Abstract
A ternary hybrid nucleating agent (THNA) powder was prepared by co-spray drying the fluid mixture of Si-MP/SNa slurry. The THNA was characterized by Fourier transform infrared and thermogravimetric analyses; the results showed that THNA was prepared successfully. The results of scanning electron microscopy (SEM) and transmission electron microscopy (TEM) showed that THNA was ring-shaped or mushroom cap-shaped and it was uniformly dispersed in the iPP matrix. With the incorporation of THNA (0.2 wt%), the crystallization peak temperature of iPP/THNA increased effectively. The nucleation efficiency and crystallinity were improved to 69% and 58%, respectively. Moreover, the flexural strength, flexural modulus, tensile strength, and impact toughness of iPP/THAN were enhanced to 49.3 MPa, 1,988 MPa, 42 MPa, and 4.93 kJ·m−2, respectively. The transparency was increased to 77.7%, and the haze was reduced to 14.1%. The compound of sodium laurate and inorganic silica/aromatic phosphate had an obvious synergistic effect.
1 Introduction
Isotactic polypropylene (iPP) has been widely used because of its exceptional good price/performance ratio (1,2,3,4,5). As a semi-crystalline polymer, iPP is usually modified by nucleating agents to promote crystallization and improve comprehensive properties (6,7). Nucleating agents for iPP mainly include inorganic, organic, and other nucleators.
Inorganic particles were often used as inorganic nucleating agents for iPP, such as inorganic silica sol, talc, calcium oxide, carbon black, calcium carbonate, mica, inorganic pigment, kaolin, and catalyst residue (1,2,4). However, an unmodified inorganic nucleating agent could not be compatible with the iPP matrix. The large aggregation scales of inorganic particles would affect the nucleation efficiency. So, compatibility and dispersibility were the key problems that should be solved for inorganic nucleating agents (7,8,9).
Aromatic phosphate, sorbitol, rosin, carboxylate, and other small organic molecule nucleating agents were commonly used for iPP (10). Organic nucleating agents exhibited higher nucleation efficiency (NE) and a significant effect on improving the comprehensive performance of polypropylene materials. However, the high price of organic nucleating agents limited their application (11,12,13).
A compound nucleating agent consists of two or more materials together to form an integrated material. Compared with the original single material, compound nucleating agents had superior performance and comprehensive effect. Nowadays, preparing a compound nucleating agent with the advantages of both inorganic and organic nucleating agents has become a new method for iPP modification. Zhang et al. prepared a compound nucleating agent of carboxylated graphene/calcium pimelate for iPP. The effects of carboxylated graphene, calcium pimelate, and carboxylated graphene/calcium pimelate nucleating agents on the crystallization behaviors of iPP were compared. The results showed that the promotion effect of carboxylated graphene on heterogeneous nucleation was insignificant and calcium pimelate had a certain promoting role in increasing the crystallization peak temperature. It was amazing that the carboxylated graphene/calcium pimelate compound system could not only improve the dispersibility and nucleation ability of carboxylated graphene in iPP matrix but also increase the crystallization peak temperature and the relative content of crystal in iPP (14). He et al. performed surface modification on calcium sulfate whiskers with silane coupling agent KH-550 and then compounded it with aryl amide nucleating agent dicyclohexyl terephthalamide. They compared the effects of modified calcium sulfate whiskers, dicyclohexyl terephthalamide, and the newly prepared compound material on crystallization behaviors of iPP; the results showed that calcium sulfate whiskers modified with KH-550 had certain nucleating ability for iPP crystallization. Moreover, the prepared compound nucleating agent had the best performance, which was obviously better than that of iPP nucleated with modified calcium sulfate whiskers or dicyclohexyl terephthalamide independently. The compound system had a certain synergistic effect on the formation of iPP crystals (15).
The commonly used nucleating agents were in a powder state, and the particle size of the powder would affect its dispersion in the polymer matrix. Spray drying is an effective method to make powder, in which the liquid is dispersed into fine droplets by an atomizer and the solvent is rapidly evaporated in the thermal drying medium to form dry powder product (16). Tabak et al. used a silane coupling agent to modify nanofibrillated cellulose by spray drying. The results showed that nanofibrillated cellulose was modified successfully. The addition of modified nanofibrillated cellulose to the polypropylene–copolymer matrix in the presence of maleic anhydride-grafted PP could improve the thermal resistance of the composite (17). Wang et al. used spray-dried to prepare cellulose nanofibrils to study the effects on the non-isothermal crystallization kinetics and thermal expansion of iPP, and the results showed that the product prepared by spray drying could promote the crystallization of iPP (18,19). Rezaei et al. prepared nano-scale polybutadiene rubber powder by spray drying irradiated rubber lattices vulcanized by 60Co radiation. The results showed the nano-scale polybutadiene rubber powder was uniformly dispersed in the polymeric matrix. The nano-scale polybutadiene rubber powder by spray drying could improve the impact resistance and tensile strength of the PP nanocomposites (20). These research studies indicated that spray drying was a good way of granulating.
In our previous research, aromatic phosphate was loaded on the silica sol to prepare a compound nucleating agent by spray drying. The average size of the obtained particle was 5 μm, and it was well dispersed in the iPP matrix. The compound nucleating agent had positive effects on crystallization and thus would improve the mechanical and optical properties of iPP composites (21).
It was usually that the compound of sodium laurate (SNa) and aryl heterocyclic phosphate hydroxyl aluminum salt (AHP-AlOH) had a good synergistic effect. Li et al. reported that, with 40% SNa in the compound system, the synergistic effect of SNa and AHP-AlOH was excellent on the iPP composite and the improvement of the compound system for mechanical and optical properties was better than that using AHP-AlOH separately (22). Liu et al. also reported that the compound of SNa and AHP-AlOH at a ratio of 4:6 had the most significant improvement in the iPP composite and the best synergistic effect (23,24). Zhang et al. studied the nucleation of the compound of AHP-AlOH and different cation laurates, and the results showed that the compound of monovalent laurates and AHP-AlOH had a synergistic effect (25).
In order to investigate the synergistic effect of SNa and the hybrid nucleating agent we prepared earlier, the following work was carried out. Referring to our previous work, Si-MP was prepared by supporting aromatic phosphate on silica sol via a chemical action with γ-aminopropyltrimethylsilane on the silica sol (26). Then, Si-MP and SNa were dispersed into distilled water to obtain spray drying precursors. A ternary hybrid nucleating agent (THNA) was prepared by the spray drying method. The nucleation effect of THNA on iPP was investigated from the viewpoint of crystallization, morphology, mechanical, and optical properties. Moreover, by changing the ratio of SNa in THNA, the synergistic effect of SNa and inorganic silica sol/aromatic phosphate was studied.
2 Experimental
2.1 Materials
Commercially available iPP (T30s) pellets were supplied by Lanzhou Petrochemical Co. (Lanzhou, China). Inorganic silica sol with an average particle size of 12 nm, a pH of 4.5, and a solid content of 30 wt% was purchased from Sigma-Aldrich Co., USA. γ-Aminopropyltrimethylsilane (KH550) was supplied by Hubei Blue Sky New Material Co., Ltd. (Wuhan, China). Anhydrous ethanol was purchased from Chongqing Chuandong Chemical Co., Ltd. (Chongqing, China). 2′,2′-Methylene bis(4,6-di-tert-butylphenyl) phosphate (MDBP-POOH) was synthesized in our laboratory. SNa was received from Linghu Xinwang Chemical Co., Ltd. (Huzhou, China).
2.2 Preparation of samples
The preparation method of Si-MP followed previous work (26). Then, certain amounts of SNa and Si-MP were dispersed in distilled water and ultrasonically dispersed for a while to obtain a homogeneous suspension. Then, the suspension was fed into a spray dryer (YC-015A; Shanghai Pilotech Instrument Equipment Co., Ltd.). In this experiment, the spray drying process was set at a solution concentration of 5.5 mg·mL−1, an atomization pressure of 0.25 MPa, and an inlet air temperature of 110°C. The dosage of SNa in THNA was set to 0, 25, 50, and 75 wt%, respectively. Also, the final collected dry powder from spray drying was THNA and denoted as S0, S1, S2, and S3, respectively.
The preparation route of THNA is shown in Scheme 1.

Schematic illustration for preparation the THNA.
The iPP pellets and one kind of THNA (0.2 wt%) were mixed fully in a mixer (SHR100, Zhangjiagang HaiChuan Machinery Co., Ltd., China) for 5 min at room temperature. The rotation speed was 950 rpm. The mixture was extruded and granulated by a corotating twin-screw extruder (CTE-20, Kopilongkeya [Nanjing] Machinery Manufacturing Co., Ltd., China) with D = 40 mm and L/D = 40. The temperature from the initial region to the nose was 160°C, 175°C, 185°C, 195°C, 205°C, and 190°C, respectively. The screw speed was 300 rpm. The feed rate was 10 rpm. The obtained granules were dried at 80°C for 24 h. Then, the above granules were injection-molded into 185 mm × 10 mm × 4 mm bars and 50 mm × 50 mm × 1 mm thick square plaques by an injection molding machine (CJ80M3V, Zhende Plastic Machinery Factory Co., Ltd., China). The temperature from the metering regions to the nozzle were 190°C, 205°C, 220°C, 230°C, and 220°C, respectively. The injection time was 5 s. The cooling time was 8 s.
2.3 Characterization
The structural investigation of the prepared samples was performed by analyzing the recorded spectra on a Fourier transform infrared (FT-IR) spectrometer (Nuxus 670, Nicolet Co., USA).
The thermal degradation behavior of the samples was examined on a TA instrument (Q50, TA Instruments Co., USA). The samples were heated from 30°C to 800°C at a rate of 10°C·min−1 under nitrogen flow.
The morphology for THNA samples was analyzed by a scanning electron microscope (SEM) (KYKY-2800B, Beijing Zhongke Instrument Technology Development Co Ltd., China). Powder samples were attached to double-sided adhesive carbon tabs mounted on SEM support, coated with a thin layer of gold, and examined with SEM at an accelerating voltage of 10 kV.
Transmission electron microscopy (TEM) (JEM 200 C, JEOL Ltd., Japan) was carried out to observe the microstructure of the polymer samples. The samples were made into ultrathin sections (60–80 nm) by a microtome (LKB-5, LKB Co., Sweden).
The melting and crystallization processes of the polymer samples were studied with a differential scanning calorimetry (DSC) (Q10, TA Instrument Co., USA). The samples were performed under a nitrogen atmosphere. First, the samples were heated from 30°C to 230°C at a heating rate of 60°C·min−1 and held at this temperature for 5 min to erase the heat history. Then, the samples were cooled to 30°C in steps of 10°C·min−1. Next, the samples were heated to 230°C at 10°C·min−1. The heat flows during cooling and heating were recorded. For the isothermal crystallization part, the specimens were first heated from 30°C to 230°C at a heating rate of 10°C·min−1 and held at this temperature for 5 min to erase the heat history from previous processes. The specimens were then cooled to the desired isothermal crystallization temperature (ranging from 126°C to 142°C) at a rate of 10°C·min−1 and held for 30 min. Heat flows of the specimens after isothermal crystallization were also recorded.
Spherulite morphologies of different samples were observed by a polarized optical microscope (POM) (E400POL, Nikon Corp., Japan). The samples were placed between two microscopy slides, melted at 230°C, and kept for 5 min to eliminate previous thermal and mechanical history. Then the samples were rapidly cooled to 130°C at 30°C·min−1 for isothermal crystallization. After completion of the crystallization, the spherulite morphologies were recorded.
The crystallization behavior of polymer samples was analyzed by a small-angle X-ray scatterometer (Bruker Nanostar U, Germany), λ at 0.154 nm, working at 50 kV and 0.6 mA. Every two-dimensional SAXS pattern was collected with a HI-STAR detector in transmission mode at room temperature.
The tensile and flexural tests were carried out using an electronic universal tensile testing machine (WdW2 10 C, Hualong Testing Instrument Co., Shanghai, China) at speeds of 50 and 2 mm·min−1 at room temperature.
The impact energy measurements were performed on a liquid crystal pendulum impact testing machine (ZBC-4B, City Xinsansi Technology Co. Ltd., Shenzhen, China), and the pendulum was loaded 2.75 J.
3 Results and discussion
3.1 FTIR analysis
Figure 1 shows the FT-IR spectra of the different samples. For SNa, –CH2– (2,850 cm−1), C═O (1,560 cm−1), and C═O (1,420 cm−1) stretching modes were assigned to their respective wavelength regions. For S1, S2, and S3, the above-said peaks appeared here. It confirmed that the functionalities of SNa were present in THNA. For S0, the peaks could be seen at around 1,230 and 1,030 cm−1 as the dispersion corresponding to P═O and P‒O, respectively. Similar peaks were seen for S1, S2, and S3. It illustrated the functionalities of MDBP–POOH was successfully grafted to modified silica sol. The results confirmed the THNA was prepared successfully by spray drying.

FT-IR spectrum of different samples.
3.2 TG analysis
To characterize the quantity of organics immobilized on the silica sol, TGA was used. As shown in Figure 2, there was no mass loss for all samples at the initial stage (≤100°C). The mass losses at the final temperature (700°C) were 9%, 17%, 30%, 46%, and 55% for silica sol, S0, S1, S2, and S3, respectively. The mass loss for silica sol was used to correct the mass loss of loaded organic compounds of other samples, and the results for S0, S1, S2, and S3 were 8%, 21%, 37%, and 46%, respectively (as shown in Table 1). Comparing S0 with S1, S2, and S3, the mass loss of the samples containing SNa were higher than that for S0. It showed that SNa was loaded on the hybrid system successfully by spray drying. From S1 to S3, the content of SNa increased, and the corrected mass loss of the samples increased correspondingly. The results confirmed that the contents of SNa in THNA were different.

TG curves of different samples.
Mass loss data from TG curves
| Sample | Mass loss (%) | Corrected mass loss (%) |
|---|---|---|
| Silica sol | 9 | — |
| S0 | 17 | 8 |
| S1 | 30 | 21 |
| S2 | 46 | 37 |
| S3 | 55 | 46 |
3.3 SEM analysis
The morphology of the different nucleating agents was observed by SEM (Figure 3). From S0 to S3, the morphology had changed. The shape of the particles changed from a solid sphere to a ring shape or a mushroom cap shape with an increase of SNa content. Some of S3 collapsed and looked wrinkled. Inorganic silica sol had too high rigidity to deform. Loading MDBP-POOH on it did not change the shape of the particle (as shown in Figure 3a). However, by loading SNa on Si-MP, the shape changed. The results suggested that SNa played an important role in influencing the particle shape of THNA. The increase of SNa content resulted in the deformation of the particle during spray drying.

SEM pictures of (a) S0, (b) S1, (c) S2, and (d) S3.
3.4 TEM analysis
The dispersion of S0, S1, S2, and S3 in the iPP matrix was investigated by TEM (Figure 4). For iPP/S0, a small number of black spots on the scale of 100–300 nm could be seen obviously. The larger black spots might be the agglomerates of S0. For iPP/S1, iPP/S2, and iPP/S3, more small black dots could be observed, and no obvious agglomerations could be seen. Also, the dispersion of the black dots was more uniform. The results suggested that SNa had a positive effect on THNA particle dispersion. In other words, the compound of SNa and Si-MP had a synergistic effect on improving the dispersibility of hybrid particle in iPP matrix. Furthermore, the small black dots for iPP/S2 were the highest number, had the smallest size, and had the best dispersion among the iPP composites, it showed that the optimum ratio of SNa in hybrid nucleating agents was 50 wt%. The result, combined with the results of SEM analysis, showed that THNA particles with a regular ring or mushroom cap structure had stronger redispersibility and could be well dispersed in the iPP matrix. This structure would have a great impact on the nucleation effect and affect the physicochemical properties of iPP composites. Then, the crystallization and physical properties of iPP composites were investigated.

TEM images of (a) iPP/S0, (b) iPP/S1, (c) iPP/S2, and (d) iPP/S3.
3.5 DSC analysis
The crystallization and melting curves of different iPP composites are shown in Figure 5. Also, the corresponding data are listed in Table 2. There was a single peak in melting curves of iPP composites, which showed that the addition of S0, S1, S2, and S3 would not affect the crystalline morphology of iPP. The melting peak temperature (T m) was around 166°C, which belonged to the typical melting peak temperature of α-iPP. However, the addition of 0.2 wt% S0, S1, S2, and S3 led to an increase of crystallization temperature (T c). The T c of iPP/S2 was the highest one among all samples. The degree of crystallinity (X c) was determined from the crystallization exotherms by using the following equation (27):
where ΔH c and ΔH 100 represented the crystallization enthalpy (J·g−1) and the overall crystallization enthalpy (J·g−1), respectively. Also, the ΔH 100 of iPP was usually taken as 209 J·g−1 (21). As shown in Table 2, the results for iPP composites were higher than those for iPP, and those for iPP/S2 were the highest. The result suggested that S0, S1, S2, and S3 could promote the crystallization of iPP. Rybnikář (28) reported that the crystallization temperature difference (ΔT c) could represent the nucleation capacity of the nucleating agent; the larger the ΔT c was, the better the nucleating capacity. As shown in Table 2, the ΔT c’s of iPP/S1, iPP/S2, and iPP/S3 were higher than that of iPP/S0, which showed that the compound of SNa and Si-MP could effectively increase the NE of the hybrid system, and the optimizing ratio of SNa was 50 wt%.
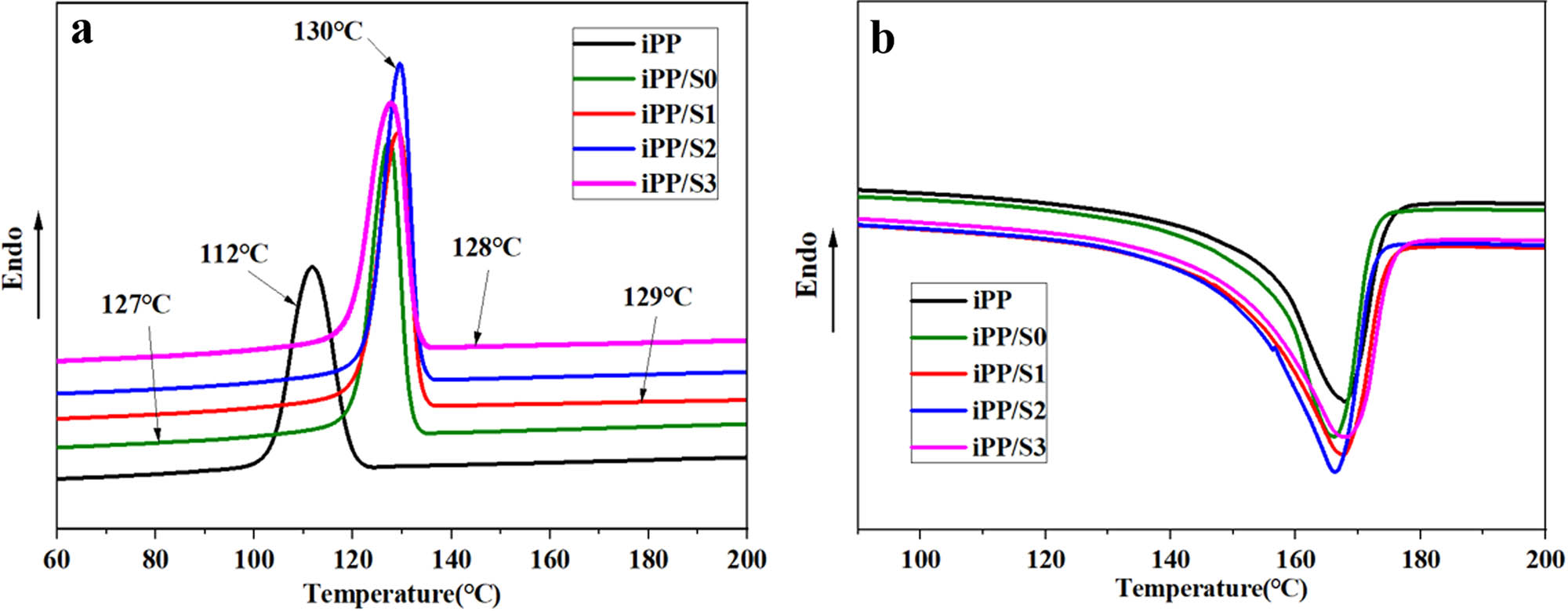
DSC patterns of iPP composites: (a) crystallization curves and (b) melting curves.
The corresponding parameters from crystallization and melting curves
| Samples | T c (°C) | T m (°C) | H c (J·g−1) | X c (%) | ΔT c (°C) | NE (%) |
|---|---|---|---|---|---|---|
| iPP | 112 | 164 | 88 | 42 | — | — |
| iPP/S0 | 127 | 164 | 115 | 55 | 15 | 58 |
| iPP/S1 | 129 | 166 | 115 | 55 | 17 | 65 |
| iPP/S2 | 130 | 167 | 121 | 58 | 18 | 69 |
| iPP/S3 | 128 | 166 | 113 | 54 | 16 | 62 |
To further verify the NE of the THNA system, the NE was calculated based on the methodology developed by Fillon et al. (29) according to the following equation:
where T c1 and T c2max are the crystallization temperature and best self-nucleated of iPP, respectively. T c is the crystallization temperature of iPP composites, which could be obtained from the DSC curves. Referring to the conclusion of Fillon et al. (30), for nucleating iPP using inorganic particles as nucleating agent, T c2max = 138°C. For iPP/S0, iPP/S1, iPP/S2, and iPP/S3, the NE results were 58%, 65%, 69%, and 62%, respectively. It was clearly that the NE values of S1, S2, and S3 was higher than that for S0, and that for S2 was the highest. The results showed that THAN system had a positively effect on crystallization and could be taken as a highly effective nucleating agent for iPP. There was a synergistic effect between SNa and Si-MP to increase the crystallization temperature, melting temperature and crystallization of NE.
3.6 Crystallization kinetics analysis
The Avrami equation (30,31,32) was an empirical equation that related crystallization kinetics of polymer. The Avrami equation is
where X(t) is the relative fraction crystallinity of polymer at time t. k is the crystallization rate constant, which is related to the nucleation and crystallization rate of polymer. The Avrami exponent n is related to the nucleating and the growing mode in the crystallization of the polymer. The logarithmic deformation of the Avrami equation could obtain the following equation:
The Avrami exponent n could be obtained from the straight slope by plotting ln[−ln(1−X(t))] against ln t, and the crystallization rate constant k was obtained by the Y axis intercept of the straight line. The relative crystallinity X(t) could be expressed as
Substituting X(t) = 50%, the half crystallization time, t 1/2, was an important data for evaluating the crystallization rate, commonly. Applying X(t) = 50% in Eq. 3, one could obtain
The isothermal crystallization curves of iPP composites at different temperatures are shown in Figure 6 and the data are listed in Table 3. It was obvious that the overall crystallized time prolonged as the crystallization temperature increased for the same material. The t 1/2 values for iPP and iPP/S0 at 130°C were 3.363 and 2.408 min, respectively. The difference in t 1/2 showed that the crystallization rate of iPP was somewhat improved by Si-MP. However, the improvement effect of Si-MP was not the best. The t 1/2 values for iPP/S1, iPP/S2, and iPP/S3 at 136°C were 1.057, 0.822, and 1.490 min, respectively. Compared with that of pure iPP/S0, the t 1/2 of iPP composites with SNa was shorter at higher temperatures. The results suggested that the crystallization rate of the iPP/THNA composite was higher than that of the iPP/Si-MP composite or pure iPP. It also suggested that the compound of Si-MP and SNa had a significant synergistic effect on the crystallization of iPP.

Isothermal crystallization curves of (a) iPP, (b) iPP/S0, (c) iPP/S1, (d) iPP/S2, and (e) iPP/S3 at different temperatures.
Isothermal crystallization data of iPP composite
| Samples | T c (°C) | n | t 1/2 (min) | k (min−n ) | n avg |
|---|---|---|---|---|---|
| iPP | 126 | 2.474 | 1.445 | 0.26748 | 2.415 |
| 128 | 2.286 | 2.352 | 0.09575 | ||
| 130 | 2.277 | 3.363 | 0.04591 | ||
| 132 | 2.621 | 7.337 | 0.00365 | ||
| iPP/S0 | 128 | 2.340 | 1.557 | 0.25436 | 2.441 |
| 130 | 2.398 | 2.408 | 0.09068 | ||
| 132 | 2.430 | 3.928 | 0.02607 | ||
| 134 | 2.595 | 6.720 | 0.00511 | ||
| iPP/S1 | 136 | 3.439 | 1.057 | 0.73126 | 2.444 |
| 138 | 3.145 | 1.622 | 0.14778 | ||
| 140 | 2.855 | 2.535 | 0.04604 | ||
| 142 | 2.738 | 4.437 | 0.01094 | ||
| iPP/S2 | 136 | 2.312 | 0.822 | 1.05812 | 2.570 |
| 138 | 2.673 | 1.280 | 0.34135 | ||
| 140 | 2.599 | 2.092 | 0.09834 | ||
| 142 | 2.695 | 3.730 | 0.01929 | ||
| iPP/S3 | 136 | 2.176 | 1.490 | 0.29639 | 2.582 |
| 138 | 2.439 | 2.797 | 0.05635 | ||
| 140 | 2.873 | 4.610 | 0.00664 | ||
| 142 | 2.838 | 8.746 | 0.00123 |
The graphic representations of ln[−ln(1 − X(t))] versus lnt for pure iPP and iPP/THNA during the isothermal crystallization were showed in Figure 7. The Avrami exponent n and crystallization rate constant k could be obtained by the straight slope and the intercept of the straight line, respectively. The average n of iPP was 2.415. It suggested that the iPP crystal coexisted in a two-dimensional and a three-dimensional growth mode in the crystallization process. The primary nucleation might be due to the impurities and residual catalysts in the polymerization process. The average n values for iPP/S0, iPP/S1, iPP/S2, and iPP/S3 were 2.441, 2.444, 2.570, and 2.582, respectively. Although n had an increasing trend, it was still less than 3. It illustrated that the crystal growth mode of iPP was not changed by adding the THNA system.
![Figure 7
Fitting curves of ln[−ln(1−X(t))] as a function of lnt during the isothermal crystallization for (a) iPP, (b) iPP/S0, (c) iPP/S1, (d) iPP/S2, and (e) iPP/S3.](/document/doi/10.1515/epoly-2022-0068/asset/graphic/j_epoly-2022-0068_fig_007.jpg)
Fitting curves of ln[−ln(1−X(t))] as a function of lnt during the isothermal crystallization for (a) iPP, (b) iPP/S0, (c) iPP/S1, (d) iPP/S2, and (e) iPP/S3.
3.7 POM analysis
The spherulites of iPP and iPP composites were observed by POM. As shown in Figure 8, the number of nuclei was very few in iPP, the size of the crystal was large, and the boundary between spherulites was obvious. However, in iPP/S0, iPP/S1, iPP/S2, and iPP/S3, the number of nuclei gradually increased, the size of the crystal became small, and the boundary between spherulites was indistinct. The results suggested that the addition of the THNA system led to an increase in the number of primary nucleation sites. Moreover, the spherulite sizes of iPP/S1, iPP/S2, and iPP/S3 were more refined than that of iPP/S0, and the spherulite size of the iPP/S2 composite was the most refined. These findings were well consistent with the results of crystallinity and NE. It indicated that the THNA system was uniformly dispersed in the iPP composite and then formed more heterogeneous nucleation sites, leading to a higher refinement degree of iPP spherulites. The results were consistent with those of TEM.

POM pictures of (a) iPP, (b) iPP/S0, (c) iPP/S1, (d) iPP/S2, and (e) iPP/S3.
3.8 SAXS analysis
As shown in Figure 9, the small-angle diffraction pattern of iPP was a regular ring. Adding the THNA system into iPP, the small-angle diffraction patterns became elliptical. The result suggested that THNA led to the transformation of crystal orientation in iPP crystallization. It further illustrated that the iPP molecular chains were regularly oriented and arranged and formed a regular crystal zone.

Small-angle diffraction patterns of (a) iPP, (b) iPP/S0, (c) iPP/S1, (d) iPP/S2, and (e) iPP/S3.
Figure 10 shows the one-dimensional correlation function curves of iPP composites. Upon analysis, the characteristic microstructure parameters were obtained (shown in Table 4), where L, L c, L tr, L a, RAF, RF, and MAF denote the crystal length, crystal layer thickness, crystal-amorphous transition region thickness, amorphous layer thickness (L a = L – L c –2L tr), volume fraction of the rigid amorphous region (RAF = 2L tr/L), volume fraction of the crystalline region (RF = L c/L), and volume fraction of the amorphous region (MAF = L a /L), respectively. It was clear that the RF and RAF of iPP/THNA composites were higher than those of iPP, and lower MAF than those of iPP. The result indicated that some conformationally ordered chain segments in the amorphous region were rearranged to the interface phase and the crystalline phase during annealing. The rearrangement led to forming ordered crystalline regions and a more perfect lamellar structure. From the comparisons of RF, RAF, and MAF in iPP/S1, iPP/S2, and iPP/S3, the improvement of iPP/S2 was the greatest. The results were consistent with the DSC results. It also explained that the THNA system could promote crystallization and increase crystallinity and orientation. The compound of SNa and Si-MP had a synergistic effect on promoting chain segment rearrangement. Also, the optimum ratio of SNa in the THNA system was 50 wt%.

One-dimensional correlation function curves of iPP composites.
Microstructure parameters of iPP composites annealed at 140°C for 1 h
| Samples | L (nm) | L tr (nm) | L a (nm) | L c (nm) | RF (vol%) | RAF (vol%) | MAF (vol%) |
|---|---|---|---|---|---|---|---|
| iPP | 12.4 | 1.6 | 6 | 3.2 | 25.8 | 25.8 | 48.4 |
| iPP/S0 | 12.1 | 1.7 | 5.4 | 3.3 | 27.3 | 28.1 | 44.6 |
| iPP/S1 | 13.6 | 2.0 | 5.7 | 3.9 | 28.7 | 29.4 | 41.9 |
| iPP/S2 | 13.5 | 2.1 | 5.4 | 3.9 | 28.9 | 31.1 | 40.0 |
| iPP/S3 | 13.0 | 1.9 | 5.5 | 3.7 | 28.5 | 29.2 | 42.3 |
For crystallizable polymer, crystallinity, spherulite size, and the interface area between the crystalline and amorphous phases were very important to the properties of materials. Next, we would examine the mechanical and optical properties of the iPP composites to verify whether THNA also improves these properties and the crystallization.
3.9 Mechanical and optical properties
Data in Table 5 revealed the effectiveness of THNA in improving the mechanical properties and optical properties of iPP. Compared with iPP, the addition of 0.2 wt% THNA led to an increase of flexural strength, flexural modulus, tensile strength, impact toughness, and transparency and the reduction of haze. The flexural strength, flexural modulus, tensile strength, and impact toughness of iPP/THNA were enhanced to 49.3 MPa, 1,988 MPa, 42 MPa, and 4.93 kJ·m−2, respectively, which were 29.0%, 27.4%, 27.3%, and 21.7% higher than that of iPP and 7.9%, 13.9%, 7.7%, and 3.4% higher than those of of iPP/Si-MP. The transparency was increased to 77.7%, which was 28.4% higher than those of iPP and 4.9% higher than those of iPP/Si-MP. The haze was reduced to 14.1%, which was 64.9% lower than that of iPP and 23.0% lower than that of iPP/Si-MP. In addition, comparing iPP/THNA composites to iPP/Si-MP composites, the results of iPP/THNA were better than those of iPP/Si-MP. The result suggested that THNA had an excellent effect on improving the mechanical and optical properties of iPP composite. Also, SNa played an important role in the THNA system. The combination of SNa and Si-MP could improve the mechanical and optical properties of iPP synergistically.
Mechanical and optical properties of iPP composites
| Samples | Tensile strength (MPa) | Flexural strength (MPa) | Flexural modulus (MPa) | Impact toughness (kJ·m−2) | Transparency (%) | Haze (%) |
|---|---|---|---|---|---|---|
| iPP | 33 | 38.2 | 1,560 | 4.1 | 60.5 | 40.2 |
| iPP/S0 | 39 | 45.7 | 1,746 | 4.8 | 74.1 | 18.3 |
| iPP/S1 | 40 | 46.4 | 1,790 | 4.8 | 76.9 | 14.4 |
| iPP/S2 | 42 | 49.3 | 1,988 | 4.9 | 77.7 | 14.1 |
| iPP/S3 | 41 | 46.1 | 1,768 | 4.8 | 76.1 | 17.8 |
In general, the crystalline morphology of iPP had a great effect on its mechanical properties. The smaller the spherulite size, the higher the crystallinity, and the better the comprehensive mechanical properties of iPP (33,34). The hybrid system with silica gel as the carrier increased the number of nucleation sites in the iPP matrix. Also, it promoted crystallization, refined the spherulite, and reduced the spherulite size. By adding SNa to the hybrid system, the compatibility of the hybrid nucleate agent with the iPP matrix was improved. The agglomeration of the hybrid system was prevented. The hybrid particles were uniformly dispersed. So, the promotion effect of the THNA hybrid system was synergistically enhanced. Moreover, the content of SNa played an important role in the hybrid system. Excessive content of SNa in the surface of Si-MP made no sense in crystallization nucleation, it diluted the nucleating agent per unit volume. And the small amount of SNa resulted in insufficient compatibility of the hybrid particles with the iPP matrix. Therefore, the content of SNa in S1 was small, resulted in poor compatibility of iPP and THNA. The content of SNa in S3 was high, the nucleation efficiency of THNA was weakened. When the SNa content was 50% in S2, the nucleation effect was the best.
Normally, the smaller the spherulites, the less the light scattering. Also, the transparency and haze of iPP composites could be attributed to the smaller spherulites. From Table 5, it was obviously that the transparency followed the order: iPP/S2 > iPP/S1 > iPP/S3 > iPP/S0 > iPP. In addition, the haze followed the order: iPP/S2 < iPP/S1 < iPP/S3 < iPP/S0 < iPP. The results of transparency and haze were consistent with the results of DSC and POM discussed above. It showed that the THNA system played an important role in promoting the nucleation of crystallization and refining the spherulite. It also suggested that the compound of SNa and Si-MP could promote iPP crystallization.
Types of nucleating agents and their dosage levels play an important role in the crystallinity of iPP, which controls the final properties of iPP composites. The existing literature work showed that there is a synergistic effect between aryl heterocyclic aluminum phosphate and aluminum phosphate (35). This phenomenon was also confirmed in our results, and the addition of the ternary nucleating agent of the inorganic nanomaterial endowed the iPP with more excellent performance.
4 Conclusions
A ternary hybrid compound nucleating agent was prepared successfully by co-spray drying the fluid mixture of Si-MP/SNa slurry, and the obtained compound powder was used as an effective nucleating agent for iPP. The obtained THNA was in the shape of a ring or mushroom cap. It was uniformly dispersed in the iPP matrix. By incorporating a small amount of THNA (0.2 wt%) into iPP, the crystallization peak temperature of iPP increased by 18°C, and the NE and crystallinity were improved to 69% and 58%, respectively. The flexural strength, flexural modulus, tensile strength, and impact toughness of iPP/THNA were enhanced to 49.3 MPa, 1,988 MPa, 42 MPa, and 4.93 kJ·m−2, respectively, which were 29.0%, 27.4%, 27.3%, and 21.7% higher than that of iPP and 7.9%, 13.9%, 7.7%, and 3.4% higher than that of iPP/Si-MP. The transparency was increased to 77.7%, which was 28.4% higher than that of iPP and 4.9% higher than that of iPP/Si-MP. The haze was reduced to 14.1%, which were 64.9% lower than that of iPP and 23.0% lower than that of iPP/Si-MP. The results showed that the THNA system played an important role in promoting the nucleation of crystallization, refining the spherulite, and improving the mechanical and optical properties of iPP. The compound of SNa and silica sol/aromatic phosphate had a synergistic effect on improving of iPP crystallization. The optimum ratio was 1:1 in this system. Also, the THNA system was an effective nucleating agent for iPP.
-
Funding information: This research was funded by National Natural Science Foundation of China, Grant No. 52063007. The Science and Technology Planning Project of Guizhou Province, Grant No. [2021]9, [2020]4Y223, [2022]058. Scientific and Technological Achievements Transformation Project of Guizhou Province, Grant No. [2022]034. Baiyun District Science and Technology Project, Guiyang, Guizhou Province, Grant No. [2019]24. Youth Fund of Guizhou Academy of Science, Guiyang, Guizhou Province, Grant No. [2021]46.
-
Author contributions: Juan Li: writing – original draft, writing – review and editing, methodology, data curation, validation – verification, formal analysis, project administration; Chunping Yang: writing – original draft, writing – review and editing, formal analysis; Nan Liu: resources, project administration; Shengbao He: resources, supervision; Tianwei Sun: writing – original draft, formal analysis; Jing Zhang: resources.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article (and the Appendix).
References
(1) Putfak N, Larpkasemsuk A. Wollastonite and talc reinforced polypropylene hybrid composites: Mechanical, morphological and thermal properties. J Met Mater Miner. 2021;31(3):92–9.10.55713/jmmm.v31i3.967Search in Google Scholar
(2) Sasimowski E, Majewski Ł, Grochowicz M. Influence of the conditions of corotating twin-screw extrusion for talc-filled polypropylene on selected properties of the extrudate. Polymers. 2019;11(9):1460. 10.3390/polym11091460.Search in Google Scholar PubMed PubMed Central
(3) Chaiwutthinan P, Suwannachot S, Larpkasemsuk A. Recycled poly(ethylene terephthalate)/polypropylene/wollastonite composites using PP-g-MA as compatibilizer: Mechanical, thermal and morphological properties. J Met Mater Miner. 2018;28(2):115–23.Search in Google Scholar
(4) Mittal P, Naresh S, Luthra P, Singh A, Dhaliwal JS, Dhaliwal JS. Polypropylene composites reinforced with hybrid inorganic fillers: Morphological, mechanical, and rheological properties. J Thermoplast Compos Mater. 2019;32(6):848–64. 10.1177/0892705718785674.Search in Google Scholar
(5) Makhlouf A, Satha H, Frihi D, Gherib S, Seguela R. Optimization of the crystallinity of polypropylene/submicronic-talc composites: The role of filler ratio and cooling rate. eXPRESS Polym Lett. 2016;10(3):237–47. 10.3144/expresspolymlett.2016.22.Search in Google Scholar
(6) Tesfaye CK. Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites. e-Polym. 2022;22:87–98. 10.1515/epoly-2022-0014.Search in Google Scholar
(7) Bao ZG, Lee E, Tao J, Sun XJ. Effect of Halloysite and maleic anhydride grafted polypropylene on the isothermal crystallization kinetics of polypropylene based composites. Polym Sci Ser A. 2015;57:889–97. 10.1134/S0965545X15060024.Search in Google Scholar
(8) Zhao XL, Huang DL, Ewulon CM, Wu M, Wang C, Huang Y. Polypropylene/graphene nanoplatelets nanocomposites with high conductivity via solid-state shear mixing. e-Polym. 2021;21:520–32. 10.1515/epoly-2021-0039.Search in Google Scholar
(9) Zhu SP, Chen JY, Zuo Y, Li HL, Cao Y. Montmorillonite/polypropylene nanocomposites: Mechanical properties, crystallization and rheological behaviors. Appl Clay Sci. 2011;52(1–2):171–8. 10.1016/j.clay.2011.02.021.Search in Google Scholar
(10) Abreu AA, Talabi SI, de Almeida Lucas A. Influence of nucleating agents on morphology and properties of injection-molded polypropylene. Polym Adv Technol. 2021;32:2197–206. 10.1002/pat.5252.Search in Google Scholar
(11) Yue Y, Yi JY, Wang L, Feng JC. Toward a more comprehensive understanding on the structure evolution and assembly formation of a bisamide nucleating agent in polypropylene Melt. Macromolecules. 2020;53(11):4381–94. 10.1021/acs.macromol.0c00019.Search in Google Scholar
(12) Liu LY, Zhao Y, Zhang CB, Dong ZY, Wang K, Wang D. Morphological characteristics of β-nucleating agents governing the formation of the crystalline structure of isotactic polypropylene. Macromolecules. 2021;54(14):6824–34. 10.1021/acs.macromol.1c01038.Search in Google Scholar
(13) An YJ, Wang SH, Li R, Shi DZ, Gao YX, Song L. Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene. e-Polym. 2019;19:32–9. 10.1515/epoly-2019-0005.Search in Google Scholar
(14) Zhang YF, Lin XF, Shuai Chen S. Preparation and nucleation effect of a novel compound nucleating agent carboxylated graphene/calcium pimelate for isotactic polypropylene. J Therm Anal Calorim. 2019;136:2363–71. 10.1007/s10973-018-7886-3.Search in Google Scholar
(15) He B, Lin XF, Zhang YF. Effect of a novel compound nucleating agent calcium sulfate whisker/β-nucleating agent dicyclohexyl-terephthalamide on crystallization and melting behavior of isotactic polypropylene. J Therm Anal Calorim. 2018;132:1145–52. 10.1007/s10973-018-7043-z.Search in Google Scholar
(16) Peng YC, Gardner D, Han Y. Drying cellulose nanofibrils: In search of a suitable method. Cellulose. 2012;19:91–102. 10.1007/s10570-011-9630-z.Search in Google Scholar
(17) Tabak C, Keskin S, Akbasak T, Ozkoc G. Polypropylene/spray dried and silane-treated nanofibrillated cellulose composites. Polym Eng Sci. 2018;60:352–61. 10.1002/pen.25290.Search in Google Scholar
(18) Wang L, Gramlich W, Gardner D, Han Y, Tajvidi M. Spray-dried cellulose nanofibril-reinforced polypropylene composites for extrusion-based additive manufacturing: Nonisothermal crystallization kinetics and thermal expansion. J Com Sci. 2018;2(1):7. 10.3390/jcs2010007.Search in Google Scholar
(19) Wang L, Gardner DJ, Bousfield DW. Cellulose nanofibril-reinforced polypropylene composites for material extrusion: Rheological properties. Polym Eng Sci. 2017;58:793–801. 10.1002/pen.24615.Search in Google Scholar
(20) Rezaei AM, Jalali-Arani A. Synergistic effects of nano-scale polybutadiene rubber powder (PBRP) and nanoclay on the structure, dynamic mechanical and thermal properties of polypropylene (PP). Iran Polym J. 2015;24:805–13. 0.1007/s13726-015-0372-x.Search in Google Scholar
(21) Li J, He WT, Long LJ, Zhang K, Xiang YS, Zhang MM, et al. A novel silica-based nucleating agent for polypropylene: Preparation, characterization, and application. J Vinyl Addit Technol. 2015;24:58–67. 10.1002/vnl.21525.Search in Google Scholar
(22) Li C, Xin Z, Feng BN. Synergistic effect of aluminum organic phosphate and sodium carboxylate composite nucleating agent on polypropylene modification. Petrochem Technol. 2008;37(2):149–52.Search in Google Scholar
(23) Liu YF, Zhang K, Ming XX, Su DW, Liu Y, Yu J. Synergistic effect of aluminum organic phosphate and sodium carboxylate on nucleating polypropylene. Plast Sci Technol. 2015;2:33–7.Search in Google Scholar
(24) Liu YF, Zhang K, Fu G, He WT, Qin SH, Yu J. Synergistic effect of aryl heterocyclic aluminum phosphate and sodium laurate on non-isothermal crystallization kinetics of isotactic polypropylene. J Thermoplast Compos Mater. 2015;30:678–90. 10.1177/0892705715610405.Search in Google Scholar
(25) Zhang K, Fu G, Zhou Y, He WT, Qin SH, Yu J. The synergistic effect of alkali metal salt of lauric acid and aromatic heterocyclic phosphate aluminum on nucleation of isotactic polypropylene. Acta Polym Sin. 2016;10:1431–8.Search in Google Scholar
(26) Li J, Qin SH, He WT, Xiang YS, Zhang Q, Zhang MM, et al. The effect of hybrid nanoparticle with silica sol as the supporter on the crystallization behavior and mechanical properties of isotactic polypropylene. J Polym Eng. 2015;35:565–73. 10.1515/polyeng-2014-0243.Search in Google Scholar
(27) Fillon B, Lotz B, Thierry A, Wittmann JC. Self-nucleation and enhanced nucleation of polymers. Definition of a convenient calorimetric “efficiency scale” and evaluation of nucleating additives in isotactic polypropylene (α phase). J Polym Sc Part B: Polym Phys. 1993;31(10):1395–405. 10.1002/polb.1993.090311014.Search in Google Scholar
(28) Rybnikář F. Character of crystallization nuclei in isotactic polypropylene. J Appl Polym Sci. 1982;27(5):1479–86. 10.1002/app.1982.070270507.Search in Google Scholar
(29) Fillon B, Thierry A, Lotz B, wittmann JC. Efficiency scale for polymer mucleating agents. J Therm Anal. 1994;42:721–31. 10.1007/BF02546745.Search in Google Scholar
(30) Fillon B, wittmann JC, Lotz B, Thierry A. Self-nucleation and recrystallization of isotactic polypropylene (α phase) investigated by differential scanning calorimetry. J Polym Sci Part B: Polym Phys. 1993;31(10):1383–93. 10.1002/polb.1993.090311013.Search in Google Scholar
(31) Cheng SZD, Lotz B. Enthalpic and entropic origins of nucleation barriers during polymer crystallization: the Hoffman–lauritzen theory and beyond. Polymer. 2005;46(20):8662–81. 10.1016/j.polymer.2005.03.125.Search in Google Scholar
(32) Van Berkel JG, Guigo N, Kolstad JJ, Sipos LK, Wang B, Dam MA, et al. Isothermal crystallization kinetics of poly (Ethylene 2,5-Furandicarboxylate). Macromol Mater Eng. 2015;300(4):466–74. 10.1002/mame.201400376.Search in Google Scholar
(33) Mai KC, Wang KF, Han ZW, Zeng HM. Study on the thermal stability of heterogeneous nucleation effect of polypropylene nucleated by different nucleating agents. J Appl Polym Sci. 2001;83:1643–50. 10.1002/app.10071.Search in Google Scholar
(34) Li J, Long LJ, He WT, Zhang K, Xiang YS, Zhang J, et al. Crystallization behavior and mechanical properties of nanosilica-reinforced isotactic polypropylene composites. Int Polym Process. 2015;30:542–7. 10.3139/217.3065.Search in Google Scholar
(35) Chi SX, Le DJ, Xu XB, Long LL. Modification of polypropylene with organophosphate nucleating agent. China Synth Resin Plast. 2006;23(5):14–6.Search in Google Scholar
© 2022 Juan Li et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

