Abstract
The marine pollution caused by traditional plastics is becoming increasingly serious, and the fundamental way to solve this problem is to look for plastic substitutes that can degrade in the marine environment. Herein, a series of high-molecular-weight poly(butylene succinate-co-diethylene glycol succinate) (PBDS) was obtained by the introduction of low-cost diethylene glycol (DEG) into the main chain of poly(butylene succinate) (PBS), which aimed to obtain the materials that can be degraded both in compost and seawater. The research showed that the increase in the DEG content reduced the crystallinity of the copolyester, which led to the decrease in mechanical strength and thermal properties of the copolyester to a certain extent. Meanwhile, the increase in hydrophilicity and the decrease in crystallinity improved the degradation rate of the material. Compared with PBS, PBDS exhibited not only a faster composting degradation rate but also a faster degradation rate in seawater.
Graphical abstract
Diethylene glycol-modified poly(butylene succinate) prepared copolyester, which can be degraded in seawater and compost at the same time, aiming to solve the problem of plastic pollution.

1 Introduction
In recent years, the problems of marine plastic pollution have become increasingly serious (1,2,3). From the perspective of reducing environmental hazards and building an environmentally friendly ecology, the use of degradable plastics to replace traditional plastics is considered to be one of the most appropriate solutions (4,5,6). Biodegradable plastics represented by poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and poly(butylene succinate) (PBS) have been investigated for nearly 30 years, which are brilliant in the field of packaging in recent years (7,8,9). Although these so-called biodegradable polyesters show excellent degradation performance under composting and soil conditions, more and more studies revealed that they are usually difficult to degrade in the marine environment. Compared with the composting environment, the marine environment is characterized by low temperature, low oxygen, and low microbial content (10,11,12,13,14,15,16).
Compared with PBAT and PLA, PBS exhibits not only excellent biodegradability but also good mechanical properties, thermal stability, higher thermal deformation temperature and outstanding processability (17,18,19). However, the high price of 1,4-butanediol (BDO), one of the raw materials of PBS, limits its widespread use (20). Therefore, it is particularly important to select a cost-effective monomer for the copolymerization and modification of PBS to greatly reduce the cost on the premise of improving the degradation performance.
Diethylene glycol (DEG) is a cheap and easily available glycol containing ether bond (21). The ether bond of DEG significantly improves the flexibility of the molecular chain when participating in copolymerization and reduces the crystallinity of the materials (22,23,24,25). At the same time, the ether oxygen atoms endow the monomer with the characteristic of hydrophilicity, which makes it easier for water molecules and microorganisms to erode the molecular chains in the process of degradation (26,27). Therefore, the introduction of ether bonds in the copolymerization process effectively improves the degradation performance of the materials. Zeng et al. investigated the influence of the DEG on the hydrophilicity of the copolymers and the results showed that the hydrophilicity was significantly improved with the increase of the DEG content (28). Gigli et al. explored the effects of the change of molecular chain structure on the enzymatic degradation performance of PBS modified with ether bond, and the results demonstrated that the amorphous phase of copolyester was more susceptible to enzymatic degradation (27). However, there are few reports on the effects of DEG on the degradation of the materials in seawater and composting.
Considering the particularity of the marine environment, the improvement of the hydrolysis is the key to preparing the materials that can be degraded in seawater (29,30), and the introduction of hydrophilic monomers in the copolymerization process is undoubtedly a favorable choice to improve the degradation performance of the materials. In this article, the hydrophilic DEG was introduced into the molecular chain of PBS by copolymerization to synthesize poly(butylene succinate-co-diethylene glycol succinate) (PBDS) copolyester and expected to get a material that can be composted and degraded by seawater at the same time. The structure of PBDS was characterized by 1H nuclear magnetic resonance (NMR), and the basic properties of the materials were analyzed by differential scanning calorimetry (DSC) and X-ray diffractometer (XRD). The effects of DEG content in copolyester on its biodegradability were investigated under the condition of composting degradation. Furthermore, the possibility of PBDS degradation in seawater was explored, and the effects of DEG on the seawater degradation performance of the materials were also studied.
2 Materials and methods
2.1 Materials
1,4-Butanediol (GC 99.0%) (BDO), tetrabutyl titanate (TBT), and deuterated chloroform (99.8% D, containing 0.03% v/v tetramethyl silane, TMS) were obtained from Budweiser Technology Co., Ltd. (Beijing, China). 1,4-Succinic acid (AR 99.5%) (SA), DEG (GC 98%) (DEG), sucrose, barium hydroxide, and discolored silica gel were purchased from McLean Biochemical Technology Co., Ltd. Phosphate-buffered solution (0.01 mol·L−1, pH = 7.2–7.4) was obtained from Regen Biology. The inoculum was purchased from Shuiguxin Company, nutrient broth was acquired from Beijing Luqiao, microcrystalline cellulose (column chromatography) was purchased from Sinopharm Group, and corn starch (reagent grade) was provided by Aladdin.
2.2 Synthesis and processing of PBDS copolyester
SA and diol (BDO/DEG = 95/5–55/45) with a molar ratio of 1/1.05 were added to a 500-mL three-necked flask equipped with an overhead mechanical stirrer. TBT with a total mass of 0.3% of the raw material was used as the catalyst. According to the molar percentage of 1,4-butanediol in the total diol content during feeding, the prepared copolyesters are named PBDS95, PBDS85, PBDS70, and PBDS55. For the prepared of PBDS55, SA (1.0 mol, 118.09 g), BDO (0.58 mol, 52.27 g), DEG (0.47 mol, 49.88 g), and TBT (0.66 g) were added into a three-mouth flask. Under a nitrogen atmosphere, the mixture was esterified at 180°C for 4 h to remove water. After esterification, the vacuum degree was gradually reduced to about 100 Pa within 1 h at a rate of about 28 Pa·s−1, and the mixture was polycondensated at 230°C for 3–5 h to obtain white to light yellow resin samples, which was directly used for subsequent tests without purification.
The copolyester was prepared into standard tensile splines with an effective length (G 0) of 25 ± 1 mm, width (b) of 6.0 ± 0.4 mm, thickness (d) of 2.0 ± 0.2 mm, and a mass of 1.5–2 g with an injection molding machine (Thermo Fisher, HAAKE minijet pro, Germany). The injection and mold temperature of PBS and PBDS copolyesters were set at 130–180°C and 30°C, respectively, with 10 s injection time and 500–800 Pa injection pressure. Then, the sample was kept under the pressure of 500 Pa for 15 s. The splines were then used for mechanical properties, crystallinity, and seawater degradation experiments.
The copolyester samples were quenched in the liquid nitrogen and ground with a pulverizer. The powder samples were screened with 20 and 40 mesh screens to obtain powders with a particle size of 0.425–0.850 mm. After drying in a 50°C vacuum oven for 48 h, the samples were used for the compost degradation experiments.
2.3 Study on degradation performance
2.3.1 Compost degradation experiment
According to the standard ISO 14855-1:2005 (31), the final aerobic biodegradability of the sample is determined by measuring the amount of CO2 emitted by the degraded sample under the condition of aerobic composting. To study the effect of DEG content on the degradation performance of compost, some PBDS samples (the interval of DEG mol% >10%) were selected for composting degradation experiment. The PBS, PBDS95, PBDS70, and PBDS55 powder samples with an equivalent molecular weight were used for composting degradation experiment, and microcrystalline cellulose served as the reference. At the same time, the blank control group was set. The three groups were set in parallel, and the degradation experiment lasted for 101 days. The performance parameters of the samples and cellulose are shown in Table 1. The nutrient solution and trace element solution were prepared according to the standard, the vermiculite and the culture solution in the proportion of 1/3 (mass/volume) were mixed, and the mixture was placed in the incubator at 52 ± 2°C and activated it for 72 h, which was used as the inoculum of composting experiment. Finally, the activated vermiculite and the sample at 4/1 were mixed and put into the composting reactor. The composting reactor was placed in a water bath of 58 ± 2°C for composting degradation experiment. Continuous saturated air was filled into the degradation reactor at a fixed flow rate. Aerobic microorganisms in the reactor used the samples for life activities to produce CO2, which was collected by a CO2 capture trap (saturated Ba(OH)2 solution). The trap was regularly updated, the concentration of Ba(OH)2 solution supernatant in the CO2 trap was titrated by a standard HCl titration method, the amount of CO2 absorbed was calculated by Eq. 1, and the mineralization rate of the sample was calculated by Eq. 2.
where (CO2)s is the average value of the mass of CO2 (g) released by the tested sample, B b is the concentration of Ba(OH)2 (g·mol−1) before CO2 absorption, B a is the concentration of Ba(OH)2 (g·mol−1) after CO2 absorption, (CO2)B is the average value of the mass of CO2 (g) released by the blank control group, W S is the total mass of the tested sample (g), and C S is the total organic carbon content (%).
Characteristics of compost samples cellulose, PBS, and PBDSs
| Sample | M n (× 104 g·mol−1) | Particle size (μm) | carbon content (%) |
|---|---|---|---|
| Cellulose | — | 25 | 42.25 |
| PBS | 4.2 | 420–840 | 49.07 |
| PBDS95 | 3.8 | 55.29 | |
| PBDS70 | 4.2 | 53.88 | |
| PBDS55 | 4.7 | 53.13 |
2.3.2 Laboratory seawater degradation experiment
The dumbbell-shaped splines were marked and weighed, six groups samples were set, each group (three splines in parallel) was put into a clean centrifuge tube, and 45 g of seawater obtained from Tianjin Bohai Sea (longitude: 117.73°; latitude: 38.99°, salinity: 28.5, pH: 8.13, December 27, 2020) was added (16). The degradation experiments were carried out at 30°C for 250 days. The samples were taken regularly to measure the changes in physical and chemical properties during the degradation of the samples. The seawater was regularly changed for every month.
2.4 Characterization
The molecular weight and distribution of the samples were measured by gel permeation chromatography GPC (Waters 1515). Chloroform served as the mobile phase with a flow rate of 1 mL·min−1, and the injection volume was 40 μL. Polystyrene was used as the standard sample. The test samples were taken from different positions of the spline surface and middle layer. The structure and composition of the copolymers were characterized by 1H NMR (Bruker amx-300, Germany). The content of BDO/DEG in the products was calculated by NMR with deuterated chloroform as the solvent and TMS as the internal standard.
The thermal properties of the copolyester samples were measured by differential scanning calorimetry (DSC 1 Mettler Toledo, Switzerland). DSC tests were conducted with a heating rate of 10°C·min−1 ranging from −80°C to 180°C, quenched to −80°C at −200°C·min−1, and then heated again. The thermal stability of the samples was tested by a thermogravimetric analyzer (TGA) (American Thermal Analysis TGA Q50 v20.10 build 36) in the temperature range from room temperature to 600°C. The crystallinities of the samples were tested by XRD (Bruker D8 focus, Germany) with Cu-Kα radiation (λ = 1.541 Å) and a scan range of 5−60° at a speed of 0.2°s·step−1. The mechanical properties of the standard tensile splines and degraded splines placed at room temperature for 1 week were tested by a universal tensile machine (WDW-10 Song dun, Shanghai) at a speed of 50 mm·min−1.
The static contact angle of the standard dumbbell sample surface was measured by OCA20 contact angle meter (DataPhysics Instruments GmbH, Germany) and calculated by the Ellipse fitting method. The water of 3 μL was dropped by microsyringe at five different positions on the sample surface, and the contact angle of the sample was the average value ± standard deviation. The salinity of the seawater was measured with a handheld salinometer (Heng Xing az8371, Taiwan), and the pH value of the seawater was measured with a pH meter (Mettler Toledo az8686, Switzerland).
The weight loss of the spline in the degradation process was detected by the gravimetric method. The samples were regularly collected and washed with deionized water and then dried in a vacuum oven at 50°C for 48 h to a constant weight for weight testing.
The organic carbon content of the samples was measured by the Dumas combustion method with a UNICUBE vario MACRO cube.
3 Results and discussion
3.1 Synthesis and structure
A series of high-molecular-weight PBDS copolyesters were synthesized by one-pot melt polycondensation with SA, butanediol, and DEG as the monomers, as shown in Figure 1. The esterification and polycondensation temperatures of the copolyester were 180°C and 230°C, respectively, which were close to the synthesis temperature of PBS. The number average molecular weight (M n) of the PBDS copolyester was above 3.6 × 104 g·mol−1, and the weight-average molecular weight (M w) was more than 8.4 × 104 g·mol−1, which showed that the synthesis conditions were suitable for copolyesters and achieved a better polymerization control. The increase of the DEG content had no significant effect on the degree of polymerization. The color of the copolyesters gradually changed from white to light yellow with the increase of the DEG content.

(a) Synthesis route of PBDS, (b) 1H NMR spectra of PBDS, and (c) partial enlargement of NMR of PBDS.
The structure of the copolyester was further characterized by 1H NMR. The proton peaks at 4.12 and 1.71 belong to the external and internal methylene peaks of butanediol in the BS sequence respectively, and the proton peaks at 3.70 and 4.25 are assigned to the methylene peaks on DEG close and away from the oxygen atom of ether bond in the DEG sequence respectively (28), which indicated that DEG was successfully introduced into the BS molecular chain. The methylene peak of SA in the BS and DEG sequences appeared at 2.62−2.67 (27,28). The peak strengths (a3 and a4) of the methylene protons on SA linked to DEG gradually increased with the increase in the DEG content. The ratio of BDO and DEG in the copolyester was calculated by the ratio of peak area at 4.12 and 3.70, which demonstrated that the actual composition of the copolyester was equal to or slightly less than the feed ratio (Table 2).
The structure and molecular weight of PBDS
| Sample | Feed ration n(BDO)/n(DEG) (%) | Actual ration n(BDO)/n(DEG) (%) | M n | M w | PDI |
|---|---|---|---|---|---|
| (g·mol−1) | (g·mol−1) | ||||
| PBS | 100/0 | 100/0 | 36,210 | 63,873 | 1.76 |
| PBDS95 | 95/5 | 94/6 | 45,043 | 85,346 | 1.90 |
| PBDS85 | 85/15 | 84/16 | 38,871 | 84,991 | 2.12 |
| PBDS70 | 70/30 | 69/31 | 36,544 | 84,991 | 2.33 |
| PBDS55 | 55/45 | 52/48 | 39,294 | 121,767 | 3.10 |
3.2 Thermal performance, mechanical properties, and hydrophilicity
The melting point (T m), melting enthalpy (ΔH m), and crystallization temperature (T c) of the PBDS copolyester gradually decreased with the increase in the DEG content (Figure 2). For example, the melting point of PBS was 116.72°C. The melting point of PBDS95 decreased to 111.87°C after adding a small amount of DEG. When the content of DEG reached more than 5%, the melting point of PBS decreased to below 100°C and the melting point of PBDS55 was only 50.84°C. Combined with the crystallinity calculated by XRD (Table 3), it can be concluded that the increase in DEG content reduced the regularity of the PBS chain structure, resulting in the decrease in crystallinity, and PBDS55 was almost amorphous. There was only the crystallization peak of PBS in the XRD pattern, indicating that the crystallization only occurred at the aggregation of BS segments (Figure 3a). The glass transition temperature of the copolyester was single, which was between pure PBS and pure PDES polyester. In addition, the glass transition temperature (T g) increased with the increase in DEG with higher T g components, which indicated that PBDS copolyester was a random copolymer. Only PBDS70 had a cold crystallization peak under the DSC test conditions used in this study, indicating that PBDS70 was not fully crystallized, and the molecular chain arrangement in some amorphous regions was regular at lower temperatures. Moreover, the introduction of DEG only slightly reduced the thermal decomposition temperature. The temperature at 5% weight loss (T d,5%) of PBDS55 was close to that of PDES, and when the content of DEG in the copolyester was less than 45 mol% of total diol content, the introduction of DEG has no significant effect on the thermal stability of the copolyester. The introduction of DEG endows the copolyester a wider processing window than PBS.
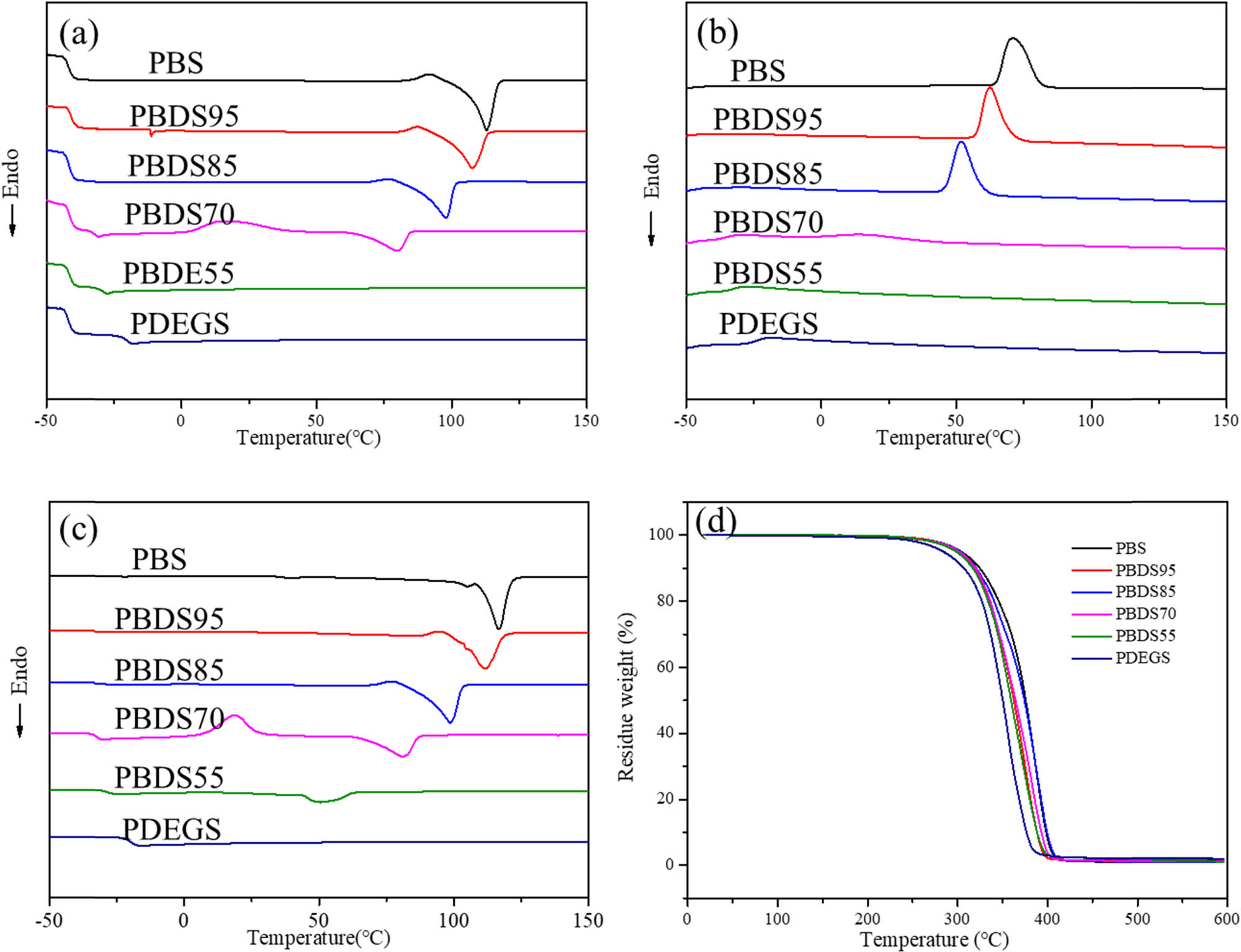
(a) First heating curve, (b) cooling curves, (c) second heating curve, and (d) TGA curves of PBDS copolyesters.
Thermal performance and thermal stability of PBDS
| Sample | DSC | TGA | XRD | CA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st heating scan | Cooling scan | 2nd heating scan | N 2 | χ c | |||||||||
| T m | −ΔH m | T c | ΔH c | T g | T cc | ΔH cc | T m | −ΔH m | T 5% | T d, max | (%) | (°) | |
| (°C) | (J·g−1) | (°C) | (J·g−1) | (°C) | (°C) | (J·g−1) | (°C) | (J·g−1) | (°C) | (°C) | |||
| PBS | 116.72 | 54.31 | 71.14 | 72.14 | −41.48 | – | – | 112.83 | 69.99 | 304.5 | 387.34 | 53.03 | 81.4 ± 0.6 |
| PBDS95 | 111.87 | 53.80 | 62.48 | 59.07 | −40.38 | – | – | 107.80 | 64.50 | 301.19 | 375.23 | 42.56 | 77.3 ± 1.5 |
| PBDS85 | 98.70 | 49.17 | 51.81 | 55.32 | −41.27 | – | – | 97.82 | 53.29 | 302.75 | 388.02 | 38.88 | 69.5 ± 1.3 |
| PBDS70 | 81.21 | 33.01 | 13.55 | 52.1 | −32.98 | 15.65 | 38.91 | 79.86 | 34.30 | 302.02 | 382.32 | 31.33 | 68.8 ± 0.6 |
| PBDS55 | 50.84 | 15.19 | – | – | −28.68 | – | – | – | – | 298.48 | 362.18 | 25.74 | 62.4 ± 0.7 |
| PDEGS | – | – | – | – | −20.19 | – | – | – | – | 284.21 | 356.43 | – | – |

(a) XRD patterns of PBDS copolyesters and (b) change of contact angle of copolyesters, and the illustrations are contact angle photos of PBS and PBDS55, respectively.
The hydrophilicity of PBS and PBDS copolyesters was characterized by static contact angle, as shown in Figure 3b. With the increase of DEG content in PBDS copolyester, the contact angle of the sample surface of copolyesters gradually decreased from 81.4° of PBS to 62.4° of PBDS55. The addition of DEG greatly improved the hydrophilicity of the surface of sample. On the one hand, the introduction of DEG leads to the reduction of the crystallinity of the material, and it is easier for water molecules to enter the loose amorphous region than the dense crystalline region. On the other hand, the ether bond on the surface of sample will interact with water molecules to produce hydrogen bond (32). The improvement of hydrophilicity will be conducive to the enhancement of the degradation ability of copolyester.
The mechanical properties of the PBS and PBDS copolyesters are shown in Figure 4 and Table 4. The tensile strength of PBDS95 was 48.5 MPa, and the ether bonds of DEG improved the flexibility of the copolyester molecular chains. Therefore, the elongation at break of the PBDS copolyester was significantly greater than that of PBS, and it increased from 413% of PBDS95 to 876% of PBDS55 with the increase in the DEG content. The introduction of DEG significantly improved the toughness of the materials, which was accompanied by slight decreases in the tensile strength. The elongation at break was greater than 800%, and the tensile strength was above 19 MPa. Compared with the conventional packaging materials (such as PBAT and LLDPE) (33), PBDS exhibited excellent comprehensive performance and had good application prospects in the field of degradable packaging.
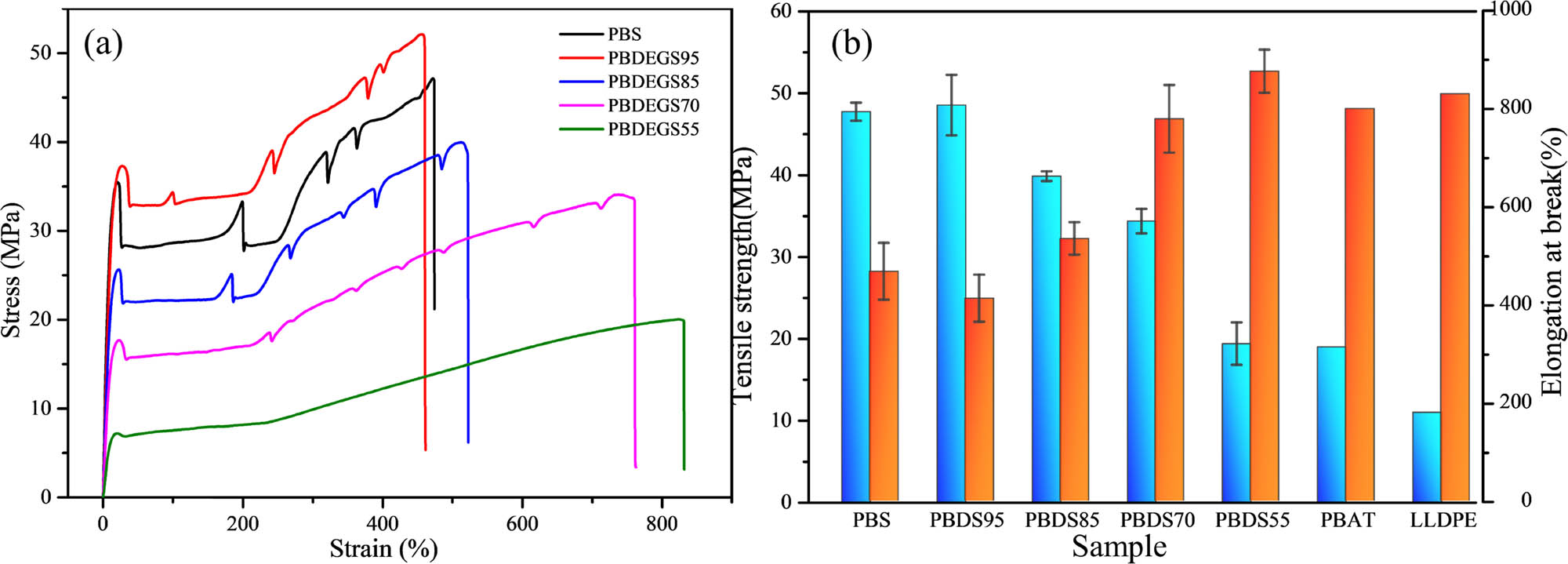
(a) Stress–strain curves of PBDS and (b) mechanical properties of PBDS and commercial packaging polyester PBAT and LLDPE.
Mechanical properties of PBDS, PBAT, and LLDPE
| Sample | Tensile strength (MPa) | Elongation at break (%) |
|---|---|---|
| PBS | 47.7 ± 1.1 | 468 ± 58 |
| PBDS95 | 48.5 ± 3.7 | 413 ± 48 |
| PBDS85 | 39.8 ± 0.6 | 535 ± 33 |
| PBDS70 | 34.3 ± 1.5 | 779 ± 69 |
| PBDS55 | 19.3 ± 2.6 | 876 ± 44 |
| PBAT | 18.9 | 800 |
| LLDPE1 | 10.9 | 830 |
1LLDPE/2045G, DOW chemical.
3.3 Degradability of PBDS copolyester
3.3.1 Compost degradation
According to the ISO 14855-1:2005 standard, the composting degradation properties of the PBS and PBDS copolyesters were compared. The composting equipment is shown in Figure 5a. The mineralization rates of PBS, PBDS95, PBDS70, and PBDS55 were obtained under the composting condition of 58°C, as shown in Figure 5b. The mineralization rate of reference cellulose was set as standard 1 to better compare the composting degradability between the samples. After 101 days of composting degradation, the relative mineralization rates of PBS, PBDS95, PBDS70, and PBDS55 (relative to the reference cellulose) reached 40.74%, 67.58%, 84.69%, and 92.71%, respectively. Compared with PBS, the biodegradability of the copolyesters containing DEG was significantly improved, and it increased with the increase in the DEG content. Figure 5c shows the increasing trend of biodegradability more intuitively. The existence of DEG reduced the crystallinity of the copolyester, and the ether bonds also increased the hydrophilicity of the polymers (28). Therefore, the increase of DEG content led to the increase in the biodegradability of the copolyester. In addition, the heterodyads were more labile than the homodyads, which was also the reason why the biodegradability of the PBDS copolyester was higher than that of PBS (27).

(a) Schematic diagram of compost degradation device, (b) the mineralization rate curves of PBS and PBDS copolyesters, and (c) variation of mineralization rate with DEG content after 101 days of compost degradation.
3.3.2 Seawater degradation
To explore the effects of the DEG content on the hydrolysis performance of the materials and the degradation possibility of the PBDS copolyesters in seawater, the seawater degradation experiments were carried out at 30°C in the laboratory. PBDS70 and PBDS55 were broken after 250 days of degradation (Figure 6). The mechanical properties of the PBDS copolyester gradually decreased as the degradation proceeded. After 250 days of degradation, the mechanical strength of PBDS95 decreased by approximately 50%, while PBDS55 and PBDS70 almost lost their mechanical strength. The elongation at break was more sensitive to the change of degradation. The elongation at break of the polyester and copolyester decreased significantly after 60 days of degradation and decreased to about zero after 100 days of degradation.

(a) Visual comparison of samples after 250 days of degradation in seawater, a1, a2, a3, a4, and a5 represent PBS, PBDS95, PBDS85, PBDS70, and PBDS55 samples, respectively, and (b–d) the weight loss, tensile strength, and elongation at break of PBS and PBDS copolyesters during 250 days of seawater degradation.
The mass loss of the materials during degradation was analyzed. After 250 days of degradation, PBS and PBDS95 exhibited only a small amount of mass loss (<1.00%), while the weight loss rate of the copolyester gradually increased with the increase in the DEG content. The mass loss of PBDS85, PBDS70, and PBDS55 was 1.46%, 2.69%, and 3.94%, respectively. The degradation rate of PBDS55 with a high DEG content was about four times that of PBS.
The essence of polymer degradation is the decrease in molecular weight caused by molecular chain fracture. GPC was conducted to test the changes of molecular weight before and after the degradation of the copolymers, which aimed to further verify the promoting effects of DEG on the degradation of PBDS. As shown in Figure 7c, the peak position of the copolyesters shifted to the left after 160 days of degradation in seawater, indicating that the molecular weight of the copolyester decreased. The decreasing proportion of molecular weight gradually increased with the increase in the DEG content, indicating that the presence of DEG promoted the breaking of the molecular chain. For example, the weight average molecular weight of PBDS95 decreased from 8.53 × 104 to 2.65 × 104 g·mol−1, decreased by 69.0%, while that of PBDS55 decreased from 12.18 × 104 to 1.77 × 104 g·mol−1, reduced by 85.5% (Figure 7a and b). In addition, the length of the molecular chain became uneven after degradation and the PDI of the sample increased after degradation. Both PBS and PBDS copolyesters showed a single peak before degradation and a double peak after degradation, and the double peak trend increased with the increase of the DEG content.

(a) Changes of weight average molecular weight before and after degradation of PBS and PBDS copolyersters, (b) percentage decrease in weight average molecular weight M w with DEG content, and (c) GPC spectra of PBDS copolyesters before and after degradation.
The rapid decrease in mechanical properties and the substantial drop in M w indicate that the copolyester mostly underwent bulk degradation (34). In the process of bulk degradation, the breaking rate of ester bond is greater than the diffusion rate of water molecules, and degradation occurs simultaneously inside and on the surface of the material (34,35). During the seawater degradation of PBDS copolyester, the weight average molecular weight decreased significantly, but the weight loss was not obvious. This may be due to the hysteresis of the diffusion of oligomers produced by the breaking of molecular chains in the material. At the same time, considering that the weight loss of copolyester increases with the increase of DEG content, we reasonably speculate that the introduction of DEG not only improves the hydrophilicity of polyester and makes the molecular chain of copolyester more prone to fracture but also improves the movement ability of molecular chain and speeds up the elimination of low polymers produced by degradation.
4 Conclusion
A series of compostable and seawater degradable PBDS copolyesters were synthesized by introducing DEG into the main chain of PBS through one-pot melt polycondensation. The melting point and crystallinity of the copolyester gradually decreased with the increase of the DEG content, but the copolyester still maintained good thermal stability. The hydrophilicity of copolyester was also improved. Compared with the traditional packaging materials (PBAT and LLDPE), PBDS copolyester had higher tensile strength and excellent toughness. The addition of DEG significantly improved the composting and seawater degradability of the materials. The mineralization rate of the PBDS copolyester increased with the increase in the DEG content, and PBDS55 has a high mineralization rate comparable to that of cellulose. The introduction of DEG promoted the breaking of the molecular chain, which endowed PBDS copolyester with a better seawater degradation performance than PBS. The weight-average molecular weight of PBDS55 decreased by 85.5% after the seawater degradation. PBDS copolyester showed a bright prospect as a cost-effective degradable packaging material. The strategy of introducing hydrophilic groups into the existing biodegradable polyester by copolymerization to improve the seawater degradation performance of materials provided an important enlightenment and a feasible route for the design and development of similar materials in the future.
-
Funding information: This work was financially supported by the National Natural Science Foundation of China (Project No. 51973228), Youth Innovation Promotion Association, CAS (2018033), DNL Cooperation Fund, CAS (DNL 180401), and Municipal Commission of Science and Technology, Beijing, China (Z211100004321005).
-
Author contributions: Tian-Yuan Liu: conceptualization, methodology, investigation, data curation, formal analysis, writing – original draft, writing – review and editing; Dan Huang: formal analysis, investigation, visualization, writing – original draft; Peng-Yuan Xu: investigation, formal analysis, validation, writing – original draft; Bo Lu: investigation, data curation, formal analysis; Zhi-Chao Zhen: investigation, data curation, formal analysis; Wei-Zhen Zheng: investigation, validation; Xiao Li: investigation, validation; Ge-Xia Wang: supervision, funding acquisition, conceptualization, methodology, writing – review and editing; and Jun-Hui Ji: supervision, funding acquisition, conceptualization, methodology, writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
(1) Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3(7):1700782. 10.1126/sciadv.1700782.Search in Google Scholar
(2) Rhodes CJ. Plastic pollution and potential solutions. Sci Prog. 2018;101(3):207–60. 10.3184/003685018x15294876706211.Search in Google Scholar
(3) Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, et al. Plastic waste inputs from land into the ocean. Science. 2015;347(6223):768–71. 10.1126/science.1260352.Search in Google Scholar
(4) Schneiderman DK, Hillmyer MA. 50th anniversary perspective: There is a great future in sustainable polymers. Macromol. 2017;50(10):3733–50. 10.1021/acs.macromol.7b00293.Search in Google Scholar
(5) Vroman I, Tighzert L. Biodegradable polymers. Mater. 2009;2(2):307–44. 10.3390/ma2020307.Search in Google Scholar
(6) Zhang Q, Song M, Xu Y, Wang W, Wang Z, Zhang L. Bio-based polyesters: Recent progress and future prospects. Prog Polym Sci. 2021;120. 10.1016/j.progpolymsci.2021.101430.Search in Google Scholar
(7) Nazareth M, Marques MRC, Leite MCA, Castro IB. Commercial plastics claiming biodegradable status: Is this also accurate for marine environments? J Hazard Mater. 2019;366:714–22. 10.1016/j.jhazmat.2018.12.052.Search in Google Scholar
(8) Gan Z, Kuwabara K, Yamamoto M, Abe H, Doi Y. Solid-state structures and thermal properties of aliphatic–aromatic poly(butylene adipate-co-butylene terephthalate) copolyesters. Polym Degrad Stab. 2004;83(2):289–300. 10.1016/s0141-3910(03)00274-x.Search in Google Scholar
(9) Thulasisingh A, Kumar K, Yamunadevi B, Poojitha N, SuhailMadharHanif S, Kannaiyan S. Biodegradable packaging materials. Polym Bull. 2021;79:4467–96. 10.1007/s00289-021-03767-x.Search in Google Scholar
(10) Bagheri AR, Laforsch C, Greiner A, Agarwal S. Fate of so-called biodegradable polymers in seawater and freshwater. Global Challen. 2017;1(4):1700048. 10.1002/gch2.201700048.Search in Google Scholar PubMed PubMed Central
(11) Deroiné M, Le Duigou A, Corre Y-M, Le Gac P-Y, Davies P, César G, et al. Accelerated ageing of polylactide in aqueous environments: Comparative study between distilled water and seawater. Polym Degrad Stab. 2014;108:319–29. 10.1016/j.polymdegradstab.2014.01.020.Search in Google Scholar
(12) Kasuya K, Takagi K, Ishiwatari S, Yoshida Y, Doi Y. Biodegradabilities of various aliphatic polyesters in natural waters. Polym Degrad Stab. 1998;59(1–3):327–32. 10.1016/s0141-3910(97)00155-9.Search in Google Scholar
(13) Wang G-X, Huang D, Ji J-H, Voelker C, Wurm FR. Seawater-degradable polymers-fighting the marine plastic pollution. Adv Sci. 2021;8(1):2001121. 10.1002/advs.202001121.Search in Google Scholar PubMed PubMed Central
(14) Nakayama A, Yamano N, Kawasaki N. Biodegradation in seawater of aliphatic polyesters. Polym Degrad Stab. 2019;166:290–99. 10.1016/j.polymdegradstab.2019.06.006.Search in Google Scholar
(15) Zhao J-H, Wang X-Q, Zeng J, Yang G, Shi F-H, Yan Q. Biodegradation of poly(butylene succinate) in compost. J Appl Polym Sci. 2005;97(6):2273–78. 10.1002/app.22009.Search in Google Scholar
(16) Wang G, Huang D, Zhang W, Ji J. Degradation performance of typical biodegradable polyesters in seawater. J Func Polym. 2020;33(5):492–99. 10.14133/j.cnki.1008-9357.20191015001.Search in Google Scholar
(17) Xu J, Guo BH. Poly(butylene succinate) and its copolymers: Research, development and industrialization. Biotechnol J. 2010;5(11):1149–63. 10.1002/biot.201000136.Search in Google Scholar PubMed
(18) Jacquel N, Freyermouth F, Fenouillot F, Rousseau A, Pascault JP, Fuertes P, et al. Synthesis and properties of poly(butylene succinate): Efficiency of different transesterification catalysts. J Polym Sci Part A Polym Chem. 2011;49(24):5301–12. 10.1002/pola.25009.Search in Google Scholar
(19) Kanemura C, Nakashima S, Hotta A. Mechanical properties and chemical structures of biodegradable poly(butylene-succinate) for material reprocessing. Polym Degrad Stab. 2012;97(6):972–80. 10.1016/j.polymdegradstab.2012.03.015.Search in Google Scholar
(20) Ratshoshi BK, Farzad S, Gorgens JF. Techno-economic assessment of polylactic acid and polybutylene succinate production in an integrated sugarcane biorefinery. Biofuels. Bioprod Bioref. 2021;15(6):1871–87. 10.1002/bbb.2287.Search in Google Scholar
(21) Rinkenbach WH. Properties of diethylene glycol. Ind Eng Chem. 1927;19(1):474–76. 10.1021/ie50208a017.Search in Google Scholar
(22) Cao A, Okamura T, Nakayama K, Inoue Y, Masuda T. Studies on syntheses and physical properties of biodegradable aliphatic poly(butylene succinate-co-ethylene succinate)s and poly(butylene succinate-co-diethylene glycol succinate)s. Polym Degrad Stab. 2002;78:107–17. 10.1016/S0141-3910(02)00124-6.Search in Google Scholar
(23) Jiang F, Qiu Z. Crystallization kinetics, mechanical properties, and hydrolytic degradation of novel eco-friendly poly(butylene diglycolate) containing ether linkages. J Appl Polym Sci. 2016;133(46). 10.1002/app.44186.Search in Google Scholar
(24) Liu G-C, Zeng J-B, Huang C-L, Jiao L, Wang X-L, Wang Y-Z. Crystallization kinetics and spherulitic morphologies of biodegradable poly(butylene succinate-co-diethylene glycol succinate) copolymers. Ind Eng Chem Res. 2013;52(4):1591–99. 10.1021/ie303016v.Search in Google Scholar
(25) Liu F-Y, Xu C-L, Zeng J-B, Li S-L, Wang Y-Z. Non-isothermal crystallization kinetics of biodegradable poly(butylene succinate-co-diethylene glycol succinate) copolymers. Thermochim Acta. 2013;568:38–45. 10.1016/j.tca.2013.06.025.Search in Google Scholar
(26) Li CT, Zhang M, Weng YX, Qin JX. Influence of ether linkage on the enzymatic degradation of pbs copolymers: Comparative study on poly (butylene succinate-co-diethylene glycol succinate) and poly (butylene succinate-co-butylene diglycolic acid). Int J Biol Macromol. 2018;118(Pt A):347–56. 10.1016/j.ijbiomac.2018.06.062.Search in Google Scholar PubMed
(27) Gigli M, Negroni A, Soccio M, Zanaroli G, Lotti N, Fava F, et al. Influence of chemical and architectural modifications on the enzymatic hydrolysis of poly(butylene succinate). Green Chem. 2012;14(10):2885. 10.1039/c2gc35876j.Search in Google Scholar
(28) Zeng JB, Huang CL, Jiao L, Lu X, Wang YZ, Wang XL. Synthesis and properties of biodegradable poly(butylene succinate-co-diethylene glycol succinate) copolymers. Ind Eng Chem Res. 2012;51(38):12258–65. 10.1021/ie300133a.Search in Google Scholar
(29) Huang D, Liu TY, Nie Y, Lu B, Zhen ZC, Xu PY, et al. Trickily designed copolyesters degraded in both land and sea - confirmed by the successful capture of degradation end product CO2. Polym Degrad Stab. 2022;196:196. 10.1016/j.polymdegradstab.2022.109817.Search in Google Scholar
(30) Liu TY, Huang D, Xu PY, Lu B, Wang GX, Zhen ZC, et al. Bio-based seawater-degradable poly(butylene succinate-l-lactide) copolyesters:exploration of degradation performance and degradation mechanism in natural seawater. ACS Sustain Chem Eng. 2022;10:3191–202. 10.1021/acssuschemeng.1c07176.Search in Google Scholar
(31) ISO 14855-1 I. Determination of the ultimate aerobic biodegradability of plastic materials under controlled composting conditions – method by analysis of evolved carbon dioxide – part 1: General method; 2005.Search in Google Scholar
(32) Zhao ML, Li FX, Yu JY, Wang XL. Preparation and characterization of poly(ethylene terephthalate) copolyesters modified with sodium-5-sulfo-bis-(hydroxyethyl)-isophthalate and poly(ethylene glycol). J Appl Polym Sci. 2014;131(3):n/a. 10.1002/app.39823.Search in Google Scholar
(33) Liu W, Liu T, Liu H, Xin J, Zhang J, Muhidinov ZK, et al. Properties of poly(butylene adipate-co-terephthalate) and sunflower head residue biocomposites. J Appl Polym Sci. 2017;134(13). 10.1002/app.44644.Search in Google Scholar
(34) Laycock B, Nikolić M, Colwell JM, Gauthier E, Halley P, Bottle S, et al. Lifetime prediction of biodegradable polymers. Prog Polym Sci. 2017;71:144–89. 10.1016/j.progpolymsci.2017.02.004.Search in Google Scholar
(35) Hofmann D, Entrialgo-Castano M, Kratz K, Lendlein A. Knowledge-based approach towards hydrolytic degradation of polymer-based biomaterials. Adv Mater. 2009;21(32–33):3237–45. 10.1002/adma.200802213.Search in Google Scholar PubMed
© 2022 Tian-yuan Liu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

