A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
-
Lin Chen
, Gaigai Duan
, Zhenzhong Liu
Abstract
Although diverse uranium (U) adsorbents have been explored, it is still a great challenge for high-efficient uranium extraction form seawater. Herein a wood-mimetic oriented porous Ti3C2T x -MXene/gelatin hydrogel (MGH) has been explored through growing directional ice crystals cooled by liquid nitrogen and subsequently forming pores by freeze-dry (Ice-template) method, for ultrafast and high-efficient U-adsorption from seawater with great enhancement by both electric field and sunlight. Different from disperse Ti3C2T x -MXene powder, this MGH not only can be easily utilized but also can own ultrahigh specific surface area for high-efficient U-adsorption. The U-adsorbing capacity of this MGH (10 mg) can reach 4.17 mg·g−1 after only 1 week in 100 kg of seawater, which is outstanding in existing adsorbents. Furthermore, on the positive pole of 0.4 V direct current source or under 1-sun irradiation, the U-adsorbing capacity of the MGH can increase by 57.11% and 13.57%, respectively. Most importantly, the U-adsorption of this hydrogel can be greatly enhanced by simultaneously using the above two methods, which can increase the U-adsorbing capacity by 79.95% reaching 7.51 mg·g−1. This work provides a new biomimetic porous MXene-based hydrogel for electric field/sunlight bi-enhanced high-efficient U-extraction from seawater, which will inspire new strategy to design novel U-adsorbents and systems.
1 Introduction
Nuclear power is an excellent alternative to replace highly polluting traditional energy (1). However, the limited uranium (U) resource on land, have become the bottleneck to vastly handle the long-term development of nuclear energy (2,3). To exploit new U resource, diverse U-enriching methods from seawater have been developed (4). Among them, including chemical/electrochemical precipitation (5), ion exchange (6), and membrane separation (7), adsorption is the most studied method with the characteristics of simple, variable, and low cost (2,8–13), and is closest to industrialize U-extraction from seawater, such as organic adsorbents (14), inorganic adsorbents (15), artificial polymeric adsorbents (16), new bio-materials (17), and so on.
In recent years, various kinds of nano-structured materials for U-adsorption (18), including covalent organic frameworks (COFs) (19), metal organic framework (MOFs) (20), porous organic polymers (POPs) (21), porous aromatic frameworks (PAFs) (22), and macroporous resins (23), have been reported. Among them, 2D transition metal carbonitrides/carbides (MXenes) (24,25) are novel and important adsorbents which have wide potential applications including catalysis (26), sensing (27), medicine (28), energy storage (29), and environmental remediation (30), owing to their specific electric/photo/magnetic/mechanical properties. Especially, MXenes can also be utilized in environmental protection including photocatalytic wastewater treatment (31,32). Furthermore, MXenes can be composited with other nanomaterials or polymers to achieve many novel functions (33,34), such as the reported MXene-graphene oxide composite hydrogel which possess high-efficient adsorption for heavy metals (35).
Nowadays, few of the MXenes have been designed for high-efficient U extraction, on account of the ultralarge specific surface area and multiple active sites for specific U-adsorption (36). In addition, the unique electric/photo performance of MXenes can be utilized to further enhance the U-adsorbing performance (37). For instance, owing to the good electro-conductivity, the U-adsorbing performance of MXenes can be significantly enhanced under a direct electric field (38,39). For another instance, because of the specific photo property, the U-adsorbing performance of MXenes can be improved by photocatalytic reduction (40,41). However, existing MXene-based adsorbents are in natural discrete powder state (42), which make them severely difficult to adsorb/desorb U conveniently for many times (43,44). Therefore, they are usually used for laboratory research and cannot reliably extract U in marine environments for a relatively long term (45,46). Most importantly, it is still a great challenge to realize electric/photo synergistic bi-enhanced MXene-based U-adsorbents, although the bi-enhancement of U-adsorption are possible in theory.
In this study, inspired by oriented channels of woods (47–51), we have explored a MXene–gelatin hydrogel (MGH) adsorbent having oriented macropores and subsequent high specific surface area, which has the capacity of electric field/sunlight bi-enhanced high-efficient U-adsorption from seawater (Scheme 1). This MGH can be obtained simply via growing directional ice crystals (52,53) cooled by liquid nitrogen and subsequently forming pores by freeze-dry (Ice-template) method (54–56), which contain evenly dispersed MXene (Ti3C2T x ) sheets and oriented macropores. First of all, compared with common MXene-based powder, this MGH can be used conveniently and without sacrificing high-efficient U-adsorbing capacity, on account of the porous integrated bulk structure with high specific surface area and hydrophilic channels. Furthermore, the U-adsorbing performance of the MGH can be further enhanced under an electric field or sunlight irradiation, owing to its good conductivity which can accelerate the immigration of uranyl ions and its high efficiency of photothermal conversion which can elevate temperature of the MGH, respectively. As a result, the U-adsorbing capacity of this MGH can reach 4.17 mg·g−1 within 7 days in seawater, and the U-adsorbing capacity can increase to 57.11%, 13.57%, and 79.95% (7.51 mg·g−1) under 0.4 V of electric field, 1-sun irradiation, or both of them, respectively, which is outstanding among the reported U-adsorbents. This work provides a new MXene-based hydrogel for electric field/sunlight bi-enhanced high-efficient U-extraction from natural seawater.
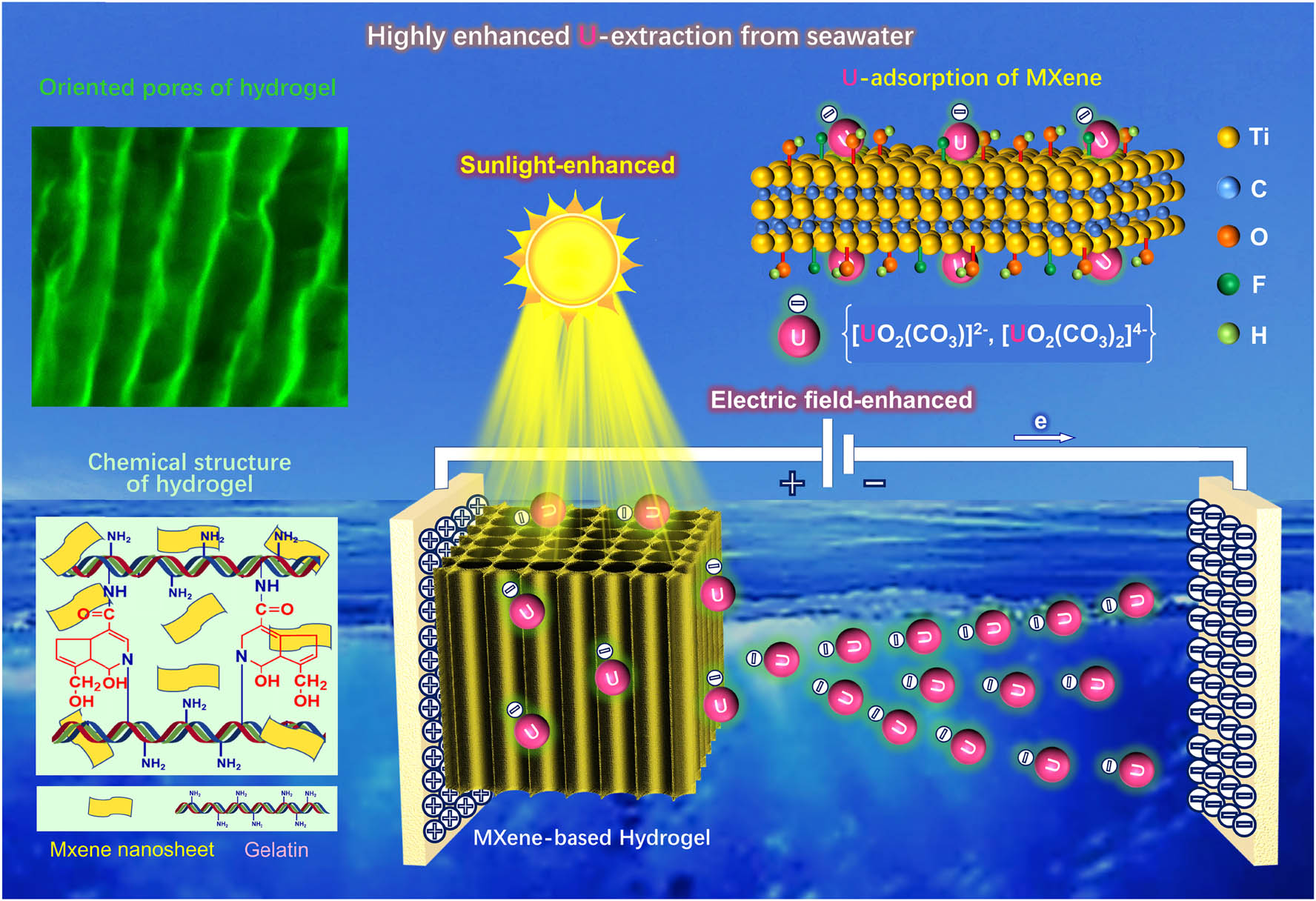
Chemical structure and highly enhanced U-adsorbing mechanism of the wood-mimetic directional macroporous MGH.
2 Materials and methods
2.1 Materials
MXene (Ti3C2T x , 1.0 wt% aqueous solution) was obtained from Shandong Kenyen New Material Co. Ltd (China). Gelatin (97.5%) was purchased from Aladdin Co. Genipin (96.0%) and arsenazo (iii) (95.5%) were bought from Macklin Co. UO2(NO3)2⋅6H2O (98.5%) was bought from the Chushengwei Chemical Co. Ltd (China). HCl (12 mol·L−1), Na2CO3 (99.5%), NaCl (99.5%), NaOH (98.5%), VOSO4·xH2O (99.0%), Cr(NO3)3·9H2O (97.5%), FeCl3·6H2O (98.0%), Mn(NO3)2·4H2O (96.5%), NiCl2·6H2O (97.0%), CuSO4·5H2O (98.0%), and Ba(NO3)2 (97.0%) were supplied by Xilong Science Co. Ltd (China). Each of the solid/liquid material was utilized directly. Sea water was fetched from the seaside of Wenchang City in China, which had been filtered with a filter membrane (0.45 μm) before use.
2.2 Characterization
The adsorption capacity of the hydrogels in highly concentrated U-added solutions was tested by using the ultraviolet-visible spectrophotometer (UV-1800PC, AuCy Instrument, China). Element-specific information was tested by the X-ray photoelectron spectroscopy (XPS; Thermo Escalab 250Xi, Thermo Fisher, USA). The microstructures and micromorphologies of the hydrogels were explored by the scanning electron microscopy (SEM; S-4800, HITACHI, Japan). Distribution of each element in the samples was observed by energy dispersive spectrometry (EDS) mapping. Monolayer morphological structures of MXenes were observed by atomic force microscopy (Dimension Icon, Bruker, USA) and high-resolution transmission electron microscopy (TEM; FEI Talos F200X, Thermo Fisher, USA). Different pH values of U-added solutions were tested by a handheld acidometer (206-pH1, Testo, Germany). The metal-adsorbing capacity of the MXene–gelatin hydrogel was measured via two types of inductively coupled plasma mass spectrometers (Agilent ICPMS 7500ce/Agilent ICPOES730, USA). Particle sizes and zeta potential of MXene sheets were determined through the Malvern Zetasizer Nano spectrometer (ZS90, Malvern Instrument Limited, UK). Specific surface area of the samples was tested based on the Brunauer–Emmett–Teller equation for the pressure P/P o range (BET, ASAP 2460, Mac, USA). The hydrophilicity of the samples was tested by a contact angle meter (OCA50, NETZSCH, Germany).
2.3 Fabrication of the MGH
The MGH was fabricated in three steps (Figure 1). First, the dispersion including 1.0 mL of MXene (1.0 wt%), 1.0 mL of gelatin (1.0 wt%), and 40 µL of genipin (1.0 wt%) was mixed uniformly and put into a hollow cylinder-shaped Teflon reactor, then the bottom of the reactor was immersed into liquid nitrogen to cool the precursor solution and form hydrogel with oriented ice crystals; second, the ice crystals were sublimated via a freeze-dryer for 24 h to obtain a wood-mimetic oriented porous structure in the gel; finally, this porous structure was reinforced by the crosslinking reaction of genipin at 37°C for 12 h to achieve the as-prepared porous MGH (Figure S2). All MGHs with 0–60 wt% of MXene in dry mass were prepared by the same method as above.

The three-step fabrication of the wood-mimetic oriented macroporous MGH.
2.4 Evaluation of U-adsorbing property of the MGH
U-adsorbing behaviors of the samples can be evaluated via Eq. 1:
where U capacity (mg·g−1) is the U-uptake weight by the unit weight of the hydrogel-based samples, W U (mg) and W dry gel (g) are adsorbed weight of U and dry weight of the samples, respectively.
The U-adsorbing properties of the MGH samples can be studied based on pseudo-first-order adsorbing kinetics and pseudo-second-order adsorbing kinetics below (Eqs. 2 and 3):
where q t and q e are the U-adsorbing efficiency (mg·g−1) of MGH at specific time-length and at equilibrium time-length, respectively; k 1 and k 2 are the rate constants of the pseudo primary and pseudo secondary adsorbing equations, respectively; T denotes the adsorbing time of the MGH (min).
The thermodynamics of U-adsorption on MGH was studied in 8 ppm of U-added pure water (pH = 6) at temperatures ranging from 298 to 328 K for 24 h under constant pH value equal to 6. According to the thermodynamic equation and the Gibbs free energy equation below (Eqs. 4 and 5):
3 Results and discussion
3.1 Characterization of MGHs
MGH was characterized on the porous structure and basic hydrogel properties. The wood-mimetic oriented porous structure of the MGH was verified by SEM images (Figure 2a), which have 15 ± 5 μm diameter and good orientation. Furthermore, the laser scanning confocal microscopy (LSCM) images (Figure 2b) showed almost the same size and orientation, which further confirmed the bio-mimetic oriented macropores of the MGH in an aqueous environmental condition. The effect of MXene content on U-adsorbing performance of MGHs was evaluated by comparing 7 sets of MGH samples with different M MXene/M hydrogel mass ratios (0/100 to 60/100) (Figures S3 and S4, Table S1 in Supplementary material). The high specific surface area and the type-IV nano-size pores of the MGH can be confirmed by the BET test within N2, on account of the composited MXene nanosheets (Figures S5 and S6), which was used in all the following tests. The nano-size and surface morphology of the MXene sheets were determined through the Malvern Zetasizer Nano spectrometer and the high-resolution TEM (Figure S7). The thickness of the MXene was approximately 1.5 nm via the atomic force microscopy, which manifested the single-layer nanostructure of the MXene sheets (Figure S8). Compared to the XPS between the blank gelatin hydrogel and the MGH, the MGH has characteristic double peaks of Ti element (461.3 and 455.5 eV), which can prove the dispersal of MXene sheets in the MGH (Figure 2c). In addition, the even and dense distribution of the Ti specific signal observed by EDS mapping further confirmed that MXene had been evenly distributed in the MGH (Figure 2d, Figures S9–S11). Moreover, the difference in thermogravimetric analysis (TGA) curves and X-Ray diffractometer (XRD) spectra between the MGH, gelatin hydrogel, and MXene also verified the MXene sheets in the MGH (Figures S12 and S13). The MGH hydrophilicity was determined by the change in water contact angle (Figure 2e and 2f): comparison of the contact angle change between blank gelatin and MGH (dry gel) from 0.10 to 3.00 s from 60.0° to 54.14° and from 48.83° to 36.89°, respectively, indicate the significant improvement in MGH hydrophilicity owing to the composited super-hydrophilic MXene sheets, which can enhance the U-adsorbing capacity of the MGH. In addition, compared with the gelatin gel, the surface free energy of the MGH increased from 45.82 to 59.08 mJ·m−2, which can further confirm the improved hydrophilicity of the MGH after composited with the MXene (Table S2).

(a) SEM images of the vertical-section and cross-section of the MGH dry gel. (b) LSCM images of the vertical-section and cross-section of the MGH wet hydrogel. (c) XPS spectra of MGH, gelatin hydrogel, and MXene. (d) Ti element EDS mappings of MXene, gelatin hydrogel, and MGH. (e and f) The comparison of the water contact angle change between MGH and gelatin hydrogel.
3.2 Confirmation and quantitative research on the U-adsorbing behavior of the MGH
The U-adsorbing process of the MGH was confirmed and quantitatively researched (Figure 3, Figures S14 and S15). Before (original MGH) and after (U-MGH) hydrogel samples immersed in 16 ppm U-spiked water for 48 h were tested by the XPS (Figure 3a): different from original MGH without the characteristic double peaks of UO2 2+, the U-MGH clearly showed the two peaks (393.0 and 392.4 eV), which confirmed its U-adsorption behavior. Furthermore, the fitting analysis of C1s peak and the two U4f peaks of U-MGH can further prove this U-adsorption of the MGH (Figures S14 and S15). The significantly different surface morphology between the MGH and the U-MGH in SEM images (Figure 3b) further confirmed the U-adsorbing behavior of the MGH. The surface morphology of the U-MGH different from the original smooth MGH observed by SEM images also indicated the U-uptake process. The strong and uniform U-signal of the U-MGH via EDS-mapping (Figure 3c, Figure S16) can further confirm the effective U-adsorption process in the whole MGH. The U-extraction capacity of MGH was tested at different temperatures (Figure 3d), which can significantly increase along with the temperature rising. The chemical U-adsorbing process can be proved by the enthalpy and entropy change via the function of ln(q e/c e):1/T (Eq. 5, Figure 3e). The U-adsorbing processes of the MGH in simulated seawater (Table S3) were evaluated by adsorbing kinetics curve, pseudo-first-order adsorption equation, and pseudo-second-order adsorption equation (Eqs. 2 and 3, Figure 3f and 3g). Soaking in simulated seawater containing 2, 8, and 16 ppm U-added solution at 25°C for 4 h, the U capacity of MGH could reach 77 ± 6, 173 ± 11, and 266 ± 13 mg·g−1, respectively; and reach 103 ± 13, 249 ± 17, and 318 ± 21 mg·g−1, respectively, after equilibrating for 120 h (Figure 3f), similar to the U-adsorption in U-added pure water (Figure S17). Furthermore, the real U-adsorption kinetic curves were found to be closer to the pseudo-secondary fitted kinetics compared to the pseudo-first-order kinetic curves (Eq. 2) and pseudo-secondary kinetics (Eq. 3) in U-spiked simulated seawater (Figure 3g, Table S4), which can further confirm the chemical U-adsorbing process of the MGH.

Comparison of (a) XPS spectra, (b) SEM images, and (c) U-element EDS mappings between the original MGH and U-MGH. (d) U-adsorbing kinetics and (e) thermodynamics on ln (q e/c e):1/T of the MGH at different temperatures within 8 ppm U-spiked pure water (pH = 6). (f) U-adsorbing kinetics of the MGH and (g) subsequent analysis via pseudo-second-order equation within U-spiked simulated seawater (pH = 6).
The pH-dependent and selectivity of MGH U-adsorption were calculated (Figures S18 and S19). The difference in the extraction capacity of MGH for 8 ppm U-added water was investigated under the same conditions over 24 h (Figure S18). The MGH showed good adsorbing capacity between pH 3–9, where the adsorption capacity increased with increasing pH in the range of pH 3–6, from 54 ± 7 to 278 ± 19 mg·g−1, but the adsorption decreased to 47 ± 6 mg·g−1 as the pH continued to increase. Thus, it was concluded that the external water environment can reach the optimum adsorption value at around pH = 6. Many kinds of metal ions can disturb the U-extracting process of the MXene-based MGH in natural seawater, so we explored whether MGH can selectively adsorb many trace ions in seawater (Figure S19, Table S5). It can be seen that although the hydrogel was slightly more selective for V than U, it was much less selective than U for other competing ions as well as Na+, K+, Ca2+, and Mg2+, which were present in large quantities in seawater.
3.3 Evaluation of the MGH U-extraction enhanced by electric field and sunlight
The enhancements of the MGH U-adsorbing capacity by the sunlight and the electric field in U-spiked solution were researched (Figure 4), on account of both the high-efficient photothermal conversion (Figure 4b) and electrical conductivity of the MGH (Figure S20). With a system for continuously circulating solution (Figure 4a), for a MGH (10 mg) in 1.00 L of 8 ppm (pH = 6) simulated seawater for 60 h, the U-capacity of the MGH largely increased by the enhancement of 1-sun irradiation, 0.4 V electric field, and both of them reaching 225 ± 12, 304 ± 13, and 363 ± 12 mg·g−1, respectively, compared with the 203 ± 11 mg·g−1 without sunlight or electric field (Figure 4c). In addition, the U-adsorbing performance of this MGH can still be maintained after five adsorption-desorption cycles (Figure 4d, Figure S21), which indicate the good reusability of the MGH. The U-extracting property of the MGH from natural seawater was further evaluated by electric field and sunlight enhancements (Figure 4e, Figure S22). The hydrogel sample (10 mg) was fixed on the white foam, and the system was then fixed on the surface of a barrel of 100 kg natural seawater for 1 week (Figures S21 and S22) to test its U-extraction capability (50 mL seawater samples were collected every 24 h, and the U capacity was calculated by the ICP-MS). Compared with U-capacity of the MGH (4.17 ± 0.19 mg·g−1) without sunlight and electric field, with the enhancement of 0.4 V of direct electric field and 1.0 sun irradiation, the U capacity can increase to 57.11% (6.56 ± 0.27 mg·g−1) and 13.57% (4.74 ± 0.21 mg·g−1), respectively. Most importantly, under both the sunlight and the electric field, the U capacity of MGH in seawater can increase to 79.95% reaching 7.51 ± 0.29 mg·g−1. The reason for the highly enhanced U-adsorbing capacity of this MGH hydrogel can be explained that the Ti3C2T x MXene has good electro-conductivity and ultrahigh efficiency of photothermal conversion, which can speed up the migration of uranyl ions onto the MGH (Figure 4b) and can rise up the temperature of the MGH respectively. Last but not least, after this bi-enhanced U-extraction, the wood-mimetic porous structure was still in good condition, which was proved by the LSCM images of the original MGH and the U-MGH (Figure 4f). In addition, this bi-enhanced U-adsorption from seawater by the MGH can be ultrafast (1.073 ± 0.041 mg·g−1·day−1), which is outstanding among various existing sorbents (Figure S23, Table S6).

(a) System and schematic diagram of U-adsorbing device enhanced by simulated sunlight and power resource of direct current. (b) Comparison of the light transmittance between the MGH and blank gelatin hydrogel over 200–2,500 nm wavelength range. (c and e) Comparison of the MGH U-adsorbing capacity from natural sea water under four different external conditions within 8 ppm U-spiked simulated seawater and seawater, respectively. (d) U-adsorbing performance (in 8 ppm U-added simulated sea water) and U-removal efficiencies during 1–5 adsorbing-desorbing cycles. (f) LSCM images of the original MGH and U-MGH after bi-enhanced U-extraction from seawater for 7 days.
4 Conclusion
In summary, we have explored a wood-mimetic oriented macroporous MGH with electric field/sunlight synergistic bi-enhanced U-adsorbing capacity, based on the Ice-template method. This MGH can provide ultrafast and ultraefficient U-extraction. In addition, the united body of this MGH hydrogel can integrate all the MXene sheets together, which can make the MGH to adsorb/desorb uranium more conveniently than the MXene powder. Furthermore, the U-adsorbing performance of the MGH can be largely enhanced under electric field and sunlight irradiation. As a result, the U-adsorbing capacity of this MGH from seawater can reach 4.17 mg·g−1, when 10 mg of MGH immersed in 100 kg of natural seawater for only 7 days. Furthermore, the U-capacity of the MGH can largely increase to 57.11%, 13.57%, and 79.95% (7.51 mg·g−1, 1.073 mg·g−1·day−1) under 0.4 V of electric field, 1.0 sun irradiation, and both of them, respectively, which is outstanding among reported U-adsorbents. This work provides a new oriented porous MXene-based hydrogel for electric field/sunlight synergistic bi-enhanced fast and high-efficient U-extraction from seawater, which will also inspire the exploration of novel U-adsorbents.
-
Funding information: This work was supported by the National Natural Science Foundations of China (No. 21965010, 22065012, 51775152, 61761016, U1967213, and 41966009), the Hainan Science and Technology Major Project (ZDKJ2020011 and ZDKJ2019013), the Hainan Provincial Natural Science Foundation of China (2019CXTD401), the Taizhou-Zhejiang University Science and Technology Program (2018NMS01), the Taizhou Innovation Centre Science Park Special Fund (QTKJ201903001), the National Key R&D program of China (2018YFE0103500), the Finance Science and Technology Project of Hainan Province (No. ZDYF2020205), and the Research Foundations of Hainan University (KYQD(ZR)1811, KYQD(ZR)1814, and KYQD(ZR)1815).
-
Author contributions: Lin Chen: writing – original draft, writing – review and editing, formal analysis, and investigation; Ye Sun: writing – review and editing and resources; Jiawen Wang: writing – review and editing; Chao Ma: writing – review and editing and methodology; Shuyi Peng: writing – review and editing and investigation; Xingyu Cao: writing – review and editing and methodology; Lang Yang: writing – review and editing and methodology; Chunxin Ma: writing – review and editing, investigation, and resources; Gaigai Duan: writing – review and editing; Zhenzhong Liu: writing – review and editing, investigation, and methodology; Hui Wang: resources; Yihui Yuan: project administration; Ning Wang: conceptualization and writing – review and editing.
-
Conflict of interest: Author states no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
(1) Xie Y, Chen CL, Ren XM, Wang XX, Wang HY, Wang XK. Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation. Prog Mater Sci. 2019;103:180–234. 10.1016/j.pmatsci.2019.01.005.Search in Google Scholar
(2) Abney CW, Mayes RT, Saito T, Dai S. Materials for the recovery of uranium from seawater. Chem Rev. 2017;117(23):13935–4013. 10.1021/acs.chemrev.7b00355.Search in Google Scholar PubMed
(3) Chi FT, Xiong J, Hu S, Hou JW, Gu M, Han J. High performance of amidoxime/amine functionalized polypropylene for uranyl (VI) from aqueous solution. e-Polym. 2013;13(1):13. 10.1515/epoly-2013-0113.Search in Google Scholar
(4) Li H, Wang S. Reaction: semiconducting MOFs offer new strategy for uranium extraction from seawater. Chem. 2021;7(2):279–80. 10.1016/j.chempr.2021.01.013.Search in Google Scholar
(5) Arendt CA, Aciego SM, Sims KWW, Robbins M. Sequential separation of uranium, hafnium and neodymium from natural waters concentrated by iron coprecipitation. Geostand Geoanal Res. 2015;39(3):293–303. 10.1111/j.1751-908x.2014.00322.x.Search in Google Scholar
(6) Zuhua Z, Xiao Y, Huajun Z, Yue C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl Clay Sci. 2009;43(2):218–23. 10.1016/j.clay.2008.09.003.Search in Google Scholar
(7) Shamsipur M, Davarkhah R, Khanchi AR. Facilitated transport of uranium(VI) across a bulk liquid membrane containing thenoyltrifluoroacetone in the presence of crown ethers as synergistic agents. Sep Purif Technol. 2010;71(1):63–9. 10.1016/j.seppur.2009.11.003.Search in Google Scholar
(8) Parker BF, Zhang Z, Rao L, Arnold J. An overview and recent progress in the chemistry of uranium extraction from seawater. Dalton Trans. 2018;47:639–44. 10.1039/c7dt04058j.Search in Google Scholar PubMed
(9) Saad EM, Elshaarawy RF, Mahmoud SA, El-Moselhy KM. New Ulva lactuca Algae Based Chitosan Bio-composites for Bioremediation of Cd(II) Ions. J Bioresour Bioprod. 2021;6(3):223–42. 10.1016/j.jobab.2021.04.002.Search in Google Scholar
(10) Wei D, Wei Z, Kaneshiro H, Wang Y, Lin H, Pen S. Preparation of bamboo charcoal pottery and its gas adsorption and humidity regulation performance. J Eng. 2020;5(1):109–13. 10.13360/j.issn.2096-1359.201905025.Search in Google Scholar
(11) Jjagwe J, Olupot PW, Menya E, Kalibbala HM. Synthesis and application of granular activated carbon from biomass waste materials for water treatment: a review. J Bioresour Bioprod. 2021;6:292–322. 10.1016/j.jobab.2021.03.003.Search in Google Scholar
(12) Wang Z, Hu C, Tu D, Zhang W, Guan L. Preparation and adsorption property of activated carbon made from Camellia olerea shells. J Eng. 2020;5(5):96–102. 10.13360/j.issn.2096-1359.202001032.Search in Google Scholar
(13) Chen Y, Hanshe M, Sun Z, Zhou Y, Mei C, Duan G, et al. Lightweight and anisotropic cellulose nanofibril/rectorite composite sponges for efficient dye adsorption and selective separation. Int J Biol Macromol. 2022;207(15):130–9. 10.1016/j.ijbiomac.2022.03.011.Search in Google Scholar PubMed
(14) Li H, He NN, Cheng C, Dong H, Wen J, Wang XL. Antimicrobial polymer contained adsorbent: A promising candidate with remarkable anti-biofouling ability and durability for enhanced uranium extraction from seawater. Chem Eng J. 2020;388(15):124273. 10.1016/j.cej.2020.124273.Search in Google Scholar
(15) Yu J, Bai HB, Wang J, Li ZS, Jiao CS, Liu Q, et al. Synthesis of alumina nanosheets via supercritical fluid technology with high uranyl adsorptive capacity. N J Chem. 2013;37:366–72. 10.1039/c2nj40514h.Search in Google Scholar
(16) Yan BJ, Ma CX, Gao JX, Yuan YH, Wang N. An ion-crosslinked supramolecular hydrogel for ultrahigh and fast uranium recovery from seawater. Adv Mater. 2020;32(10):1906615. 10.1002/adma.201906615.Search in Google Scholar PubMed
(17) Yuan YH, Yu QH, Wen J, Li CY, Guo ZH, Wang XL, et al. Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber. Angew Chem Int Ed Engl. 2019;58(34):11785–90. 10.1002/anie. 201906191.Search in Google Scholar
(18) Zubair M, Daud M, McKay G, Shehzad F, Al-Harthi MA. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl Clay Sci. 2017;143:279–92. 10.1016/j.clay.2017.04.002.Search in Google Scholar
(19) Zhong X, Liu YX, Wang S, Zhu YL, Hu BW. In-situ growth of COF on BiOBr 2D material with excellent visible-light-responsive activity for U(VI) photocatalytic reduction. Sep Purif Technol. 2021;279(15):119627. 10.1016/j.seppur.2021.119627.Search in Google Scholar
(20) Amini A, Khajeh M, Oveisi AR, Daliran S, Ghaffari-Moghaddam M, Delarami HS. A porous multifunctional and magnetic layered graphene oxide/3D mesoporous MOF nanocomposite for rapid adsorption of uranium(VI) from aqueous solutions. J Ind Eng Chem. 2021;93(25):322–32. 10.1016/j.jiec.2020.10.008.Search in Google Scholar
(21) Yavuz CT. Reaction: porous organic polymers for uranium capture. Chem. 2021;7(2):276–7. 10.1016/j.chempr.2021.01.011.Search in Google Scholar
(22) Yuan Y, Yang YJ, Zhu GS. Molecularly imprinted porous aromatic frameworks for molecular recognition. ACS Cent Sci. 2020;6(7):1082–94. 10.1021/acscentsci.0c00311.Search in Google Scholar PubMed PubMed Central
(23) Wen SX, Sun Y, Liu RR, Chen L, Wang JW, Peng SY, et al. Supramolecularly poly(amidoxime)-loaded macroporous resin for fast uranium recovery from seawater and uranium-containing wastewater. ACS Appl Mater Interfaces. 2021;13(2):3246–58. 10.1021/acsami.0c21046.Search in Google Scholar PubMed
(24) Gogotsi Y, Anasori B. The rise of MXenes. ACS Nano. 2019;13(8):8491–4. 10.1021/acsnano.9b06394.Search in Google Scholar PubMed
(25) Naguib M, Barsoum MW, Gogotsi Y. Ten years of progress in the synthesis and development of MXenes. Adv Mater. 2021;33(39):2103393. 10.1002/adma.202103393.Search in Google Scholar PubMed
(26) Naguib M, Kurtoglu M, Presser V, Lu J, Niu JJ, Heon M, et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater. 2011;23(37):4248–53. 10.1002/adma.201102306.Search in Google Scholar PubMed
(27) Riazi H, Nemani SK, Grady MC, Anasori B, Soroush M. Ti3C2 MXene–polymer nanocomposites and their applications. J Mater Chem A. 2021;9(13):8051–98. 10.1039/d0ta08023c.Search in Google Scholar
(28) VahidMohammadi A, Rosen J, Gogotsi Y. The world of two-dimensional carbides and nitrides (MXenes). Science. 2021;372(6547). 10.1126/science.abf1581.Search in Google Scholar PubMed
(29) Anasori B, Lukatskaya MR, Gogotsi Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat Rev Mater. 2017;2(2):16098. 10.1038/natrevmats.2016.98.Search in Google Scholar
(30) Rasool K, Pandey RP, Rasheed PA, Buczek S, Gogotsi Y, Mahmoud KA. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes). Mater Today. 2019;30:80–102. 10.1016/j.mattod.2019.05.017.Search in Google Scholar
(31) Rosales M, Garcia A, Fuenzalida VM, Espinoza-González R, Song GC, Wang B, et al. Unprecedented arsenic photo-oxidation behavior of few- and multi-layer Ti3C2Tx nano-sheets. Appl Mater Today. 2020;20:100769. 10.1016/j.apmt.2020.100769.Search in Google Scholar
(32) González-Poggini S, Rosenkranz A, Colet-Lagrille M. Two-dimensional nanomaterials for the removal of pharmaceuticals from wastewater: a critical review. Processes. 2021;9(12):2160. 10.3390/pr9122160.Search in Google Scholar
(33) Quero F, Rosenkranz A. mechanical performance of binary and ternary hybrid mxene/nanocellulose hydro- and aerogels – a critical review. Adv Mater Interfaces. 2021;8(18):2100952. 10.1002/admi.202100952.Search in Google Scholar
(34) Wyatt BC, Rosenkranz A, Anasori B. 2D MXenes: tunable mechanical and tribological properties. Adv Mater. 2021;33(17):2007973. 10.1002/adma.202007973.Search in Google Scholar PubMed
(35) Shang TX, Lin ZF, Qi CS, Liu XC, Li P, Tao Y, et al. 3D macroscopic architectures from self-assembled MXene hydrogels. Adv Funct Mater. 2019;29(33):1903960. 10.1002/adfm.201903960.Search in Google Scholar
(36) Wang L, Song H, Yuan LY, Li ZJ, Zhang YJ, Gibson JK, et al. Efficient U(VI) reduction and sequestration by Ti2CTx MXene. Env Sci Technol. 2018;52(18):10748–56. 10.1021/acs.est.8b03711.Search in Google Scholar PubMed
(37) Hantanasirisakul K, Gogotsi Y. Electronic and optical properties of 2D transition metal carbides and nitrides (MXenes). Adv Mater. 2018;30(52):1804779. 10.1002/adma.201804779.Search in Google Scholar PubMed
(38) Zhang YZ, Zhou J, Wang D, Cao RY, Li JX. Performance of MXene incorporated MOF-derived carbon electrode on deionization of uranium(VI). Chem Eng J. 2022;430(Part 2):132702. 10.1016/j.cej.2021.132702.Search in Google Scholar
(39) Deng H, Li ZJ, Wang L, Yuan LY, Lan JH, Chang ZY, et al. Nanolayered Ti3C2 and SrTiO3 composites for photocatalytic reduction and removal of uranium(VI). ACS Appl Nano Mater. 2019;2(4):2283–94. 10.1021/acsanm.9b00205.Search in Google Scholar
(40) Yu KF, Jiang PY, Wei JC, Yuan HB, Xin Y, He R, et al. Enhanced uranium photoreduction on Ti3C2Tx MXene by modulation of surface functional groups and deposition of plasmonic metal nanoparticles. J Hazard Mater. 2021;426:127823. 10.1016/j.jhazmat.2021.127823.Search in Google Scholar PubMed
(41) Li ST, Wang Y, Wang JJ, Liang JJ, Li YQ, Li P. Modifying g-C3N4 with oxidized Ti3C2 MXene for boosting photocatalytic U(VI) reduction performance. J Mol Liq. 2021;346:117937. 10.1016/j.molliq.2021.117937.Search in Google Scholar
(42) Wei LS, Deng WJ, Li SS, Wu ZG, Cai JH, Luo JW. Sandwich-like chitosan porous carbon Spheres/MXene composite with high specific capacitance and rate performance for supercapacitors. J Bioresour Bioprod. 2022;7(1):63–72. 10.1016/j.jobab.2021.10.001.Search in Google Scholar
(43) Yang YD, Liu GX, Wei YC, Liao SQ, Luo MC. Natural rubber latex/MXene foam with robust and multifunctional properties. e-Polym. 2021;21(1):179–85. 10.1515/epoly-2021-0017.Search in Google Scholar
(44) Cao XX, Wu MQ, Zhou AG, Wang Y, He XF, Wang LB. Non-isothermal crystallization and thermal degradation kinetics of MXene/linear low-density polyethylene nanocomposites. e-Polym. 2017;17(5):373–81. 10.1515/epoly-2017-0017.Search in Google Scholar
(45) Yang HC, Guo XJ, Chen RR, Liu Q, Liu JY, Yu J, et al. A hybrid sponge with guanidine and phytic acid enriched surface for integration of antibiofouling and uranium uptake from seawater. Appl Surf Sci. 2020;525:146611. 10.1016/j.apsusc.2020.146611.Search in Google Scholar
(46) Gill GA, Kuo LJ, Janke CJ, Park J, Jeters RT, Bonheyo GT, et al. The uranium from seawater program at the pacific northwest national laboratory: overview of marine testing, adsorbent characterization, adsorbent durability, adsorbent toxicity, and deployment studies. Ind Eng Chem Res. 2016;55(15):4264–77. 10.1021/acs.iecr.5b03649.Search in Google Scholar
(47) Wang N, Zhao XM, Wang JW, Yan BJ, Wen SX, Zhang JC, et al. Accelerated chemical thermodynamics of uranium extraction from seawater by plant-mimetic transpiration. Adv Sci (Weinh). 2021;8(24):2102250. 10.1002/advs.202102250.Search in Google Scholar PubMed PubMed Central
(48) Hu XH, Li D, Zhou F, Gao CY. On the structure alteration of crosslinkable gelatin coupled with methacrylic acid and its hydrogel. e-Polym. 2012;12(1):151. 10.1515/epoly.2012.12.1.151.Search in Google Scholar
(49) Wang F, Liu XL, Duan GG, Yang HQ, Cheong JY, Lee JY, et al. Wood-derived, conductivity and hierarchical pore integrated thick electrode enabling high areal/volumetric energy density for hybrid capacitors. Small. 2021;17(35):2102532. 10.1002/smll.202102532.Search in Google Scholar PubMed
(50) Wang F, Cheong JY, Lee J, Ahn J, Duan GG, Chen HL, et al. Pyrolysis of enzymolysis-treated wood: hierarchically assembled porous carbon electrode for advanced energy storage devices. Adv Funct Mater. 2021;31(31):2101077. 10.1002/adfm.202101077.Search in Google Scholar
(51) Li YQ, Huang XB, Lv JJ, Wang F, Jiang SH, Wang G. Enzymolysis-treated wood-derived hierarchical porous carbon for fluorescence-functionalized phase change materials. Composites, Part B. 2022;234:109735. 10.1016/j.compositesb.2022.109735.Search in Google Scholar
(52) Chen YM, Li SJ, Li XL, Mei CT, Zheng JJ, Duan ESJ, et al. Liquid transport and real-time dye purification via lotus petiole-inspired long-range-ordered anisotropic cellulose nanofibril aerogels. ACS Nano. 2021;15(12):20666–77. 10.1021/acsnano.1c10093.Search in Google Scholar PubMed
(53) Yao KQ, Song CH, Fang H, Wang F, Chen L, Jiang SH, et al. Robust and fatigue resistant polyimide fibrous aerogels by freezing-extraction/vacuum-drying and their composites with enhanced fire-retardance. Engineering. 2021;8(24). 10.1016/j.eng.2021.08.024.Search in Google Scholar
(54) Joukhdar H, Seifert A, Jungst T, Groll J, Lord MS, Rnjak-Kovacina J. Ice templating soft matter: fundamental principles and fabrication approaches to tailor pore structure and morphology and their biomedical applications. Adv Mater. 2021;33(34):2100091. 10.1002/adma.202100091.Search in Google Scholar PubMed
(55) Huang CJ, Peng JS, Wan SJ, Du Y, Dou SX, Wagner HD, et al. Ultra-Tough Inverse Artificial Nacre Based on Epoxy-Graphene by Freeze-Casting. Angew Chem Int Ed Engl. 2019;131(23):7718–22. 10.1002/ange.201902410.Search in Google Scholar
(56) Chen Y, Luo H, Guo H, Liu K, Mei C, Li Y, et al. Anisotropic cellulose nanofibril composite sponges for electromagnetic interference shielding with low reflection loss. Carbohydr Polym. 2022;276:118799. 10.1016/j.carbpol.2021.118799.Search in Google Scholar PubMed
© 2022 Lin Chen et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

