Abstract
A series of hyper-crosslinked polymers (HCPs) with connected hierarchical porous structures were synthesized from phenyl-based precursors of benzene (BEN), benzyl alcohol, aniline, biphenyl, and 1,3,5-triphenylbenzene (TPB) via the knitting method. The porous structures of the HCPs were greatly influenced by substituent groups and BEN ring number in the precursors. HCPs prepared from TPB had the largest surface area and pore volume with multiscale porosity. The porous structure of the HCPs could also be adjusted by the crosslinker amount. Insufficient crosslinking led to incomplete pore architecture, while excessive crosslinking resulted in a considerable decrease in the pore volume. With these HCPs as adsorbents, the BEN yield in the cigarette smoke could be largely reduced due to the connected multiscale porosity and π–π aromatic stacking interaction that facilitated the smoke aerosol passing and the small aromatic molecules absorbing, showing great potential of these HCPs as adsorbents for effective removal of BEN from cigarette smoke.
1 Introduction
Cigarette smoke is a complex aerosol composed of more than 5,000 chemicals generated in a burning cigarette, containing approximately 150 toxicants such as ammonia, hydrogen cyanide (HCN), benzene (BEN), phenol, and low-weight aldehydes (1,2,3). Prolonged exposure to cigarette smoke may result in tremendous harm to the respiratory system and nervous system, leading to lung cancer, chronic obstructive pulmonary disease, cardiovascular disease, leukemia, and many other serious diseases (4,5). BEN, one of the group 1 carcinogens classified by the International Agency for Research on Cancer, may increase the risk of leukemia, bladder cancer, congenital disabilities, neurocognitive impairment, and damage to liver tissue under long-term exposure (6,7). Considering the severe damage and high smoke yield (∼50 μg·cig−1) of BEN in cigarette smoke, BEN has been assigned as one of the 18 priority toxicants in cigarette smoke for reporting and regulation by the World Health Organization (WHO) (8,9,10). Therefore, the effective removal of the toxicant yield in cigarette smoke, such as the BEN yield in smoke, is of great significance for both human health and environmental protection.
Many attempts have been carried out to reduce the harmful component yield in cigarette smoke, amongst which the most used technique is adsorption of the toxic chemicals by various adsorbents. The adsorption is challenging in cigarette smoke due to that the adsorbents need to operate under a very high flow rate (17.5 mL·s−1 for a typical ISO machine-smoke regime) at the gas-solid interface in the presence of thousands of other chemicals that cover a range of chemical groups and functionalities. For example, the most used adsorbent in cigarette filters is active carbon, which could reduce a relatively broad range of volatile smoke components. However, the adsorption of BEN in cigarette smoke by active carbon showed a much less effective performance in the extremely complex aerosol ambient (11). Porous carboxymethyl cellulose/cellulose composite microspheres loaded with cupric ions were used to minimize the HCN yield in cigarette smoke, reducing 23–50% HCN due to the high complexing ability of cupric ions for HCN and the connected porous structure (12). Yang et al. prepared poly (high internal phase emulsion) monoliths of glycidyl methacrylate, which could reduce the release of phenol by up to 56% through the potential reaction between the epoxy groups and phenol (13). The carboxymethyl starch-grafted-polyacrylic acid copolymers showed high adsorption of ammonia and phenol, and the ionic interactions and hydrogen bonding interactions of carboxylate with ammonia and phenol were considered as the main reason (14).
BEN has a strong π-conjugation effect, hydrophobicity, and small dynamic size, which requires the absorbents to have corresponding characteristics such as sizeable π-conjugated structure, hydrophobic interaction, and micropore size distribution (15). Many porous materials with sizeable π-conjugated structures have been investigated for the removal of BEN in water and the atmospheric environment. For instance, porous carbons derived from the metal organic framework and microporous polymers with extended π-structures showed remarkable adsorption performance for BEN vapor (16,17). However, the extremely complex aerosol ambient of cigarette smoke makes the adsorption for BEN in cigarette smoke much more challenging (18).
Hyper-crosslinked polymers (HCPs) are a series of microporous polymer materials with remarkable advantages such as extremely high surface areas and porosity, easy functionalization, low-cost reagents, mild operating conditions, and outstanding adsorption properties (15,19,20,21). Due to these advantages, HCPs have received increasing interest in recent years. Many HCPs with diverse chemical functionalities and controlled porous structures were prepared from an extensive range of organic precursors via facile template-free chemical processes and used to adsorb volatile organic compounds (VOCs) in the atmosphere and water (4,22,23,24). For instance, a HCP with a specific surface area of 1,345 m2·g−1 and micro/mesopores showed excellent adsorption performance and high selectivity for BEN vapor in humidity gas streams (25). The tetraphenyl methane-based HCPs showed a much higher adsorption capacity (about eight times) for VOCs than the commercial HSZ-Y zeolite (26). The π-conjugation effect and porous structure were the critical factors for improving the adsorption capacity of these materials for VOCs. However, to our best knowledge, study on the impact of the porous structure of HCPs on the adsorption of BEN in cigarette smoke has not been conducted.
In this work, a series of HCPs with controlled multiscale porosity were synthesized by Friedel–Crafts alkylation reaction of phenyl-based precursors and the external crosslinker of formaldehyde dimethyl acetal (FDA) via the knitting method. The effect of the chemical structure of precursors and the amount of the external crosslinkers on the porous structures of the obtained HCPs were investigated. Finally, the reduction of BEN yield in cigarette smoke using these HCPs as adsorbents was investigated.
2 Materials and methods
2.1 Materials
BEN, biphenyl (BPH), 1,3,5-triphenylbenzene (TPB), and aniline (ANI) were purchased from Shanghai Macklin Reagent Co., Ltd. Benzyl alcohol (BA), FDA, anhydrous ferric chloride (FeCl3), and active carbon were obtained from Aladdin Chemical Reagent Co., Ltd. Methanol, 1,2-dichloroethane (DCE), and isopropanol were of HPLC grade and purchased from Sigma-Aldrich. All chemicals were of analytical grade and used as received without further purification unless otherwise specified.
2.2 Synthesis of HCPs
HCPs were prepared by the Friedel–Crafts alkylation reaction of phenyl-based precursors and the external crosslinker of FDA under the catalysis of FeCl3 (27). BEN, BA, ANI, BPH, and TPB were used as precursors, and the obtained HCPs were denoted as HCP-BEN, HCP-BA, HCP-ANI, HCP-BPH, and HCP-TPB, respectively. Typically, the precursor of TPB (0.01 mol, 3.06 g) and the crosslinker of FDA (0.09 mol, 6.84 g) were dissolved in DCE (40 mL) in a 100 mL flask. After stirring for 10 min, the catalyst of FeCl3 (0.09 mol, 14.60 g) was charged slowly to the mixture. The reaction was performed at 45°C for 3 h and then 80°C for 10 h before being quenched by methanol. The solid product obtained by filtration was washed with methanol and deionized water until the filtrate was colorless, subsequently washed thoroughly by Soxhlet extraction using methanol as a solvent for 48 h to eradicate FeCl3 before being dried under reduced pressure at 60°C.
2.3 Characterization of HCPs
The Fourier transform infrared spectra (FT-IR) were measured on a Bruker Tensor 27 Spectrometer using the KBr method in the range of 500–4,000 cm−1. The solid-state 13C cross-polarization magic angle spinning (CP/MAS) NMR spectrum was conducted using a Bruker AVANCE III 400 WB equipped with a 4 mm double-resonance MAS probe and a spinning frequency of 8 kHz. ZEISS Gemini 300 field emission electron scanning microscope (SEM) and FEI TF20 transmission electron microscope (TEM) were used to observe the microscopic morphology of HCPs. N2 adsorption/desorption analysis was measured to be 77.3 K using a Micrometrics Tristar II 3,020 surface area and porosity analyzer. Before the analysis, the samples were outgassed at 100°C for 8 h under vacuum. The specific surface area was determined using the standard Brunauer–Emmet–Teller (BET) method. The total pore volume was evaluated from the amount of nitrogen adsorbed at P/P 0 = 0.99, the micropore volume was derived from the t-plot method, and the pore size distribution was obtained from nonlocal density functional theory (NLDFT) model.
2.4 Removal of BEN from cigarette smoke
The removal of BEN from cigarette smoke using these HCPs was evaluated on a homemade device linked to the smoking machine (Figure 1). The added amounts of HCPs and/or active carbon are 15 mg for a cigarette. The cigarettes used in the test were made up of a 59 mm length tobacco rod containing Virginia-style tobacco and a 25 mm length cellulose acetate filter. After being equilibrated for 48 h under 22°C and a relative humidity of 60%, the mainstream smoke yield was measured using the standard ISO smoking regime with a puff volume of 35 mL, puff duration of 2 s, and puff interval of 60 s on a Filtrona SM-450 linear smoking machine. BEN in the gas phase of the cigarette smoke was collected by passing the whole mainstream smoke stream through an impinger of methanol in a cold trap. The concentration of captured BEN was measured by Agilent 5977A GC-MS.

Device to simulate the addition of absorbents to the cigarette filter; 1 – cigarette, 2 – homemade device, 3 – cigarette holder, 4 – 120 mesh sieves, 5 – samples, 6 – Cambridge filter pad, 7 – smoking machine, 8 – absorption flask with 15 mL of methanol, 9 – cold trap containing isopropanol and drikold.
3 Results and discussion
3.1 Preparation and characterization of HCPs
HCPs were prepared by the Friedel–Crafts alkylation reaction of phenyl-based precursors and the external crosslinker of FDA under the catalysis of FeCl3 (Scheme 1). First, carbo-cationic species were formed in FDA by FeCl3 catalyst, phenyl rings in the precursors attacked the generated carbo-cationic species to form intermediates. The highly reactive intermediate groups were further converted to methylene linkages by reacting with other BEN-contained precursor molecules and resulted in rigid HCP networks (28). BEN, BA, ANI, BPH, and TPB were used as precursors. The resultant HCPs were light brown or dark brown solids. They were insoluble in conventional organic solvents such as methanol, acetone, and dichloromethane, indicating that the hyper-crosslinked structure was formed (29).

Synthetic pathway of HCPs.
Figure 2a shows the FT-IR spectra of HCP-TPB, the precursor of TPB, and the crosslinker of FDA. The bands at 1,600, 1,500, and 1,450 cm−1 were attributed to the –C═C– vibration of the aromatic ring, and the vibration peak at 3,025 cm−1 was identified as the C–H stretching vibration of the aromatic rings in the precursor of TPB (30). The characteristic adsorption of C–H stretching vibration bands of –CH2– at 2,925 cm−1 was evidently observed after the hyper-crosslinked procedure in the spectrum of HCP-TPB, indicating the presence of hyper-crosslinked structure in HCP-TPB (31). The occurrence of the hyper-crosslinked process was further proved by the 13C CP/MAS NMR spectrum of HCP-TPB in Figure 2b. The resonance peaks near 133 and 124 ppm were assigned to the substituted aromatic carbons and the unsubstituted aromatic carbons. The carbon signal near 32 ppm was generated by a methylene linker derived from the FDA via Friedel–Crafts reaction (32).

(a) FT-IR spectra of TPB, FDA, and HCP-TPB; (b) 13C CP/MAS NMR spectrum of HCP-TPB.
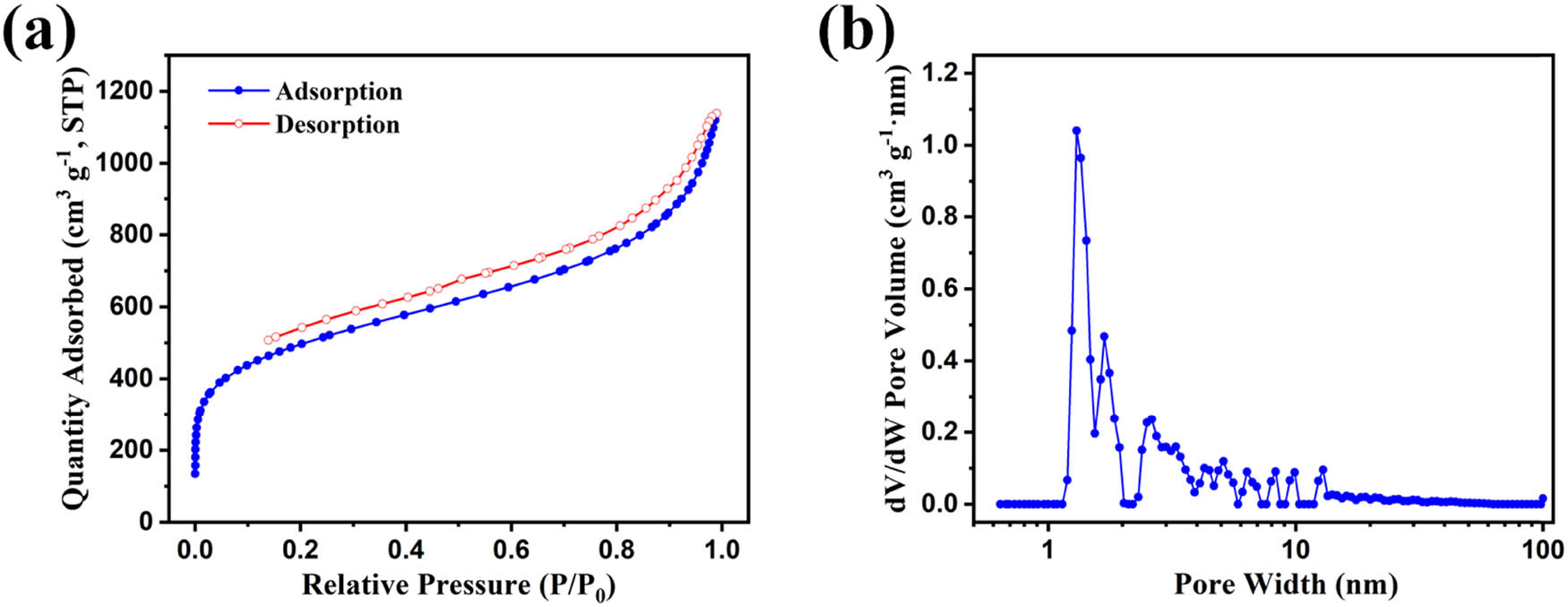
(a) Nitrogen adsorption/desorption isotherms and (b) pore size distributions of HCP-TPB simulated by nonlocal density functional theory model.
Figure 3a shows the nitrogen adsorption/desorption isotherms of a typical HCP of HCP-TPB. Nitrogen uptake at low relative pressure (P/P 0 = 0–0.001) in the adsorption isotherms indicated the presence of abundant micropores in the resultant HCPs, while the sharp upward trend at medium and high-pressure regions (P/P 0 = 0.8–1.0) reflected the presence of mesopores and macropores. In the desorption curve, an H3-type hysteresis loop was observed, which was regarded as the presence of slit-shaped mesopores. The pore size distributions of HCP-TPB, simulated by the NLDFT model, showed a hierarchical pore size distribution of abundant micropores and mesopores (Figure 3b), which would facilitate the passing of smoke aerosol and the absorbing of small aromatic molecules. The multiscale porosity of the obtained HCPs resulted from the highly solvated three-dimensional network that formed during the Friedel–Crafts alkylation reaction of phenyl-based precursors and the external crosslinkers (19). The diffused solvent molecules were filled in the voids of the network with a low-density. After the solvent was removed, the rigid phenyl-based precursor structure and crosslink bridge locked the polymer chains to ensure that the network would not collapse, resulting in the multi-pore structure of the HCPs.
Figure 4 shows the SEM and TEM images of HCP-TPB. HCP-TPB had a macropore-mesopore-micropore hierarchical porous structure, which was accumulated of irregular spherules with a rough surface. The rough surface of the irregular spherules could be the source of the micro- and mesoporous structures (29), and the pores formed by the accumulation of irregular spherules might be the source of macropores in the hyper-crosslinked structures. TEM images in Figure 4b also proved that the HCPs were composed of irregular nanoparticle spheres and nanoscale pores.

(a) SEM and (b) TEM images of HCP-TPB.
3.2 Effect of precursors on the HCP structures
BEN, BA, ANI, BPH, and TPB were used as precursors to obtain HCPs. The effect of substituent groups and the number of BEN rings in the precursors on the obtained HCP structures was investigated. The molar amount of the crosslinker used were 3, 2.5, 2.5, 5, and 9 times of the precursors for BEN, BA, ANI, BPH, and TPB, respectively, leading to a consistent molar ratio of the reactive sites in the crosslinkers to the precursor (Table 1).
Preparation parameters and characterization of the HCPs
| Sample | Precursor | C/P 1 | BET Surface area (m2·g−1) | Pore volume (cm3·g−1) | |
|---|---|---|---|---|---|
| Total pore volume | Micropore volume | ||||
| HCP-BEN | BEN | 3.0/1 | 1,290 | 1.93 | 0.30 |
| HCP-BA | BA | 2.5/1 | 781 | 0.59 | 0.18 |
| HCP-ANI | ANI | 2.5/1 | 7 | — | — |
| HCP-BPH | BPH | 5.0/1 | 727 | 0.43 | 0.28 |
| HCP-TPB | TPB | 9.0/1 | 1,798 | 1.76 | 0.61 |
| HCP-TPB-1 | TPB | 4.5/1 | 1,446 | 2.20 | 0.36 |
| HCP-TPB-2 | TPB | 9.0/1 | 1,798 | 1.76 | 0.61 |
| HCP-TPB-3 | TPB | 13.5/1 | 1,289 | 0.83 | 0.57 |
| HCP-TPB-4 | TPB | 18.0/1 | 1,058 | 0.71 | 0.44 |
- 1
C/P, the molar ratio of crosslinker to precursor.
Figure 5 displays the nitrogen adsorption/desorption isotherms and the pore size distributions of the HCPs prepared from different precursors. Sharp upward trends in both the low relative pressure and the high relative pressure were observed in the absorption curve of BEN-HCP, indicating the presence of abundant micropores and mesopores/macropores. Wide pore distribution in the range of micropores, mesopores, and macropores was observed for HCP-BEN, facilitating the passing of smoke aerosol and the absorbing of small aromatic molecules. The upward trend in low relative pressure in the absorption curve of HCP-BA was not as sharp as that of HCP-BEN, indicating the micropores’ volume was lower than that of HCP-BEN. The conspicuous H4-type hysteresis ring in the desorption curve of HCP-BA implied the presence of a large amount of mesopores. However, an almost non-porous structure-based nitrogen adsorption isotherm was observed for HCP-ANI. The BET surface areas and pore volumes calculated from the nitrogen adsorption isotherms are summarized in Table 1. For the comparison of the HCPs prepared from BEN, BA, and ANI with different functionality, the HCP-BEN had the highest BET surface area of 1,290 m2·g−1 and pore volume of 1.93 cm3·g−1, and slightly lower BET surface areas and pore volumes were observed for HCP-BA, and an almost non-porous structure for HCP-ANI. The significant difference in porous structure might be due to the different reaction activities caused by the functional groups (27,33,34). As for the HCPs prepared from the precursors with increasing BEN ring number, the HCP-TPB showed a similar nitrogen adsorption/desorption isotherm as compared to HCP-BEN, a sharper upward trend was observed in the low relative pressure range (Figure 5c), which indicated that there were more micropores in HCP-TPB as compared to HCP-BEN, resulting in a higher BET surface area of 1,798 m2·g−1 (Table 1). However, the HCP-BPH has much lower upward trends in the low relative pressure range, and the upward trend in the high relative pressure range almost disappeared, indicating a much lower pore volume and BET surface areas. These porous structure differences were probably due to the steric hindrance effect produced by short links between rings (27).

(a and c) Nitrogen adsorption/desorption isotherms and (b and d) pore size distributions of HCPs prepared from verified precursors.
HCP prepared from different precursors had a pretty different micromorphology (Figure 6). HCP-BEN, HCP-BA, and HCP-TPB had porous structures accumulated of irregular spherules with a rough surface. Stacked small irregular spherical particles of sizes of 50–150 nm were observed in the SEM images of HCP-BEN and HCP-BA due to their small structural units, and a larger particle size of 500–1,000 nm was observed for HCP-TPB, which could be due to the large structural unit of TPB as compared to BEN. HCP-BPH had a tightly crosslinked structure with a flower-like form with large inter-wrinkled distances, which is similar to the report of Varyambath (28). The micromorphology of HCP-BPH is quite different from that of HCP-BEN and HCP-TPB, consistent with their difference in nitrogen adsorption/desorption results. Unlike the porous structures of the above HCPs, HCP-ANI had a relatively smooth morphology, consistent with the non-porous structure in nitrogen adsorption/desorption characterization.

SEM images of HCPs prepared from verified precursors: (a) HCP-BEN, (b) HCP-BA, (c) HCP-ANI, (d) HCP-BPH, and (e) HCP-TPB.
3.3 Effect of the amount of the crosslinker on the HCP structures
The amount of crosslinker exerted an important effect on the HCPs’ porous structures. As shown in Figure 7a, all HCP prepared from TPB had sharp upward trend in the lower pressure range in the nitrogen adsorption isotherm, indicating that they all had abundant micropores and high BET surface areas (Table 1). The upward trend in the low pressure range increased when the molar ratio of crosslinker to precursor (C/P) increased from 4.5/1 to 9/1 and then decreased with a higher crosslinker amount (e.g., C/P = 13.5/1 or 18/1). The HCP-TPB with a crosslinker amount of C/P = 9/1 has the largest BET surface area (1,798 m2·g−1) and micropore volume (0.61 cm3·g−1). The upward trend in the high pressure range was obviously observed for HCP-TPB with lower crosslinker amount (e.g., 4.5/1 or 9.0/1), but became flat while the crosslinker amount increased to 13.5/1 or 18.0/1, indicating that HCP-TPB had abundant meso/macropores when the crosslinker amount is 4.5/1 and 9.0/1, but much less meso/macropores in the HCP-TPB with more enormous crosslinker amount, which meant that the total pore volume decreased vastly, while the crosslinker amount increased (Table 1). The pore size distributions of HCPs in Figure 7b also showed that HCPs prepared from TPB had a high amount of micropores, but the HCP with a higher crosslinker amount had relatively fewer mesopores. The change in pore distribution resulted from the difference in the degree of crosslinking. When the crosslinker amount was 4.5/1, insufficient crosslinking reaction could lead to low density and incomplete pore architectures, resulting in a high amount of meso/macropores but a relatively lower amount of micropores. The crosslinking degrees would increase when increasing the crosslinker amount to 9.0/1, resulting in more complete pore architectures and a high amount of micropores and meso/macropores, and a higher BET surface area. However, with a larger crosslinker amount (e.g., 13.5/1 or 18.0/1), the crosslinked reaction was rapid and violent, resulting in excessive crosslinking in the HCPs. The crosslinked process was limited to a concentrated area, and the extension of chains was restricted. As a result, the meso/macropore volume and the total pore volume decreased largely in HCP-TPB with the largest crosslinker amount of 18.0/1.

(a) Nitrogen adsorption/desorption isotherms and (b) pore size distributions of HCPs prepared with the increasing amount of crosslinker.
Figure 8 displayed the SEM images of HCP-TPB prepared with increasing amount of crosslinker. They all showed porous structures accumulated of irregular spherules with a rough surface. With the crosslinker amount increased, the volume of spherules increased, and the pellets became regular and dense, which could result in a decrease in meso/macropores. These morphologies change following the results of nitrogen adsorption.

SEM images of HCPs prepared with the increasing amount of crosslinker: (a) HCP-TPB-1, (b) HCP-TPB-2, (c) HCP-TPB-3, and (d) HCP-TPB-4.
3.4 Evaluation of the BEN removal efficiency of HCPs in cigarette smoke
BEN, one of the 18 priority toxicants in cigarette smoke proposed by WHO, causes enormous damage to humans. In order to evaluate the removal efficiency of HCPs for BEN in cigarette smoke, cigarette with 15 mg·cig−1 of HCPs and with an empty cavity as blank control were smoked under the ISO smoking regime. For comparison, the most common used filter adsorbent of active carbon was also evaluated for the removal of BEN in cigarette smoke. The BET surface area of the tested active carbon was ca. 900 m2·g−1. The smoke yields of BEN were analyzed, and the removal efficiency was calculated as compared to the blank control, which was summarized in Table 2.
Benzene removal efficiency of HCPs in cigarette smoke
| Sample | Benzene yield (μg·cig−1) | Benzene removal efficiency (%) |
|---|---|---|
| Blank control | 49.5 | / |
| Active carbon | 46.6 | 5.9 |
| HCP-BEN | 30.1 | 39.2 |
| HCP-BA | 39.3 | 20.7 |
| HCP-ANI | 47.1 | 5.0 |
| HCP-BPH | 39.2 | 21.1 |
| HCP-TPB | 27.6 | 44.2 |
| HCP-TPB-1 | 35.8 | 27.8 |
| HCP-TPB-2 | 27.6 | 44.2 |
| HCP-TPB-3 | 31.1 | 37.3 |
| HCP-TPB-4 | 39.6 | 20.0 |
The yield of BEN in the mainstream cigarette smoke was 49.5 μg·cig−1. However, the BEN yield for cigarettes with HCPs (except HCP-ANI) as filter additives was 39.3–27.7 μg·cig−1, which was much lower than that of the blank control. The BEN removal efficiency of HCPs was 20.7–44.2%. However, the active carbon could only reduce 5.9% of BEN at the same dosage, showing a much lower removal efficiency than the HCPs in this study (Table 2). The high BEN reduction rate of HCPs might be related to both the porous structure and the π–π aromatic stacking interaction between the HCPs and the BEN in the cigarette smoke.
The precursors greatly influenced the BEN removal efficiency of HCPs. HCP-TPB and HCP-BEN had the highest removal efficiency for BEN in cigarette smoke. They could remove about 40% BEN from the cigarette smoke due to the π–π aromatic stacking interaction between the aromatic ring in the HCPs and the BEN in the cigarette smoke. However, the removal efficiency decreased largely with the usage of the precursors of BA and BPH instead of BEN or TPB, and even lost the BEN removal ability using the ANI precursor. Both the HCP-TPB and HCP-BEN had the largest BET surface areas and pore volumes, and they had a wide pore size distribution in the range of micropores, mesopores, and macropores, which would facilitate the passing of smoke aerosol and the absorbing of small aromatic molecules. HCP-BA and HCP-BPH had medium-level BET surface areas and pore volumes, and thus the BEN removal ability is lower than that of HCP-TPB and HCP-BEN. Besides the porous structure difference between the HCPs, the π–π aromatic stacking interaction could be weakened, while using the precursors of BPH and BA instead of BEN and TPH.
The crosslinker amount could also significantly affect the porous structure of HCPs and thus affect the reduction in BEN in cigarette smoke. An insufficient crosslinking (e.g., crosslinker amount 4.5/1) led to incomplete pore architectures with lower micropore volume, while excessive crosslinking (e.g., crosslinker amounts 13.5/1 and 18.0/1) could greatly decrease the meso/macropore volume, resulting in a relatively lower BEN removal efficiency as compared to the appropriate crosslinking with the crosslinker amount of 9.0/1. In conclusion, both the π–π aromatic stacking interaction and the porous structure played critical roles in removing BEN from the cigarette smoke. The π–π aromatic stacking interaction and micropores were critical to BEN adsorption, and the mesopores/macropores were favorable to the passage of smoke aerosol particles.
4 Conclusion
A series of HCPs with controlled multiscale porosity were synthesized by the reaction of phenyl-based precursors and the external crosslinker of FDA via knitting method. BEN, BA, ANI, BPH, and TPB were used as the precursors to obtain HCPs with a controlled porous structure. The multiscale porosity of HCPs could be well adjusted by using varied precursors with different substituent groups and BEN ring number. HCPs prepared for the precursor of TPB had the largest BET surface areas and pore volumes with a multiscale pore size distribution. The porous structure of the HCPs could also be adjusted by varying the amount of external crosslinker. Insufficient crosslinking reaction could lead to low density and incomplete pore architectures, but excessive crosslinking in the HCPs resulted in a large decrease in the total pore volume. Finally, using these HCPs as adsorbents, the BEN yield in the cigarette smoke could be largely reduced up to 20.7–44.2% due to the π–π aromatic stacking interaction and the connected hierarchical porous structure, showing the great potential of HCPs in effective removal of BEN from cigarette smoke.
-
Funding information: This research was funded by the Science and Technology Project of CNTC (Grant No. 110202002002) and Natural Science Foundation of Henan Province (Grant No. 212300410314).
-
Author contributions: Xiaochen Xia: investigation, writing – original draft, and visualization; Peijian Sun: conceptualization, funding acquisition, writing – review and editing, and project administration; Xuehui Sun: investigation, resources, and writing – original draft; Yipeng Wang: investigation and data curation; Song Yang: investigation and writing – review and editing; Yunzhen Jia: investigation and data curation; Bin Peng: investigation; Cong Nie: conceptualization, funding acquisition, writing – review and editing, and supervision.
-
Conflict of interest: Authors state no conflict of interest.
References
(1) Hoffmann D, Hoffmann I, El-Bayoumy K. The less harmful cigarette: a controversial issue. A tribute to Ernst L. Wynder. Chem Res Toxicol. 2001;14(7):767–90. 10.1021/tx000260u.Search in Google Scholar PubMed
(2) Fowles J. Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke. Tob Control. 2003;12(4):424–30. 10.1136/tc.12.4.424.Search in Google Scholar PubMed PubMed Central
(3) Xie J, Marano KM, Wilson CL, Liu H, Gan H, Xie F, et al. A probabilistic risk assessment approach used to prioritize chemical constituents in mainstream smoke of cigarettes sold in China. Regul Toxicol Pharmacol. 2012;62(2):355–62. 10.1016/j.yrtph.2011.10.017.Search in Google Scholar PubMed
(4) Subri NNS, Jamil SNAM, Cormack PA, Abdullah LC, Kamaruzaman S, Adeyi AA. The synthesis and characterisation of porous and monodisperse, chemically modified hypercrosslinked poly(acrylonitrile)-based terpolymer as a sorbent for the adsorption of acidic pharmaceuticals. E-Polymers. 2020;20(1):328–45. 10.1515/epoly-2020-0037.Search in Google Scholar
(5) Shukla S, Ward C, Walters EH. Mechanistic insights on EMT and smoking-related COPD. Stem Cell Rev Rep. 2021;17(4):1503–4. 10.1007/s12015-021-10152-8.Search in Google Scholar PubMed
(6) Rafiee A, Delgado-Saborit JM, Sly PD, Amiri H, Hoseini M. Lifestyle and occupational factors affecting exposure to BTEX in municipal solid waste composting facility workers. Sci Total Env. 2019;656(15):540–6. 10.1016/j.scitotenv.2018.11.398.Search in Google Scholar PubMed
(7) Blount B, Kobelski R, Mcelprang D, Ashley D, Morrow J, Chambers D, et al. Quantification of 31 volatile organic compounds in whole blood using solid-phase microextraction and gas chromatography-mass spectrometry. J Chromatogr B. 2006;832(2):292–301. 10.1016/j.jchromb.2006.01.019.Search in Google Scholar PubMed
(8) Chambers DM, Ocariz JM, McGuirk MF, Blount BC. Impact of cigarette smoking on volatile organic compound (VOC) blood levels in the U.S. population: NHANES 2003–2004. Env Int. 2011;37(8):1321–8. 10.1016/j.envint.2011.05.016.Search in Google Scholar PubMed
(9) Uchiyama S, Hayashida H, Izu R, Inaba Y, Nakagome H, Kunugita N. Determination of nicotine, tar, volatile organic compounds and carbonyls in mainstream cigarette smoke using a glass filter and a sorbent cartridge followed by the two-phase/one-pot elution method with carbon disulfide and methanol. J Chromatogr A. 2015;1426(24):48–55. 10.1016/j.chroma.2015.11.058.Search in Google Scholar PubMed
(10) WHO. The scientific basis of tobacco product regulation: second report of a WHO Study Group. Geneva: WHO; 2008.Search in Google Scholar
(11) Ji H, Zhang L, Liu J, Wangle F, Liu N, Man J. Filtration efficiency of cigarette filter to benzene and its homologues in mainstream cigarette smoke (in Chinese). Tob Sci Technol. 2016;49(05):45–53. 10.16135/j.issn1002–0861.20160000.Search in Google Scholar
(12) Sun P, Yang S, Sun X, Wang Y, Pan L, Wang H, et al. Functional porous carboxymethyl cellulose/cellulose acetate composite microspheres: preparation, characterization, and application in the effective removal of HCN from cigarette smoke. Polymers. 2019;11(1):181. 10.3390/polym11010181.Search in Google Scholar PubMed PubMed Central
(13) Yang S, Wang Y, Jia Y, Sun X, Sun P, Qin Y, et al. Tailoring the morphology and epoxy group content of glycidyl methacrylate-based polyHIPE monoliths via radiation-induced polymerization at room temperature. Colloid Polym Sci. 2018;296(6):1005–16. 10.1007/s00396-018-4307-x.Search in Google Scholar
(14) Haroon M, Yu H, Wang L, Ullah RS, Haq F, Teng L. Synthesis and characterization of carboxymethyl starch-g-polyacrylic acids and their properties as adsorbents for ammonia and phenol. Int J Biol Macromol. 2019;138(1):349–58. 10.1016/j.ijbiomac.2019.07.046.Search in Google Scholar PubMed
(15) Li X, Zhang L, Yang Z, Wang P, Yan Y, Ran J. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: a review. Sep Purif Technol. 2020;235(18):116213. 10.1016/j.seppur.2019.116213.Search in Google Scholar
(16) Wang C, Yin H, Tian P, Sun X, Pan X, Chen K, et al. Remarkable adsorption performance of MOF-199 derived porous carbons for benzene vapor. Env Res. 2020;184:109323. 10.1016/j.envres.2020.109323.Search in Google Scholar PubMed
(17) Yang SJ, Ding X, Han BH. Conjugated microporous polymers with extended π-structures for organic vapor adsorption. Macromolecules. 2018;51(3):947–53. 10.1021/acs.macromol.7b02515.Search in Google Scholar
(18) Goel R, Bitzer ZT, Reilly SM, Bhangu G, Trushin N, Elias RJ, et al. Effect of charcoal in cigarette filters on free radicals in mainstream smoke. Chem Res Toxicol. 2018;31(8):745–51. 10.1021/acs.chemrestox.8b00092.Search in Google Scholar PubMed PubMed Central
(19) Huang J, Turner SR. Hypercrosslinked polymers: a review. Polym Rev. 2018;58(1):1–41. 10.1080/15583724.2017.1344703.Search in Google Scholar
(20) Tan L, Tan B. Hypercrosslinked porous polymer materials: design, synthesis, and applications. Chem Soc Rev. 2017;46(11):3322–56. 10.1039/C6CS00851H.Search in Google Scholar PubMed
(21) Šálek P, Horák D. Hypercrosslinked polystyrene microspheres by suspension and dispersion polymerization. E-Polymers. 2011;11(1):1–12. 10.1515/epoly.2011.11.1.688.Search in Google Scholar
(22) Wang WQ, Wang J, Chen JG, Fan XS, Liu ZT, Liu ZW, et al. Synthesis of novel hyper-cross-linked polymers as adsorbent for removing organic pollutants from humid streams. Chem Eng J. 2015;281(1):34–41. 10.1016/j.cej.2015.06.095.Search in Google Scholar
(23) Cai Y, Wen X, Wang Y, Song H, Li Z, Cui Y, et al. Preparation of hyper-crosslinked polymers with hierarchical porous structure from hyperbranched polymers for adsorption of naphthalene and 1-naphthylamine. Sep Purif Technol. 2021;266(1):118542. 10.1016/j.seppur.2021.118542.Search in Google Scholar
(24) Zhou L, Chai K, Yao X, Ji H. Enhanced recovery of acetophenone and 1-phenylethanol from petrochemical effluent by highly porous starch-based hypercrosslinked polymers. Chem Eng J. 2021;418(15):129351. 10.1016/j.cej.2021.129351.Search in Google Scholar
(25) Wang J, Wang WQ, Hao Z, Wang G, Li Y, Chen JG, et al. A superhydrophobic hyper-cross-linked polymer synthesized at room temperature used as an efficient adsorbent for volatile organic compounds. RSC Adv. 2016;6(99):97048–54. 10.1039/C6RA18687D.Search in Google Scholar
(26) Paul G, Begni F, Melicchio A, Golemme G, Bisio C, Marchi D, et al. Hyper-cross-linked polymers for the capture of aromatic volatile compounds. ACS Appl Polym Mater. 2020;2(2):647–58. 10.1021/acsapm.9b01000.Search in Google Scholar
(27) Li B, Gong R, Wang W, Huang X, Zhang W, Li H, et al. A new strategy to microporous polymers: knitting rigid aromatic building blocks by external cross-linker. Macromolecules. 2011;44(8):2410–4. 10.1021/ma200630s.Search in Google Scholar
(28) Varyambath A, Song WL, Singh S, Kim JS, Kim I. Tunable construction of biphenyl-based porous polymeric nanostructures and their synergistically enhanced performance in pollutant adsorption and energy storage. Micropor Mesopor Mater. 2021;312:110800. 10.1016/j.micromeso.2020.110800.Search in Google Scholar
(29) Liu Y, Chen X, Jia X, Fan X, Zhang B, Zhang A, et al. Hydroxyl-based hyper-cross-linked microporous polymers and their excellent performance for CO2 capture. Ind Eng Chem Res. 2018;57(50):17259–65. 10.1021/acs.iecr.8b05004.Search in Google Scholar
(30) Yang Y, Tan B, Wood CD. Solution-processable hypercrosslinked polymers by low cost strategies: a promising platform for gas storage and separation. J Mater Chem A. 2016;4(39):15072–80. 10.1039/C6TA05226F.Search in Google Scholar
(31) Cerón MR, Izquierdo M, Alegret N, Valdez JA, Rodríguez-Fortea A, Olmstead MM, et al. Novel fullerene-based porous materials constructed by solvent kniƫng strategy. Chem Commun. 2016;52(1):64–7. 10.1039/C5CC07416A.Search in Google Scholar
(32) Ma H, Zhang QM, Cheng G, Wang Z, Zong QS, Tan B, et al. Heteroatom engineering of hyper-cross-linked polymers for iodine capture. ACS Appl Polym Mater. 2021;3(1):209–15. 10.1021/acsapm.0c01047.Search in Google Scholar
(33) Dawson R, Ratvijitvech T, Corker M, Laybourn A, Khimyak YZ, Cooper AI, et al. Microporous copolymers for increased gas selectivity. Polym Chem. 2012;3(8):2034–8. 10.1039/c2py20136d.Search in Google Scholar
(34) Luo Y, Zhang S, Ma Y, Wang W, Tan B. Microporous organic polymers synthesized by self-condensation of aromatic hydroxymethyl monomers. Polym Chem. 2013;4(4):1126–31. 10.1039/C2PY20914D.Search in Google Scholar
© 2022 Xiaochen Xia et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

