Abstract
Nowadays, with the continuous understanding of the pathogenic mechanism of bacterium, the demand for antibacterial plastic products had significantly increased. Besides that, many counties issued mandatory standards for plastic products, which imposed strict requirements on ash content to prevent the addition of excessive inorganic matter to plastics in order to avoid weakening the properties of plastics and deteriorating the recyclable property. Based on this, the development of composites with organic fiber-loaded antibacterial agents is of practicable value and urgency. We used an open-ring addition reaction to modified aramid fiber (AF) by utilizing epoxypropyltrimethoxysilane to react with the reactive groups on the surface of AFs. Subsequently, the modified fibers were surface loaded with silver ionic glass beads. After that, a series of high-density polyethylene composites with excellent mechanical properties and antibacterial properties were prepared using melt mixing method. It was shown that the composite had a low ash value (1.88 wt%) even at a higher filling concentration (7 wt%) and the fibers could change the crystalline properties and morphology of the composite. Because of the fiber reinforcement and crystallization induction effects, the tensile strength and elasticity module of the composites could be improved by 141% and 136%, respectively. In addition, the composites had excellent long-lasting contact antibacterial effects against the inhibition of E. coli. The proposed organic fiber loading technique and antibacterial composites will provide a method for designing and preparing eco-friendly and high-performance plastic products.
1 Introduction
As an important component of the ecological environment, the existence of bacteria is susceptible to various human diseases or infections (1). With the continuous understanding of the pathogenic mechanism of bacterium, there is a great increase in concern for plastic products with antibacterial properties. As of 2021, the annual plastics usage reached 13 million tons in China (2). In particular, plastic products that came into close contact with the human body, such as plastic water pipes, consumer electronics, plastic tableware, and fabrics, generated surface bacterial adhesion and proliferation after long-term use, which raised the risk of bacterial infections in humans and threatens health. Therefore, it is of great significance to develop antibacterial polymer composites.
In composite, fiber-reinforced polymer matrices had been studied extensively because of their unique and efficient toughening and stiffening properties (3). Traditionally, inorganic fibers such as glass fiber, basalt fiber, and carbon fiber were commonly used as reinforcing phases (4,5). These fibers were highly rigid, cheap, and convenient to process. However, for the requirement of high-quality development of the plastic industry, many current standards for plastic products, such as pipes, films, and sheets, had strict requirements for the ash content after high-temperature burning (6,7). It was mainly to improve the plastic recycling ability and to limit the addition of an excessive number of inorganic substances to plastics, which resulted in poor performance of plastics. The requirement of ash content was normally below 1 wt%, and even some standards required less than 0.1 wt% (8). Inorganic fibers were difficult to vaporize and decompose at the burning temperature of 700–1,000°C. Thus, the adding fibers in the composite formed the main component of ash content. The challenge is that the effective addition for fiber reinforcement in composites is usually more than the value restricted in many standards. The higher the fiber amount, the more obvious the reinforcement effect was, but the higher the ash content was. It is difficult to meet both the requirements of the mandatory standards for plastic products and final performances.
In order to reconcile the contradiction between the high ash content and the fiber reinforcement, the development of natural fibers or synthetic organic fibers with high modulus and high strength as reinforcing phase has become a research hotspot, recently (9,10,11). Aramid fiber (AF) is a kind of common organic fibers with excellent performances (12). According to Li’s study, in addition to its advantages of low density, high strength, and heat resistance, AF has a large number of benzene rings on its molecular chains, which provides high rigidity (13). Also, there are a significant number of carbonyl and amine groups on its molecular chains, and such reactive groups are beneficial for modification and further functionalization of the pristine fiber. The surface modification of AF can improve adhesion to plastic matrix. There are many interesting and nascent AF surface modification methods reported, such as chemical treatment (14), surface grafting (15), γ-ray treatment (16), ultrasonic treatment (17), and ultraviolet treatment (18). The principle of these technologies was to endow the fiber surface with abundant reactive groups. With these surface modification methods, it was not only to provide fibers with strong interfacial adhesion but also to further load functional particles. The modified fibers could realize the composite materials with functions such as electrical conductivity, electromagnetic shielding, flame retardant, or antibacterial. As mentioned earlier, the antibacterial property is of great importance for bulk plastic products in daily life.
Therefore, designing AF with broad antibacterial effects used in plastics has become a well-established strategy (19). The common antibacterial agents for plastics could be classified into three categories: quaternary ammonium compounds, metal nanoparticles, and metal ion compounds. In the case of an antibacterial agent of nano-metal particles, it represented by silver nanoparticles had a good antibacterial effect and was commonly used in medical materials. However, current research revealed that the antibacterial performance of metal nanoparticles was closely related to the particle size. The smaller the size, the better the antibacterial effect was. Unfortunately, the phenomenon of small size brought the problem of easy agglomeration, which led to a decrease in long-lasting antibacterial performance. Adegboyega et al. found small sizes of metal nanoparticles also exhibited cytotoxicity to animals (20). The case of quaternary ammonium compounds represented by didecyldimethylammonium chloride and alkyldimethylbenzylammonium was proved to be toxic to mitochondria and nerve root myelin in recent studies (21,22). In contrast, although metal ion antibacterial agents represented by Ag ion compounds had a slow-release rate and relatively weak antibacterial effect than the former antibacterial agents, it was reliable and had long-lasting effect.
Therefore, we tried to develop a simple and feasible surface modification technique to prepare an efficient antibacterial fiber by utilizing the feature that AF had numerous reactive groups. The surface chemical modification of the fibers was achieved using the reaction of γ-glyceryl ether oxypropyl trimethoxysilane (GEOTES) with the reactive side groups on AF. Subsequently, Ag ions glass beads (AgGB) could be loaded on the surface of modified AF. The obtained AgGB@AF were induced to high-density polyethylene (HDPE) to prepare a series of composites. The composites not only possessed excellent mechanical properties but also had low ash content and long-lasting safe contact antibacterial function via Ag ions slowly migration to the surface of composites. The prepared material is conducive to the realization of antibacterial and high-strength plastics, which meets the product standards in close contact with the human body in daily life.
2 Materials and methods
2.1 Materials
HDPE resin, grade 7600 M, was obtained by Yanshan Petrochemical Company. AgGB, grade B120 and was purchased from Langyi Functional Materials Company. The particle diameter of AgGB was around 0.1–5 μm, and its Ag concentration was about 1 wt%. AF, grade 1414, was supplied by Shenzhen Teli Chemical Fiber Company. The average length of AF was around 5–20 mm, and its diameter was 10 μm, respectively. GEOTES was obtained from Sigma-Aldrich Company. E. coli strain, grade ATCC 25922, was supplied by Qingdao Hope Biotechnology Company.
2.2 Composites preparations
The reaction route of preparing AgGB@AF is shown in Figure 1. First, 500 mL of deionized water was added to a beaker and formic acid was added to reduce the pH value of the aqueous solution to 5.0. Subsequently, 20 g of GEOTES was dropped into the deionized water. It was reflux heated to 50°C and stirred for 4 h using a magnetic stirrer to achieve the hydrolysis of GEOTES.

Reaction route of preparing AgGB@AF.
Second, 20 g of AF was added to the hydrolyzed GEOTES aqueous solution. It was reflux heated to 60°C and stirred for 2 h. The surface chemical modification of AF was achieved during this process. The amino and carbonyl groups on the molecular chain of the AF surface reacted with the epoxy group of GEOTES, which allowed GEOTES to attach to the surface of AF.
Last, a mixture of H2SO4/H2O2 (70/30, v/v) was prepared (23). The pristine AgGB particles were added to the mixture to prepare a solution with a concertation of 0.5 g·mL−1. The solution was stirred at room temperature for 2 h to remove the organic substances and to hydroxylate AgGB. Subsequently, the modified AgGB particles were filtered and washed with deionized water. After that, 20 g of the modified AgGB was added to the surface-modified AF solution and stirred at 60°C for 2 h to achieve the surface-loaded AF. The obtained solution was extracted and filtered. The extracted substance was washed with deionized water and dried at 80°C for 4 h to obtain the final loaded fiber named AgGB@AF.
The HDPE composites were prepared by using an internal mixer. Different concentrations of AgGB@AF and HDPE resins were put into the mixer. The HDPE composites with mass ratio of AgGB@AF 0, 1, 3, 5, and 7 wt% were coded as 1#, 2#, 3#, 4#, and 5#, respectively. To ensure a uniform dispersion, the temperature of the mixer was set to 190°C, and the speed and the mixing time were set to 100 rpm and 10 min. The obtained HDPE composites were then pressed into sheets with 2 mm thickness by using a hot press for subsequent tests of antibacterial properties and mechanical properties.
2.3 Characterizations
2.3.1 Fiber structure characterizations
The morphology of pristine AF and AgGB@AF structures were characterized by optical microscopy (Olympus, BX-51, Japan) and scanning electron microscopy (SEM: FEI, Nova Nano 450, USA). The magnification of the optical microscope was 500×. The acceleration voltage of SEM was set to 10 kV, and the magnifications were 500× and 2,000×. The elemental composition of AgGB@AF was analyzed by using energy-dispersive spectroscopy (EDS) of SEM.
2.3.2 Thermal properties characterizations
The thermal stability of HDPE composites was characterized by using a thermogravimetric analyzer (TGA) (Netzsch, STA449, Germany). Before testing, all samples were dried and subsequently placed in the TGA and heated from room temperature to 600°C with a heating rate of 20°C·min−1. Then, the thermogravimetric curves of HDPE composites were recorded.
The ash content of HDPE composites was characterized by using a muffle furnace. Before testing, the samples were dried at 80°C for 4 h and subsequently placed in ceramic dishes. And the samples and ceramic dishes were weighed and recorded by an electronic balance. Subsequently, the samples and ceramic dishes were placed in a muffle furnace heated to 850°C and held for 30 min. After that, the samples were removed from the muffle furnace. The ash and ceramic dishes were weighed and recorded. The ash content of HDPE composites could be calculated by Eq. 1:
where w 1, w 2, and w 3 represent the mass of sample, the ceramic dishes, and ash and the ceramic dishes after burning, respectively.
The crystallization properties of HDPE composites were evaluated by using a differential scanning calorimeter (DSC; Netzsch, 204 P, Germany). The sample was first rapid heated up to 180°C and held for 2 min to eliminate thermal history. The crystallization behavior of HDPE composites was observed by cooling from 180°C to room temperature with a cooling rate of 10°C·min−1. Then, the HDPE composites were heated up from room temperature to 180°C at a heating rate of 10°C·min−1 to observe the melting behavior. Also, the crystallization and melting behaviors of HDPE composites under 5.0 MPa pressure were tested at the same thermal conditions as atmospheric pressure. The crystallinity of HDPE composites was calculated by Eq. 2 (24,25):
where ΔH m represents the melt enthalpy and w f represents the mass proportion of AgGB@AF in HDPE composites. According to the literature, the standard melt enthalpy of HDPE used in Eq. 2 was 293 J·g−1 (26).
The crystalline morphology of HDPE composites was observed using an optical microscope, with a special polarizing lens that allowed the observation of the spherical crystal morphology formed in crystallization. The test conditions were performed by placing the sample between two thin glass sheets. The samples were heated to 200°C with a heating stage at a rate of 20°C·min−1 and held for 5 min to allow complete melting of crystals. Subsequently, based on the results of DSC characterization, the temperature was decreased to 110°C at a rate of 20°C·min−1 and maintained for 20 min for isothermal crystallization. The spherical crystal morphology could be subsequently observed. The magnification was 500×.
2.3.3 Mechanical property characterizations
The mechanical properties of HDPE composites were tested by using an electronic universal testing machine (Xinsansi, CTM6000, China). The tests were performed according to the standard of ASTM D638. The tensile rate was set to 50 mm·min−1. Each sample was tested five times, and the mechanical strength and elasticity module were recorded by taking its average value.
2.3.4 Antibacterial property characterizations
The water contact angle and oil contact angle of HDPE composites were characterized by using a contact angle tester (Yunfan Instrument, V5, China). Deionized water and pentane were used as testing liquids. For each sample, three different areas were tested, and their average value was taken as the water contact angle and the oil contact angle.
Liquid Luria–Bertani (LB) media preparation: 100 mL of deionized water was poured into a reagent bottle; 2.5 g of LB media (A507002) was weighed, then dissolved with stirring, and sterilized in an autoclave (Hirayama, HVE-50, Japan) at 121°C for 15 min for the following use.
Solid LB medium preparation: 100 mL of deionized water was poured into a reagent bottle; 2.5 g of LB medium and 1.5 g of agar powder (A505255) were added in and dissolved with stirring, sterilized in an autoclave at 121°C for 15 min. Subsequently, the medium was cooled to 40°C, and 15 mL of the media was placed in a disposable sterile Petri dish using a pipette.
Preparation of bacterial suspension: Two bacterial culture tubes were used for each sample; 3 mL of liquid LB media was added, and a colony from E. coli solid media was induced into the liquid LB media. The other one was used as a blank control sample. The bacterial culture tubes were placed in a thermostatic shaker (Crystal Technology and Industries, IS-RDV1, USA) and incubated at 37°C for 15 h.
The antibacterial properties of HDPE composites were characterized using the optical density value at 600 nm method (OD600). The difference in transmittance of 600 nm was depended on the energy absorbed by the test object. There was a quantitative relationship between the concentration of bacteria and the transmittance at the fixed wavelength. The HDPE composites were placed in bacterial suspensions. The E. coli bacterial solution was diluted to a concentration of 106 CFU·mL−1. 2.5 mL of the diluted bacterial solution was added to the bacterial culture tube and incubated at 37°C for 18 h in a constant temperature shaker. The culture solution was added into a cuvette, and the OD600 value of each sample was measured on an UV-visible light spectrometer (Shanghai Yuanxi, UV5800, China) at a wavelength of 600 nm every 3 h, and the average value of samples was taken three times as its OD600 value.
Ag ion concentration test: First, a series of Ag ion solutions with concentrations of 1, 3, 5, 7, and 9 mg·mL−1 was prepared. Then, an atomic absorption spectrometer (Shimadzu, AA7000, Japan) was used to record the absorbances of the solutions, and a standard curve was obtained. After that, the HDPE composites with the size of 2 cm × 2 cm × 2 mm were soaked in deionized water for 24 h. The soaking solutions were taken to measure the Ag ion concentration by atomic absorption spectrometer.
Inhibition circle test: The E. coli solution was diluted with PBS buffer solution to 106 CFU·mL−1 concentration. The sample was clamped into a Petri dish and 100 μL of the dilution was evenly spread on LB solid media. The Petri dishes were placed in a constant temperature incubator at 37°C and incubated for 24 h. Subsequently, it was removed to measure the size of the inhibition circle and to observe the bacterial growth on the surface of HDPE composites.
3 Results and discussion
3.1 AgGB@AF morphology characterizations
The structure of the prepared AgGB@AF was displayed in Figure 2. It was obvious that pristine AF appeared to be straight and rigid with a smooth surface, and the AgGB particles were cubic matters. The diameter of AF was about 12 μm, while the size of AgGB particles ranged from 0.3 to 4 μm. Figure 2c and d shows the loaded structure of AgGB@AF. The viscous filamentous matters on the surface of AF could be deduced as GEOTES. By the surface modification of GEOTES, AgGB particles could be efficiently loaded on AF due to the coupling mechanism. The epoxy group at one end of GEOTES molecular reacted with AF, while the groups at the other end were affinity to AgGB particles. It could also observe that the AgGB particles with different sizes could be loaded, which was beneficial to give an important antibacterial performance for AF. To further prove the successful synthesis of AgGB@AF, EDS image and optical microscopy were used as shown in Figure 2e and f. The oxygen, silicon, and carbon elements reflected the existing of AF and GEOTES, while the sodium, magnesium, and calcium elements proved the existence of AgGB. The optical microscopic image displayed similar structural features to the SEM image. Accordingly, the photos could determine the Ag ions fibers that were prepared.

SEM images of (a) pristine AF, (b) AgGB particles, (c) AgGB@AF, (d) AgGB@AF in partially enlarged view, (e) EDS image of AgGB@AF, and (f) optical microscope image of AgGB@AF.
The TGA curves reflected the important thermal stability of HDPE composites. As shown in Figure 3a, HDPE composites began to thermal decomposed at a temperature of about 400°C. However, the addition of AgGB@AF only slightly affected the onset temperature of thermal decomposition, which ensured the thermal stability of HDPE composites. Interestingly, the ash content of HDPE composites could be decreased to as low as 1.88 wt% even with a high adding amount of 7 wt% AgGB@AF heated under 850°C. Its residual weight was only 16% compared with that of basalt fiber (27) as displayed in Figure 3b. This was attributed to that AF belonged to organic fiber, which could be completely thermal decomposed at a high temperature of 850°C. The lower ash content of HDPE composites was expected to meet the low ash content requirement in various standards.

(a) TGA curves of HDPE composites, (b) ash content curves of HDPE/AgGB@AF and HDPE/AgGB@AF composites.
3.2 Thermal property characterizations
Figure 4 and Table 1 display the DSC curves and thermal parameters of HDPE composites under normal and high pressures. A melting peak and a crystallization peak in HDPE composites could be observed. The T m and T c values of HDPE were not affected by the addition of AgGB@AF, which remained at about 124°C and 106°C, respectively. However, the crystallinity of HDPE composites was increased with the addition of AgGB@AF due to the heterogeneous nucleation effect. The nucleation mechanism would be proved in the following crystalline morphology studies. The higher crystallinity brought higher stiffness of HDPE composites. In other aspects, high pressure would influence crystallization. Thus, we tested the thermal properties of HDPE under a pressure of 5.0 MPa. As shown in Figure 4c and d, the T m and T c of HDPE composites decreased by 1–2°C compared with that of normal pressure. And the crystallinities of HDPE were also reduced under high-pressure conditions. This was due to the high pressure condition restricting the mobility of molecular chain and crystallization nucleation rate (28); thus, it reduced the crystallinity. According to Zou’s study, the crystalline formed under high pressure had many defects, so it could be melted at a relatively low temperature (29). On the whole, the high-pressure condition had an obvious effect on the crystallinity of HDPE composites rather than the thermal temperatures.

DSC curves of the HDPE composites with (a) cooling rate of 10°C·min−1 under normal pressure, (b) heating rate of 10°C·min−1 under normal pressure, (c) cooling rate of 10°C·min−1 under high pressure, and (d) heating rate of 10°C·min−1 under high pressure.
Thermal property parameters of HDPE composites
| Samples | Pressure (MPa) | T c (°C) | T m (°C) | H m (J·g−1) | X c (%) |
|---|---|---|---|---|---|
| 1# | 0.1 | 106.4 | 124.5 | 34.01 | 11.61 |
| 3.0 | 104.2 | 123.4 | 23.92 | 8.16 | |
| 2# | 0.1 | 106.9 | 123.8 | 34.94 | 12.05 |
| 3.0 | 104.5 | 123.2 | 21.19 | 7.30 | |
| 3# | 0.1 | 107.7 | 124.3 | 35.09 | 12.34 |
| 3.0 | 104.3 | 125.3 | 27.60 | 9.70 | |
| 4# | 0.1 | 107.1 | 123.4 | 29.36 | 10.54 |
| 3.0 | 103.7 | 126.3 | 16.87 | 5.99 | |
| 5# | 0.1 | 106.4 | 125.9 | 31.17 | 11.44 |
| 3.0 | 103.4 | 126.2 | 21.99 | 8.07 |
Since HDPE was a typical semi-crystalline polymer, its crystalline morphology would impact the final performance significantly. As shown in Figure 5, numerous spherulites formed in pure HDPE. And the spherulite nucleation sites were dispersed uniformly. Pure HDPE had an average spherulite diameter of 4.25 μm. However, with the addition of AgGB@AF, the spherulite morphology of HDPE changed obviously. The black fibrous matters in the POM photos belonged to the AgGB@AFs. It is worth noting that the spherulites next to the fibers had a smaller diameter compared with that in pure HDPE. Its average diameter was only 0.16 μm. This implied that the existence of AgGB@AFs could promote heterogeneous nucleation of HDPE. The higher the AgGB@AF content, the more spherulites of small diameter were, which was beneficial to improve the mechanical properties of HDPE composites.
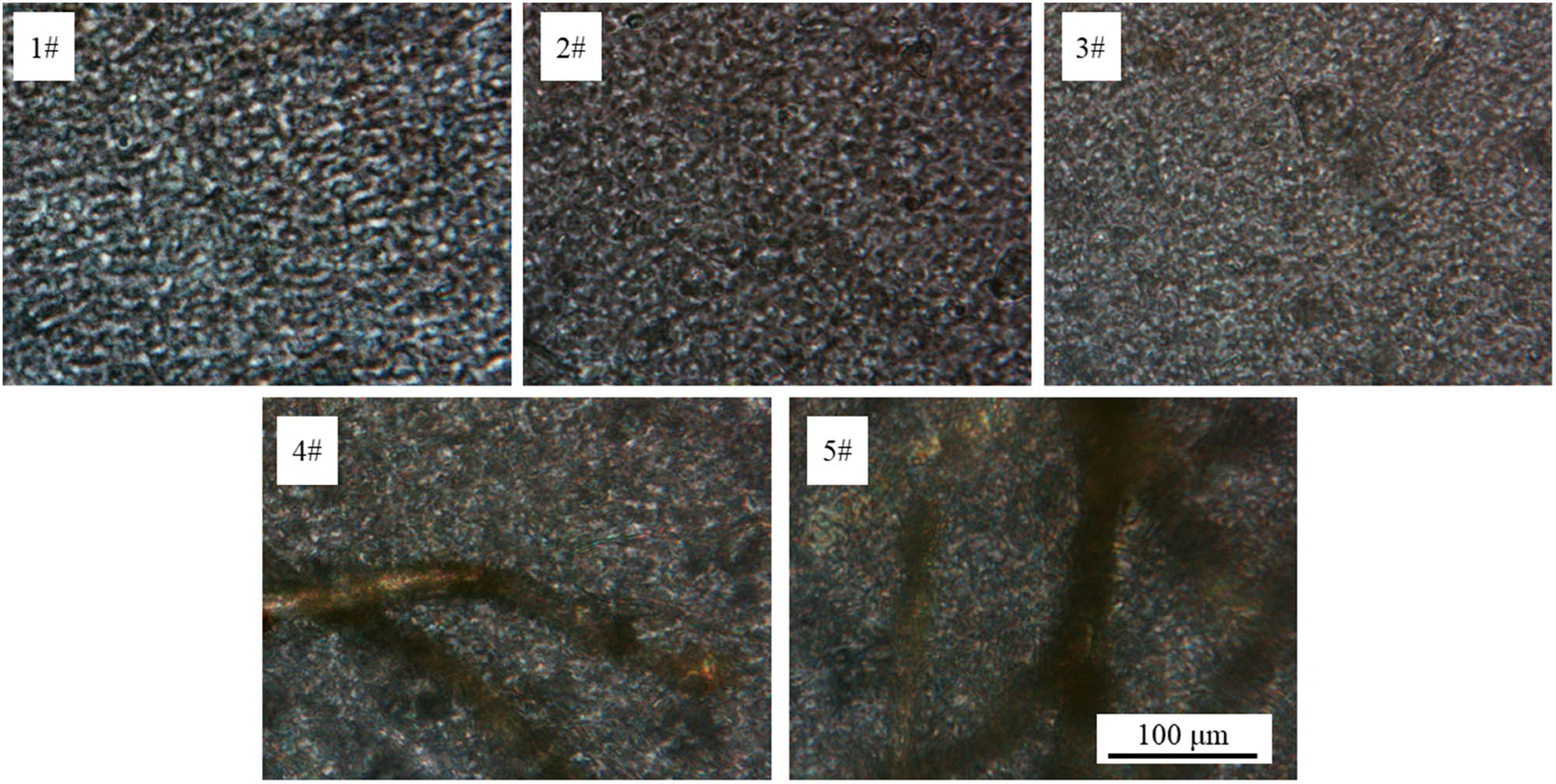
POM images of HDPE composites.
3.3 Mechanical property characterization
Figure 6a and b shows the mechanical properties of HDPE composites. The tensile curve of pure HDPE exhibited a typical ductile fracture with a low tensile strength of 21.63 MPa. With the addition of AgGB@AF, the tensile strength of HDPE composites significantly increased to 29.44 MPa, which was 1.36 times that of pure HDPE. Besides that, the elasticity of HDPE composites also increased from 0.980 to 1.385 GPa. However, with the increasing AgGB@AF content, the elongation ratio of HDPE composites was reduced, which was induced by the cavitation between AgGB@AF and HDPE matrix. The stiffening effect of HDPE composites could be attributed to two aspects: the fiber-reinforcing mechanism and the fine crystalline morphology (30). The tensile strength results were consistent with the crystalline morphology and thermal parameters, where HDPE composites displayed a spherulite morphology with bimodal size distribution and high crystallinity. On the other hand, AF with numerous benzene rings on its chains was a rigid polymer, which had high tensile strength and elasticity module. A crack could not propagate through the composites because it needed to break excessive stiffness fibers before the ultimate failure occurred, which is known as the energy dissipation mechanism (31). And, it is worth noting that the modified AF had excellent adhesion to the HDPE matrix, which was good for improving tensile strength as shown in Figure 6c and d. The mechanical properties indicated that the synthesized AgGB@AF could efficiently improve the stiffness of HDPE composites.

(a) Tensile curves and (b) tensile strength, (c) surface, and (d) fracture surface of HDPE composites.
3.4 Surface wettability and antibacterial property characterizations
Figure 7 depicts the water contact angles and oil contact angles of HDPE composites with various AgGB@AF contents. It was clear that pure HDPE had low water contact angle and oil contact angle, which were 79.72° and 27.19°, respectively. With the addition of AgGB@AF, the water contact angle and the oil contact angle of the HDPE composite were gradually increased up to 90.02° and 50.80°. The change in surface wettability could be attributed to the existence of AgGB@AF dispersed on the surface and the improved surface roughness as shown in Figure 6c. The phenomenon was beneficial to improve antibacterial properties. In detail, bacterial contamination on plastic surfaces could be divided into adhesion phase, colonization phase, and proliferation phase. The adhesion of bacteria to the surface of materials depended on the hydrophobicity or electrostatic interactions of the surface (32). In general, a hydrophobic and oleophobic surface was expected to have greatly anti-fouling and antibacterial properties by relieving the adhesion phenomena of bacteria compared with that of pure HDPE.

(a) Water contact angle and (b) pentane contact angle of HDPE composites.
Figure 8 shows the OD600 values and the bacterial inhibition zone photos of HDPE composites with different AgGB@AF contents. Clearly, pure HDPE had the highest OD600 values about 1.147. Besides, its OD600 value increased very fast in 18 h as shown in Figure 8b. With further increasing AgGB@AF content to 3 wt%, the OD600 value was dramatically decreased to 0.130, which was the percolation threshold for antibacterial properties of HDPE composites. Therefore, the minimum inhibitory concentration of HDPE/AgGB@AF composites against E. coli was deduced to be 3 wt%. The study also implied that increasing the concentration of AgGB@AF could enhance the antibacterial ability of HDPE composites, and the AgGB@AF was provided to efficiently kill the common bacteria of E. coli. Figure 8c displays the contact antibacterial effect of AgGB@AF. With the increase of AgGB@AF content, the diameter of growth inhibition zones was not significantly changed. However, the bacterial colony was absent on the surface of HDPE/AgGB@AF composites. This was due to fact that Ag ions could migrate to the surface of HDPE composites and it had an efficient antibacterial performance. In order to further study the migration amount of Ag ions, the Ag ions in the immersion solution were measured as shown in Figure 8d. The results demonstrated that the concentration of Ag ions was related to the AgGB@AF content. When the content of AgGB@AF reached 5 wt%, the Ag ion concentration was as high as 1.0 mg·mL−1. Simply put, HDPE composites with only 5 wt% AgGB@AF would generate absolute contact antibacterial effect.

(a) OD600 values after 18 h, (b) OD600 curves, (c) photos of inhibition ring, and (d) Ag ion concertation of various HDPE composites.
3.5 Mechanisms
Figure 9 displays the mechanisms of AF surface modification and surface loading technologies, as well as the antibacterial mechanism of HDPE composites. AF and AgGB could act as reinforce and antibacterial agents, respectively. However, these two fillers would not spontaneously form a hybrid structure. Hence, we used GEOTES as a coupling agent. In the coupling reaction, GEOTES was hydrolyzed via hydroxyl ions and –O–C– nucleophilic addition reaction to produce numerous hydroxyl groups. The ethylene oxide group on GEOTES had high reactivity. And the epoxy groups had a small bond angle, which generated a strong tendency to open rings. In addition, the electron cloud density near the oxygen atoms was high, while the electron cloud density attached to the carbon atoms was low. Therefore, the carbon atoms on GEOTES were easily attached by nucleophilic groups. In the case of AF, a large number of tertiary amino groups on its chains had unshared electron pairs. This electron’s structure made the tertiary amino groups nucleophilic. Thus, GEOTES could readily react with AF to form numerous hydroxyl groups on its surface. This reaction mechanism had been discussed in relevant research (33,34). Then, the AgGBs with positively charged were efficiently loaded on AF’s surface via electrostatic interaction to form the AgGB@AF as shown in Figure 2. After that, the HDPE/AgGB@AF composites were prepared by melt-mixing technology. The Ag ions on the AgGB@AF slowly migrated to the surface of HDPE composites, which acted as an antibacterial function. The antibacterial mechanism of AgGB@AF could be attributed to two factors. One was due to the feature of negatively charged membranes of E. coli, which would generate the Coulombic attraction effect with the positively charged HDPE surface. The attraction effect caused cell inactivation. Another was due to the protein coagulation effect induced by Ag ions. When E. coli contacted the materials’ surface, Ag ions penetrated through cytomembranes and went into the intracellular. Protein coagulation occurred because the sulfhydryl groups of E. coli’s intracellular protein reacted with Ag ions (35,36). The coagulation reaction destroyed the synthetase’s activity and interfered with the synthesis of DNA for E. coli proliferation. As well as for the rough surface, E. coli could not adhere, split, and proliferate on the surface of HDPE composites. The antibacterial mechanism also determine the contact antibacterial effect of HDPE/AgGB@AF composites as shown in Figure 8.

Schematic of mechanisms for preparing AgGB@AF and antibacterial of HDPE composites.
4 Conclusions
In general, a nascent hybrid fiber, AgGB@AF, and a series of HDPE composites with high stiffness and tensile strength, antibacterial, as well as low ash content were prepared. Since AF was an organic substance, it generated an ash content of HDPE composites as low as 1.88 wt% even at the high adding dosage of 7 wt%. On the premise of meeting several national standards for plastic products, this was conducive to expanding the application range of fiber-reinforced HDPE composites, even for some polyolefins. On the other hand, the addition of AgGB@AF would greatly change the crystalline morphology of HDPE. With increasing AgGB@AF content, more spherulites with small diameters formed. Accompanied by the fiber-reinforced effect, the tensile strength, and elasticity module of HDPE composites were both increased, which reached 29.44 MPa and 1.385 GPa. Besides, with the addition of 7 wt% AgGB@AF, HDPE composites’ water contact angle and oil contact angle were increased to 112.85% and 186.83%, respectively. The enhanced hydrophobic and oleophobic surface of HDPE composites had an anti-fouling effect, which could relieve bacterial adhesion. Moreover, the effective antibacterial concentration of AgGB@AF was proved to be 5 wt%. The Ag ions on the AgGB@AF slowly migrated to the materials’ surface; thus, the HDPE composites had a long-lasting and contact antibacterial effect.
-
Funding information: We acknowledge the support by Guizhou Provincial Science and Technology Projects (QianKeHeJiChu-ZK[2022]YiBan179), National Natural Science Foundation of China (52263004, 51863003), and Research Start-up Foundation for Advanced Talents of Guizhou Institute of Technology (XJGC20190657).
-
Author contributions: Wei Liu: formal analysis and writing – original draft; Xian Wu: methodology, investigation, data curation; Yang Li: methodology, investigation, data curation; Shan Liu: writing – review and editing; Yunwei Lv: resources and formal analysis; Chun Zhang: supervision.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: Data available on request from the authors.
References
(1) Marini M, De Niederhausern S, Iseppi R, Bondi M, Sabia C, Toselli M, et al. Antibacterial activity of plastics coated with silver-doped organic-inorganic hybrid coatings prepared by sol-gel processes. Biomacromolecules. 2007;8:1246–54.10.1021/bm060721bSearch in Google Scholar PubMed
(2) Ma Z, Niu G, Lu S. China plastics industry (2021). China Plast. 2022;6:142–8.Search in Google Scholar
(3) Gogoi R, Maurya A, Manik G. A review on recent development in carbon fiber reinforced polyolefin composites. Compos Part C. 2022;8:100279–300.10.1016/j.jcomc.2022.100279Search in Google Scholar
(4) Bilotta A, Lignola G. Effect of fiber-to-matrix bond on the performance of inorganic matrix composites. Compos Struct. 2021;265:113655–65.10.1016/j.compstruct.2021.113655Search in Google Scholar
(5) Zhang Y, Pontikes Y, L, Lessard, Vuure A. Recycling and valorization of glass fibre thermoset composite waste by cold incorporation into a sustainable inorganic polymer matrix. Compos Part B. 2021;223:109120–30.10.1016/j.compositesb.2021.109120Search in Google Scholar
(6) ASTM D5630. Standard test method for ash content in plastics; 2006.Search in Google Scholar
(7) EN ISO 3451. Plastics-determination of ash; 2019.Search in Google Scholar
(8) GB/T 1366313663. Polyethylene (PE) piping systems for water supply; 2018.Search in Google Scholar
(9) Yang X, Tu Q, Shen X, Yin Q, Pan M, Jiang C, et al. Enhancing the interfacial adhesion with rubber matrix by grafting polydopamine-carbon nanotubes onto poly(P-phenylene terephthalamide) fibers. Polymers. 2019;11:1231–43.10.3390/polym11081231Search in Google Scholar PubMed PubMed Central
(10) Arshad M, Kaur M, Ullah A. Green biocomposites from nanoengineered hybrid natural fiber and biopolymer. ACS Sustain Chem Eng. 2016;4:1785–93.10.1021/acssuschemeng.5b01772Search in Google Scholar
(11) Cheung H, Ho M, Lau K, Cardona F, Hui D. Natural fibre-reinforced composites for bioengineering and environmental engineering applications. Compos Part B. 2009;40:655–63.10.1016/j.compositesb.2009.04.014Search in Google Scholar
(12) Yu S, Li J, Dong J, Liao J, Shen P, Liu C, et al. Organic Solvent-Resistant and Robust Kevlar Nanofiber-Based Cation-Exchange Membranes for Improved Electrodialysis Performance. ACS Appl Polym Mater. 2022;4:889–98.10.1021/acsapm.1c01385Search in Google Scholar
(13) Li Y, Luo Z, Yang L, Luo Y, Li Q, Zhang L, et al. Influence of polyamide acid coating reaction on the properties of aramid fibre. Polymer. 2019;178:121550–60.10.1016/j.polymer.2019.121550Search in Google Scholar
(14) Liu T, Zheng Y, Hu J. Surface modification of aramid fibers with novel chemical approach. Polym Bull. 2011;66:259–75.10.1007/s00289-010-0313-ySearch in Google Scholar
(15) Zhang B, Lian T, Shao X, Tian M, Ning N, Zhang L, et al. Surface coating of aramid fiber by a graphene/aramid nanofiber hybrid material to enhance interfacial adhesion with rubber matrix. Ind Eng Chem Res. 2021;60:2472–80.10.1021/acs.iecr.0c05794Search in Google Scholar
(16) Zhang Y, Huang Y, Liu L, Cai K. Effects of γ-ray radiation grafting on aramid fibers and its composites. Appl Surf Sci. 2008;254:3153–61.10.1016/j.apsusc.2007.10.081Search in Google Scholar
(17) Li L, Huang Y, Zhang Z, Jiang Z, Wu L. Ultrasonic treatment of aramid fiber surface and its effect on the interface of aramid/epoxy composites. Appl Surf Sci. 2008;28:2954–99.10.1016/j.apsusc.2007.09.091Search in Google Scholar
(18) Ghosh L, Fadhilah M, Kinoshita H, Ohmae N. Synergistic effect of hyperthermal atomic oxygen beam and vacuum ultraviolet radiation exposures on the mechanical degradation of high-modulus aramid fibers. Polymer. 2006;47:6836–42.10.1016/j.polymer.2006.07.029Search in Google Scholar
(19) Murali G, Lee M, Modigunta J, Kang B, Kim J, Park E, et al. Ultraviolet-ozone-activation-driven Ag nanoparticles grown on plastic substrates for antibacterial applications. ACS Appl Nano Mater. 2022;5:8767–74.10.1021/acsanm.2c00319Search in Google Scholar
(20) Adegboyega N, Sharma V, Siskova K, Vecerova R, Kolar M, Zbořil R, et al. Enhanced formation of silver nanoparticles in Ag-NOM- + Iron(II, III) systems and antibacterial activity studies. Environ Sci Technol. 2014;48:3228–35.10.1021/es405641rSearch in Google Scholar PubMed
(21) Datta S, He G, Tomilov A, Sahdeo S, Denison M, Cortopassi G. In vitro evaluation of mitochondrial function and estrogen signaling in cell lines exposed to the antiseptic cetylpyridinium chloride. Environ Health Perspect. 2017;22:87015–22.10.1289/EHP1404Search in Google Scholar PubMed PubMed Central
(22) Herron J, Reese R, Tallman K, Narayanaswamy R, Porter N, Xu L. Identification of environmental quaternary ammonium compounds as direct inhibitors of cholesterol biosynthesis. Toxicol Sci. 2016;151:261–70.10.1093/toxsci/kfw041Search in Google Scholar PubMed PubMed Central
(23) Wei Z, Hui Y, Liu F. Molecular interactions between DOPA and surfaces with different functional groups: A chemical force microscopy study. RSC Adv. 2017;7:32518–27.10.1039/C7RA04228KSearch in Google Scholar
(24) Wang Z, Ding X, Zhao M, Wang X, Xu G, Xiang A, et al. A cooling and two-step depressurization foaming approach for the preparation of modified HDPE foam with complex cellular structure. J Supercrit Fluids. 2017;125:22–30.10.1016/j.supflu.2017.01.011Search in Google Scholar
(25) Liu W, Wu X, Chen X, Liu S, Zhang C. Flexibly controlling the polycrystallinity and improving the foaming behavior of polylactic acid via three strategies. ACS Omega. 2022;7:6248–60.10.1021/acsomega.1c06777Search in Google Scholar PubMed PubMed Central
(26) Zhang Q, Lan L, Zheng Z, Liu P, Wu H, Guo S, et al. Constructing highly oriented and condensed shish-kebab crystalline structure of HDPE/UHMWPE blends via intense stretching process: Achieving high mechanical properties and in-plane thermal conductivity. Polymer. 2022;241:124532–42.10.1016/j.polymer.2022.124532Search in Google Scholar
(27) Liu W, Wu X, Liang J, Ding P, Lv Y, Zhang C. Preparation of antibacterial and strengthened high-density polyethylene composites via compounding with silver ion glass microbead-loaded basalt fiber. J Mater Eng Perform. 2022;31:1493–502.10.1007/s11665-021-06222-0Search in Google Scholar
(28) Zou L, Zhang W. Molecular dynamics simulations of the effects of entanglement on polymer crystal nucleation. Macromolecules. 2022;12:4899–906.10.1021/acs.macromol.2c00817Search in Google Scholar
(29) Yousefi N, Saghez B, Pettipas R, Kelly T, Kaake L. Physical supercritical fluid deposition of polymer films: controlling the crystallinity with pressure. Mater Chem Front. 2021;5:1428–37.10.1039/D0QM00403KSearch in Google Scholar
(30) Romera M, Lou X, Schill J, Huurne G, Fransen P, Voets I, et al. Strain-stiffening in dynamic supramolecular fiber networks. J Am Chem Soc. 2018;140:17547–55.10.1021/jacs.8b09289Search in Google Scholar PubMed PubMed Central
(31) Beckett L, Lewis J, Tonge T, Korley L. Enhancement of the mechanical properties of hydrogels with continuous fibrous reinforcement. ACS Biomater Sci Eng. 2020;6:5453–73.10.1021/acsbiomaterials.0c00911Search in Google Scholar PubMed
(32) Liang X, Qin L, Wang J, Zhu J, Zhang Y, Liu J. Facile construction of long-lasting antibacterial membrane by using an orientated halloysite nanotubes interlayer. Ind Eng Chem Res. 2018;57:3235–45.10.1021/acs.iecr.7b04725Search in Google Scholar
(33) Chai D, Xie Z, Wang Y, Liu L, Yum Y. Molecular dynamics investigation of the adhesion mechanism acting between dopamine and the surface of dopamine-processed aramid fibers. ACS Appl Mater Interfaces. 2014;6:17974–84.10.1021/am504799mSearch in Google Scholar PubMed
(34) Sa R, Yan Y, Wei Z, Zhang L, Wang W, Tian M. Surface modification of aramid fibers by bio-inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl Mater Interfaces. 2014;6:21730–8.10.1021/am507087pSearch in Google Scholar PubMed
(35) Zheng K, Setyawati M, Lim T, Seong D, Xie J. Antimicrobial cluster bombs: Silver nanoclusters packed with daptomycin. ACS Nano. 2016;10:7934–42.10.1021/acsnano.6b03862Search in Google Scholar PubMed
(36) Lin F, Qi Q, Zhang J, Zhou W, Zhang J, Fu P, et al. From unimolecular template to silver nanocrystal clusters: An effective strategy to balance antibacterial activity and cytotoxicity. ACS Appl Mater Interfaces. 2021;13:39806–18.10.1021/acsami.1c07986Search in Google Scholar PubMed
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

