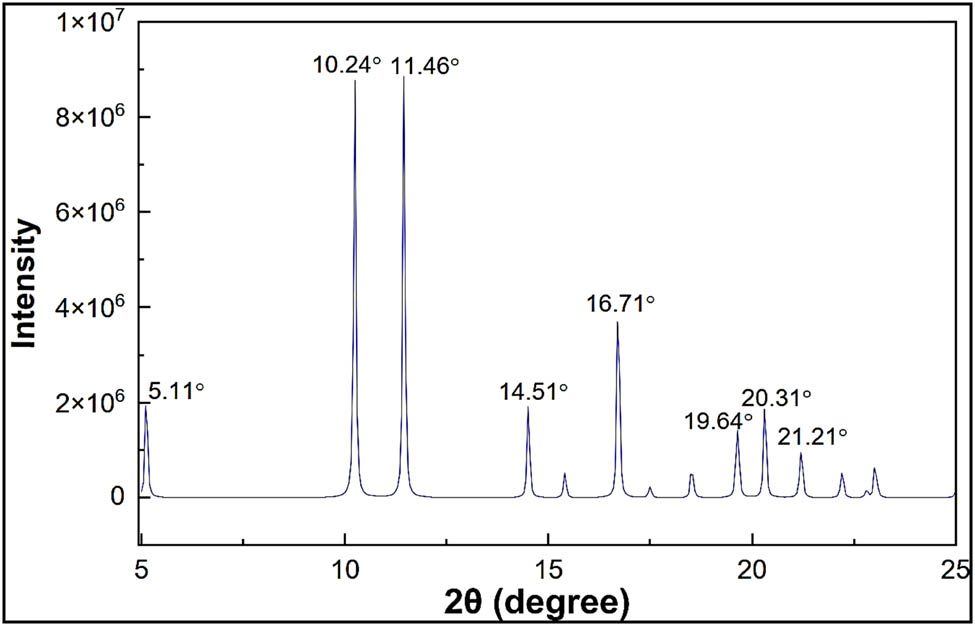
XRD spectra of nylon 66 structure.
Debye–Scherrer equation (Eq. 1) can be used to predict the particle sizes (34). The additional diffraction peaks at scattering angles 2θ = 5.11°, 10.24°, 11.46°, 14.51°, 16.71°, 19.64°, 20.31°, and 21.21° of the nylon 66 are observed.
where D – particle diameter (nm), λ – X-ray radiation wavelength (λ = 0.154 nm), K – a constant and typically accepted as 0.9, β – full width at half maximum (FWHM) (rad), θ – Bragg angle of the peak.
2.3 HOMO–LUMO bandgap
The HOMO–LUMO energy gap of nylon 66, which indicates the molecule’s redox potential, was calculated using the B3LYP/6-31 level. The ability to obtain an electron is represented by the electron acceptor LUMO, whereas the ability to supply an electron is represented by the electron donor HOMO (35).
Nylon 66 polymer is produced by chemically reacting adipic acid [HOOC(CH2)4COOH] n with hexamethylene diamine [H2N(CH2)6NH2] n in a condensation polymerization (30). Nylon 66’s thermophysical characteristics might change depending on the composition. Table 1 displays the values of nylon 66’s properties.
| Property | Units | Value | Test method |
|---|---|---|---|
| Crystalline melting point | °C | 260 | ASTM D3418 |
| Density | g·cm−3 | 1.42 | ASTM D792 |
| Dielectric strength 1 mm | kV·mm−1 | 15.7 | ASTM D149 |
| Flexural modulus | GPa | 3.10 | ASTM D790 |
| Glass transition temperature | °C | 50 | ASTM E1356 |
| Modulus of elasticity | MPa | 3,300 | ASTM D53457 |
| Specific gravity | g·cm−3 | 1.15 | ASTM D792 |
| Specific heat capacity | J·g−1·K−1 | 1.5 | ASTM C351 |
| Specific volume resistance | Ω·cm | 1014 | ASTM D257 |
| Tensile strength at break | MPa | 82.7 | ASTM D638 |
| Thermal conductivity | W·m−1·K−1 | 0.245 | ASTM C177 |
The potentials were estimated using Koopmans’ theorem, with the oxidation potential being 1.86 eV and the reduction potential being −4.13 eV. The oxidation potential is calculated using the HOMO energy, whereas the reduction potential is computed using the LUMO energy. Figure 4 illustrates how to calculate the band gap by deducting the LUMO value from the HOMO value.
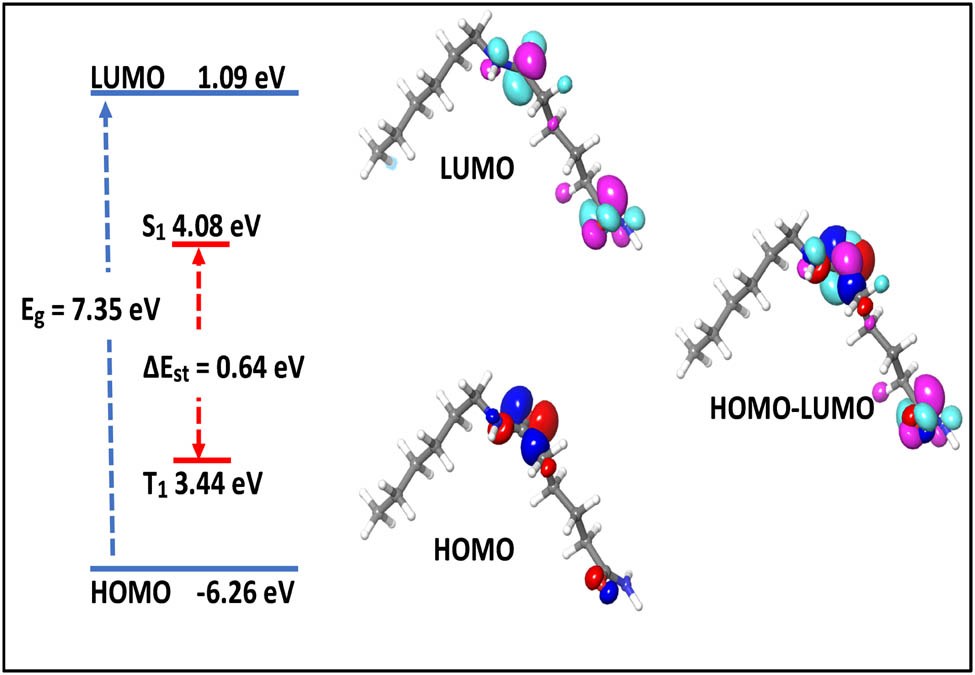
Highest and lowest molecular orbital electronic distributions, energy level, and bandgaps (Eg), the lowest singlet (S1), and triplet (T1) of nylon 66.
3 Results and discussion
3.1 XRD analysis
On the basis of the XRD results shown in Table 2, it is expected that the nylon 66 particle average sizes are close to 98.154 nm.
Crystallites (grain) size D determination using Debye–Scherrer equation
| 2θ Degree | FWHM | D (nm) |
|---|---|---|
| 5.11 | 0.08244 | 96.4604 |
| 10.24 | 0.07343 | 108.6220 |
| 11.46 | 0.07014 | 113.8321 |
| 14.51 | 0.07335 | 109.1807 |
| 16.71 | 0.08378 | 95.8404 |
| 19.64 | 0.10217 | 78.9118 |
| 20.31 | 0.08195 | 98.4835 |
| 21.21 | 0.09633 | 83.9026 |
It has been shown that the key to turning on nanoparticles’ catalytic activities is to regulate their diameter. It has been discovered that making particles smaller causes them to have a negative redox potential (40).
Sometimes the degree of crystallinity makes it possible to pinpoint the root of flaws like insufficient rigidity, cracks, whitening, and other defects. The XRD spectra are used in Eq. 2 to calculate the degree of crystallinity (42)
The distribution curve of nylon 66 was illustrated in Figure 5 depending on the SEM image determined the total area of the crystalline peaks, which is 1,497,886.462, and the total area of the crystalline and amorphous peaks is 3,227,005.373, using the XRD spectra displayed in Figure 3. The degree of crystallinity estimated using Eq. 2 is 46.44%.
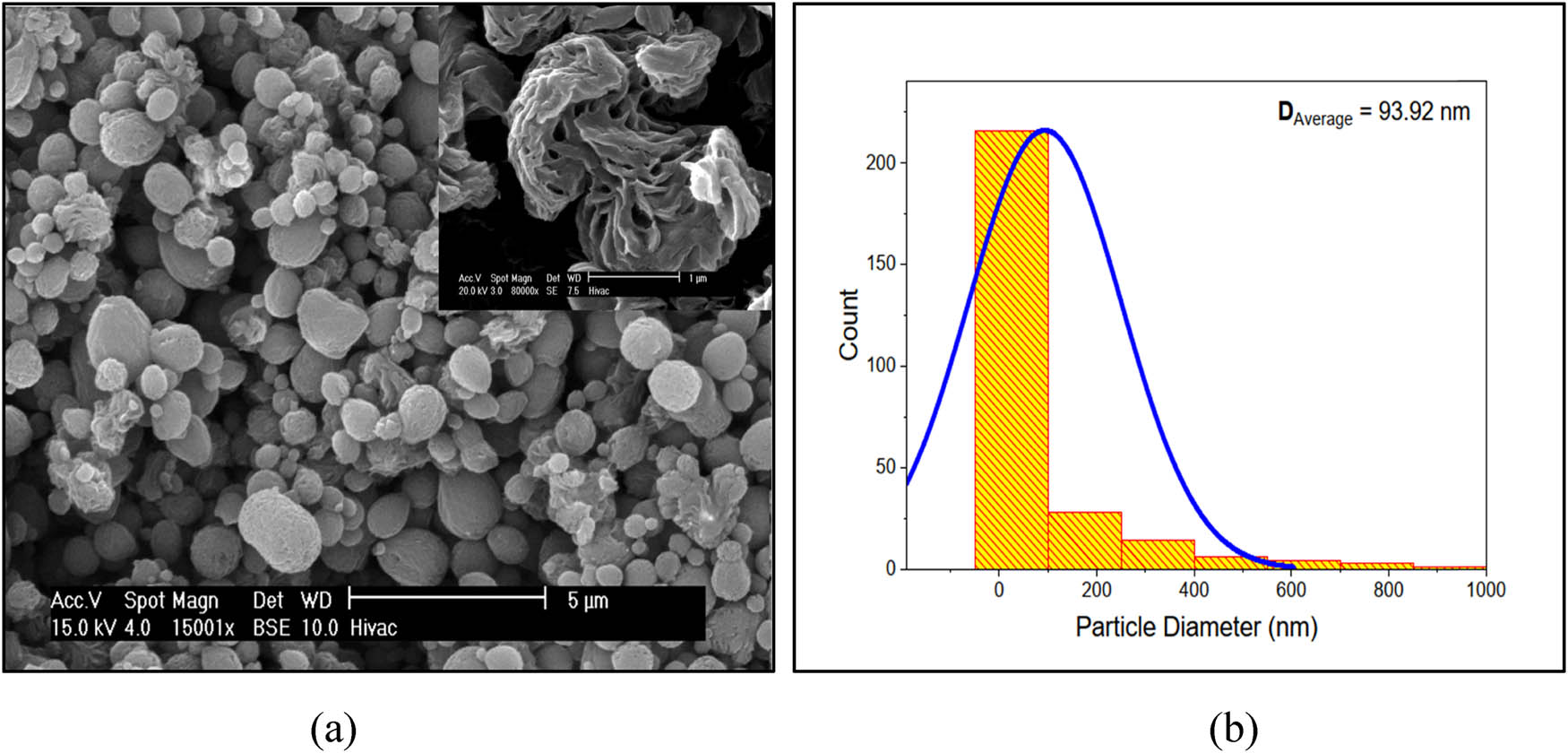
(a) SEM micrograph of nylon 66 spherulite (41). (b) Nylon 66 crystallites’ (grains) diameter distribution curve.
Franco et al. obtained the degree of crystallinity using DSC of nylon 66 as 39.45% (43). Chen et al. calculated that the degree of crystallinity was 33.6% from the relation (ΔH m/ΔH m°), where ΔH m – the fusion enthalpy, 65.8 J·g−1; ΔH m° – the fusion enthalpy of pure crystalline nylon 66, 196 J·g−1 (44). And when doped with micro-CaCO3 and nano-CaCO3, according to Ghadami and Ghadam using a thermal analysis apparatus (DSC-60 Shimadzu, Japan), the degree of crystallinity decreased from 44.78% to 41.38% when 3% micro-CaCO3 was doped into nylon 66 and 39.54% when it was doped with 3% nano-CaCO3 (45). According to the research done by Lu et al. using a Q-100 thermal analysis apparatus, the degree of crystallinity increased from 29.6% to 33.7% when 3% nano-SiO2 was doped into nylon 66 (46).
Crystallization (T c) and melting (T m), first-order transitions, show peaks in the exothermic and endothermic directions, respectively. Figure 6 depicts the DTA curve (differential thermal analysis) for nylon 66.
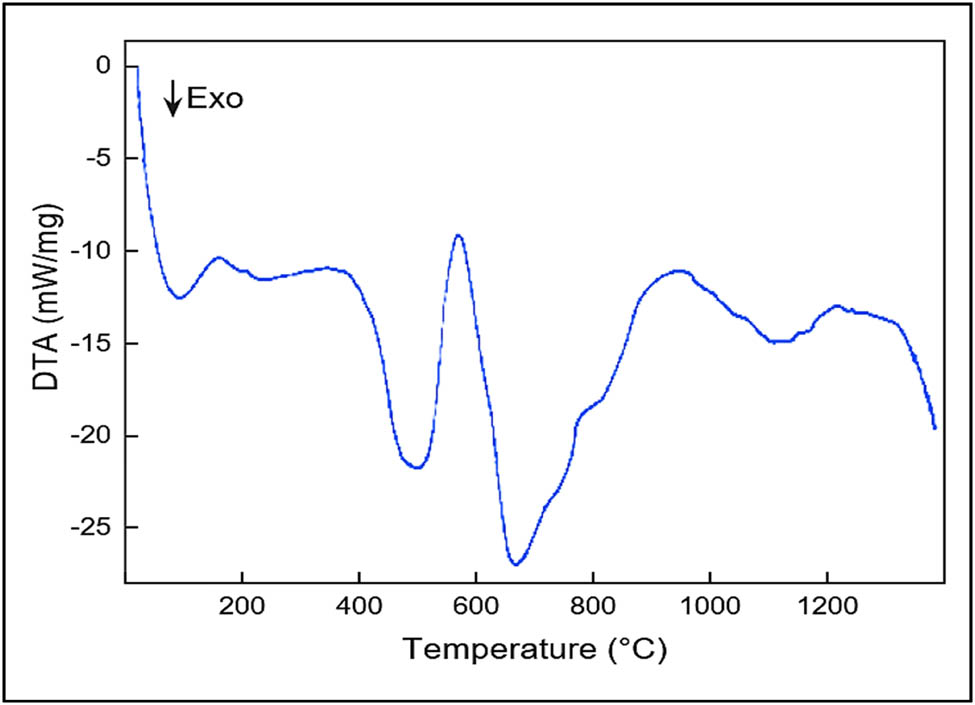
Differential thermal analysis thermogram of nylon 66.
The material may undergo breakdown processes at higher temperatures (T d) after melting, producing wide peaks. The melting behavior is influenced by the polymer’s heating rate and crystallization temperature (47,48,49). For nylon 66, the glass transition temperature, T g = 50°C, and the Brill transition temperature occurs between 170°C and 220°C, well below the T m, which is in the range of 250–272°C. The value of T m depends upon the molar mass and degree of chain branching in a particular type of polymer (50,51,52).
3.2 HOMO–LUMO bandgap analysis
The HOMO energy of nylon 66 is −6.26 eV, while the LUMO energy is 1.09 eV. The HOMO–LUMO band gap is 7.35 eV. The energy gap between the HOMO and LUMO explains the eventual charge transfer interactions that occur within the molecule (53). The increased reactivity and decreased stability result from the lower band gap energy. Also, as illustrated in Figure 7, the lowest singlet (S 1) value for nylon 66 is 4.08 eV, and the highest triplet (T 1) value is 3.44 eV. Thus, the energy difference between them is 0.64 eV. By confining the S 1 state in nylon 66 monomers, the gap between the lowest singlet and the highest triplet can be increased.
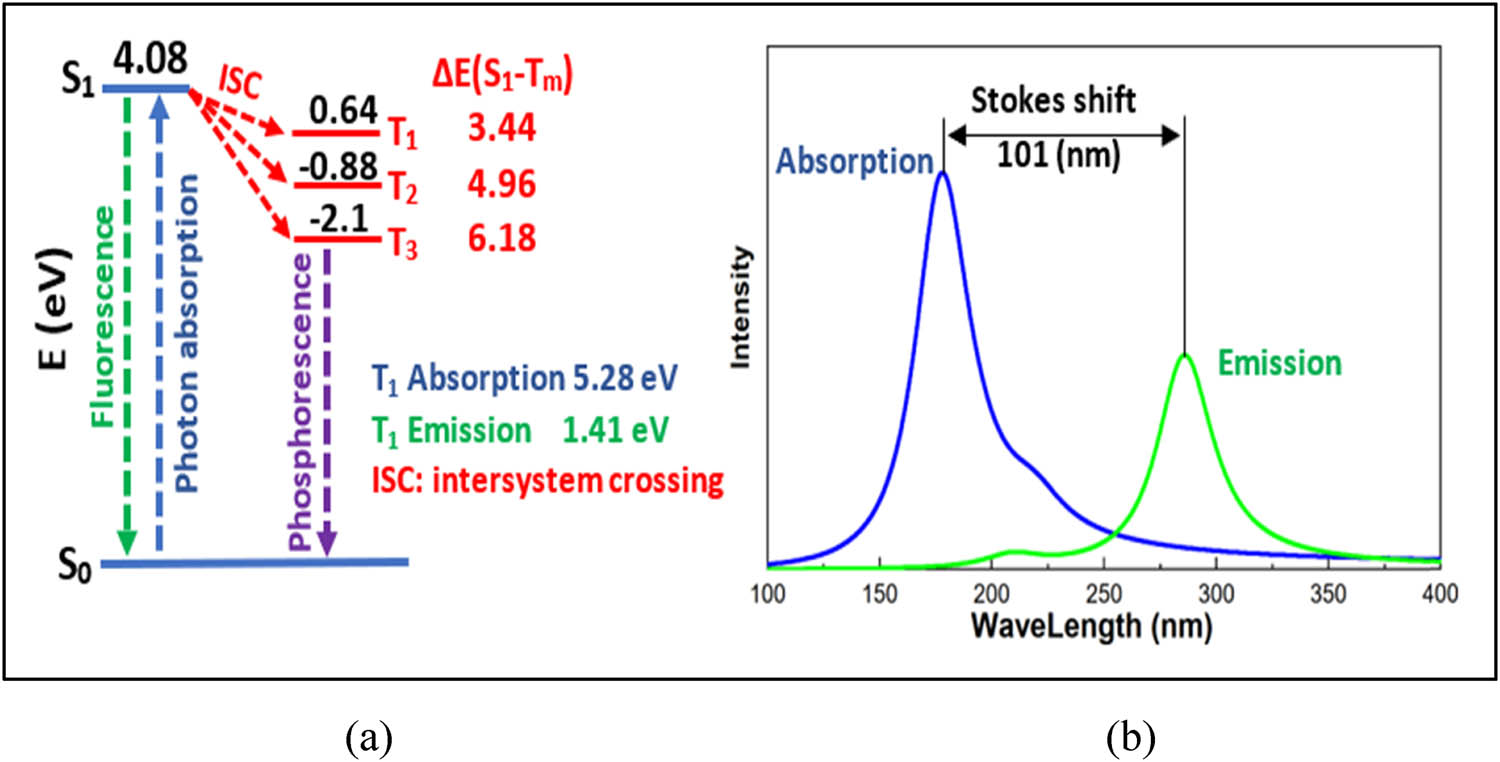
(a) The Jablonski energy diagram. (b) Ensemble excitation and emission spectra of nylon 66.
Calculation of molecules’ additional electronic characteristics must take into account their ionization potential and electron affinities. While the electron affinity has a negative LUMO energy value, the ionization potential has a negative HOMO energy value. Chemical hardness, softness, and electronegativity of nylon 66 are 3.675, 0.136, and 2.585 eV, respectively. The electrophilicity index may be computed by
which equals 0.909 eV (54).
As seen in Figure 4, the Jablonski diagram is a partial energy diagram that shows the various energy levels of a photoluminescent molecule. T 1, T 2, and T 3 triplet states have lower energies than the singlet state S1. Absorption transitions can occur rapidly from S0 to various vibrational levels in the singlet stimulated vibrational states. Additionally, emission transitions from the triplet to singlet states take longer to complete (phosphorescence) than those from the electronic singlet state to the ground electronic singlet state (fluorescence). When the vibration levels of two states overlap, the possibility of intersystem crossover increases. Because the interaction between the spin and orbital increases, the spin becomes more favorable. According to the Jablonski diagram, Shi et al. claimed that the amount of emission energy is always less than the amount of absorption energy (55), notwithstanding the Stokes shift between the nylon 66 absorption transition and emission transitions in Figure 7b.
3.3 Tauc optical bandgap energy
The Tauc relation, as illustrated in Eq. 4, is a popular technique for evaluating the bandgap. The optical bandgap of nylon 66 is estimated by extrapolating the linear region to the (αhυ)1/n vs photon energy curve, according to Figure 8 (56).
where α – absorption coefficient, h – Planck constant, υ – incident photon frequency, β – band tailing parameter constant, E g – optical bandgap energy, n – the power factor of the transition mode.
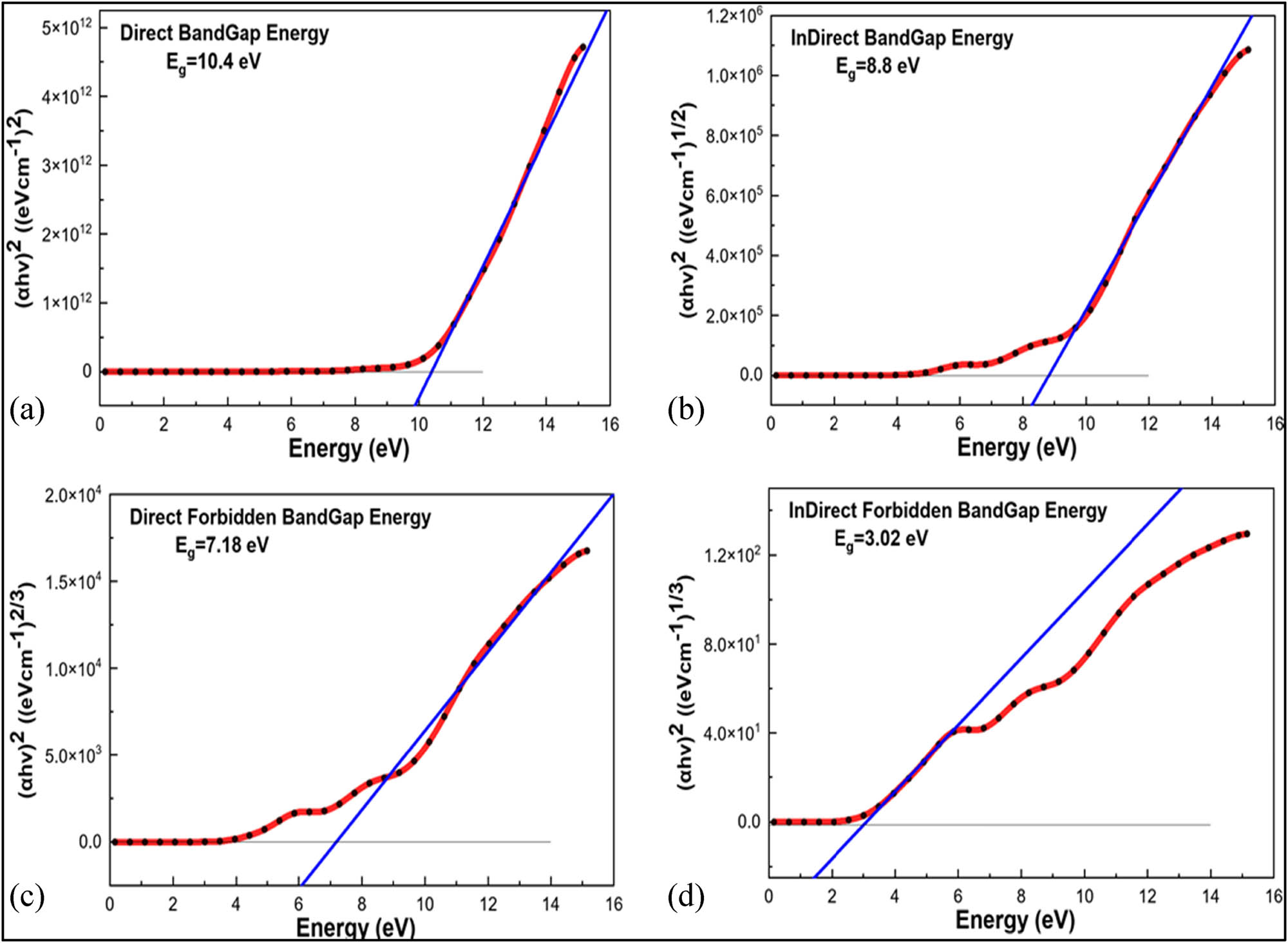
A schematic Tauc plot demonstrating the band gap for nylon 66 using several transition modes: (a) direct bandgap, (b) indirect bandgap, (c) direct forbidden bandgap, and (d) indirect forbidden bandgap.
Gupta et al. used UV-Vis spectroscopy in the 200–800 nm range to examine the optical properties of nylon 66 specimens. As shown in Eq. 5, the optical bandgap energy for direct transitions was calculated using the Tauc relation, which led to a link between the quantity of carbon atoms per conjugation length (N) and bandgap energy (E g) (57).
The simulated value of the direct bandgap energy for nylon 66 using the Tauc relation is 10.4 eV, whereas the experimental value is 9.9 eV while using Eq. 4. The percentage error between the simulated and experimental values was 5.05%. When the result of the HOMO–LUMO simulated bandgap of 7.35 eV is compared with the forbidden direct bandgap of 7.18 eV using the Tauc relation, the percentage error is 2.36%.
3.4 Density of states
Partial density of states and local density of states (LDOS) are semi-qualitative methodologies for evaluating the electronic structure. LDOS in optical relates to the conditions that a photon can occupy. Different elements in the nylon 66 structure have various local densities and influence spontaneous emission differently. For oxygen and nitrogen elements, very low LDOS is expected. That induces the inhibition of the emission of photons. When evaluating the physical properties of materials, the density of states (DOS) is critical.
The electrical band structure of nylon 66 is demonstrated in this study, along with the state’s examined partial and total densities at standard pressure. Figure 9 depicts the partial and total densities of states of nylon 66. As H: 1s1, C: 2s2 2p2, N: 2s2 2p3, and O: 2s2 2p4 were all considered valence electrons in this experiment, the valence band between −7.0 eV and −10.0 eV is constituted of C: 2s, N: 2s 2p, O: 2s 2p states, with H: 1s, C: 2p states dominating. However, the C: 2p state is the most critical in generating this band.
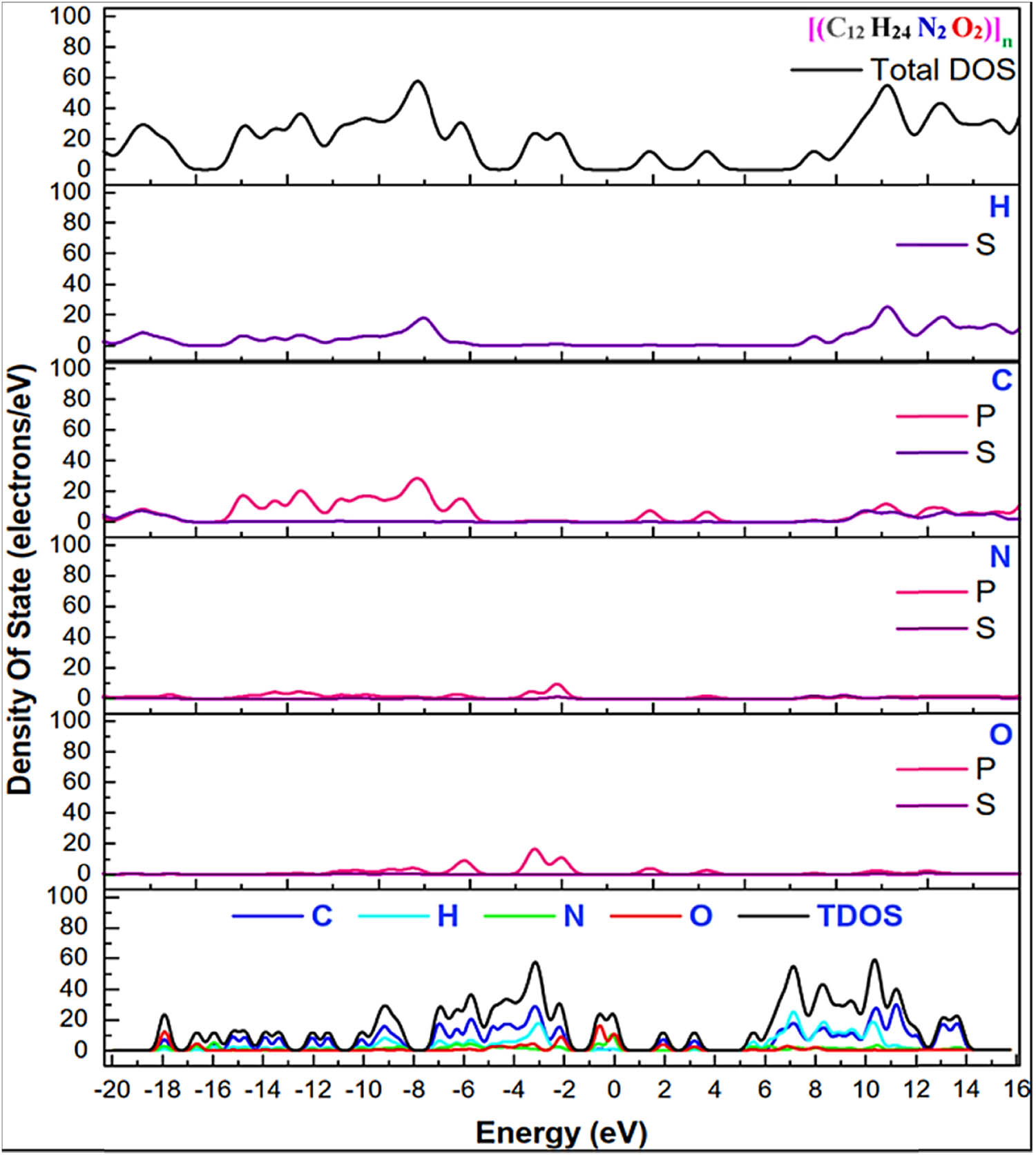
Total and local density of states for nylon 66 in Brillouin zone using GGA-PBE.
3.5 Optical properties of nylon 66
Czichos et al. defined and explained the concept of optical properties (58). This study focused on nylon 66 absorption (ω), dielectric function ε(ω), refractive index n(ω), and conductivity σ(ω). The fluctuation of the optical coefficients of the nylon 66 structure as a function of photon energy was investigated from 0 to 20 eV.
Figure 10 shows the simulated absorption spectra, which have two peaks with variable strengths. The two low-intensity peaks, representing two crucial transitions between the electronic states of nylon 66, are located at 5.9 and 15 eV, respectively. The transition from the occupied n to the unoccupied n* state is represented by the conduction bands’ initial peak. The transition of π–π* states for C bonds in 2s2 2p2 is indicated by the area of large second peak. Kuila et al. calculated the absorption coefficient (ω) mathematically using Eq. 6 (59). Figure 10 depicts the simulated and mathematically calculated absorption spectra
where ω is the light frequency, ε 1(ω) and ε 2(ω) are the real and imaginary dielectric function.
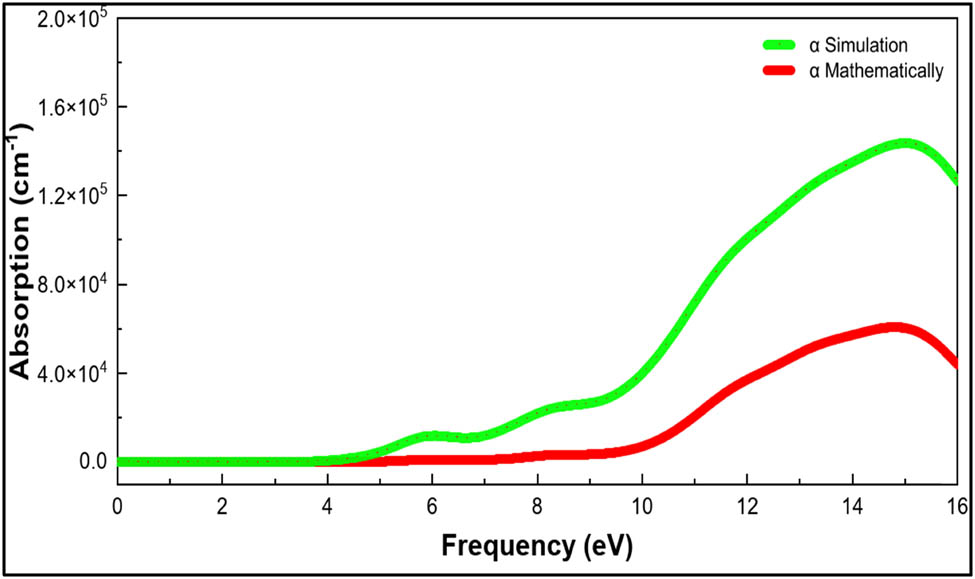
Mathematical and simulation absorption vs frequency of nylon 66.
The optical properties of materials and the interactions that take place when light travels through matter can be explained using the simulated dielectric function spectra in Figure 11. It affects both the absorption effects and the dispersion effects via its imaginary and real components, respectively (dielectric loss and dielectric constant) (60). Figure 11b illustrates the total of the dielectric function components. The electron transition energy between the conduction and valence bands is reflected in the dielectric function (61). The outcome was an increase in reflectance and an increase in the absorption coefficient (60). Figure 11a depicts the hypothetical dielectric function, which has three peaks between 0 and 16 eV that are always associated with electron excitation. The imaginary dielectric portion has a low value for incident photons with energy below 4 eV. Additionally, a real dielectric function value greater than zero and an imaginary dielectric function equal to zero in the 0–3.5 eV range denote a transparent zone. This suggests that there is no low-energy absorption since nylon 66’s valence electrons are not readily available.
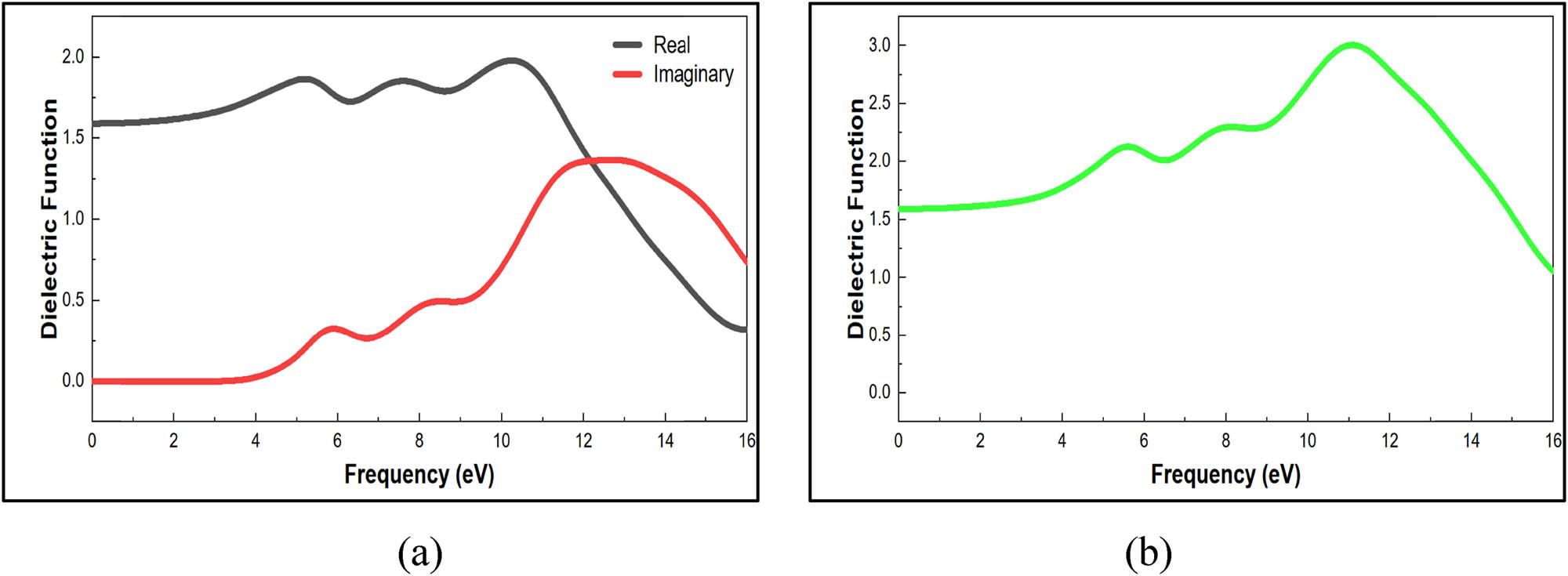
(a) Real and imaginary dielectric function optical properties of nylon 66. (b) Dielectric function optical properties of nylon 66 structure.
According to Figure 11b, the dielectric function is 3 at 11 eV. According to the MatWeb website, the dielectric function ranges from 3 to 8 (62). At high frequencies, ion oscillations could be the only source of loss, which lowers the dielectric loss value, although at low and intermediate frequencies, ion migration conduction loss and ion polarization loss give the dielectric loss a significant amount of weight (63). A method that connects the energy bandgap to the dielectric constant was developed by Brza et al. in 2019 (64). Propagation in absorbing materials can be described by the value of the refractive index, which consists of the summation of the real refractive index (ω) and the imaginary refractive index (ω) as illustrated in Figure 12a. From the preceding values, when frequency equals zero, n(0) = 1.25 is shown in Figure 12b. The dielectric function and refractive index validate the relation n =
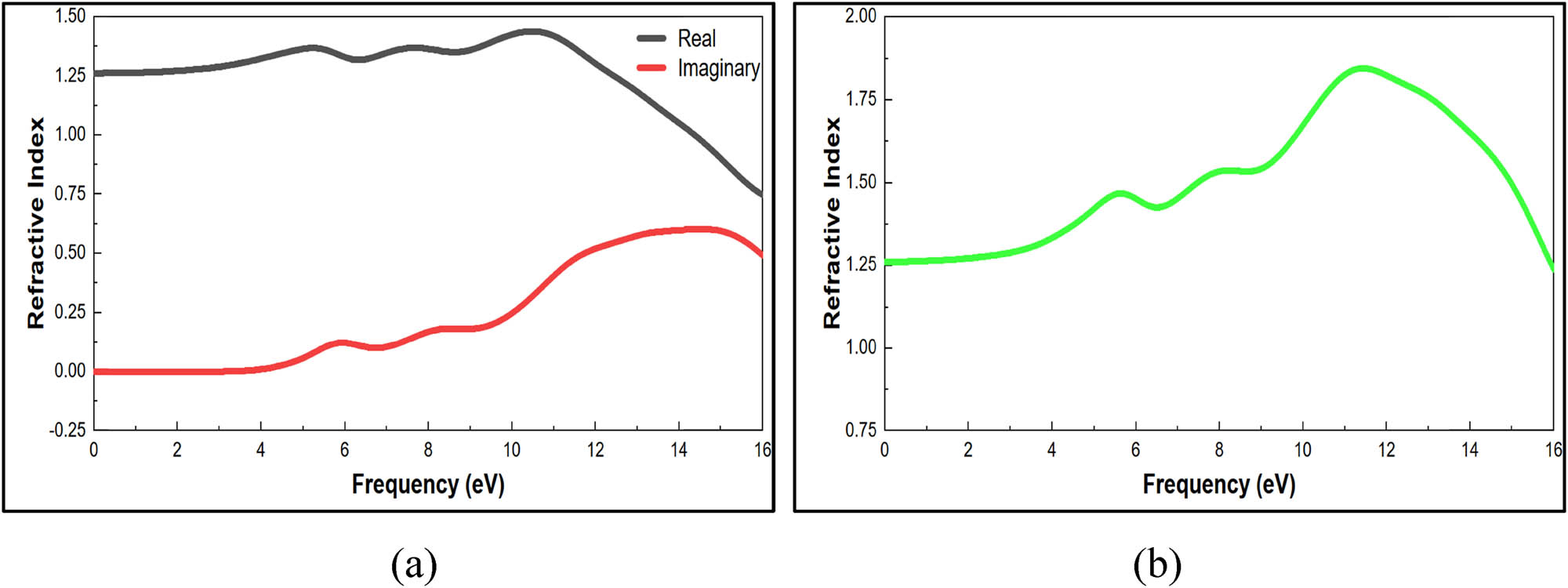
(a) Real and imaginary refractive index optical properties of nylon 66. (b) Refractive index optical properties of nylon 66 structure.
The average simulated refractive index from 0 to 16 eV was 1.502. According to Shackelford, polymer refractive indices ranged from 1.4 to 1.6, with nylon 66 having an average theoretical refractive index of 1.53 (65). El-Nicklawy et al. measured that the refractive index value for nylon 66 ranged from 1.513 to 1.569, with an approximate error of 5% (66).
The optical conductivity is estimated by summating the real conductivity σ1(ω) and the imaginary conductivity σ2(ω), Figure 13a illustrates the real and imaginary optical conductivity components as a function of frequency.
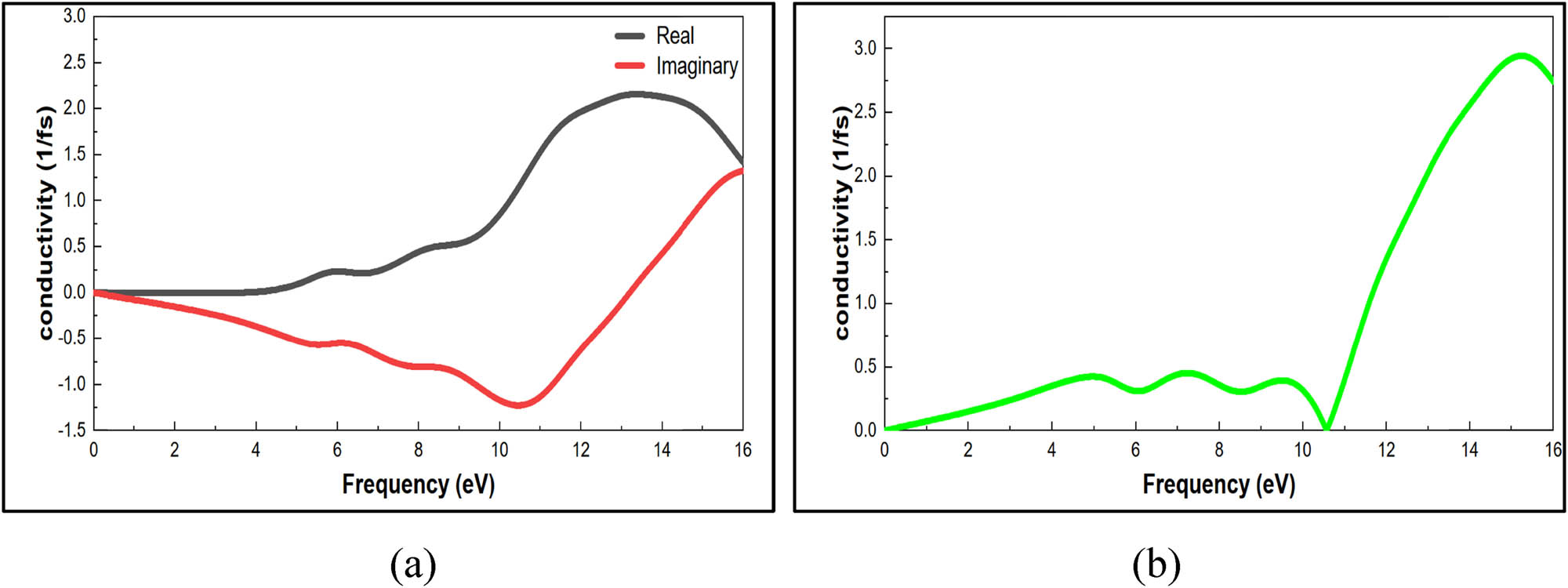
(a) Real and imaginary optical conductivity properties of nylon 66. (b) Optical conductivity properties of nylon 66.
The genuine peak at 5.93 eV corresponds to the fundamental bandgap because of the inter-band transitions. In the 0–13.2 eV range, imaginary peak has a negative value, indicating that the charge is well dispersed. Furthermore, real conductivity equals zero (1/fs) in the 0–2.11 eV range, due to the absorption at low frequency, because the electrical field cannot react with the valence electrons inside the nylon 66 structure.
3.6 Nonlinear optical property
The second-order polarizability (first-order hyperpolarizability (β)), dipole moment (µ), and polarizability (α) were computed using the finite-field technique and the B3LYP/6-31G basis. The equations for computing the magnitude of β, µ, and α are as follows:
The second-order polarizability (first order hyperpolarizability (β)) in the (A.U) unit is as follows: β xxx = −61.384, β xyy = 35.870, β xzz = 6.829, β yyy = 7.493, β yzz = 4.661, β yxx = −14.567, β zzz = 4.616, β zxx = 28.209, β zyy = −5.598. Using Eq. 7, the β tot is 33.11 A.U, the calculated value of the second-order polarizability (β) have been converted into electrostatic units (esu) by multiplying with 8.6393 × 10−33 esu. Then, the results are β = 2.86 × 10−31 esu.
Using Eq. 8, the overall dipole moment is calculated to be 0.26789 Debye from the individual dipole moments in the X, Y, and Z components, which are, respectively, 0.1224, 0.0004, and 0.2383 Debye. The dielectric constant is determined by the ensuing dipole moment, which depends on the amount of displacement and the number of charges. Thus, materials having ionic bonds and polymers like nylon 66 that include polar groups −OH− and −NH− have greater dielectric constants than others (67).
When a dipole moment arises in the core of a molecule that has positive and negative charge, some core molecules with multiple positive and negative charges, such as quadrupole moments, octupole moments, and hexadecapolar moments, form (68). A molecular quadrupole moment results from an uneven charge distribution within the molecule. Quadrupole moments cannot be explained by two-point charges separated by a distance, in contrast to molecular dipole moments. The second-order term in a molecule’s multipole expansion of an electric field is called a quadrupole.
Quadrupole moment resulting in the Debye-Ang unit are as follows: q xx = −78.9185, q yy = −106.2437, q zz = −99.3962, q xy = −4.8355, q xz = −21.1584, q yz = −8.3433, when using the 3 × 3 symmetric matrix as described in Eq. 9, a molecular quadrupole is −779,723.34 Debye-Ang (69). The quadrupole moment has six different values. However, the traceless quadrupole moments are used to omit the spherical component.
The values of the polarizabilities in αxx, αyy, and αzz, respectively, in the A.U unit are 190.874, 132.906, and 132.038 when using Eq. 10. The polarizability (α) is 151.9393; therefore, the anisotropy of the polarizability (∆α), using Eq. 11, is calculated to be 41.3; the computed value of polarizability (α) and anisotropy of the polarizability (∆α) have been converted into electrostatic units (esu) by multiplying with 0.1482 × 10−12 esu. Then, the results are α = 2.25 × 10−11 esu and ∆α = 6.12 × 10−12 esu.
The dipole of a molecule is not a magnetic dipole that generates a magnetic field, but an electric dipole with an intrinsic electric field. Numerous compounds have these dipole moments as a result of uneven distribution of positive and negative charges on certain atoms.
4 Conclusion
In this work, DFT and finite-field techniques were used to analyze the optoelectronic and spectroscopic properties of nylon 66 polymer. Based on the XRD results, this study determined that nylon 66’s average particle size is roughly 98.154 nm. The distribution curve in the SEM micrograph is 93.920 nm, while the average crystallite size is 96.037 nm. The bandgap widens as the crystal size shrinks because the energy band structure varies with size. Nylon 66 crystallizes to a degree of 46.44%. The percentage inaccuracy is 2.36% when the restricted bandgap is contrasted with the simulated bandgap of 7.35 eV. The difference in inaccuracy between the experimental and simulated values was 5.05%. The absorption coefficient value, however, shows that while the refractive index and dielectric function remain stable, the optical conductivity rises. The output results are particularly effective in the area of nylon 66’s spectroscopic and optoelectronic properties.
Due to the importance of the effects of linear and nonlinear optical properties, more research is needed in this area. Nylon 66 is a significant and extremely desirable thermoplastic polymer with good optoelectronic characteristics. It might be possible to create novel materials if optical properties can be anticipated using computer simulation based on knowledge of their chemical structure and composition. The output findings and recommendations of this study are highly useful for understanding the spectroscopic characteristics of nylon 66 and optoelectronics. Therefore, there are still a lot of industrial and technological potential for research regarding the characteristics and applications of nylon 66 polymers.
Acknowledgment
The author would like to thank the Faculty of Engineering Technology for providing the laboratory facilities to accomplish this work.
-
Funding information: The author states no funding involved.
-
Author contributions: Ali F. Al-Shawabkeh: writing – original draft, writing – review and editing, methodology, data curation, formal analysis, software, and validation.
-
Conflict of interest: The author states no conflict of interest.
-
Data availability statement: The author confirms that the data findings of this study are original and was carried out at Department of Physics and Basic Sciences, Faculty of Engineering Technology, Al-Balqa Applied University, Amman, Jordan.
References
(1) Song L, Hu Y, He Q, You F. Study on crystallization, thermal and flame retardant properties of nylon 66/organoclay composites by in situ polymerization. J Fire Sci. 2008;26:475–92. doi: 10.1177/0734904108092115.10.1177/0734904108092115Search in Google Scholar
(2) Lin DJ, Chang CL, Lee CK, Cheng LP. Fine structure and crystallinity of porous nylon 66 membranes prepared by phase inversion in the water/formic acid/Nylon 66 system. Eur Polym J. 2006;42:356–67. doi: 10.1016/j.eurpolymj.2005.07.007.10.1016/j.eurpolymj.2005.07.007Search in Google Scholar
(3) Suzuki A, Chen Y, Kunugi T. Application of a continuous zone-drawing method to nylon 66 fibres. John Wiley & Sons, Inc. J Polym Sci B: Polym Phys. 1998;36:473–81. doi: 10.1002/(SICI)1099-0488(199802)36:3 < 473: AID-POLB10 > 3.0.CO,2-D.10.1002/(SICI)1099-0488(199802)36:3<473::AID-POLB10>3.0.CO;2-DSearch in Google Scholar
(4) Randhawa KS, Patel AD. A review on tribo-mechanical properties of micro-and nanoparticulate-filled nylon composites. J Polym Eng. 2021;41(5):339–55. doi: 10.1515/polyeng-2020-0302.10.1515/polyeng-2020-0302Search in Google Scholar
(5) Dynisco, Understanding Plastics and Polymers - The Different Types of Plastic: 2019; AZO Materials. https://www.azom.com/article.aspx? ArticleID = 17477.Search in Google Scholar
(6) Carraher CE. Polymer chemistry. Revised and expanded. Vol. 10016. 6th edn. New York, NY: Marcel Dekker, Inc; 2003. http://www.dekker.com.Search in Google Scholar
(7) Bicerano J. Prediction of polymer properties. New York: Dekker; 2002. p. 784. doi: 10.1201/9780203910115.10.1201/9780203910115Search in Google Scholar
(8) Chow WS, Mohd Ishak ZA. Polyamide blend-based nanocomposites: A review. Exp Polym Lett. 2015;9(3):211–32. doi: 10.3144/expresspolymlett.2015.22.10.3144/expresspolymlett.2015.22Search in Google Scholar
(9) Gilbert M. Chapter 18 - Aliphatic Polyamides. Brydson’s Plastics Materials. 8th edn. Butterworth-Heinemann: Elsevier; 2017. p. 487–511. doi: 10.1016/B978-0-323-35824-8.00018-9.10.1016/B978-0-323-35824-8.00018-9Search in Google Scholar
(10) Randhawa KS, Patel AD. Influence of boric anhydride reinforcement on mechanical properties and abrasive wear of nylon 6. Mater Res Express. 2020;7(5):055303. doi: 10.1088/2053-1591/ab8ee4.10.1088/2053-1591/ab8ee4Search in Google Scholar
(11) Vaidya MJ, Raycha IM, Trivedi DP, Shah JP, Randhawa KS. Tribo-mechanical characterisation of MoS2 and H-BN reinforced PA66 composite. Aust J Mech Eng. 2022;1–10. doi: 10.1080/14484846.2022.2073019.10.1080/14484846.2022.2073019Search in Google Scholar
(12) Qin H, Su Q, Zhang S, Zhao B, Yang M. Thermal stability and flammability of polyamide 66/montmorillonite nanocomposltes. Polymer. 2003;44:7533–8. doi: 10.1016/j.polymer.2003.09.014.10.1016/j.polymer.2003.09.014Search in Google Scholar
(13) Araujo EM, Araujo KD, Paz RA, Gouveia TR, Barbosa R, Ito EN. Polyamide 66/Brazilian clay nanocomposites. J Nanomater. 2009;1–5. Article ID 136856. doi: 10.1155/2009/136856.10.1155/2009/136856Search in Google Scholar
(14) Song L, Hu Y, He QL, You F. Study on crystallization, thermal and flame retardant properties of Nylon 66/organoclay nanocomposites by in situ polymerization. J Fire Sci. 2008;26:475–92. doi: 10.1177/0734904108092115.10.1177/0734904108092115Search in Google Scholar
(15) Modern Plastic Encyclopedia Handbook. Modern Plastics Magazine. New York: McGraw hill; 1994. ASIN: B000HA2KN8.Search in Google Scholar
(16) Chang HH, Beltsios K, Lin DJ, Cheng LP. Formation of polyamide 12 membranes via thermal-nonsolvent induced phase separation. J Appl Polym Sci. 2013;130:14–24. doi: 10.1002/app.39138.10.1002/app.39138Search in Google Scholar
(17) Shih CH, Gryte CC, Cheng LP. Morphology of membranes formed by the isothermal precipitation of polyamide solutions from water/formic acid systems. J Appl Polym Sci. 2005;96:944–60. doi: 10.1002/app.21545.10.1002/app.21545Search in Google Scholar
(18) Zeni M, Riveros R, de Souza JF, Mello K, Meireles C, Filho GR. Morphologic analysis of porous polyamide 6,6 membranes prepared by phase inversion. Desalination. 2008;221:294–7. doi: 10.1016/j.desal.2007.03.012.10.1016/j.desal.2007.03.012Search in Google Scholar
(19) Vallés‐Lluch A, Camacho W, Ribes‐Greus A, Karlsson S. Influence of water on the viscoelastic behavior of recycled nylon 6, 6. J Appl Polym Sci. 2002;85:2211–8. doi: 10.1002/app.10838.10.1002/app.10838Search in Google Scholar
(20) Randhawa KS, Patel A. The effect of environmental humidity/water absorption on tribo-mechanical performance of polymers and polymer composites – A review. Ind Lubr Tribol. 2021;73(9):1146–58. doi: 10.1108/ILT-02-2021-0045.10.1108/ILT-02-2021-0045Search in Google Scholar
(21) Randhawa KS, Patel A. Influence of moisture/water absorption on mechanical and thermal properties of polyamide6/boric oxide composites. Pigment Resin Technol. 2022;51(3):354–63. doi: 10.1108/PRT-03-2021-0031.10.1108/PRT-03-2021-0031Search in Google Scholar
(22) Nguyen TN, Moon J, Kim JJ. Microstructure and mechanical properties of hardened cement paste including Nylon 66 nanofbers. Constr Build Mater. 2020;232:117–34. doi: 10.1016/j.conbuildmat.2019.117134.10.1016/j.conbuildmat.2019.117134Search in Google Scholar
(23) Yanilmaz M, Zhu J, Lu Y, Ge Y, Zhang X. High-strength, thermally stable nylon 6,6 composite nanofiber separators for lithium-ion batteries. J Mater Sci. 2017;52:5232–41. doi: 10.1007/s10853-017-0764-8.10.1007/s10853-017-0764-8Search in Google Scholar
(24) Elzein T, Brogly M, Schultz J. Crystallinity measurements of polyamides adsorbed as thin films. J Polym. 2002;43:4811–22. doi: 10.1016/S0032-3861(02)00239-2.10.1016/S0032-3861(02)00239-2Search in Google Scholar
(25) Vasanthan N, Kotek R, Jung D-W, Shin D, Tonelli AE, Salem DR. Lewis acid–base complexation of polyamide 66 to control hydrogen bonding, extensibility and crystallinity. Polymer. 2004;45:4077–85. doi: 10.1016/j.polymer.2004.03.074.10.1016/j.polymer.2004.03.074Search in Google Scholar
(26) Bera D, Chatterjee R, Banerjee S. Aromatic polyamide nonporous membranes for gas separation application. e-Polymers. 2021;21:108–30. doi: 10.1515/epoly-2021-0016.10.1515/epoly-2021-0016Search in Google Scholar
(27) Ma H, Gao B, Wang M, Yuan Z, Shen J, Zhao J, et al. Strategies for enhancing thermal conductivity of polymer-based thermal interface materials: A review. J Mater Sci. 2021;56:1064–86. doi: 10.1007/s10853-020-05279-x.10.1007/s10853-020-05279-xSearch in Google Scholar
(28) Lin Y, Jia Y, Alva G, Fang G. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage. Renew Sustain Energy Rev. 2018;82:2730–42. doi: 10.1016/j.rser.2017.10.002.10.1016/j.rser.2017.10.002Search in Google Scholar
(29) Huang C, Qian X, Yang R. Thermal conductivity of polymers and polymer nanocomposites. Mater Sci Eng R Rep. 2018;132:1–22. doi: 10.1016/j.mser.2018.06.002.10.1016/j.mser.2018.06.002Search in Google Scholar
(30) Ellis B, Smith R, editors. Polymers: a property database. 2nd edn. CRC Press: Taylor & Francis Group; 2008. p. 1200. doi: 10.1201/9781420005707.10.1201/9781420005707Search in Google Scholar
(31) Pal S. Fundamentals of molecular structural biology. 1st edn. UK: Academic Press; 2019. p. 138. doi: 10.1016/C2017-0-02189-4.10.1016/C2017-0-02189-4Search in Google Scholar
(32) Guo L, Ding S, Yuan S, Gou X, Cai F, Wang D, et al. Study on the thermal properties and insulation resistance of epoxy resin modified by hexagonal boron nitride. e-Polymers. 2021;21(1):681–90. doi: 10.1515/epoly-2021-0069.10.1515/epoly-2021-0069Search in Google Scholar
(33) Clark SJ, Segall MD, Pickard CJ, Hasnip PJ, Probert MI, Refson K, et al. First principles methods using CASTEP. Z Für Kristallographie-Crystalline Mater. 2005;220(5–6):567–70. doi: 10.1524/zkri.220.5.567.65075.10.1524/zkri.220.5.567.65075Search in Google Scholar
(34) Bokuniaeva AO, Vorokh AS. Estimation of particle size using the Debye equation and the Scherrer formula for polyphasic TiO2 powder. J Phys Conf Ser IOP Publish. 2019;1410(1):012057. doi: 10.1088/1742-6596/1410/1/012057.10.1088/1742-6596/1410/1/012057Search in Google Scholar
(35) İzzet KA, Kiraz AÖ, Cetişli H, Donat R, Kolsuz N. Theoretical calculation on a compound formed by methyl alcohol and simmondsin. Int J Secondary Metabolite. 2016;3(1):39–48. doi: 10.21448/ijsm.240701.10.21448/ijsm.240701Search in Google Scholar
(36) Lee JE, Han DH, Kim YM, Choi KM. Comparison of mechanical and thermal properties of polyamide-66/rubber/fiber alloy composites. Asian J Chem. 2013;25(9):5237–44. doi: 10.14233/ajchem.2013.F25.10.14233/ajchem.2013.F25Search in Google Scholar
(37) Eltahir YA, Saeed HAM, Chen Y, Xia Y, Wang Y. Effect of hot drawing on the structure and properties of novel polyamide 5,6 fibers. Text Res J. 2014;84(16):1700–7. doi: 10.1177/0040517514527378.10.1177/0040517514527378Search in Google Scholar
(38) Polymer Properties Database, Nylon 6-6, Thermo-Physical Properties: Experimental/ Literature Data, Free Polymer Information Source; 2022. https://polymerdatabase.com/polymers/nylon6.html.Search in Google Scholar
(39) Zytel Nylon Resins, Product Guide and Properties, E. I. du Pont de Nemours & Co., Inc., Wilmington, Del.; 1998. http://plastics.dupont.com.Search in Google Scholar
(40) Chanda M. Introduction to polymer science and chemistry: a problem-solving approach. CRC Press: Taylor & Francis Group; 2013. p. 11–2.10.1201/b14577Search in Google Scholar
(41) Cheval N, Gindy N, Flowkes C, Fahmi A. Polyamide 66 microspheres metallised with in situ synthesised gold nanoparticles for a catalytic application. Nanoscale Res Let. 2012;7(182):1–9. doi: 10.1186/1556-276X-7-182.10.1186/1556-276X-7-182Search in Google Scholar PubMed PubMed Central
(42) Randhawa KS, Patel AD. Enhancing tribo-mechanical properties and thermal stability of nylon 6 by hexagonal boron nitride fillers. e-Polymers. 2020;20(1):733–45. doi: 10.1515/epoly-2020-0069.10.1515/epoly-2020-0069Search in Google Scholar
(43) Franco E, Dussán R, Amú M, Navia D. Statistical optimization of the sol–gel electrospinning process conditions for preparation of polyamide 6/66 nanofiber bundles. Nanoscale Res Leters. 2018;13(230):1–7. doi: 10.1186/s11671-018-2644-9.10.1186/s11671-018-2644-9Search in Google Scholar PubMed PubMed Central
(44) Chen YH, Ranganathan P, Lee YH, Rwei SP. New strategy and polymer design to synthesize polyamide 66 (PA66) copolymers with aromatic moieties from recycled PET (rPET). ACS Sustain Chem & Eng. 2021;9(9):3518–28. doi: 10.1021/acssuschemeng.0c08242.10.1021/acssuschemeng.0c08242Search in Google Scholar
(45) Ghadami A, Ghadam J. Influence of CaCO3 micro- and nano-particles on the structure and properties of Nylon-66. Bulg Chem Comm, Bulgarian Acad Sciences, Union Chem Bulgaria. 2015;47:19–34.Search in Google Scholar
(46) Lu H, Xu X, Li X, Zhang Z. Morphology, crystallization and dynamic mechanical properties of PA66/nano-SiO2 composites. Bull Mater Sci. 2006;29(5):485–90. doi: 10.1007/BF02914079.10.1007/BF02914079Search in Google Scholar
(47) Spells SJ. Characterization of Solid Polymers-New Techniques and developments. UK: Chapman & Hall; 1994. p. 368. doi: 10.1002/sca.4950160612.10.1007/978-94-011-1262-8Search in Google Scholar
(48) Singh RP, Desai SM, Pathak G. Thermal decomposition kinetics of photooxidized nylon 66. J Appl Polym Sci. 2003;87:2146–50. doi: 10.1002/app.11589.10.1002/app.11589Search in Google Scholar
(49) Young RJ, Lovell PA. Introduction to polymers. 2nd edn. UK: Nelson Thornes Ltd; 1991. p. 454. ISBN13 9780748757404.10.1007/978-1-4899-3176-4Search in Google Scholar
(50) Ramesh C, Keller A, Eltink S. Studies on the crystallization and melting of nylon-66: 1. The dependence of the Brill transition on the crystallization temperature. Polymer. 1994;35:2483. doi: 10.1016/0032-3861(94)90367-0.10.1016/0032-3861(94)90367-0Search in Google Scholar
(51) Seeger A, Freitag D, Freidel F, Luft G. Melting point of polymers under high pressure: Part I: Influence of the polymer properties. Thermochim Acta. 2004;424(1–2):175-18115. doi: 10.1016/j.tca.2004.05.025.10.1016/j.tca.2004.05.025Search in Google Scholar
(52) Xenopoulos A. Thermal analysis and studies of conformational disorder in aliphatic polyamides. PhD thesis. Troy, NY: Rensselaer Polytechnic Institute, Chemistry; 1990.Search in Google Scholar
(53) Jaramillo-Fierro X, Hernández K, González S. Cu(C3H3N3S3)3 adsorption on to ZnTiO3/TiO2 for coordination-complex sensitized photochemical applications. Materials. 2022;15(9):3252. doi: 10.3390/ma15093252.10.3390/ma15093252Search in Google Scholar PubMed PubMed Central
(54) Das R, Vigneresse JL, Chattaraj PK. Chemical reactivity through structure‐stability landscape. Int J Quantum Chem. 2014;114(21):1421–9. doi: 10.1002/qua.24706.10.1002/qua.24706Search in Google Scholar
(55) Shi Y, Hu Y, Jiang N, Yetisen AK. Fluorescence sensing technologies for ophthalmic diagnosis. ACS sensors. 2022;7(6):1615–33. doi: 10.1021/acssensors.2c00313.10.1021/acssensors.2c00313Search in Google Scholar PubMed PubMed Central
(56) Tripathi SL, Alvi PA, Subramaniam U. Electrical and electronic devices, circuits, and materials, technological challenges and solutions. 1st edn. John Wiley & Sons; 2021. p. 267–70.10.1002/9781119755104Search in Google Scholar
(57) Gupta SK, Singh P, Kumar R. PALS and physico-chemical study of swift heavy ions and gamma radiation irradiated polyamide nylon 66 polymer. Vacuum. 2015;121:177–86. doi: 10.1016/j.vacuum.2015.08.015.10.1016/j.vacuum.2015.08.015Search in Google Scholar
(58) Czichos H, Saito T, Smith L. Springer handbook of materials measurement methods. Berlin: Springer; 2006. p. 399–429. doi: 10.1007/978-3-540-30300-8.10.1007/978-3-540-30300-8Search in Google Scholar
(59) Kuila SK, Gorai DK, Gupta B, Gupta AK, Tiwary CS, Kundu TK. Lanthanum ions decorated 2-dimensional g-C3N4 for ciprofloxacin photodegradation. Chemosphere. 2021;268:128780. doi: 10.1016/j.chemosphere.2020.128780. [PMID: 33187655.10.1016/j.chemosphere.2020.128780Search in Google Scholar PubMed
(60) Zhou G, Tian Y, Xue S, Zhou G, Song C, Zhou L, et al. Enhancement of the load capacity of high-energy laser monocrystalline silicon reflector based on the selection of surface lattice defects. Materials. 2020;13(18):4172. doi: 10.3390/ma13184172.10.3390/ma13184172Search in Google Scholar PubMed PubMed Central
(61) Al-Shawabkeh AF, Elimat ZM, Abushgair KN. Effect of non-annealed and annealed ZnO on the optical properties of PVC/ZnO nanocomposite films. J Thermoplast Compos Mater. 2021;038631. doi: 10.1177/08927057211038631.10.1177/08927057211038631Search in Google Scholar
(62) Online Materials Information Resource, MatWeb, Data sheets for metals, plastics, ceramics, and composites. LLC, Kraft Drive, Suite, 3005, Blacksburg, VA 24060 USA; 2020. info@matweb.com.Search in Google Scholar
(63) Hosni HMMA. On some physical properties of GeSe3-Sb2 Se3-ZnSe thin films and their radiation response. PhD thesis. Faculty of Science: Cairo University; 2010. p. 124.Search in Google Scholar
(64) Brza MA, Aziz SB, Anuar H, Al Hazza MHF. From green remediation to polymer hybrid fabrication with improved optical band gaps. Int J Mol Sci. 2019;20:3910. doi: 10.3390/ijms20163910.10.3390/ijms20163910Search in Google Scholar PubMed PubMed Central
(65) Shackelford JF. Introduction to materials science for engineers. 8th edn. Upper Saddle River, NJ: College Div, University of California, Davis. Pearson Higher Education, Inc; 2015. p. 07458. ISBN-13: 978-0-13-382665-4. ISBN-10: 0-13-382665-1.Search in Google Scholar
(66) El-Nicklawy MM, Hassan AF, El-Hagary M, Adel A. Variations in refractive optical properties of nylon 66 fibres under different thermal conditions. Ukrainian J Phys Opt. 2010;11(3):138–46. doi: 10.3116/16091833/11/3/138/2010.10.3116/16091833/11/3/138/2010Search in Google Scholar
(67) Ashby MF, Ferreira P, Schodek DL. Nanomaterials, nanotechnologies and design: an introduction for engineers and architects. Butterworth-Heinemann: Elsevier; 2009. p. 125.10.1016/B978-0-7506-8149-0.00003-9Search in Google Scholar
(68) Yao C, Ding A, Li H, Wei Y. Determination of luminescent characteristics of organometallic complex in land and coal mining. Open Phys. 2022;20(1):538–47. doi: 10.1515/phys-2022-0044.10.1515/phys-2022-0044Search in Google Scholar
(69) Tatum J. Electricity and magnetism, quadrupole moment. University of Victoria, Open Education Resource (OER), LibreTexts; 2022. p. 62–5. https://phys.libretexts.org/@go/page/5765.Search in Google Scholar
© 2022 Ali F. Al-Shawabkeh, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

