Abstract
Reasonable design of inorganic nano-base materials to promote the anticorrosion performance of waterborne epoxy resin (EP) coating remains a great challenge. Herein, we describe the preparation method and anticorrosion properties of zeolitic imidazolate framework (ZIF-67) composite coatings. ZIF-67 could be used as both anticorrosion filler and pigment for the coatings. The addition of ZIF-67 improved the impermeability of the coating, thus slowing down the chemical reaction on the surface of the substrate. The long-term corrosion-resistant performance of composite coatings (ZIF-67@EP) in 3.5% NaCl solution was evaluated comparatively. Before immersion, the low-frequency impedance of 1% ZIF-67@EP coating was 2.5 times of 0% ZIF-67@EP coating, and after 14 days of immersion, the low-frequency impedance of 1% ZIF-67@EP coating was still 1.8 times of 0% ZIF-67@EP coating. The corrosion rate of 1% ZIF-67@EP coating was decreased by 177 times.
1 Introduction
Metal materials that come into contact with oxygen water molecules in the environment can undergo a chemical or electrochemical reaction at the interface of the metal, which is called metal corrosion. Corrosion shifts the metal to an oxidized (ionic) state. This can significantly reduce the strength, plasticity, toughness, and other mechanical properties of metal materials, destroy the geometry of metal components, affect the physical properties of electricity and optics, shorten the service life of the equipment, and even cause catastrophic accidents such as fires and explosions. Therefore, it is considered to be an important cause of global energy loss and economic loss (1,2). Preparing coatings on metal surfaces is an effective method of corrosion protection. Recently, metal coatings (3,4,5), polymer coatings (6,7,8), and organic–inorganic composite coatings (9,10,11,12) are commonly used coatings, which exhibit different degrees of anticorrosion properties according to different protection mechanisms. In recent years, nanomaterials have shown their potential for application in anticorrosion coating systems for metals (13,14,15,16,17,18,19,20). Nanoparticles such as ZnO (13,14,15), TiO2 (16), CuO (17), V2O5 (18), Fe2O3 (19), and Fe3O4 (20) were dispersed in the coating, exhibiting excellent anticorrosion performance and wear resistance of the coating. However, these conventional inorganic metal nanoparticles have some problems, such as poor dispersion and easy agglomeration, which seriously affect the use-value of the coating.
Metal organic frameworks (MOFs) are types of nanomaterials that have flourished in the last decade for their rich variety, adjustable morphology, high stability, and high specific surface area. MOFs are composed of two main components, metal ions or clusters, and organic ligands, which are assembled through coordination bonds. Researchers have successfully created thousands of MOFs, including metal ions or clusters and organic ligands (21,22,23,24). As a subfamily of metal-organic frameworks, zeolitic imidazole framework (ZIFs) materials integrate metals, nitrogen, and carbon into an ordered crystal structure, giving them excellent physical and chemical properties (such as good crystallinity, structural diversity) and outstanding stability (25,26,27,28,29). Therefore, researchers tried to add ZIFs to polymers for the preparation of new anticorrosive materials with excellent anticorrosive properties. Zhang and Liu (30) prepared ZIF-8-based anticorrosive coatings with different microstructures by growing double hydroxide buffer layers (ZnAl-NO3) by a simple hydrothermal method followed by solvent heat treatment with different formulations of ZIF-8 precursor solutions. By the ratios of different precursors, the defects at the coating interface are reduced and the corrosion resistance of the coating is improved. Ren et al. (31) introduced a corrosion prevention strategy to encapsulate corrosion inhibitors into the matrix of MOFs and chose the environmentally friendly corrosion inhibitor zinc gluconate (ZnG) as a metal source to be embedded in ZIF-8. By characterization, it was confirmed that ZnG was loaded into the ZIF-8 matrix. The prepared ZnG@ZIF-8/epoxy resin (EP) coating improved the barrier properties, corrosion resistance, and durability of the corrosion inhibitor of the EP coating. ZIF-67 is a nitrogen-doped cobalt-based MOFs assembled from cobalt nitrate as a metal salt and dimethylimidazole as a linker, which can be used as an inhibitor to improve the corrosion resistance of the coating due to its high stability and nonconductivity. Besides, the cobalt-based ZIFs with certain magnetic properties can better enhance the adhesion between the coating and the metal. Meanwhile, as a nano-scale particle, ZIF-67 displays a filling role in the coating. Currently, there are few reports on composite coatings doped with ZIF-67 nanoparticles.
On the basis of the previous work and the positive effect of nano-ZIF-67, we prepared anticorrosion ZIF-67@EP coating by synthesizing nano-ZIF-67 and dispersing it in waterborne EP coating based on previous reports (32) (Scheme 1). The structure of ZIF-67 nanoparticles was characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The dispersion of ZIF-67 particles in the waterborne resin matrix was discussed. The corrosion resistance of the prepared ZIF-67@EP composite coating was researched through the impermeability test, polarization analysis, and electrochemical impedance analysis. Due to the uniform distribution of ZIF-67 nanoparticles in the waterborne epoxy coating and the excellent properties exhibited, and the ZIF-67@EP coating would be used as a multifunctional coating.
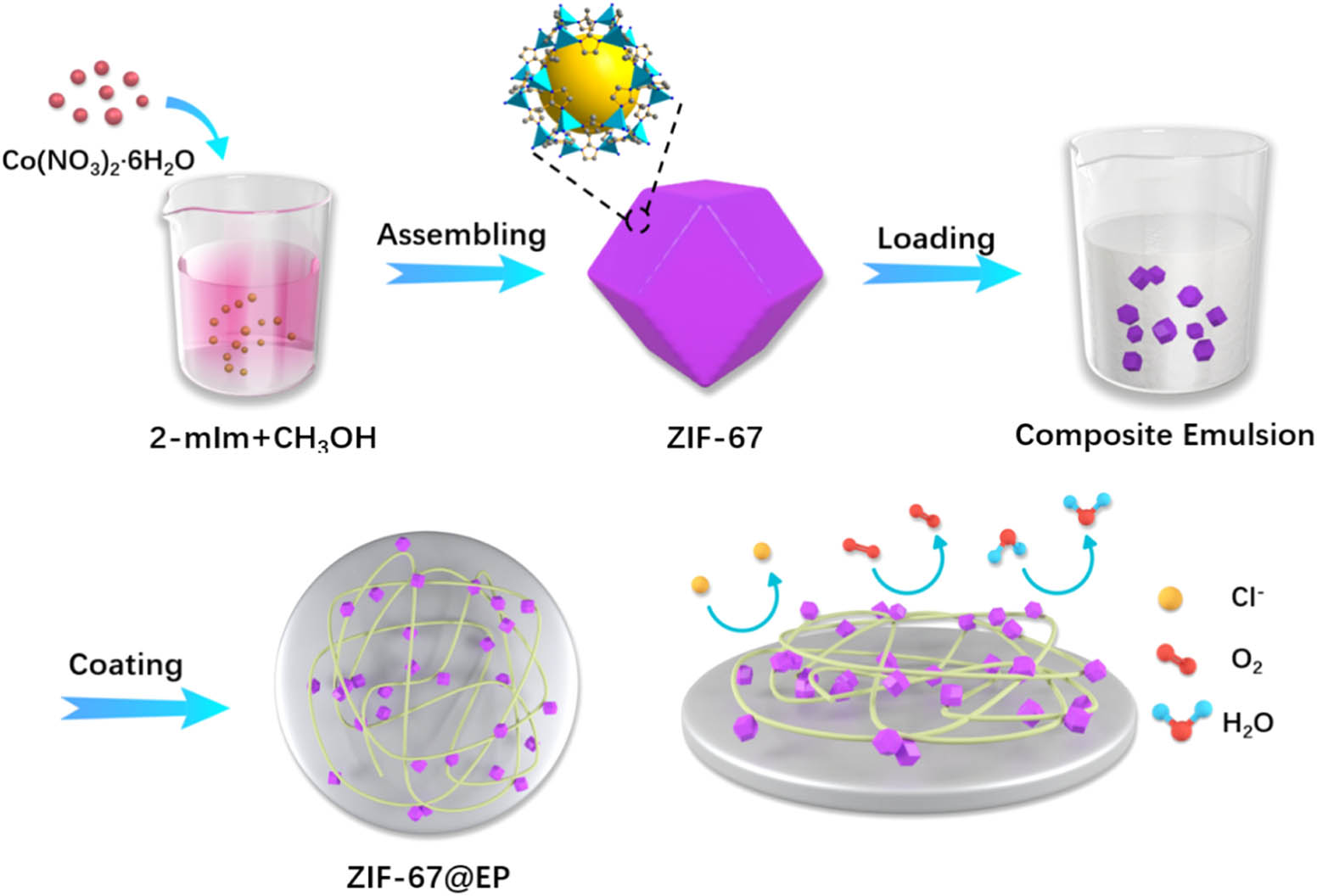
Schematic diagram of the preparation of ZIF-67@EP coating and the anticorrosive mechanism of the anticorrosive coating.
2 Experimental section
2.1 Preparation of ZIF-67
Typically, 2.4 mmol of Co(NO3)2·6H2O was dissolved in 30 mL of methanol under stirring. Then, a methanol solution of 30 mL of 1.84 g of 2-methylimidazole was added to the aforementioned solution and aged at 25°C for 3 days. The purple precipitate was collected by centrifugation, washed several times with methanol, and then dried overnight at 65°C.
2.2 Preparation of ZIF-67@EP composite films
In our previous work, we prepared waterborne EP (32). Here, 20 g of waterborne resin was weighed, and then a certain amount of ZIF-67 was weighed and added to the EP, which was ultrasonically dispersed for 1 h. The composite emulsion was poured into the poly tetra fluoro ethylene plate, dried naturally for 5 days, dried under vacuum at 60°C for 2 h, and placed the film in a drier. According to the difference in the quantity of ZIF-67, the samples were named as 0% ZIF-67@EP, 0.5% ZIF-67@EP, 1% ZIF-67@EP, 2% ZIF-67@EP, and 3% ZIF-67@EP. The mass fraction of ZIF-67 is represented as 0.5%, 1%, 2%, and 3%.
2.3 Preparation of ZIF-67@EP composite coatings
The aforementioned composite emulsion is spread evenly on carbon steel. Before coating, it is polished with silicon carbide paper, ultrasonically cleaned with deionized water, then cleaned with ethanol absolute, and then dried. The compound emulsion is uniformly sprayed on the substrate, dried naturally for 7 days, and finally placed into a vacuum dryer at 60°C for 2 h and then placed in the film in the dryer. According to the difference in the quantity of ZIF-67, the samples were named as 0% ZIF-67@EP coating, 0.5% ZIF-67@EP coating, 1% ZIF-67@EP coating, 2% ZIF-67@EP coating, and 3% ZIF-67@EP coating. We selected a sample with a composite film thickness of 120 ± 2 μm for the performance measurement.
2.4 Characterization
SEM (FEI Verios 460) was used to observe the microscopic morphology of the samples. XRD (D8 Advance, Bruker) was applied to detect the structure of ZIF-67. Composite emulsion particle size was measured by the laser particle scattering method (Panalytical, Malvern). The test laser wavelength was 633 nm, the test temperature was 25°C, and the laser angle is 90°. For the composite emulsion, the transmittance and stability were measured by a stability tester using near-infrared light (λ = 880 nm) to test the sample for 1 h. The water vapor transmission rate (WVTR) of coatings was measured by a water vapor permeability tester (WVTR-W6, Pubtester). WVTR measures were performed at 25°C and 90% relative humidity. The oxygen transmission rate (OTR) of the coatings was measured by an OTR tester (OTR-O1, Pubtester). All OTR tests were conducted at 25°C and 50% relative humidity, 1 atm. Electrochemical tests were performed in a PARSTAT MC (Princeton) electrochemical workstation. The tests were performed using a three-electrode system, with platinum foil as the counter electrode, a saturated calcium-aluminum electrode as the reference electrode, and all test samples as the working electrode with an exposed area of 1 cm−2 and an electrolyte of 3.5% NaCl(aq). The open circuit potential (OCP) should be made to reach equilibrium before the measurement. Electrochemical impedance spectroscopy (EIS) measurements were performed at 100,000–0.01 Hz. EIS results were fitted with ZsimpWin software, and the corresponding corrosion parameters were recorded. The polarization curve measurements were performed at −1.000 to +2.000 V with a scan rate of 0.01 V·s−1 (33,34,35,36).
3 Results and discussion
3.1 Characterization of ZIF-67 and composite coatings
ZIF-67 as inorganic nanoparticles doped into waterborne EP, and it is crucial to disperse uniformly in the waterborne EP. The process steps of ZIF-67 in the doping process and the amount of doping were reasonably controlled to make ZIF-67 uniformly dispersed in the waterborne EP coating. Figure 1a and b shows the SEM images of ZIF-67 nanoparticles, which is a rhombic dodecahedral structure with a slick surface and an average particle size of 500 nm. Figure A1 (in Appendix) shows the XRD pattern of ZIF-67, which proves the synthesis of ZIF-67. Figure 1c and d shows no significant aggregation phenomenon and nanoparticle morphology change during ZIF-67 doping with uniform particles, which is due to the uniform dispersion of ZIF-67 in the coating. In addition, SEM image analysis of ZIF-67@EP coating sections further demonstrated that ZIF-67 was uniformly distributed in the coating. Figure A2 shows the uniform distribution of elements of ZIF-67 nanoparticles on the EP matrix by SEM-EDS (energy dispersive spectroscopy) mapping. All SEM images reflect the uniform dispersion of ZIF-67 in the EP coating, illustrating the feasibility of our strategy.

SEM images of (a and b) ZIF-67 and (c and d) ZIF-67@EP.
3.2 Characterization of stability and particle size of composite emulsions
Figure 2a shows the Turbsican stability index (TSI) curve of emulsion dispersion stability index for analyzing the effect of aging time on the stability of composite emulsions. With the addition of ZIF-67, the TSI value of the composite emulsion showed a rising state, which was caused by the addition of nano ZIF-67. The overall TSI values were all controlled within 1, showing good emulsion stability (37). Figure 2b shows the delta transmission of the composite emulsion to characterize the dispersibility of the composite emulsion. The left part, middle part, and right part of the curve are distributed to represent the bottom, middle, and top of the composite emulsion in the sample bottle, respectively. The composite emulsion shows variation in transmittance at the bottom and good dispersion at the middle and top, the variation at the bottom is due to the deposition of ZIF-67 under gravity (38).

(a) TSI plots for composite coatings and (b) ΔT plots for composite coatings.
The average particle size of the composite emulsion is shown in Figure 3. The average particle size showed a small increasing trend, all within 80 nm. This is caused by the addition of ZIF-67, and the absence of excessive particle size in the composite emulsion also proves the good dispersion of ZIF-67 in the waterborne resin, without the accumulation of ZIF-67.

Particle size distribution of composite emulsions.
3.3 Characterization of impermeability of composite films
The impermeability of the coating is critical to its corrosion resistance. In general, higher impermeability leads to better corrosion-resistant coating (39). Therefore, the OTR and WVTR of the prepared composite films were measured. Figure 4a shows that the OTR value (Table A1 in Appendix) decreases with the addition of ZIF-67, and the 1% ZIF-67@EP film has the lowest OTR value, with a 19.1% decrease compared to the EP film. Besides, as shown in Figure 4b, the WVTR value (Table A1) of 1% ZIF-67@EP film decreased by about 16.7%, which is the lowest of all samples. The OTR and WVTR results indicate that 1% ZIF-67@EP film has better impermeability than EP and other composite films. The improved impermeability is due to the good compatibility of ZIF-67 with EP, as well as the filling effect that enhances the defects of the composite film and inhibits the diffusion path of water molecules and oxygen in the EP matrix. These results also demonstrated that the 1% ZIF-67@EP film is a more compatible metal protection film than other composite films.

OTR (a) and WVTR (b) of different composite membranes.
3.4 Open-circuit potential of coated substrate
Figure 5 shows the OCP curves of the substrate in 3.5% NaCl(aq). Table A2 presents the values of OCP. The OCP of coated steel is determined by a combination of the resistance of the coating and the corrosion potential of the steel. The higher the OCP value, the higher the coating resistance and the more beneficial the protection of the substrate (7). As shown in Figure 5 and Table A2, during 14 days of immersion in 3.5% NaCl(aq), all OCP of ZIF-67@EP-coated steel was higher than that of EP-coated steel. As the ZIF-67 increases, the OCP value of the composite coating generally shifts to a more inert value. The more negative the OCP value, the greater the resistance of the coating. In other words, 0% ZIF-67@EP coating has the lowest resistance, while 1% ZIF-67@EP coating has the highest resistance after being immersed in 3.5% NaCl(aq). In addition to the effect of the resistance of the coating, a higher OCP is associated with a lower corrosion sensitivity (40). The OCP of the coated substrate by the 1% ZIF-67@EP coating was −0.08857, −0.08245, and −0.07826 after 0, 7, and 14 days of immersion in 3.5% NaCl(aq), respectively, which displays the least-negative OCP among all of the coated samples.

OCP of the substrates protected by different coatings after 0, 7, and 14 days of immersion in 3.5% NaCl(aq).
3.4.1 Potentiodynamic polarization of coated substrate
The potential curve of the dynamic potential (Figure 6) of the substrate coated with EP and its composite coating with immersion time after 14 days at 3.5% NaCl(aq). The ZSimWin software was used to fit the corrosion potential (E corr) and the corrosion current density (i corr). The inhibition efficiency (IE) was calculated by Eq. 1 (8):
where i sub and i corr represent the corrosion current density of the substrate electrode and the corrosion current density of the coating-coated electrode, respectively.

Potentiodynamic polarization plots of the substrates protected by different coatings.
The corrosion penetration rates (CPRs) were calculated according to Eq. 2 (41):
where M is the weight of the formula, i corr is the corrosion current density, n is the chemical valence of Fe, ρ is the carbon electrode density, and F is the Faraday constant. The fitted electrochemical parameters are presented in Table 1.
Polarization parameters of the coating in 3.5% NaCl(aq)
| Sample | Potential (V) | Current density (A·cm−2) | IE% | CPR (mm·year−1) |
|---|---|---|---|---|
| 0% ZIF-67@EP | −0.65786 | 1.09 × 10−9 | 99.28 | 1.27 × 10−5 |
| 0.5% ZIF-67@EP | −0.39464 | 1.12 × 10−10 | 99.92 | 1.30 × 10−6 |
| 1% ZIF-67@EP | −0.09071 | 1.80 × 10−11 | 99.98 | 2.09 × 10−7 |
| 2% ZIF-67@EP | −0.29322 | 4.83 × 10−11 | 99.96 | 5.62 × 10−7 |
| 3% ZIF-67@EP | −0.50231 | 2.88 × 10−10 | 99.81 | 3.35 × 10−6 |
Different from the OCP, E corr obtained from the potential polarization measurement reflects the corrosion of the substrate samples (8,9). In Table 1, E corr of 0% ZIF-67@EP coating is −0.65786 V, and E corr of composite coating (ZIF-67 = 0.5%, 1%, 2%, 3%) is −0.39464, −0.09071, −0.29322, and −0.50231 V, respectively. 1% ZIF-67@EP coating has the highest E corr value, which means that 1% ZIF-67@EP coating has the best protection effect on the substrate. The values of the CPR (Table 1) of the composite coating (ZIF-67 = 0%, 0.5%, 1%, 2%, and 3%) were distributed as 1.27 × 10−5, 1.30 × 10−6, 2.09 × 10−7, 5.62 × 10−7, and 3.35 × 10−6 mm·year−1. These CPR results show that at 3.5% NaCl(aq), the corrosion rate of the 1% ZIF-67@EP-coated substrate specimens was 61 times slower than that of the EP specimens. For the IE, 1% ZIF-67@EP coating had the highest value of 99.98%, showing that the addition of 1% ZIF-67 to the EP could provide better substrate protection. The potential polarization results showed that the corrosion resistance of the composite coating was correlated with the content of ZIF-67 doping. When appropriate ZIF-67 (1%) was added, it effectively reduced the defects of the coating and improved the impermeability of the coating. When an excessive amount of ZIF-67 is added, the integrity of the coating is compromised and further promotes corrosion resistance.
E corr, IE, and CPR can illustrate the effect of ZIF-67 on the anticorrosive properties of composite coatings, and the data of some related studies are presented in Table 2. In comparison, 1% ZIF-67@EP coating has a small E corr, a large IE value, and a large CPR value. It shows that 1% ZIF-67@EP has excellent corrosion resistance.
Comparison with other inorganic filler-based polymer anticorrosion coatings studied
| Sample | E corr (V) | i corr (A·cm−2) | IE% | CPR (mm·year−1) | Ref. |
|---|---|---|---|---|---|
| 1% ZIF-67@EP | −0.09071 | 1.80 × 10−11 | 99.98 | 2.09 × 10−7 | This study |
| EpAc-BMF-Fe3O4-2.5 | −0.694 | 2.15 × 10−7 | 98.21 | 1.21 × 10−3 | (17) |
| ESODG-PA-NiO-2 | +0.1032 | 3.42 × 10−11 | — | 3.99 × 10−7 | (18) |
| EP/MoS2-CNFs (0.8%) | −0.53389 | 6.76 × 10−8 | — | — | (42) |
| MoS2@PDA/EP | −0.0820 | 5.838 × 10−10 | — | — | (43) |
| Zn/Chit-PTA | −0.860 | 0.87 | 97.91 | — | (44) |
3.5 EIS measurements of composite coatings
The composite coating’s protective properties and electrochemical behavior were studied by EIS tests (Figure 7a). The EIS test is conducted to investigate the protective effect of the composite coating on the substrate. In general, a wider semicircle diameter in the Nyquist diagram represents a coating with better corrosion resistance (9,45). From Figure 7, the Nyquist plots with 0% ZIF-67@EP coating had the smallest semicircular diameter and those with 1% ZIF-67@EP coating had the largest diameter (0% ZIF-67@EP < 3% ZIF-67@EP < 0.5% ZIF-67@EP < 2% ZIF-67@EP < 1% ZIF-67@EP), regardless of the immersion time. As the immersion time increased, the semicircular diameter of the Nyquist plot of the 1% ZIF-67@EP coating was always larger than that of the other coatings. After immersing for 14 days (Figure 7d), the Nyquist plot diameter of the 1% ZIF-67@EP coating was 15 times larger than that of the nonimmersion 1% ZIF-67@EP coating, and the radius of the Nyquist diagram of the 1% ZIF-67@EP coating was more than twice that of the 2% ZIF-67@EP coating and 150 times that of the 0% ZIF-67@EP coating, proving that the addition of ZIF-67 improves the anticorrosion performance of the coating.

Three-electrode system (a), Nyquist plots of composite coatings at different immersion times: 0 day (b), 7 days (c), and 14 days (d).
To further explain the anticorrosion behavior of the coating, the electrochemical data were fitted using ZSimWin software to obtain the impedance and phase angle (PA) of the Bode plots. Figure 8 shows the frequency-dependent changes in bode impedance and PA of the EP and composite coatings after 14 days of immersion in 3.5 wt% NaCl(aq). The low-frequency (|Z|0.01 Hz) impedance is a semi-quantitative indicator of the coating’s protective performance. Higher bode impedance at low frequencies exhibits better corrosion resistance (9). Figure 8a, c, and e and Table A3 show the bode impedance at low frequencies (0.01) for the unimmersed 0% ZIF-67@EP, 0.5% ZIF-67@EP, 1% ZIF-67@EP, 2% ZIF-67@EP, and 3% ZIF-67@EP coatings of 1.54 × 107, 7.56 × 108, 2.52 × 109, 1.05 × 109, and 6.30 × 107 Ω·cm−2, respectively, with the 1% ZIF-67@EP coating exhibiting the highest bode impedance, two orders of magnitude higher than the 0% ZIF-67@EP coating. The 1% ZIF-67@EP coating exhibits the highest bode impedance, which is two orders of magnitude higher than the 0% ZIF-67@EP coating. It shows good corrosion resistance.

Bode impedance and bode phase plots of composite coatings at different immersion times: 0 day (a and b), 7 days (c and d), and 14 days (e and f).

EEC models for composite coatings.
Figure 8b, d, and f shows the change in PA with frequency for the samples immersed in 3.5% NaCl(aq) for 14 days. The PA at high frequencies is considered to characterize the capacitance of the coating. The ideal PA of the coating is −90°, indicating that the coating is a purely nonconductive capacitor, and a PA close to −90° implies a highly capacitive behavior of the coating (45–47). As shown in Figure 8b, d, and f and Table A4, 1% ZIF-67@EP coating presents the lowest PA, best corrosion resistance, while the other coatings show an intermediate PA value. After 14 days of immersion of the composite coating, the PA of 0% ZIF-67@EP, 0.5% ZIF-67@EP, 1% ZIF-67@EP, 2% ZIF-67@EP, and 3% ZIF-67@EP coating at high frequencies is reduced from −84.87°, −87.81°, −89.68°, −88.09°, and −87.47° to −75.94°, −80.13°, −83.90°, −81.26°, and −78.32°, respectively. The PA of 1% ZIF-67@EP coating is still closest to −90°, indicating that the coating has long-term stability. Therefore, this is consistent with the results of the |Z|0.01Hz measurements.
An equivalent circuit (EEC) model shown in Figure 9 was used to fit the EIS data to obtain the fitted parameters, which were used to explain the behavior of the different interfaces of the coated substrate and to demonstrate the importance of the ZIF-67 composite coating on the corrosion of the substrate. In the EEC model, R s is the solution resistance, which is negligible with respect to the other parameters. For this case, Q c is the coating capacitance and Q dl is the double-layer capacitance at the substrate/electrolyte interface. R c denotes the coating resistance and R ct denotes the charge transfer resistance (45,48,49). The changes in the electrical parameters (R c, R ct, Q c, and Q dl) during the immersion of the coating in 3.5% NaCl(aq) are shown in Figure 10. R c and R ct values of all samples showed a decreasing trend with the increasing immersion time, indicating a decrease in the barrier capacity. As shown, all the composite coatings had higher R c (Figure 10a) and R ct (Figure 10b) values than the EP coatings. After 0, 7, and 14 days of immersion, the R c values of the 1% ZIF-67@EP coating were 1.69, 1.63, and 1.60 times higher than those of the 0% ZIF-67@EP coating, respectively. The R ct values of 1% ZIF-67@EP were 1.76, 1.83, and 1.87 times higher than those of 0% ZIF-67@EP, respectively. After immersion for 7 and 14 days, the R c of 0% ZIF-67@EP coating decreased by 5.08% and 14.69%, respectively, and the Rct decreased by 13.65% and 23.62%, respectively, while the R c of 1% ZIF-67@EP coating decreased by 8.69% and 19.39%, respectively, and the R ct decreased by 10.25% and 19.04%, respectively. At different immersion times, the RC and RCT of 1% ZIF-67@EP coating were much greater than 0% ZIF-67@EP coating and the other composite coatings. The increase in R c and R ct values implies decreased coating porosity and improved coating integrity, which inhibits electron transport in the coating. The results show that after 14 days of immersion, Q c and Q dl values of all the samples are reduced. The Q c value of 1% ZIF-67@EP coating is lower than that of other composite coatings (Figure 10c) and much lower than that of 0% ZIF-67@EP coating. The results indicate that the incorporation of ZIF-67 into the substrate can effectively inhibit the coating’s penetration behavior and improve the coating’s impermeability. Similarly, in Figure 10d, the same trend is observed for Q dl. Based on the high R and low Q values of 1% ZIF-67@EP coating, it can be concluded that ZIF-67 enhances the barrier properties of the coating by inhibiting the electron transfer process of the electrochemical process and the penetration of the corrosive medium, which significantly improved corrosion protection of EP coatings.

Electrical parameters (a) R c, (b) R ct, (c) Q c, and (d) Q dl of composite coatings after 0, 7, 14 days of immersion in 3.5% NaCl(aq). From left to right, 0% ZIF-67@EP coating, 0.5% ZIF-67@EP coating, 1% ZIF-67@EP coating, 2% ZIF-67@EP coating, and 3% ZIF-67@EP coating, respectively.
3.6 Microscopic morphology of the corrosion area
Figure 11 shows that 0% ZIF@EP coating (Figure 11a and b), 0.5% ZIF@EP coating (Figure 11c and d), and 3% ZIF@EP coating (Figure 11e and f) protect the surface of the substrate with a wide area of corrosion. The surface of the substrate showed a large area of peeling phenomenon, and 0% ZIF@EP coating (Figure 11a and b) corrosion is the deepest. 1% ZIF@EP coating (Figure 11g and h) and 2% ZIF@EP coating (Figure 11i and j) protection of the substrate surface results in a relatively smooth and smaller area of corrosion, and 1% ZIF@EP coating presented the best anticorrosion effect after 14 days of immersion.
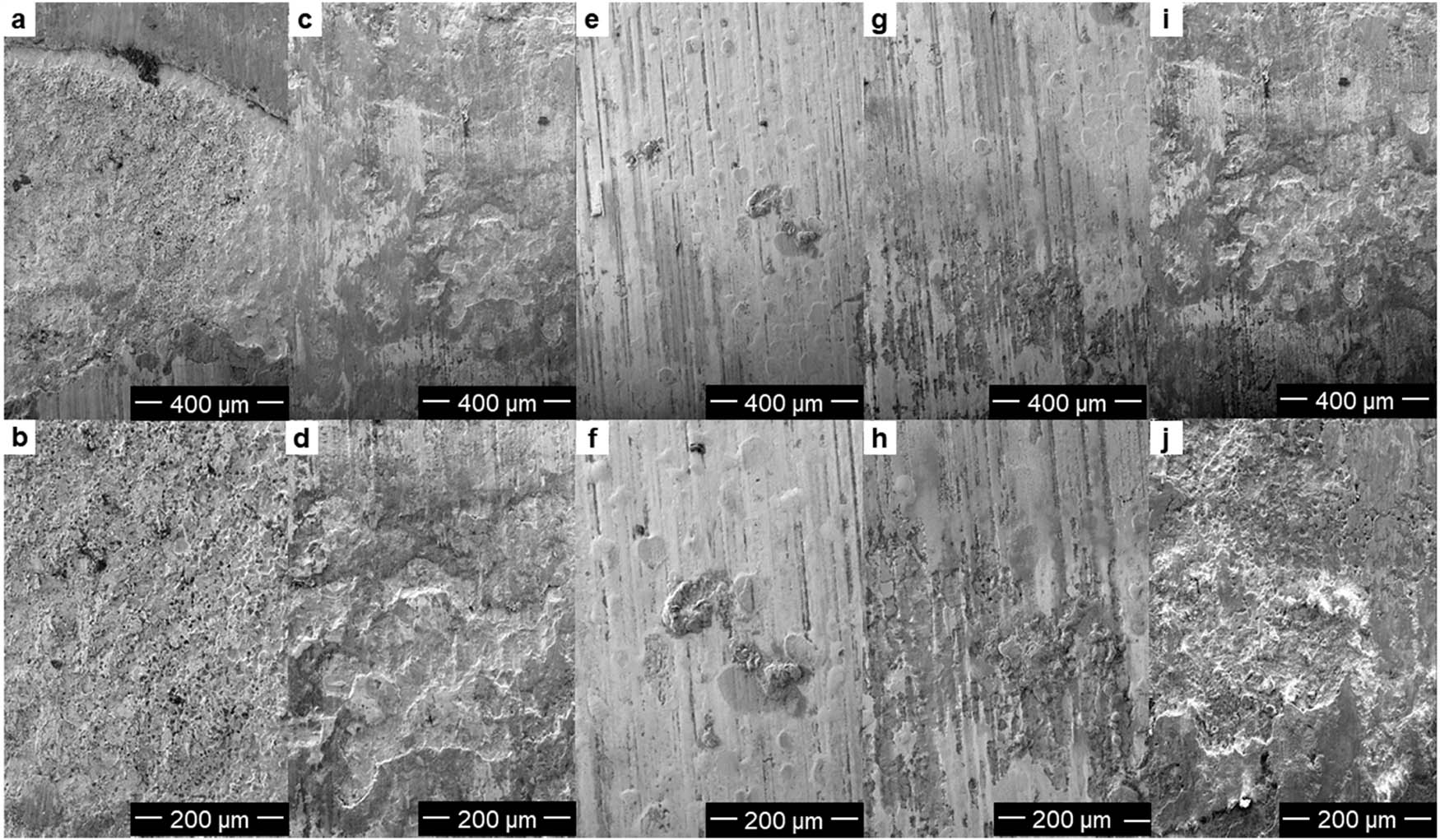
SEM images of the corrosion area of 0% ZIF-67@EP (a and b), 0.5% ZIF-67@EP (c and d), 1% ZIF-67@EP (e and f), 2% ZIF-67@EP (g and h), and 3% ZIF-67@EP (i and j) coatings after 14 days of immersion in 3.5% NaCl(aq).
3.6.1 Anticorrosion mechanism of coatings
Corrosion of composite coatings is mainly caused by electrochemical or chemical reactions occurring at the interface between the substrate and the coating (45). The reactions at the interface are mainly two half-reactions, the anodic half-reaction (Eq. 3) and the cathodic half-reaction (Eq. 4). The final reaction that forms the corrosion product is shown in Eq. 5.
In addition, the addition of chloride ions accelerates the corrosion process, and the reaction can be represented by Eqs. 6 and 7. Therefore, the entry of H2O, O2, and Cl− into the interface is the key factor affecting the whole corrosion process. Compared with the EP coating, the composite coating is filled with nano-scale ZIF-67 in the epoxy coating, which reduces the defects of the epoxy coating and slows down the penetration of O2, H2O, and Cl−. The large number of microporous defects in the EP coating provides multiple paths for O2, H2O, and Cl− to reach the interface and cause corrosion on the surface of the substrate. With the addition of ZIF-67, the microporous defects are filled, and the anticorrosion ability is improved, where 1% ZIF-67@EP coating exhibits the best addition of ZIF-67 and shows the best anticorrosion performance.
4 Conclusions
In this article, the zeolitic imidazolate framework ZIF-67 was prepared by the hydrothermal method as anticorrosion filler and color filler for the coating. The excellent resin compatibility, stability, and dispersion of ZIF-67 were proved by particle size and stability tests. The well-dispersed ZIF-67 compensates for the defects of the coating and inhibits the channels for water and oxygen diffusion, further improving the impermeability and corrosion resistance of the EP coating. The corrosion resistance of the composite coating was evaluated by electrochemistry. The corrosion resistance of the composite coating improved with increasing the usage of ZIF-67, and when ZIF-67 = 1 wt%, the composite coating exhibited the best electrochemical corrosion resistance. 1% ZIF-67@EP coating shows the best protection performance by SEM observation of the corrosion area of the coating. This composite coating of mental organic framework material provides a new approach and idea for the development of polymeric nanocomposites.
-
Funding information: This work was supported by the National Natural Science Foundation of China (22001156), Key Laboratory Project of Science and Technology overall Planning and Innovation Project of Shaanxi Province (2013SZS10-Z01), and the open Foundation of State Key Laboratory of Structural Chemistry (20180024). Agricultural Project of Science and Technology Department of Shaanxi Province (S2020-YF-YBNY-0235).
-
Author contributions: Li Li: writing – original draft; Gang Cheng: visualization; Xuyong Chen: writing – review and editing, methodology, and formal analysis.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The raw data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
(1) Shi X, Liu Y, Mooney M, Berry M, Hubbard B, Nguyen TA. Laboratory investigation and neural networks modeling of deicer ingress into portland cement concrete and its corrosion implications. Corros Rev. 2011;28:105–153.10.1515/CORRREV.2010.28.3-4.105Search in Google Scholar
(2) Ikechukwu EE, Pauline EO. Environmental impacts of corrosion on the physical properties of copper and aluminium: A case study of the surrounding water bodies in Port Harcourt. Open J Soc Sci. 2015;3(2):143–50.10.4236/jss.2015.32019Search in Google Scholar
(3) Sun J, Du DX, Lv HF, Zhou L, Wang YG, Qi CG. Microstructure and corrosion resistance of pulse electrodeposited Ni–Cr coatings. Surf Eng. 2015;31:406–11.10.1179/1743294414Y.0000000392Search in Google Scholar
(4) Chuang H-C, Sanchez J. Feasibility study on sc-Ar electroplating for metal coating fabrication. Surf Eng. 2017;34:440–5.10.1080/02670844.2017.1358482Search in Google Scholar
(5) Cai F, Jiang CH. Characterisation of electrodeposited Ni–Zr coatings prepared at different current densities. Surf Eng. 2015;31:245–50.10.1179/1743294414Y.0000000389Search in Google Scholar
(6) Xavier JR, Nallaiyan R. Application of EIS and SECM Studies for Investigation of anticorrosion properties of epoxy coatings containing ZrO2 nanoparticles on mild steel in 3.5% NaCl solution. J Fail Anal Preven. 2016;16:1082–91.10.1007/s11668-016-0187-xSearch in Google Scholar
(7) Shi X, Nguyen TA, Suo Z, Liu Y, Avci R. Effect of nanoparticles on the anticorrosion and mechanical properties of epoxy coating. Surf Coat Technol. 2009;204:237–45.10.1016/j.surfcoat.2009.06.048Search in Google Scholar
(8) Pourhashem S, Vaezi MR, Rashidi A, Bagherzadeh MR. Exploring corrosion protection properties of solvent based epoxy-graphene oxide nanocomposite coatings on mild steel. Corros Sci. 2017;115:78–92.10.1016/j.corsci.2016.11.008Search in Google Scholar
(9) Liu D, Zhao W, Liu S, Cen Q, Xue Q. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf Coat Technol. 2016;286:354–64.10.1016/j.surfcoat.2015.12.056Search in Google Scholar
(10) Ghosal A, Iqbal S, Ahmad S. NiO nanofiller dispersed hybrid Soy epoxy anticorrosive coatings. Prog Org Coat. 2019;133:61–76.10.1016/j.porgcoat.2019.04.029Search in Google Scholar
(11) Sumi VS, Arunima SR, Deepa MJ, Ameen Sha M, Riyas AH, Meera MS, et al. PANI-Fe2O3 composite for enhancement of active life of alkyd resin coating for corrosion protection of steel. Mater Chem Phys. 2020;247.10.1016/j.matchemphys.2020.122881Search in Google Scholar
(12) Jing Y, Wang P, Yang Q, Wang Q, Bai Y. MoS2 decorated with ZrO2 nanoparticles through mussel-inspired chemistry of dopamine for reinforcing anticorrosion of epoxy coatings. Colloids Surf A: Physicochem Eng Asp. 2021;608:125625.10.1016/j.colsurfa.2020.125625Search in Google Scholar
(13) Balananda S, Mariaa MJ, Rajan TPD, Mohamed AP, Ananthakumar S. Bulk processing of ZnO nanostructures via microwave assisted oxidation of mechanically seeded Zn dust for functional paints and coatings. Chem Eng J. 2016;284:657–67.10.1016/j.cej.2015.08.163Search in Google Scholar
(14) Chiplunkar PP, Pratap AP. Utilization of sunflower acid oil for synthesis of alkyd resin. Prog Org Coat. 2016;93:61–7.10.1016/j.porgcoat.2016.01.002Search in Google Scholar
(15) Selim MS, Shenashen MA, Elmarakbi A, EL-Saeed AM, Selimd MM, El-Safty SA. Sunflower oil-based hyperbranched alkyd/spherical ZnO nanocomposite modeling for mechanical and anticorrosive applications. RSC Adv. 2017;7:21796–808.10.1039/C7RA01343DSearch in Google Scholar
(16) Hochmannova L, Vytrasova J. Photocatalytic and antimicrobial effects of interior paints. Prog Org Coat. 2010;67:1–5.10.1016/j.porgcoat.2009.09.016Search in Google Scholar
(17) Ong HR, Khan MdMR, Ramli R, Rahmana MdW, Yunus RM. Tailoring base catalyzed synthesis of palm oil based alkyd resin through CuO nanoparticles. RSC Adv. 2015;5:95894–902.10.1039/C5RA19575FSearch in Google Scholar
(18) Natalio F, Andre R, Hartog AF, Stoll B, Jochum KP, Wever R, et al. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nat Nanotechnol. 2012;7:530–5.10.1038/nnano.2012.91Search in Google Scholar PubMed
(19) Jeyasubramanian K, Benitha VS, Parkavi V. Nano iron oxide dispersed alkyd coating as an efficient anticorrosive coating for industrial structures. Prog Org Coat. 2019;132:76–85.10.1016/j.porgcoat.2019.03.023Search in Google Scholar
(20) Ur Rahman O, Kashif M, Ahmad S. Nanoferrite dispersed waterborne epoxy-acrylate: Anticorrosive nanocomposite coatings. Prog Org Coat. 2015;80:77–86.10.1016/j.porgcoat.2014.11.023Search in Google Scholar
(21) Kitao T, Zhang Y, Kitagawa S, Wang B, Uemura T. Hybridization of MOFs and polymers. Chem Soc Rev. 2017;46:3108–33.10.1039/C7CS00041CSearch in Google Scholar
(22) Zhang Y, Feng X, Li H, Chen Y, Zhao J, Wang S, et al. Photoinduced postsynthetic polymerization of a metal-organic framework toward a flexible stand-alone membrane. Angew Chem Int Ed Engl. 2015;54:4259–63.10.1002/anie.201500207Search in Google Scholar PubMed
(23) Xie K, Fu Q, He Y, Kim J, Goh SJ, Nam E, et al. Synthesis of well dispersed polymer grafted metal-organic framework nanoparticles. Chem Commun (Camb). 2015;51:15566–69.10.1039/C5CC06694HSearch in Google Scholar
(24) Wu T, Shen L, Luebbers M, Hu C, Chen Q, Ni Z, et al. Enhancing the stability of metal-organic frameworks in humid air by incorporating water repellent functional groups. Chem Commun (Camb). 2010;46:6120–2.10.1039/c0cc01170cSearch in Google Scholar PubMed
(25) Huang W, Li X, Yu D, Yang X, Wang L, Liu P, et al. CoMo-bimetallic N-doped porous carbon materials embedded with highly dispersed Pt nanoparticles as pH-universal hydrogen evolution reaction electrocatalyst. Nanoscale. 2020;12:19804–13.10.1039/D0NR04418KSearch in Google Scholar PubMed
(26) Huang WH, Li X-M, Yang X-F, Zhang H-Y, Liu P-B, Ma Y-M, et al. CeO2-embedded mesoporous CoS/MoS2 as highly efficient and robust oxygen evolution electrocatalyst. Chem Eng J. 2020;127595.10.1016/j.cej.2020.127595Search in Google Scholar
(27) Huang W, Zhang X, Zhao Y, Zhang J, Liu P. Hollow N-doped carbon polyhedrons embedded Co and Mo2C nanoparticles for high-efficiency and wideband microwave absorption. Carbon. 2020;167:19–30.10.1016/j.carbon.2020.05.073Search in Google Scholar
(28) Abazari R, Yazdani E, Nadafan M, Kirillov AM, Gao J, Slawin AM, et al. Third-order nonlinear optical behavior of an amide-tricarboxylate zinc (II) metal–organic framework with two-fold 3D + 3D interpenetration. Inorg Chem. 2021;60(13):9700–8.10.1021/acs.inorgchem.1c00997Search in Google Scholar PubMed
(29) Xu H, Zhong F, Chen F, Luan TX, Li P, Xu S, et al. A Zr-MOF nanoflower sensor and its mixed-matrix membrane for the highly sensitive detection of nitroaromatics. J Mater Chem C. 2022;10(19):7469–75.10.1039/D2TC00920JSearch in Google Scholar
(30) Zhang M, Liu Y. Enhancing the anti-corrosion performance of ZIF-8-based coatings via microstructural optimization. N J Chem. 2020;44:2941–6.10.1039/C9NJ05998ASearch in Google Scholar
(31) Ren B, Chen Y, Li Y, Li W, Gao S, Li H, et al. Rational design of metallic anti-corrosion coatings based on zinc gluconate@ZIF-8. Chem Eng J. 2020;384:123389.10.1016/j.cej.2019.123389Search in Google Scholar
(32) Chen X, Li X, Yang K, Zhang Q, Zhu H, Li K. Polar migration behavior of phosphonate groups in phosphonate esterified acrylic grafted epoxy ester composites and their role in substrate protection. e-Polymers. 2020;20:636–50.10.1515/epoly-2020-0066Search in Google Scholar
(33) Liu C, Du P, Zhao H, Wang LJAANM. Synthesis of l-histidine-attached graphene nanomaterials and their application for steel protection. ACS Appl Nano Mater. 2018;1:1385–95.10.1021/acsanm.8b00149Search in Google Scholar
(34) Ding J, Zhao H, Xu B, Zhao X, Su S, Yu HJASC. Engineering, Superanticorrosive graphene nanosheets through π deposition of boron nitride nanodots. ACS Sustain Chem Eng. 2019;7:10900–11.10.1021/acssuschemeng.9b01796Search in Google Scholar
(35) Bertuoli PT, Baldissera AF, Zattera AJ, Ferreira CA, Alemán C, Armelin E. Polyaniline coated core-shell polyacrylates: control of film formation and coating application for corrosion protection. Prog Org Coat. 2019;128:40–51.10.1016/j.porgcoat.2018.12.007Search in Google Scholar
(36) Wu C, Huang X, Wu X, Qian R, Jiang PJAM. Mechanically flexible and multifunctional polymer-based graphene foams for elastic conductors and oil-water separators. Adv Mater. 2013;25:5658–62.10.1002/adma.201302406Search in Google Scholar PubMed
(37) Bonaccorso A, Musumeci T, Serapide MF, Pellitteri R, Uchegbu IF, Puglisi G. Nose to brain delivery in rats: Effect of surface charge of rhodamine B labeled nanocarriers on brain subregion localization. Colloids Surf B Biointerfaces. 2017;154:297–306.10.1016/j.colsurfb.2017.03.035Search in Google Scholar PubMed
(38) Wang H, Qin S, Yang X, Fei G, Tian M, Shao Y, et al. A waterborne uniform graphene-poly(urethane-acrylate) complex with enhanced anticorrosive properties enabled by ionic interaction. Chem Eng J. 2018;351:939–51.10.1016/j.cej.2018.06.151Search in Google Scholar
(39) Ding J, Zhao H, Xu B, Zhao X, Su S, Yu H. Superanticorrosive Graphene Nanosheets through π Deposition of Boron Nitride Nanodots. ACS Sustain Chem & Eng. 2019;7:10900–11.10.1021/acssuschemeng.9b01796Search in Google Scholar
(40) Schem M, Schmidt T, Gerwann J, Wittmar M, Veith M, Thompson GE, et al. CeO2-filled sol–gel coatings for corrosion protection of AA2024-T3 aluminium alloy. Corros Sci. 2009;51:2304–15.10.1016/j.corsci.2009.06.007Search in Google Scholar
(41) Gu L, Liu S, Zhao H, Yu H. Facile preparation of water-dispersible graphene sheets stabilized by carboxylated oligoanilines and their anticorrosion coatings. ACS Appl Mater Interfaces. 2015;7:17641–8.10.1021/acsami.5b05531Search in Google Scholar PubMed
(42) Wu Y, He Y, Chen C, Li H, Xia Y, Zhou T. MoS2-CNFs composites to enhance the anticorrosive and mechanical performance of epoxy coating. Prog Org Coat. 2019;129:178–86.10.1016/j.porgcoat.2019.01.021Search in Google Scholar
(43) Xia Z, Liu G, Dong Y, Zhang Y. Anticorrosive epoxy coatings based on polydopamine modified molybdenum disulfide. Prog Org Coat. 2019;133:154–60.10.1016/j.porgcoat.2019.04.056Search in Google Scholar
(44) Szőke ÁF, Szabó G, Simó Z, Hórvölgyi Z, Albert E, Végh AG, et al. Chitosan coatings ionically cross-linked with ammonium paratungstate as anticorrosive coatings for zinc. Eur Polym J. 2019;118:205–12.10.1016/j.eurpolymj.2019.05.057Search in Google Scholar
(45) Zhang Y, Tian J, Zhong J, Shi X. Thin nacre-biomimetic coating with super-anticorrosion performance. ACS Nano. 2018;12:10189–200.10.1021/acsnano.8b05183Search in Google Scholar PubMed
(46) Zhong J, Zhou G-X, He P-G, Yang Z-H, Jia D-C. 3D printing strong and conductive geo-polymer nanocomposite structures modified by graphene oxide. Carbon. 2017;117:421–6.10.1016/j.carbon.2017.02.102Search in Google Scholar
(47) Armelin E, Whelan R, Martinez-Triana YM, Aleman C, Finn MG, Diaz DD. Protective coatings for aluminum alloy based on hyperbranched 1,4-polytriazoles. ACS Appl Mater Interfaces. 2017;9:4231–43.10.1021/acsami.6b14174Search in Google Scholar PubMed
(48) Suleiman RK, Kumar AM, Adesina AY, Al-Badour FA, Meliani MH, Saleh TA. Hybrid organosilicon-metal oxide composites and their corrosion protection performance for mild steel in 3.5% NaCl solution. Corros Sci. 2020;169:108637.10.1016/j.corsci.2020.108637Search in Google Scholar
(49) Sadeghi Erami R, Amirnasr M, Meghdadi S, Talebian M, Farrokhpour H, Raeissi K. Carboxamide derivatives as new corrosion inhibitors for mild steel protection in hydrochloric acid solution. Corros Sci. 2019;151:190–7.10.1016/j.corsci.2019.02.019Search in Google Scholar
Appendix
A1 Materials
EP (E20) was obtained from Jining Huakai Resin Co., Ltd (Jining, China). Linoleic acid was purchased from Anhui Rifende Oil Deep Processing Co., Ltd. (Langxi, China). Hydroxypropyl acrylate (HPA), methyl methacrylate (MMA), methacrylic acid (MAA), butyl acrylate (BA), and styrene (St) were purchased from Tianjin Hedong Hongyan Reagent Plant (Tianjin, China). The aforementioned monomers are chemically pure. Dibenzoyl peroxide (BPO), tert-butyl peroxybenzoate (TBPO), and tetrabutylammonium bromide (TBAB) were purchased from Kingchemical Co., Ltd (Shanghai, China).
A2 Synthesis of waterborne resin (EP)
E20 (0.1 mol) was taken in a three-necked round bottom flask, tetrabutylammonium bromide was added as an initiator, linoleic acid (0.2 mol) was added into the reaction flask with continuous stirring at 130°C, and the reaction occurred for 4 h. Then, HPA (0.1 mol) etherified graft at 90°C for 3 h, followed by the addition of MMA, MAA, BA, and St for graft copolymerization, with BPO (0.5 wt%) as the initiator and reaction time for 3 h at 80°C, and then the temperature was increased to 110°C, TBPO (0.5 wt%) was added, and the reaction mixture was stirred for 2 h. Then, it was neutralized with DMEA to 0.8 and emulsified with deionized water to produce an emulsion with 40% solid content for the preparation of emulsion films and coatings. The EP reaction equation is as follows (Scheme A1).

XRD pattern of ZIF-67.

SEM image (a) and corresponding element mappings of (b) ZIF-67@EP, demonstrating the distributions of (c) C, (d) N, (e) O and (f) Co.

Reaction equation.
Water vapor and OTR values of composite films
| OTR (cc·m−2·h−1) | WVTR (g·m−2·h−1) | |
|---|---|---|
| 0% ZIF-67@EP | 0.80 | 9.19 |
| 0.5% ZIF-67@EP | 0.70 | 7.78 |
| 1% ZIF-67@EP | 0.67 | 7.44 |
| 2% ZIF-67@EP | 0.68 | 7.61 |
| 3% ZIF-67@EP | 0.72 | 7.74 |
OCP values of the substrates protected by different coatings after immersion in 3.5 wt% NaCl(aq)
| OCP (V) | |||
|---|---|---|---|
| 0 day | 7 days | 14 days | |
| 0% ZIF-67@EP | −0.64 | −0.59 | −0.56 |
| 0.5% ZIF-67@EP | −0.38 | −0.36 | −0.34 |
| 1% ZIF-67@EP | −0.089 | −0.082 | −0.072 |
| 2% ZIF-67@EP | −0.29 | −0.27 | −0.25 |
| 3% ZIF-67@EP | −0.49 | −0.45 | −0.43 |
Bode impedance values of composite coatings at different immersion times
| Bode impedance |Z|0.01 (Ω·cm2) | |||
|---|---|---|---|
| 0 day | 7 days | 14 days | |
| 0% ZIF-67@EP | 1.54 × 10−7 | 1.20 × 10−7 | 2.17 × 10−6 |
| 0.5% ZIF-67@EP | 7.56 × 10−8 | 6.27 × 10−8 | 6.42 × 10−7 |
| 1% ZIF-67@EP | 2.52 × 10−9 | 1.97 × 10−9 | 2.09 × 10−8 |
| 2% ZIF-67@EP | 1.05 × 10−9 | 8.77 × 10−8 | 1.21 × 10−8 |
| 3% ZIF-67@EP | 6.29 × 10−7 | 4.94 × 10−7 | 1.02 × 10−7 |
Bode phase values of composite coatings at different immersion times
| PA (degree) | |||
|---|---|---|---|
| 0 day | 7 days | 14 days | |
| 0% ZIF-67@EP | 84.87 | 79.37 | 75.94 |
| 0.5% ZIF-67@EP | 87.81 | 81.27 | 80.13 |
| 1% ZIF-67@EP | 89.68 | 85.80 | 83.90 |
| 2% ZIF-67@EP | 88.09 | 81.89 | 81.26 |
| 3% ZIF-67@EP | 87.47 | 80.78 | 78.32 |
© 2022 Li Li et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

