Abstract
The substitutions of the chlorine atoms in p-dichlorobenzene (DCB) or the chlorine end-groups of polyphenylene sulfide (PPS) by sodium N-methyl-4-aminobutyrate (SMAB) are important reactions in the polymerization of PPS. The reaction process of DCB with SMAB was studied under conditions similar to the polymerization of PPS. The molecular structures of the products were analyzed through high-performance liquid chromatography, liquid chromatography-mass spectroscopy, gas chromatography-mass spectrometry, and other methods. Several pairs of para- and meta-isomer products were found, which indicates the coexistence of both nucleophilic addition–elimination (SN2Ar) mechanism and aryne intermediate mechanism. Based on mechanism analysis, the networks of reactions of DCB with SMAB were proposed and the effects of the ratio of SMAB to DCB, water content, and temperature on the reaction process were studied.
1 Introduction
Polyphenylene sulfide (PPS) is widely used because of its excellent heat tolerance and chemical corrosion resistance (1,2,3). PPS is usually prepared with the sodium sulfide method, in which sodium sulfide and p-dichlorobenzene (DCB) polymerize at elevated temperature and pressure in the polar aprotic solvent N-methyl-2-pyrrolidone (NMP) (J. T. Edmonds and H. W. Hill, November 1967, Production of polymers from aromatic compounds, U.S. patent 3354129). In the pre-polymerization stage, sodium sulfide is hydrolyzed to produce sodium hydrosulfide and sodium hydroxide; sodium hydroxide can react with NMP to produce sodium N-methyl-4-aminobutyrate (SMAB) that forms a complex with sodium hydrosulfide, SMAB-NaSH, which is soluble in NMP at high temperatures, allowing sulfur elements to dissolve in the organic phase and participate in polymerization (4). However, SMAB can engage in nucleophile substitution with the chlorine atoms in DCB or the chlorine end-groups of PPS, leading to a decrease in the activity of DCB or termination of PPS chain growth (4,5). This substitution is one of the most important reactions in the polymerization of PPS. Utilizing matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) to analyze PPS molecular chains, Gies et al. (5) found PPS molecular chains with SMAB as end-groups, which provided sufficient evidence for this substitution. Fahey et al. found sodium N-(4-chlorophenyl)-N-methyl-4-aminobutyrate (p-SCMAB), one of the reaction products of DCB with SMAB, in PPS filtrate through HPLC analysis (D. R. Fahey, O. H. Decker, C. E. Ash, J. F. Geibel, F. C. Vidaurri, L. E. Scoggins, et al., August 1995, Process for preparing poly (arylene sulfide) polymers, U.S. patent 5438115). They also studied the reaction kinetics of DCB with SMAB by gas chromatography and obtained a reaction activation energy of 167.43 kJ·mol−1 (6). Through reaction models and computer simulation, Fahey et al. studied chain propagation, cyclization, and substitution, the three primary reactions in the polymerization of PPS, and they agreed that nucleophilic addition–elimination (SN2Ar) is the reaction mechanism of the substitution (4,6). Nevertheless, Fahey and Gies (4,5,6) neither analyzed the reaction products of DCB with SMAB nor noticed the reactions related to the hydrolysis of DCB.
In this article, considering the PPS chlorine end-groups content is pretty low, the reaction of DCB with SMAB was chosen as a model reaction to analyze the substitution in PPS polymerization, and the products were carefully analyzed through several detection methods for the first time (Figure 1). After the detection, the reaction mechanism and networks of DCB with SMAB were proposed. Finally, the effects of the ratio of SMAB to DCB, water content, and temperature on the reaction process were studied.

The reaction of DCB with (b) SMAB was carried out under conditions similar to the polymerization of (a) PPS.
2 Experiments
2.1 Materials
DCB (99%) was purchased from Shanghai Macklin Biochemical Co., Ltd. (China). NMP (99.5%) was purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (China). Sodium hydroxide (NaOH, 96%), dichloromethane (99.5%), and chloroform (99%) were purchased from Sinopharm Chemical Reagent Co., Ltd. (China). Silica gel for column chromatography (300–400 mesh) was purchased from Qingdao Marine Chemical Co., Ltd. (China). HPLC-grade reagents, water, and methanol, were purchased from Sigma-Aldrich (Shanghai, China).
2.2 Preparation of samples
2.2.1 Preparation of SMAB
To a 250 mL three-necked flask, 110 g (1.1 mol) NMP and 100 mL (40%) aqueous NaOH were added, and the reaction proceeded at 110°C for 3 h under a slow flow of nitrogen (R. W. Campbell, February 1975, Aryne sulfide polymers, U.S. patent 3867356). A clarified amber solution was obtained at the end of the reaction. Water and residual NMP were evaporated through vacuum rotary evaporation at 180℃. The solid obtained was dried at 200℃ for 5 h under a vacuum. Nuclear magnetic resonance (NMR) characterization indicated that the resulting white solid was SMAB. This SMAB prepared was used as a reactant in the reaction with DCB.
2.2.2 Preparation of p-SCMAB
The scheme to synthesize p-SCMAB is similar to the protocol (7,8) reported by Chen et al. With the facilitation of 12.3 g (0.15 mol) acid-binding agent sodium acetate, and catalyzation of 0.1 g iodine monomer, 9.7 g (0.09 mol) N-methyl-4-chloroaniline reacted with 14 g (0.072 mol) ethyl 4-bromobutyrate at 120°C for 12 h. After the reaction was completed and cooled to room temperature, 50 mL water was added to dissolve the salts, and then, 20 mL ethyl acetate was added to extract the organic products. The extraction was repeated three times, the organic phase was combined, and ethyl acetate was removed. Twenty milliliters (17.5%) of aqueous NaOH was added to the condensed extracts, and the mixture was refluxed at 100°C for 1 h. After the water was completely removed, a yellow-white solid was obtained. The p-SCMAB was purified through antisolvent crystallization; 20 mL heated anhydrous methanol was added to the solid, the mixture was filtered to remove insoluble impurities, and then, 300 mL antisolvent acetonitrile was added dropwise. The precipitated white solid was filtered, and the filter cake was washed with 50 mL acetonitrile. The filter cake was dried, and 13.4 g white solid was obtained with a total yield of 75.2%. The white solid was identified as p-SCMAB by NMR characterization. The synthesized p-SCMAB was used as reference material.
2.2.3 Preparation of 4,4′-dichlorodiphenyl ether
The 4,4′-dichlorodiphenyl ether was prepared according to the protocol reported by Maiti D and Buchwald SL (9); 6.8 g (0.0285 mol) 4-chloro-1-iodobenzene, 4.4 g (120 mol%, relative to 4-chloro-1-iodobenzene) p-chlorophenol, 0.27 g (5 mol%) of catalyst cuprous iodide, 0.35 g (10 mol%) ligand 2-pyridinecarboxylic acid, 15 g (250 mol%) acid-binding agent potassium phosphate and 100 mL solvent dimethyl sulfoxide (DMSO) were added to a 250 mL three-necked flask. The reaction proceeded at 90°C for 48 h under a slow flow of nitrogen. After the reaction was completed, 50 mL water was added and mixed well. Then, 20 mL of ethyl acetate was added to extract the organic contents. The extraction was repeated three times, the organic phase was combined, and washed with 50 mL saline. After ethyl acetate was removed, the concentrate was dissolved by adding an appropriate amount of n-hexane. The 4,4′-dichlorodiphenyl ether was isolated from the solution by column chromatography using 200–300 mesh silica gel as the stationary phase and ethyl acetate (5 vol%)-n-hexane as the mobile phase. Finally, 4.54 g clear and transparent oily liquid was obtained with a total yield of 66.57%, and NMR characterization identified it as 4,4′-dichlorodiphenyl ether. The synthesized 4,4′-dichlorodiphenyl ether was used as reference material.
2.2.4 Reaction of DCB with SMAB
Add 250 mL NMP, 0.1 mol DCB, a certain amount of SMAB and water (if needed) into a 500 mL autoclave and let nitrogen flow slowly through the autoclave for 30 min to replace air (10). Heat to a predetermined temperature, start the timer, and keep the reaction for 12 h. Take 0.1 mL product solution and add HPLC-grade methanol to dilute to 10 mL for analysis.
2.3 Detection instruments
2.3.1 HPLC
Instrument: Shimadzu Prominence LC-20A with SPD-M20A diode array detector; stationary phase: Shimadzu Shim-pack VP-ODS, 260 mm × 4.6 mm; mobile phase: methanol and 0.01 mol·L−1 aqueous monopotassium phosphate (adjust pH to 2.6 with phosphoric acid); elution mode: gradient elution; injection volume: 10 μL; column temperature: 40°C; detection wavelength: 265 nm; data processing software: LC solution.
2.3.2 Liquid chromatography-mass spectroscopy and gas chromatography-mass spectrometry
The instrument of the liquid chromatograph of LC-MS was Thermofisher UltiMate 3000, and the mass spectrometer was Thermofisher Q Exactive equipped with an electrospray ionization (ESI) source. The instrument for GC-MS analysis was Agilent 6890-5973GCMS equipped with a 70 eV EI source.
3 Results and discussion
3.1 Product analysis
3.1.1 HPLC detection results
The gradient elution curve and chromatogram of the reaction products of DCB with SMAB are shown in Figure 2a. Fahey et al. (6) thought that p-SCMAB is the only reaction product of DCB with SMAB; however, Figure 2a shows several other products. The absorption peaks of DCB and sodium p-chlorophenolate were determined by comparing them with the retention times of reference materials. The absorption peak of sodium p-chlorophenolate is pretty low if water is not added to the autoclave, which suggests that sodium p-chlorophenolate is generated from the hydrolysis of DCB catalyzed by alkaline SMAB. So, in this article, the reaction of DCB with SMAB contains two kinds of reactions: reactions related to the substitution of chlorine atoms in DCB by SMAB and reactions related to the hydrolysis of DCB.

The gradient elution curve and chromatogram of the reaction products of DCB with (a) SMAB; the molecular structure of (b) products I, II, III, IV, and V.
Except for DCB and sodium p-chlorophenolate, there are five other products whose contents were also relatively high, and the five products (absorption peaks) are recorded as I, II, III, IV, and V in the order of retention time. There is an interference peak between peak IV and peak V, which may be caused by the rapid gradient change of the mobile phase but does not interfere with the identification of the other peaks. By comparing with the retention time of reference material, product II with the highest content was identified as p-SCMAB. The molecular structures of the four other products were determined using LC-MS and GC-MS.
3.1.2 LC-MS detection results
Under alkaline, high-temperature conditions, aryl halides may eliminate the hydrogen adjacent to the halogen on the aromatic ring to produce aryne intermediates (11). In the reaction system, considering that SMAB is alkaline, and the reaction temperature is above 260℃, aromatic chlorides may generate aryne intermediates, and additions of nucleophilic reagents to the intermediates can form positional isomers. The results of LC-MS detection are detailed in Table 1. The molecular ion weights of product III and product II are the same, indicating that III and II are a pair of isomers, and it is reasonable to presume that product III is sodium N-(3-chlorophenyl)-methyl-N-4-aminobutyrate (m-SCMAB), the positional isomer concerning product II. Based on the difference in the formulas of the hydrogenated molecular ions of product I and product II, product I can be the product of substitutions of both chlorine atoms in DCB by SMAB, and these two substituent groups may be in the para- or meta- position.
LC-MS detection results
| Product* | Retention time (min) | Molecular cation weight (Da) | Molecular cation formula |
|---|---|---|---|
| I | 16.87 | 309.18041 | C16H25O4N2 |
| II | 45.65 | 228.07826 | C11H15O2NCl |
| III | 52.49 | 228.07823 | C11H15O2NCl |
| IV | 71.20 | 320.10480 | C17H19O3NCl |
| V** | 74.68 | — | — |
*The product in the table represents the corresponding hydrogenated molecular cation.
**Product V cannot be detected by LC-MS with an ESI ion source.
3.1.3 Identification of product Ⅲ
Product Ⅲ was isolated from the product mixture, and NMR characterization was adopted to verify the presumption that product Ⅲ is m-SCMAB.
Since the products containing SMAB fragments have carboxylic acid anion and amino groups, they can transform between carboxylic acid anion, carboxylic acid, and quaternary ammonium salt under different pH conditions. The solubility of different chemical forms in specific solvents is different, so the initial separation can be achieved by extraction after adjusting the pH (Figure 3). After the reaction of DCB with SMAB, water of equal volume was added, and the pH of the mixture was about 12 as measured by a pH meter. Dichloromethane was added to extract the low-polarity substances indicated by the long retention times in chromatogram, such as DCB and product V. Then, hydrochloric acid is added to adjust the pH to 8 and dichloromethane is added to extract p-chlorophenol and product IV. Finally, adjust the pH to 5, and add dichloromethane to extract products I, II, and III, more precisely, the conjugate acids of products I, II, and III.

The initial separation of the reaction products was done by extraction after adjusting pH, and the mobile phase of the column chromatography for further separation was screened by TLC: (a) CHCl3 100 vol%; (b) CHCl3 95 vol% + MeOH 5 vol%; (c) CHCl3 95 vol% + MeOH 5 vol% + HCOOH; (d) CHCl3 85 vol% + MeOH 15 vol% + HCOOH; (e) CHCl3 50 vol% + ethyl acetate 50 vol% + HCOOH; and (f) n-hexane 50 vol% + CHCl3 25 vol% + ethyl acetate 25 vol% + HCOOH.
The conjugated acids of products I, II, and III were further separated by column chromatography. The stationary phase was 300–400 mesh chromatographic silica gel, and the mobile phase was screened by thin-layer chromatography (TLC); the results are shown in Figure 3. The extracted conjugate acid mixture was eluted with chloroform (85 vol%)-methanol, (15 vol%)-formic acid, and (0.5–1% of the volume of chloroform and methanol) to obtain the mixture of the conjugate acids of products II and III; then, the mixture was eluted with hexane (50 vol%)-chloroform (25 vol%)-ethyl acetate (25 vol%)-formic acid (0.5–1% of the volume of n-hexane, chloroform, and ethyl acetate) to obtain the conjugate acid of product III and the conjugate acid of product II, respectively. NMR characterization of the conjugate acid of product III indicated that product III is m-SCMAB.
3.1.4 GC-MS detection results
Reactant DCB, product Ⅴ, and other low-polarity substances cannot be detected by LC-MS because they cannot be ionized in the ESI ion source; however, they are easy to vaporize, and they can be detected by GC-MS. The GC-MS detection results of the extracts at pH = 12 are shown in Figure A2. Product V was verified to be 4,4′-dichlorodiphenyl ether by GC-MS spectral analysis and comparison with the HPLC retention time of its reference material. Then, considering the difference in the molecular ion weights of products Ⅳ and Ⅴ, it is presumed that product Ⅳ is the product of the substitution of a chlorine atom in 4,4′-dichlorodiphenyl ether by SMAB.
Phenols, diphenyl ethers, triphenyl ethers as well as dechlorinated products of ethers were also detected by GC-MS. However, the contents of some of these substances are low, and their absorption peaks are not obvious in the HPLC chromatogram. The phenols are generated by the alkali-catalyzed hydrolysis of DCB, and the phenyl ethers are products produced by the further condensation of DCB with phenols. Bottini et al. and other authors (12,13,14) studied the hydrolysis of aromatic halides at high temperatures. They also found these products such as chlorophenol, diphenyl ethers, and corresponding dechlorinated derivatives (12,13,14). The GC-MS results agree with the conclusions of Bottini et al.
3.2 Reaction mechanism and networks
Bottini et al. (12) found the presence of multiple pairs of positional isomers when they studied the hydrolysis of aromatic halides, and they suggested the coexistence of SN2Ar mechanism and aryne intermediate mechanism in the hydrolysis (12). In this work, we found at least four pairs of positional isomers in the reaction products of DCB with SMAB: sodium p-chlorophenolate and sodium m-chlorophenolate, p-SCMAB and m-SCMAB, dichlorobiphenyl ether at para- and meta-positions, and dichlorotriphenyl ether at para- and meta-positions. Besides, the content of the para-isomer is usually much higher than that of corresponding meta-isomer, which is only generated through aryne intermediate mechanism. Therefore, it is reasonable to conclude that there are two mechanisms, the SN2Ar mechanism and the aryne intermediate mechanism, in the reaction system of DCB with SMAB.
When Lenz et al. (15) studied the polymerization of PPS by self-condensation of metal salts of p-halothane thiophenols, they ruled out the possibility that the condensation proceeded by aryne intermediate mechanism and concluded that the reaction mechanism was SN2Ar. Fahey et al. (4,6) also concluded that the SN2Ar mechanism was the reaction mechanism for the polymerization of PPS by the sodium sulfide method. The reaction of DCB with SMAB studied in this article was carried out under conditions similar to the polymerization of PPS by sodium sulfide method, so the aryne intermediate mechanism may also occur during the polymerization of PPS.
As indicated by the HPLC chromatogram, the content of p-SCMAB is much higher than that of m-SCMAB, and this is because p-SCMAB could be produced through the SN2Ar mechanism and aryne intermediate mechanism, while m-SCMAB could only be produced through aryne intermediate mechanism. Product I is produced by further reaction of p-SCMAB or m-SCMAB with SMAB. The benzene rings of p-SCMAB and m-SCMAB contain electron-giving amino groups, and they are difficult to react with through SN2Ar mechanism. However, p-SCMAB and m-SCMAB can generate product I through an aryne intermediate mechanism, and thus, product I is a mixture of para- and meta-isomers, although there is only one absorption peak in the HPLC chromatogram. If the pH of the buffer solution of the elution phase gets lower, the absorption peak of product I will split into two adjacent shoulder peaks. The ortho-isomer of product I is not generated because of steric hindrance. Through the analysis earlier, the reaction networks shown in Figure 4 are proposed for the reaction process of DCB with SMAB under conditions similar to the polymerization of PPS.

The networks of reactions related to (a) the substitution reaction and (b) the hydrolysis reaction of DCB.
3.3 Effect factors of the reaction process
DCB can react with SMAB through the SN2Ar mechanism and aryne intermediate mechanism. The ratio of SMAB to DCB, water content, and reaction temperature were found to have effects on the reaction process. Because the reaction was carried out under conditions similar to the polymerization of PPS, the conclusions concerning the effect factors can be generalized to the PPS polymerization system.
3.3.1 Effect of ratio of SMAB to DCB
As can be seen from Figure 5a, the higher the ratio of SMAB to DCB, the higher the rate of DCB conversion during the reaction. This is because SMAB will make the reaction system more alkaline, and so the rate of reaction of DCB with SMAB through the aryne intermediate mechanism increases. In the dehydration process before PPS polymerization, NaOH is usually added as a polymerization helper (J. T. Edmonds and H. W. Hill, November 1967, Production of polymers from aromatic compounds, U.S. patent 3354129). From the law shown in Figure 5a, it is not advisable to add too much NaOH to the polymerization system; otherwise, there will be excessive SMAB, which will increase the reaction of DCB with SMAB and reduce the conversion rate of DCB into PPS.
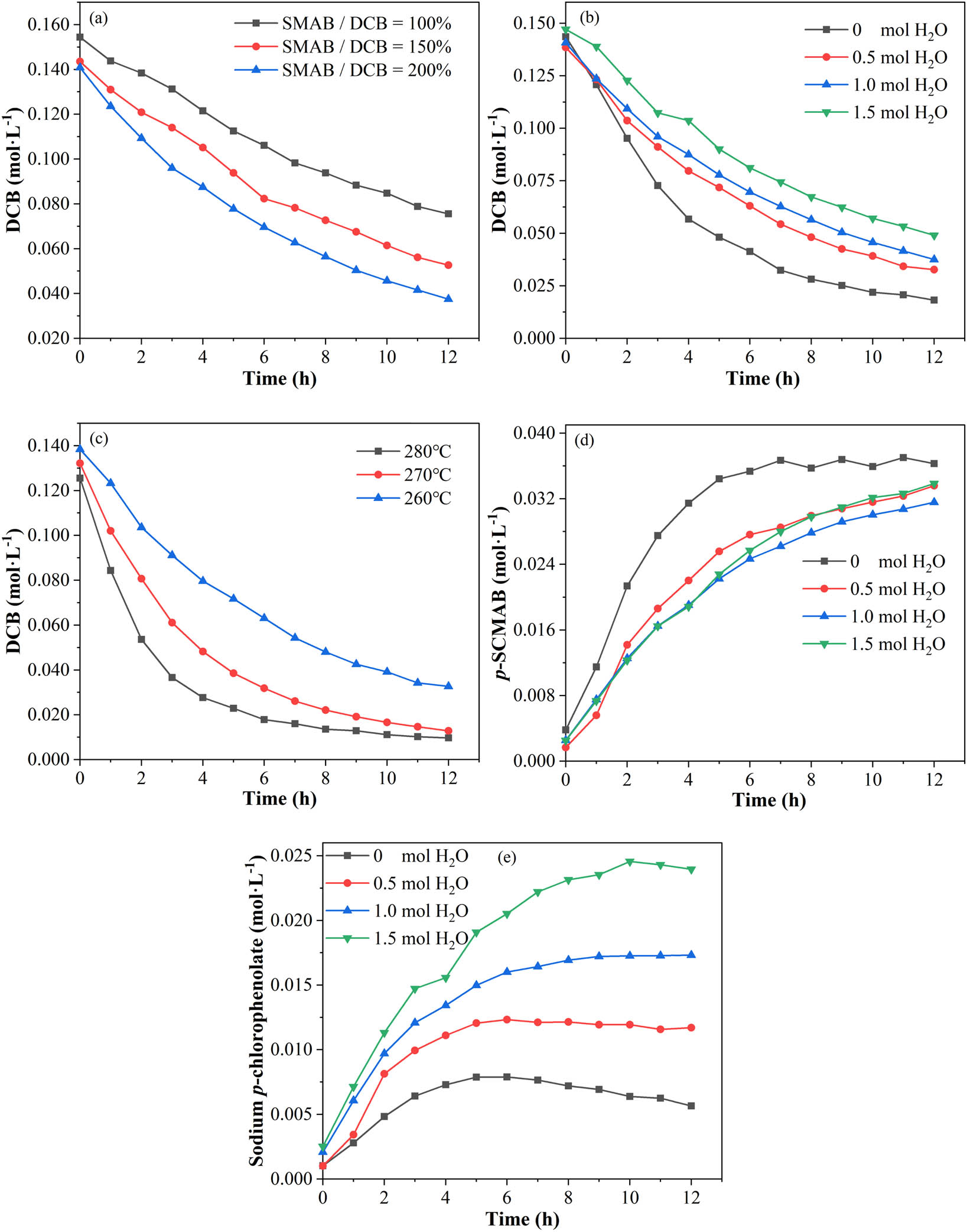
The effects of (a) the ratio of SMAB to DCB, (b) water content, and (c) reaction temperature on the conversion of DCB; (d) the solvation effect of water is significant; (e) water is favorable to the formation of sodium p-chlorophenolate.
3.3.2 Effect of water content
As can be seen from Figure 5b, the higher the water content in the system, the lower the rate of conversion of DCB during the reaction. The difference in the reaction rates between the reactions carried out with and without water is significant (Figure 5d). The water in this reaction system may have a solvation effect on SMAB, which hinders the participation of SMAB in the reaction. Thus, a certain amount of water in the PPS polymerization system can reduce the reaction degrees of DCB with SMAB. However, the higher the water content, the greater the content of phenols produced by the hydrolysis of DCB (Figure 5e). Therefore, the water content in the polymerization system needs to be controlled within an appropriate range (16).
3.3.3 Effect of reaction temperatures
As can be seen from Figure 5c, the higher the reaction temperature, the greater the conversion rate of DCB during the reaction. High temperature can significantly increase the reaction rate of DCB with SMAB. In the late stage of PPS polymerization, there is still a small amount of DCB involved in the chain growth reaction (4,6,17), and the temperature needs to be increased to prevent the precipitation of PPS molecular chains from NMP (17). From the law shown in Figure 5c, the polymerization temperature should not be too high in the late stage of polymerization; otherwise, it will accelerate the reaction of DCB with SMAB.
4 Conclusions
In this article, the reaction between DCB and SMAB was investigated under conditions similar to the polymerization of PPS by the sodium sulfide method, and the structures of the reaction products were identified by HPLC, LC-MS, GC-MS, and other methods. The reaction mechanisms were studied, and the reaction networks were proposed. The effect factors of the reaction process were studied, and some suggestions were put forward for the polymerization of PPS. The following are the conclusions:
The substitution of DCB with SMAB and the hydrolysis of DCB can occur in the reaction of DCB with SMAB.
Multiple pairs of para- and meta-isomers were detected in the reaction system, indicating the coexistence of SN2Ar and aryne intermediate mechanisms.
High temperature and a higher ratio of SMAB to DCB can increase the reaction rate; water hinders the substitution reaction of DCB with SMAB but is beneficial to the hydrolysis of DCB.
Acknowledgements
The authors thank Department of Chemistry, Zhejiang University for NMR tests and Zhejiang NHU Co., Ltd. for LC-MS test.
-
Funding information: The authors state no funding involved.
-
Author contributions: Hong Yin: writing – review and editing, methodology, data curation, validation – verification, formal analysis; Yao Shen: writing – original draft, writing – review and editing, investigation, formal analysis; Zhirong Chen: project administration, supervision formal analysis; Shenfeng Yuan: writing – review and editing, methodology; Xiaofeng Chen: resources, validation; Shihui Wang: investigation, formal analysis; Yanyu Jia: resources, investigation, data curation; Jie Jiang: resources, investigation; Jinbo Jia: investigation; Hangjun Deng: supervision.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Appendix
The equipment for NMR tests is the 500 MHz Bruker AVANCE III 500. The following are the NMR characterization results of SMAB, p-SCMAB, m-SCMAB, and 4,4′-dichlorodiphenyl ether.
SMAB: 1H NMR (500 MHz, deuterium oxide) δ 2.47–2.41 (m, 2H), 2.22 (d, J = 1.1 Hz, 3H), 2.13–2.07 (m, 2H), 1.68–1.58 (m, 2H); 13C NMR (126 MHz, deuterium oxide) δ 183.28, 50.18, 35.35, 34.49, 25.43.
p-SCMAB: 1H NMR (500 MHz, deuterium oxide) δ 7.21–7.14 (m, 2H), 6.81–6.75 (m, 2H), 3.23–3.15 (m, 2H), 2.74 (s, 3H), 2.07 (t, 2H), 1.71–1.61 (m, 2H); 13C NMR (126 MHz, deuterium oxide) δ 182.87, 148.38, 128.93, 122.27, 115.90, 52.76, 37.99, 34.85, 21.87.
m-SCMAB: 1H NMR (500 MHz, methanol-d4) δ 7.10 (t, 1H), 6.68 (t, 1H), 6.67–6.62 (m, 1H), 6.58 (d, 1H), 3.39–3.33 (m, 2H), 2.91 (s, 3H), 2.33 (t, 2H), 1.84 (p, 2H); 13C NMR (126 MHz, methanol-d4) δ 177.03, 151.91, 136.10, 131.17, 116.69, 112.81, 111.55, 52.63, 38.52, 31.90, 22.96.
4,4′-dichlorodiphenyl ether: 1H NMR (500 MHz, methanol-d4) δ 7.27–7.20 (m, 2H), 6.90–6.83 (m, 2H); 13C NMR (126 MHz, methanol-d4) δ 157.17, 131.00, 129.79, 121.35 (Figure A1).
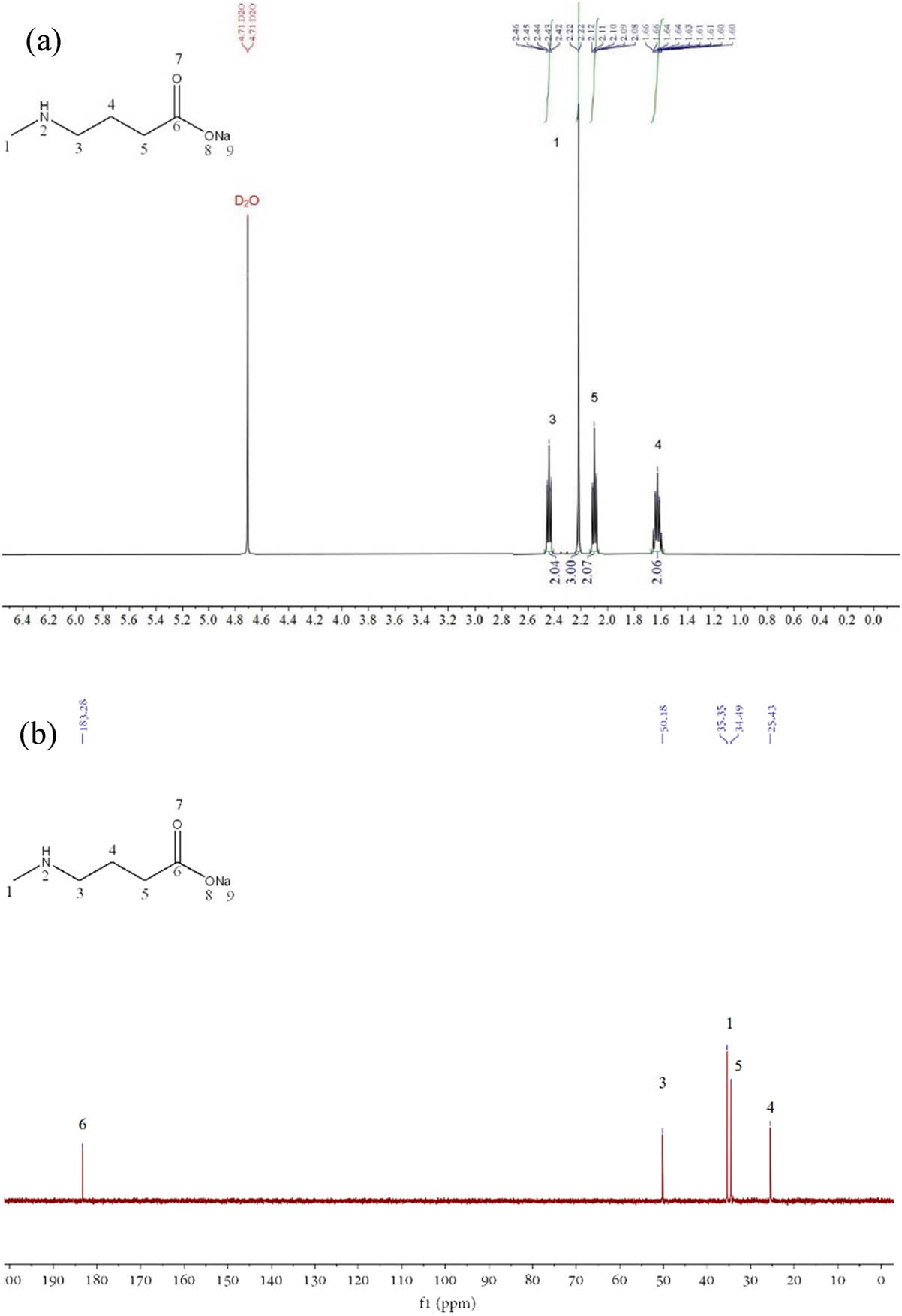


(a) 1H-NMR of SMAB, (b) 13C-NMR of SMAB, (c) 1H-NMR of p-SCMAB, (d) 13C-NMR of p-SCMAB, (e) 1H-NMR of m-SCMAB, (f) 13C-NMR of m-SCMAB, (g) 1H-NMR of 4,4′-dichlorodiphenyl ether, and (h) 13C-NMR of 4,4′-dichlorodiphenyl ether.
The GC-MS results are shown in Figure A2.

The GC-MS detection results of low-polar substances.
References
(1) Zuo P, Tcharkhtchi A, Shirinbayan M, Fitoussi J, Bakir F. Overall investigation of poly (phenylene sulfide) from synthesis and process to applications–a review. Macromol Mater Eng. 2019;304(5):1800686. 10.1002/mame.201800686.Search in Google Scholar
(2) Rahate AS, Nemade KR, Waghuley SA. Polyphenylene sulfide (PPS): state of the art and applications. Rev Chem Eng. 2013;29(6):471–89. 10.1515/revce-2012-0021.Search in Google Scholar
(3) Yu Y, Xiong S, Huang H, Zhao L, Nie K, Chen S, et al. Fabrication and application of poly (phenylene sulfide) ultrafine fiber. React Funct Polym. 2020;150:104539. 10.1016/j.reactfunctpolym.2020.104539.Search in Google Scholar
(4) Fahey DR, Ash CE. Mechanism of poly (p-phenylene sulfide) growth from p-dichlorobenzene and sodium sulfide. Macromol. 1991;24(15):4242–9. 10.1021/ma00015a003.Search in Google Scholar
(5) Gies AP, Geibel JF, Hercules DM. MALDI-TOF MS study of poly (p-phenylene sulfide). Macromol. 2010;43(2):943–51. 10.1021/ma902117u.Search in Google Scholar
(6) Fahey DR, Hensley HD, Ash CE, Senn DR. Poly (p-phenylene sulfide) synthesis: a step-growth polymerization with unequal step reactivity. Macromol. 1997;30(3):387–93. 10.1021/ma961015d.Search in Google Scholar
(7) Chen H, Herkstroeter WG, Perlstein J, Law K-Y, Whitten DG. Aggregation of a surfactant squaraine in Langmuir-Blodgett films, solids, and solution. J Phys Chem. 1994;98:5138–46. 10.1021/j100070a033.Search in Google Scholar
(8) Dombrowski GW, Dinnocenzo JP, Zielinski PA, Farid S, Wosinska ZM, Gould IR. Efficient unimolecular deprotonation of aniline radical cations. J Org Chem. 2005;70(10):3791–800. 10.1021/jo047813g.Search in Google Scholar PubMed
(9) Maiti D, Buchwald SL. Cu-catalyzed arylation of phenols: synthesis of sterically hindered and heteroaryl diaryl ethers. J Org Chem. 2010;75(5):1791–4. 10.1021/jo9026935.Search in Google Scholar PubMed PubMed Central
(10) Berrueco C, Álvarez P, Venditti S, Morgan TJ, Herod AA, Millan M, et al. Sample contamination with NMP-oxidation products and byproduct-free NMP removal from sample solutions. Energy Fuels. 2009;23:3008–15. 10.1021/ef900036m.Search in Google Scholar
(11) Xing Q, Pei W, Xu R, Pei J. Basic organic chemistry. 4th edn. Beijing: Peking University Press; 2005.Search in Google Scholar
(12) Bottini AT, Roberts JD. Mechanisms for liquid phase hydrolyses of chlorobenzene and halotoluenes. J Am Chem Soc. 1957;79(6):1458–62. 10.1021/ja01563a050.Search in Google Scholar
(13) Dalman GW, Neumann FW. The mechanism of the formation and hydrolysis of phenyl ether in the basic hydrolysis of chlorobenzene. J Am Chem Soc. 1968;90(6):1601–5. 10.1021/ja01008a033.Search in Google Scholar
(14) Zoratti M, Bunnett JF. Mechanism of the non-aryne hydroxydehalogenation of unactivated aryl halides. J Org Chem. 1980;45(10):1769–76. 10.1021/jo01298a006.Search in Google Scholar
(15) Lenz RW, Handlovits CE, Smith HA. Phenylene sulfide polymers. Ⅲ. The synthesis of linear polyphenylene sulfide. J Polym Sci. 1962;58(166):351–67. 10.1002/pol.1962.1205816620.Search in Google Scholar
(16) Ito MM, Onda M, Ona S, Inoue H. PPS preparation. A kinetic study and the effect of water on the polymerization. Bull Chem Soc Jpn. 1990;63(5):1484–8. 10.1246/bcsj.63.1484.Search in Google Scholar
(17) Rajan CR, Ponrathnam S, Nadkarni VM. Poly (phenylene sulfide): polymerization kinetics and characterization. J Appl Polym Sci. 1986;32(4):4479–91. 10.1002/app.1986.070320416.Search in Google Scholar
© 2022 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes
Articles in the same Issue
- Research Articles
- The effect of isothermal crystallization on mechanical properties of poly(ethylene 2,5-furandicarboxylate)
- The effect of different structural designs on impact resistance to carbon fiber foam sandwich structures
- Hyper-crosslinked polymers with controlled multiscale porosity for effective removal of benzene from cigarette smoke
- The HDPE composites reinforced with waste hybrid PET/cotton fibers modified with the synthesized modifier
- Effect of polyurethane/polyvinyl alcohol coating on mechanical properties of polyester harness cord
- Fabrication of flexible conductive silk fibroin/polythiophene membrane and its properties
- Development, characterization, and in vitro evaluation of adhesive fibrous mat for mucosal propranolol delivery
- Fused deposition modeling of polypropylene-aluminium silicate dihydrate microcomposites
- Preparation of highly water-resistant wood adhesives using ECH as a crosslinking agent
- Chitosan-based antioxidant films incorporated with root extract of Aralia continentalis Kitagawa for active food packaging applications
- Molecular dynamics simulation of nonisothermal crystallization of a single polyethylene chain and short polyethylene chains based on OPLS force field
- Synthesis and properties of polyurethane acrylate oligomer based on polycaprolactone diol
- Preparation and electroactuation of water-based polyurethane-based polyaniline conductive composites
- Rapeseed oil gallate-amide-urethane coating material: Synthesis and evaluation of coating properties
- Synthesis and properties of tetrazole-containing polyelectrolytes based on chitosan, starch, and arabinogalactan
- Preparation and properties of natural rubber composite with CoFe2O4-immobilized biomass carbon
- A lightweight polyurethane-carbon microsphere composite foam for electromagnetic shielding
- Effects of chitosan and Tween 80 addition on the properties of nanofiber mat through the electrospinning
- Effects of grafting and long-chain branching structures on rheological behavior, crystallization properties, foaming performance, and mechanical properties of polyamide 6
- Study on the interfacial interaction between ammonium perchlorate and hydroxyl-terminated polybutadiene in solid propellants by molecular dynamics simulation
- Study on the self-assembly of aromatic antimicrobial peptides based on different PAF26 peptide sequences
- Effects of high polyamic acid content and curing process on properties of epoxy resins
- Experiment and analysis of mechanical properties of carbon fiber composite laminates under impact compression
- A machine learning investigation of low-density polylactide batch foams
- A comparison study of hyaluronic acid hydrogel exquisite micropatterns with photolithography and light-cured inkjet printing methods
- Multifunctional nanoparticles for targeted delivery of apoptin plasmid in cancer treatment
- Thermal stability, mechanical, and optical properties of novel RTV silicone rubbers using octa(dimethylethoxysiloxy)-POSS as a cross-linker
- Preparation and applications of hydrophilic quaternary ammonium salt type polymeric antistatic agents
- Coefficient of thermal expansion and mechanical properties of modified fiber-reinforced boron phenolic composites
- Synergistic effects of PEG middle-blocks and talcum on crystallizability and thermomechanical properties of flexible PLLA-b-PEG-b-PLLA bioplastic
- A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater
- Simultaneously enhance the fire safety and mechanical properties of PLA by incorporating a cyclophosphazene-based flame retardant
- Fabrication of two multifunctional phosphorus–nitrogen flame retardants toward improving the fire safety of epoxy resin
- The role of natural rubber endogenous proteins in promoting the formation of vulcanization networks
- The impact of viscoelastic nanofluids on the oil droplet remobilization in porous media: An experimental approach
- A wood-mimetic porous MXene/gelatin hydrogel for electric field/sunlight bi-enhanced uranium adsorption
- Fabrication of functional polyester fibers by sputter deposition with stainless steel
- Facile synthesis of core–shell structured magnetic Fe3O4@SiO2@Au molecularly imprinted polymers for high effective extraction and determination of 4-methylmethcathinone in human urine samples
- Interfacial structure and properties of isotactic polybutene-1/polyethylene blends
- Toward long-live ceramic on ceramic hip joints: In vitro investigation of squeaking of coated hip joint with layer-by-layer reinforced PVA coatings
- Effect of post-compaction heating on characteristics of microcrystalline cellulose compacts
- Polyurethane-based retanning agents with antimicrobial properties
- Preparation of polyamide 12 powder for additive manufacturing applications via thermally induced phase separation
- Polyvinyl alcohol/gum Arabic hydrogel preparation and cytotoxicity for wound healing improvement
- Synthesis and properties of PI composite films using carbon quantum dots as fillers
- Effect of phenyltrimethoxysilane coupling agent (A153) on simultaneously improving mechanical, electrical, and processing properties of ultra-high-filled polypropylene composites
- High-temperature behavior of silicone rubber composite with boron oxide/calcium silicate
- Lipid nanodiscs of poly(styrene-alt-maleic acid) to enhance plant antioxidant extraction
- Study on composting and seawater degradation properties of diethylene glycol-modified poly(butylene succinate) copolyesters
- A ternary hybrid nucleating agent for isotropic polypropylene: Preparation, characterization, and application
- Facile synthesis of a triazine-based porous organic polymer containing thiophene units for effective loading and releasing of temozolomide
- Preparation and performance of retention and drainage aid made of cationic spherical polyelectrolyte brushes
- Preparation and properties of nano-TiO2-modified photosensitive materials for 3D printing
- Mechanical properties and thermal analysis of graphene nanoplatelets reinforced polyimine composites
- Preparation and in vitro biocompatibility of PBAT and chitosan composites for novel biodegradable cardiac occluders
- Fabrication of biodegradable nanofibers via melt extrusion of immiscible blends
- Epoxy/melamine polyphosphate modified silicon carbide composites: Thermal conductivity and flame retardancy analyses
- Effect of dispersibility of graphene nanoplatelets on the properties of natural rubber latex composites using sodium dodecyl sulfate
- Preparation of PEEK-NH2/graphene network structured nanocomposites with high electrical conductivity
- Preparation and evaluation of high-performance modified alkyd resins based on 1,3,5-tris-(2-hydroxyethyl)cyanuric acid and study of their anticorrosive properties for surface coating applications
- A novel defect generation model based on two-stage GAN
- Thermally conductive h-BN/EHTPB/epoxy composites with enhanced toughness for on-board traction transformers
- Conformations and dynamic behaviors of confined wormlike chains in a pressure-driven flow
- Mechanical properties of epoxy resin toughened with cornstarch
- Optoelectronic investigation and spectroscopic characteristics of polyamide-66 polymer
- Novel bridged polysilsesquioxane aerogels with great mechanical properties and hydrophobicity
- Zeolitic imidazolate frameworks dispersed in waterborne epoxy resin to improve the anticorrosion performance of the coatings
- Fabrication of silver ions aramid fibers and polyethylene composites with excellent antibacterial and mechanical properties
- Thermal stability and optical properties of radiation-induced grafting of methyl methacrylate onto low-density polyethylene in a solvent system containing pyridine
- Preparation and permeation recognition mechanism of Cr(vi) ion-imprinted composite membranes
- Oxidized hyaluronic acid/adipic acid dihydrazide hydrogel as cell microcarriers for tissue regeneration applications
- Study of the phase-transition behavior of (AB)3 type star polystyrene-block-poly(n-butylacrylate) copolymers by the combination of rheology and SAXS
- A new insight into the reaction mechanism in preparation of poly(phenylene sulfide)
- Modified kaolin hydrogel for Cu2+ adsorption
- Thyme/garlic essential oils loaded chitosan–alginate nanocomposite: Characterization and antibacterial activities
- Thermal and mechanical properties of poly(lactic acid)/poly(butylene adipate-co-terephthalate)/calcium carbonate composite with single continuous morphology
- Review Articles
- The use of chitosan as a skin-regeneration agent in burns injuries: A review
- State of the art of geopolymers: A review
- Mechanical, thermal, and tribological characterization of bio-polymeric composites: A comprehensive review
- The influence of ionic liquid pretreatment on the physicomechanical properties of polymer biocomposites: A mini-review
- Influence of filler material on properties of fiber-reinforced polymer composites: A review
- Rapid Communications
- Pressure-induced flow processing behind the superior mechanical properties and heat-resistance performance of poly(butylene succinate)
- RAFT polymerization-induced self-assembly of semifluorinated liquid-crystalline block copolymers
- RAFT polymerization-induced self-assembly of poly(ionic liquids) in ethanol
- Topical Issue: Recent advances in smart polymers and their composites: Fundamentals and applications (Guest Editors: Shaohua Jiang and Chunxin Ma)
- Fabrication of PANI-modified PVDF nanofibrous yarn for pH sensor
- Shape memory polymer/graphene nanocomposites: State-of-the-art
- Recent advances in dynamic covalent bond-based shape memory polymers
- Construction of esterase-responsive hyperbranched polyprodrug micelles and their antitumor activity in vitro
- Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes

