Abstract
In the framework of this research, four new herbicidal ionic liquids (HILs) comprising chlorsulfuron as the anion were synthesized and characterized. The new salts with chlorsulfuron contained the following cations: tetramethylammonium, didecyldimethylammonium, benzyltrimethylammonium and cholinium. All products were obtained with high yields exceeding 90% via acid–base reaction or ion exchange reaction, by the use of environment-friendly solvents. The structures of all synthesized HILs were confirmed by FT-IR, 1H NMR and 13C NMR analyses. Their efficacy against weeds has been studied under field conditions in fiber flax. All HILs showed herbicidal activity but efficiency was highly dependent on the type of cation and weed species. There were no statistically significant differences in the effectiveness of HILs toward common lambsquarters compared to the reference herbicide, except for salt with cholinium cation that showed significantly lower efficiency. As regards barnyard grass control, all HILs exhibited significantly lower efficacy than that of the reference herbicide, except for didecyldimethylammonium salt that showed similar activity. The synthesized products did not cause damage to flax plants. The obtained results confirmed that the herbicidal effectiveness of the active ingredient (chlorsulfuron) in the form of an ionic liquid can be adjusted by the selection of an appropriate cation in the synthesis.
1 Introduction
Chlorsulfuron is a selective herbicide belonging to the group of sulfonylureas that was commercialized in the 1980s. It is mainly utilized in cereal crops for controlling broad-leaf weeds and some grasses. This herbicide is also characterized by high selectivity for flax plants (Linum usitatissimum L.) and is known to be a potential agent for the protection of this crop. Flax is one of the oldest cultivated crops used for fiber and seed [1,2]. The advantages of chlorsulfuron, such as low effective dose (approx. 10–40 g per hectare), relatively low toxicity (LD50, rat, orally = 5.545 g/kg) and high popularity worldwide, make it a promising source of anion in the synthesis of new forms of this compound [3]. Currently, on the basis of the risk analysis related to the use of chlorsulfuron, the European Commission has decided to withdraw this substance from the market. The process of reviewing the pesticidally active substances lasts in the European Union for almost 30 years. The main basis for this action was a Directive 91/414/EEC, released in 1991. However, the policy regarding the use of chemicals has changed radically after the adoption of the REACH regulation in 2006. Subsequent legal acts such as Regulation No. 1107/2009 and Directive 128/2009/EC resulted in the implementation of even more drastic restrictions in the use of plant protection products. As a result, a large number of active substances in plant protection products have been withdrawn from the market and further restrictions are being planned in the near future. This action is also strongly related to the adopted strategy of the European Green Deal. The deterioration in the availability of the chemical plant protection products makes the farmers in the European Union extremely difficult to ensure plant health and high-quality of plant production. Nevertheless, due to the fact that other methods focused on the protection of agricultural plants against weeds are less effective and more expensive, the use of chemical herbicides is nowadays the basic approach and the most probably this situation will not change in the near future. It is indisputable that the use of herbicides, in addition to the obvious benefits, may have a negative impact on the environment and therefore we should focus our effort to minimize this phenomenon. In a current situation, when registration of new herbicidally active substances is much more difficult, the research on the improvement of existing substances, even if they have been removed from the market, is justified. This strategy indicates a new direction in the preparation of herbicidal formulations, which complies with the principles of “green chemistry.” This is the reason why it is important to develop its novel herbicidal formulations that are characterized with even lower environmental impact and greater efficacy. One of the possibilities to reduce the negative environmental impact of herbicides is their transformation into ionic liquids (ILs) exhibiting herbicidal activity commonly known as herbicidal ionic liquids (HILs).
HILs belong to ILs which beside a melting temperature below 100°C contain at least one ion exhibiting herbicidal activity [4]. Their discovery in 2011 led to the formation of new chemical compounds from third-generation ILs [5], characterized by increased herbicidal activity and multifunctional properties. This strategy demonstrates the possibility to develop more effective formulations starting from the commercially available herbicides, such as the derivatives of phenoxyacetic acid (2,4-Dichlorophenoxyacetic acid [2,4-D], 4-chloro-2-methylphenoxyacetic acid [MCPA], 2-(4-Chloro-2-methylphenoxy)propanoic acid [MCPP], 2-(2,4-dichlorophenoxy)propanoic acid [2,4-DP] and 4-(4-chloro-2-methylphenoxy)butanoic acid [MCPB]) [4,6,7,8,9,10], benzoic acid (dicamba) [11], clopyralid [12], fomesafen [13], glyphosate [14,15], metsulfuron methyl [16], bentazone [17], nicosulfuron [18], nonanoic acid [19] and picloram [20]. One of the most crucial advantages of HILs compared to the currently applied herbicidal formulations refers to their limited volatility [11]. This eliminates one of the most significant problems of pesticides, which leads to the contamination of neighboring areas through air emissions. Furthermore, utilization of HILs allows for a minimization of the herbicide dose per hectare, decrease of toxicity (toxic herbicides from the group of phenoxy acids were found to be nontoxic as HILs [4]) and introduction of specific physicochemical properties (such as surface activity, thermal stability and solubility in water). Additionally, the environmental impact including compounds’ toxicity and biodegradability may be directly regulated by proper modification of the structure of the cation of HILs [21,22]. Previous publications demonstrate synthesis and characterization of HILs comprising the following anions: metsulfuron-methyl [16], iodosulfuron-methyl [23] and nicosulfuron [18]. However, HILs comprising chlorsulfuron as the anion have not been published so far.
Furthermore, tetraalkylammonium cations selected as the counterions for chlorsulfuron are commonly known, cost-effective and commercially available. It should also be emphasized that one of them are substance of natural origin. Choline (2-hydroxyethyltrimethylammonium) cation is an essential component of animal cells’ membrane lipids and lipoproteins. However, due to insufficient production of choline in organism, this naturally occurring nutrient has to be provided by diet [24,25]. Additionally, choline derivatives were successfully utilized as blood preservatives [26], insect feeding deterrents [26], herbicides [27], antimicrobials [28] or as substrates for chemical synthesis [29]. It could be stressed that living organisms utilize choline to produce betaine and acetylcholine neurotransmitter, which mobilize muscles to motion thereby regulate breathing, heart rate and control skeletal muscles [24]. Interestingly, the cholinergic system is also found in plants [23] and non-neural tissues such as erythrocytes and placental cells [30]. Its stimulating effect on enzymes, which can affect metabolism [23,30,31], makes it an interesting source of cation for biologically active HILs.
2 Materials and methods
2.1 Materials
All materials were used as supplied unless otherwise noted. Chlorsulfuron (98%) was purchased from Pestinova. A 40% methanolic solution of tetramethylammonium hydroxide, 40% aqueous solution of benzyltrimethylammonium hydroxide, choline (2-hydroxyethyltrimethylammonium) chloride (98%) and all solvents (methanol (98%), dimethyl sulfoxide (DMSO; 98%), acetonitrile (99%), acetone (99%), 2-propanol (98%), ethyl acetate (99%), chloroform (98%), toluene (99.5%) and hexane (98%), were purchased from Sigma-Aldrich. A 50% isopropanol/water (2:3) solution of didecyldimethylammonium chloride was purchased from Merck. Water deionized (conductivity < 0.1 µS cm−1) with demineralizer HLP Smart 1000 (Hydrolab) was used.
2.2 General
The IR spectra were recorded with a ReactIR iC15 (Mettler Toledo) spectrometer equipped with an MCT detector and a 9.5 mm AgX probe with a diamond tip in a range from 3,000 to 650 cm−1 at 8 cm−1 resolution. The iCIR 4.3 software was used to process the collected spectra. NMR spectra were recorded using tetramethylsilane (TMS) as the internal standard with the use of Mercury Gemini 300 spectrometer operating at 300 and 400 MHz (in the case of 1H NMR spectra) or at 75 and 100 MHz (in the case of 13C NMR spectra). The Karl Fisher TitroLine KF Trace coulometric titrator (SI Analytics) was utilized to analyze the water content in the obtained products.
2.3 Synthesis
2.3.1 Method A
In a 50 cm3 round-bottom flask equipped with a magnetic stirring bar, 0.01 mol of the solution of the corresponding tetraalkylammonium hydroxide (tetramethylammonium hydroxide, benzyltrimethylammonium hydroxide and choline hydroxide) was mixed with 5 cm3 of methanol. Subsequently, an equimolar amount of chlorsulfuron herbicide (2-chloro-N-((4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoyl)benzenesulfonamide) was added to the flask. The mixture was then stirred for 10 min at room temperature and the progress of neutralization was monitored with Mettler Toledo SevenExcellence pH meter S400. Next, after evaporation of the solvent in a rotary evaporator, products (1, 3 and 4) were dried under vacuum (10 mbar) at 50°C for 48 h and stored over P4O10.
2.3.2 Method B
First, the potassium salt of chlorsulfuron herbicide (2-chloro-N-((4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoyl)benzenesulfonamide) was obtained. After addition of equimolar amounts of chlorsulfuron to 20% methanolic solution of potassium hydroxide (0.04 mol), the progress of neutralization reaction was monitored with Mettler Toledo SevenExcellence pH meter S400. Then, the methanol was evaporated in a rotary evaporator and the product was dried under reduced pressure at 50°C for 48 h.
Subsequently, in a 50 cm3 round-bottom flask equipped with a magnetic stirring bar, 0.01 mol of the corresponding tetraalkylammonium chloride (didecyldimethylammonium chloride) was dissolved in 10 cm3 of methanol (2). Then, an equimolar amount of the potassium salt of chlorsulfuron herbicide (2-chloro-N-((4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoyl)benzenesulfonamide) was added to the flask. The obtained mixture was stirred for 1 h at 40°C temperature, and the solvent was removed using a rotary evaporator. Then, 20 cm3 of anhydrous acetone was added. After filtration of the precipitated sediment of inorganic by-product, the solvent was evaporated from the filtrate. Finally, the product (2) was dried under reduced pressure at 50°C for 48 h and stored over P4O10.
2.4 Spectroscopic data
2.4.1 Tetramethylammonium chlorsulfuron (1)
1H NMR (401.13 MHz, DMSO-d6) δ [ppm] = 2.26 (s, 3H,
13C NMR (100.87 MHz, DMSO-d6) δ [ppm] = 25.1, 54.1, 54.4(3), 126.2, 130.40, 130.6, 130.8, 131.2, 143.5, 154.6, 165.6, 170.6, 177.5.
IR νmax [cm−1] = 764, 792, 825, 861, 945, 1,038, 1,084, 1,109, 1,025, 1,135, 1,156, 1,221, 1,256, 1,308, 1,364, 1,392, 1,418, 1,480, 1,562, 1,655.
2.4.2 Didecyldimethylammonium chlorsulfuron (2)
1H NMR (300.41 MHz, DMSO-d6) δ [ppm] = 0.82–0.92 (m, 6H, CH2–C
13C NMR (75.55 MHz, DMSO-d6) δ [ppm] = 14.0, 21.7, 22.1, 25.1, 25.8, 28.5, 28.7, 28.8, 28.9, 31.3, 50.0, 54.1, 62.8, 126.1, 130.4, 130.5, 130.9, 131.1, 143.5, 154.5, 165.6, 170.6, 177.4.
IR νmax [cm−1] = 758, 818, 881, 956, 1,040, 1,086, 1,105, 1,122, 1,142, 1,200, 1,234, 1,357, 1,467, 1,508, 1,554, 1,670, 1,711, 2,853, 2,925.
2.4.3 Benzyltrimethylammonium chlorsulfuron (3)
1H NMR (399.91 MHz, DMSO-d6) δ [ppm] = 2.27 (s, 3H, CC
13C NMR (100.57 MHz, DMSO-d6) δ [ppm] = 25.1, 51.7, 54.1, 67.7, 126.2, 128.4, 128.8, 130.2, 130.4, 130.5, 130.9, 131.2, 132.8, 143.4, 154.7, 165.7, 170.6, 177.4.
IR νmax [cm−1] = 760, 818, 885, 954, 982, 1,040, 1,101, 1,107, 1,124, 1,200, 1,230, 1,342, 1,360, 1,366, 1,452, 1,470, 1,508, 1,552, 1,662.
2.4.4 Choline chlorsulfuron (4)
1H NMR (402.65 MHz, DMSO-d6) δ [ppm] = 2.29 (s, 3H, CC
13C NMR (101.25 MHz, DMSO-d6) δ [ppm] = 25.5, 53.6(2), 53.7, 54.6, 55.7, 67.4, 126.7, 130.9, 131.0, 131.3, 131.7, 143.7, 155.0, 166.0, 171.0, 177.9.
IR νmax [cm−1] = 759, 773, 812, 897, 958, 1,030, 1,040, 1,092, 1,105, 1,120, 1,193, 1,228, 1,280, 1,146, 1,340, 1,370, 1,414, 1,457, 1,508, 1,570, 1,680.
2.5 Differential scanning calorimetry (DSC)
Mettler Toledo Stare DSC1 (Mettler Toledo) unit was utilized to analyze thermal transition temperatures of the synthesized salts. In the experiment, samples of products in a range from 4.5 to 7.0 mg were placed in aluminum pans and heated under nitrogen at a heating rate of 10°C min−1 from 25 to 120°C. Subsequently, pans were cooled at a cooling rate equal to 10°C min−1 to the temperature of −100°C and then heated again to 120°C.
2.6 Thermal gravimetric analysis (TGA)
Samples (approx. 4.0–5.0 mg) were weighed in aluminum pans and introduced into Mettler Toledo Stare TGA/DSC1 unit (Mettler Toledo). In the experiment, pans were heated under nitrogen at a constant heating rate (10°C min−1) starting from 30 to 450°C and the instrument recorded the mass loss during the process of heating.
2.7 Solubility
The following solvents, arranged in the order of decreasing Snyder polarity index: water, 9.0; methanol, 6.6; DMSO, 6.5; acetonitrile, 6.2; acetone, 5.4; chloroform, 4.4; isopropanol, 4.3; ethyl acetate, 4.3; toluene, 2.3; and hexane, 0.0, were selected for the test which was performed according to the methodology described in Vogel’s Textbook of Practical Organic Chemistry. The sample of ILs (0.1 ± 0.0001 g) was weighed in a vial and then a specific volume of solvent was introduced at 25°C. Compounds that dissolved in 1 cm3 of the solvent (>10.0% m/v) were marked with a term “good solubility,” whereas “medium solubility” was applied for compounds that dissolved in 3 cm3 of the solvent (between 3.33 and 10.0% m/v). The term “low solubility” was utilized in the case of products that did not dissolve in 3 cm3 of the solvent (<3.33% m/v).
2.8 Field studies
The biological activity of new IL forms of chlorsulfuron was examined under field conditions in the fiber flax. The plots were located at the experimental station of Institute of Natural Fibres and Medicinal Plants in Pętkowo, Poland (DMS: 52°12′N, 17°15′E). The target weeds are the following two species: common lambsquarters (Chenopodium album L.) and common barnyard grass (Echinochloa crus-galli L.). The experiment was established on 13.5 m2 plots arranged in a completely randomized setup with three replications. Fiber flax variety Modran has been sown in a quantity of 120 kg ha−1 with a row distance of 15 cm. HILs and reference product (Glean 75 WG, 75% of chlorsulfuron; Cheminova Polska, Warszawa, Poland) were applied at the dose of 10 g ha−1. A knapsack sprayer equipped with a TeeJet 1102 flat-fan nozzle delivering 200 L ha−1 of spray solution at 0.2 MPa operating pressure was utilized for all the treatments. The plots were sprayed when flax plants were 8−12 cm tall and the weeds reached 4–6 leaves. The efficacy of herbicides was assessed visually 4 weeks after application and demonstrated as percent of weed control in comparison to control (non-treated plants) using a scale of 0 (no herbicide effects) to 100% (complete weed destruction). The influence of the tested compounds on the growth of flax plants was evaluated twice, 2 and 4 weeks after spraying. The assessment of herbicide selectivity for flax plants consisted of a visual comparison of the growth of herbicide-treated plants with plants from control plots (non-treated). After harvesting, the influence of the used substances on yielding was also determined. For this purpose, 20 randomly selected flax plants from each experimental plot were harvested. The mass of straw after ginning and root removal was assumed as the straw yield. The mass of seeds after extracting from seed bolts was assumed as the seed yield.
In order to study the effect of applied forms of chlorsulfuron on the yield of fiber flax and the weed reduction, a one-way analysis of variance (ANOVA) was performed. The analysis was preceded by the Fligner–Killeen test of homogeneity of variances. If the null hypothesis of ANOVA was rejected, multiple Tukey’s post hoc test (α = 0.05) was applied to compare the studied treatments.
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
In this research, four new quaternary ammonium salts of chlorsulfuron were synthesized via acid–base reaction or by ion exchange reaction in methanol or methanol–water solution. The new salts with chlorsulfuron contained the following cations: tetramethylammonium (1), didecyldimethylammonium (2), benzyltrimethylammonium (3) and cholinium (4) (Figure 1 and Table 1). All products were synthesized with high yields exceeding 90%, with the use of environment-friendly solvents, which can be easily regenerated by distillation and reused in the process. Additionally, all reactions were carried out in mild conditions, wherein the temperature was equal to 40°C or slightly lower. It should also be emphasized that salt 4 comprises a cation of natural origin, which directly participates in the metabolism of living organisms, including plants and humans. Interestingly, compounds comprising smaller cations (1, 3 and 4) turned out to possess a glassy appearance at room temperature, while one compound with two long alkyl chains present in the cation (2) turned out to be a liquid.
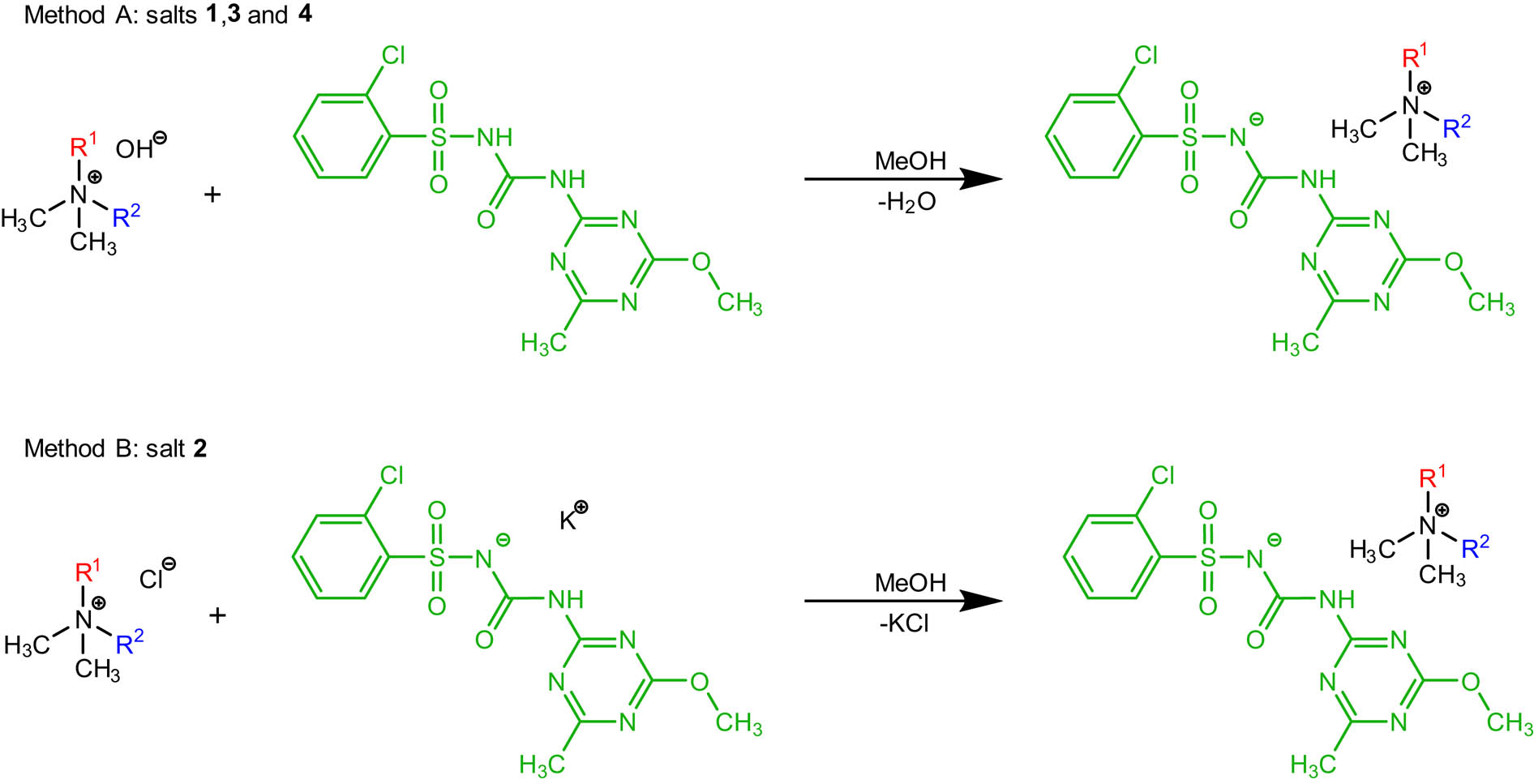
Synthesis of new salts comprising various tetraalkylammonium cations and chlorsulfuron as the anion (1–4).
Obtained chlorsulfuron-based salts (1–4)
| Salt | Code | R1 | R2 | State at 25°C | Method of synthesis | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | [TMA[CHS] | CH3 | CH3 | Glass | A | 95 |
| 2 | [DDA][CHS] | C10H21 | C10H21 | Liquid | B | 94 |
| 3 | [BTMA][CHS] | CH3 | Ph-CH2 | Glass | A | 99 |
| 4 | [CHOL][CHS] | CH3 | HOCH2CH2 | Glass | A | 90 |
The water content in the dried products, examined by Karl Fischer titration, was less than 2,000 ppm. Additionally, all the products were found to be stable in contact with water and tested organic solvents as well as during storage in air. FT-IR and NMR spectra descriptions for compounds 1–4 are provided in the Supporting Information (Figures S1–S12).
The NMR analysis revealed that all characteristic peaks originating from the chlorsulfuron anion were present in the collected 1H NMR spectra. Hence, in the 1H NMR spectrum of cholinium chlorsulfuron (4) (Figure 2), we can distinguish three singlets at 2.27 ppm (2) (protons from the methyl group), 3.81 ppm (1) (protons from the methoxyl group) and 9.05 ppm (3) (proton from the amide group), one multiplet at 7.39 ppm (4) (three protons from the aromatic ring) and one doublet at 8.01 ppm (5) (one proton from the aromatic ring, J = 6.9 Hz) for this particular herbicide. Interestingly, detachment of the proton from the nitrogen atom of the sulfonamide group decreased the peak shift from the second proton from the urea group from 11 ppm to approximately 9 ppm. The signals originating from three methyl groups of the cholinium cation appeared at 3.12 ppm (9), whereas peaks from two methylene groups occurred at 3.41 ppm (8) and 3.84 ppm (7) (overlapping with protons from the methyl group of the anion), respectively. Additionally, we can find a broad singlet at 5.32 ppm (6), which was attributed to the hydroxyl substituent present in the cation.
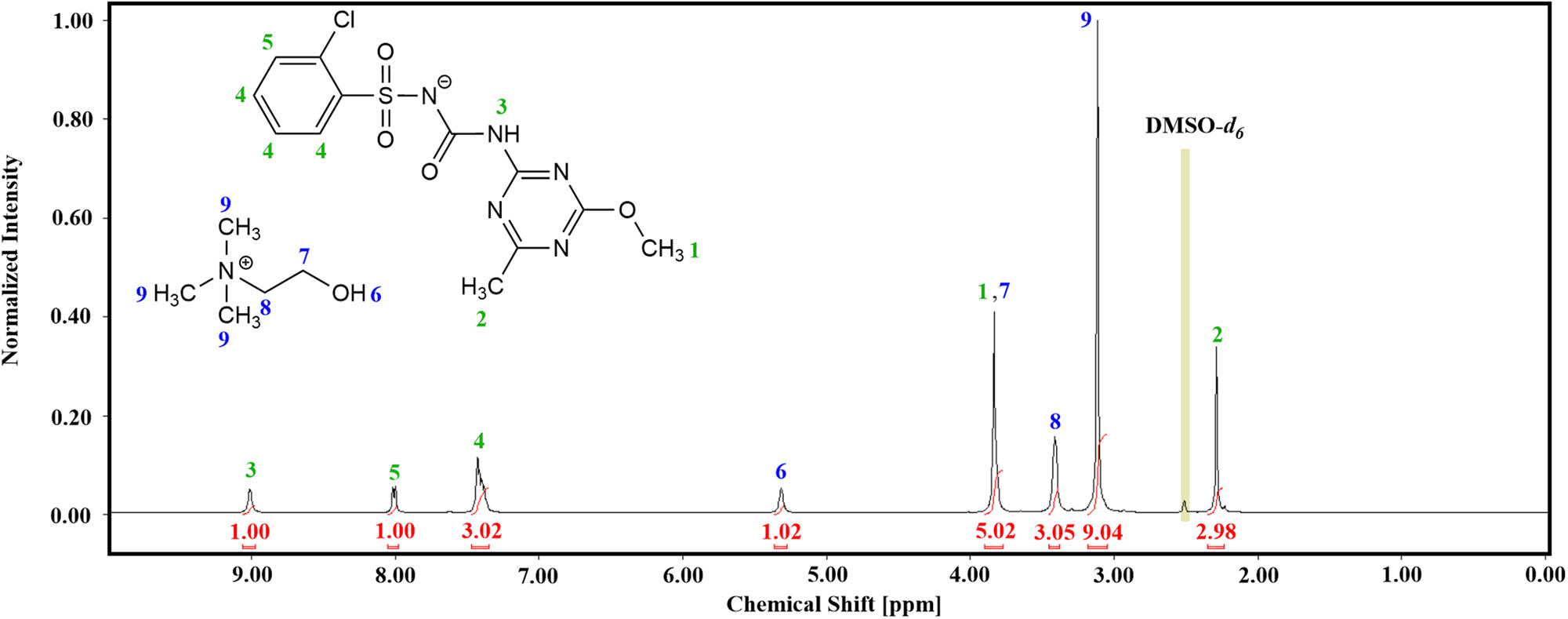
1H NMR spectrum of salt comprising cholinium cation and chlorsulfuron as the anion (4).
Direct comparison of FT-IR spectra of the potassium salt of chlorsulfuron [K][CHS] and IL comprising a didecyldimethylammonium cation (2) is presented in Figure 3. The most distinctive bands for the chlorsulfuron occurred in the range 1,720–750 cm−1. Therefore, in both spectra we can distinguish a strong peak from the amide group (ν ∼ C═O) at 1,670 cm−1 (2), which overlapped with peak originating from in-plane stretching vibrations from C═C conjugated bonds [32]. We can also observe a characteristic peak originating from a sulfonamide group [32] (ν (as) ∼ S═O) at 1,357 cm−1 (3), which occurred at slightly shorter values of wavelength for both analyzed salts compared to chlorsulfuron in the non-ionized form [33]. Moreover, both salts exhibited very strong signals at 1,200 and 1,234 cm−1 (4), which may be assigned to stretching vibrations from the aryl-methyl ether group. A group of peaks between 1,150 and 1,080 cm−1 (5) originate from the following vibrations: S═O symmetrical stretching [32], C–H aromatic in-plane bending [32] and C–N stretching from the triazine ring [34]. In Figure 3, we can also note a characteristic peak for sulfonamides [32,35], which occurred at 881 cm−1 (ν (s) ∼ C–N) (7). Bands appearing at 818 and 758 cm−1 (8) are due to C–H out of-plane bending vibrations from aromatic rings [32]. All the aforementioned peaks that originate from chlorsulfuron anion are also clearly visible in the FT-IR spectra of the other salts (1, 3 and 4), which serves as a proof of the presence of this herbicide in the structure of the obtained products. It should also be stressed that in the spectrum of IL 2 there are two broad, strong peaks at 2,925 and 2,853 cm−1 (1), which can be attributed to alkyl chains in didecyldimethylammonium cation (ν ∼ CH2). Furthermore, additional asymmetric stretching vibrations of the C–N group (ν (as) ∼ C–N) present in the utilized quaternary ammonium cation caused the increase in intensity of signal at 881 cm−1 (6) [36].
![Figure 3 FT-IR spectra of IL comprising a didecyldimethylammonium cation (2) and potassium salt of chlorsulfuron [K][CHS].](/document/doi/10.1515/chem-2020-0165/asset/graphic/j_chem-2020-0165_fig_003.jpg)
FT-IR spectra of IL comprising a didecyldimethylammonium cation (2) and potassium salt of chlorsulfuron [K][CHS].
3.1 Thermal properties
The thermal properties of products (1–4) are demonstrated in Table 2 and Figure 4. The lack of melting or crystallization events within the analyzed temperature range allows us to classify all salts as ILs. However, all of them exhibited a glass transition temperature (Tg) ranging from −3.7°C (for 2) to 74.8°C (for 1). Glass transition temperature noted for product with cholinium cation (47.4°C) (4) was much higher than glass transition temperature of structurally similar HIL containing a metsulfuron-methyl anion (Tg = 4°C) [16]. IL with longest alkyl chain (−3.7°C) (2) possessed the lowest glass transition temperature, whereas the highest value (74.8°C) was demonstrated by HIL with shortest possible alkyl chain – methyl group (1), which complies with available data [16].
Thermal properties of the obtained salts (1–4)
| Salt | Tga (°C) | Tmb (°C) | Tcc (°C) | Steps of degradation | T5%d (°C) | T50%e (°C) |
|---|---|---|---|---|---|---|
| 1 | 74.8 | — | — | 1 | 204 | 276 |
| 2 | −3.7 | — | — | 2 | 200 | 258 |
| 3 | 55.6 | — | — | 1 | 204 | 277 |
| 4 | 47.4 | — | — | 2 | 188 | 260 |
- a
Tg – glass transition temperature.
- b
Tm – melting point.
- c
Tc – temperature of crystallization.
- d
T5% – decomposition temperature of 5% sample.
- e
T50% – decomposition temperature of 50% sample.
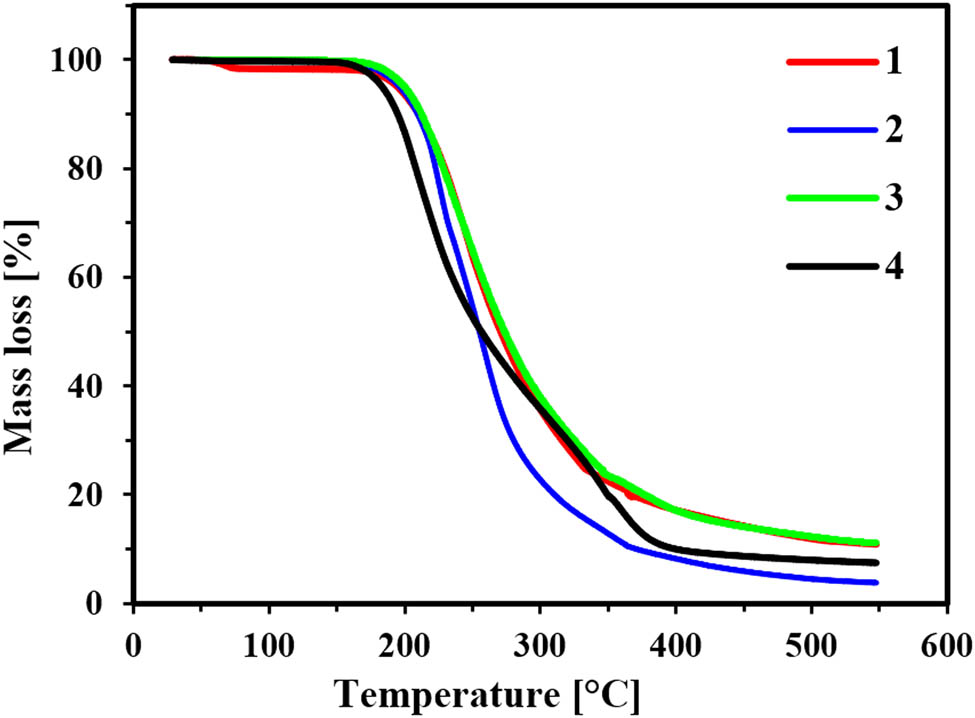
Thermal stability of the obtained salts (1–4).
According to thermogravimetric analysis, simple thermal decomposition with a single decomposition step was noted only for two of the examined salts (1 and 3) (Figure 4). On the other hand, products containing didecyldimethylammonium (2) and cholinium (4) cations exhibited a two-step decomposition characteristics. Such differences may be caused by the partial fragmentation of long alkyls (in the case of 2) [8] or the presence of functional (hydroxyl) group (in the case of 4) that are known to alter the thermal stability of HILs comprising tetraalkylammonium cation [19]. As shown in Table 2, choline salt (4) possessed the lowest initial decomposition temperature (T5% = 188°C), whereas products 1 and 3 (T5% = 204°C) exhibited the greatest value. Salt 2 possessed initial decomposition temperature equal to approx. 200°C. Interestingly, 4 proved to be more resistant to thermal decomposition than HIL containing the same cholinium cation and metsulfuron-methyl as the anion, which showed a decomposition of 5% at 150°C [16]. Molecule of metsulfuron-methyl differs from chlorsulfuron in the presence of methoxycarbonyl group instead of chlorine atom. Hence, we may conclude that the structure of sulfonylurea anion plays a crucial role in susceptibility of this group of HILs on thermal decomposition and glass transition temperature.
According to data in Table 2, the decomposition temperatures of 50% sample (T50%) were found to be higher for products comprising the shortest alkyl or alkylaryl substituents in the cation (1, 3), for which the decomposition temperature exceeded 275°C. Salts with decyl and 2-hydroxyethyl substituents (2 and 4) exhibited slightly lower temperature of decomposition (approx. 260°C). Additionally, notably greater mass loss of 2 in a temperature range of 250–350°C may be explained by a successive degradation of long alkyl groups and evaporation of volatile products of their decomposition. This assumption can be supported by literature data, according to which didecyldimethylammonium-based ILs exhibited very similar values of thermal stability parameters [37,38].
3.1.1 Solubility
The procedure described by Vogel was used to examine the solubilities of chlorsulfuron, its potassium salt as well as the prepared chlorsulfuron-based quaternary ammonium salts (1–4) [39]. As shown in Table 3, solvents characterized by diverse polarity were selected for the analysis. The collected data revealed that chlorsulfuron ([H][CHS]) was found to be soluble in DMSO, acetonitrile and chloroform, while its potassium salt ([K][CHS]) was characterized with good affinity only with water and DMSO. Interestingly, HIL comprising didecyldimethylammonium cation (2) was characterized by exceptionally good affinity with the majority of utilized solvents. This can be attributed to the presence of two long alkyl chains in the cation, which leads to the enhancement of compound’s surface activity and amphiphilicity. In effect, 2 turned out to be insoluble only in the most polar (water) and most non-polar (hexane). On the contrary, the other synthesized salts were found to be soluble in solvents possessing the highest polarity such as methanol, DMSO and water. Additionally, salts comprising the most polar cations (1 and 4) were found to be soluble only in these three solvents, while 3 exhibited affinity with semi-polar solvents as well. Regarding the less polar solvents, two of the obtained salts (2 and 3) were soluble in chloroform, while only one (2) was miscible with toluene and none dissolved in hexane. The explanation of this observation lies in the fact that solvents with low dielectric constants are generally poorly miscible with ILs (which are equal to 4.4 for chloroform, 2.3 for toluene and 0.0 for hexane) [40,41]. Generally, the differences between solubility of the synthesized chlorsulfuron-based salts lead to conclusion that it is possible to influence this parameter by simple modification of particular substituents in the cation.
Solubility of the prepared chlorsulfuron-based HILs (1–4) at 25°C
| Salt | Water | Methanol | DMSO | Acetonitrile | Acetone | Chloroform | Isopropanol | Ethyl acetate | Toluene | Hexane |
|---|---|---|---|---|---|---|---|---|---|---|
| 9.0a | 6.6 | 6.5 | 6.2 | 5.4 | 4.4 | 4.3 | 4.3 | 2.3 | 0.0 | |
| 1 | + | + | + | − | − | − | − | − | − | − |
| 2 | − | + | + | + | + | + | + | + | + | − |
| 3 | + | + | + | + | + | + | − | − | − | − |
| 4 | + | + | + | − | − | − | − | − | − | − |
| [H][CHS]b | − | − | + | ± | − | + | − | − | − | − |
| [K][CHS]c | + | − | + | − | − | − | − | − | − | − |
- a
Snyder polarity index [41]; “+”: good solubility; “±”: medium solubility; “−”: low solubility.
- b
Chlorsulfuron.
- c
Potassium salt of chlorsulfuron.
3.2 Herbicidal effectiveness
The efficiency of four synthesized HILs (1–4) has been studied under field conditions in the cultivation of flax variety Modran. This variety has a dominate structure of flax fiber in Poland. Generally, it can be concluded that all tested HILs demonstrated herbicidal activity, but their efficiency was highly influenced by the structure of each cation as well as weed species. The one-way ANOVA has shown the significant differences in mean effects of the action of the tested HILs toward both common lambsquarters (see F = 7.194; p-value = 0.00537 in Table S2) and common barnyard grass (see F = 39.61; p-value = 4.2 × 10−6 in Table S2). The most active turned out to be HILs with didecyldimethylammonium (2) and benzyltrimethylammonium (3) cations (Figure 5, Figures S13 and S14). The post hoc comparisons using the Tukey HSD test (α = 0.05) indicated that the mean reduction of common barnyard grass treated with these two HILs was significantly higher from the effects of tetramethylammonium (1) and cholinium (4). There were no statistically significant differences in the effectiveness of HILs toward common lambsquarters compared to the reference herbicide, except for cholinium (4) that showed significantly lower activity. As regards barnyard grass control, only salt 2 showed similar efficacy to the reference herbicide, while the other HILs showed significantly lower efficacy (Figure 5 and Table S2).
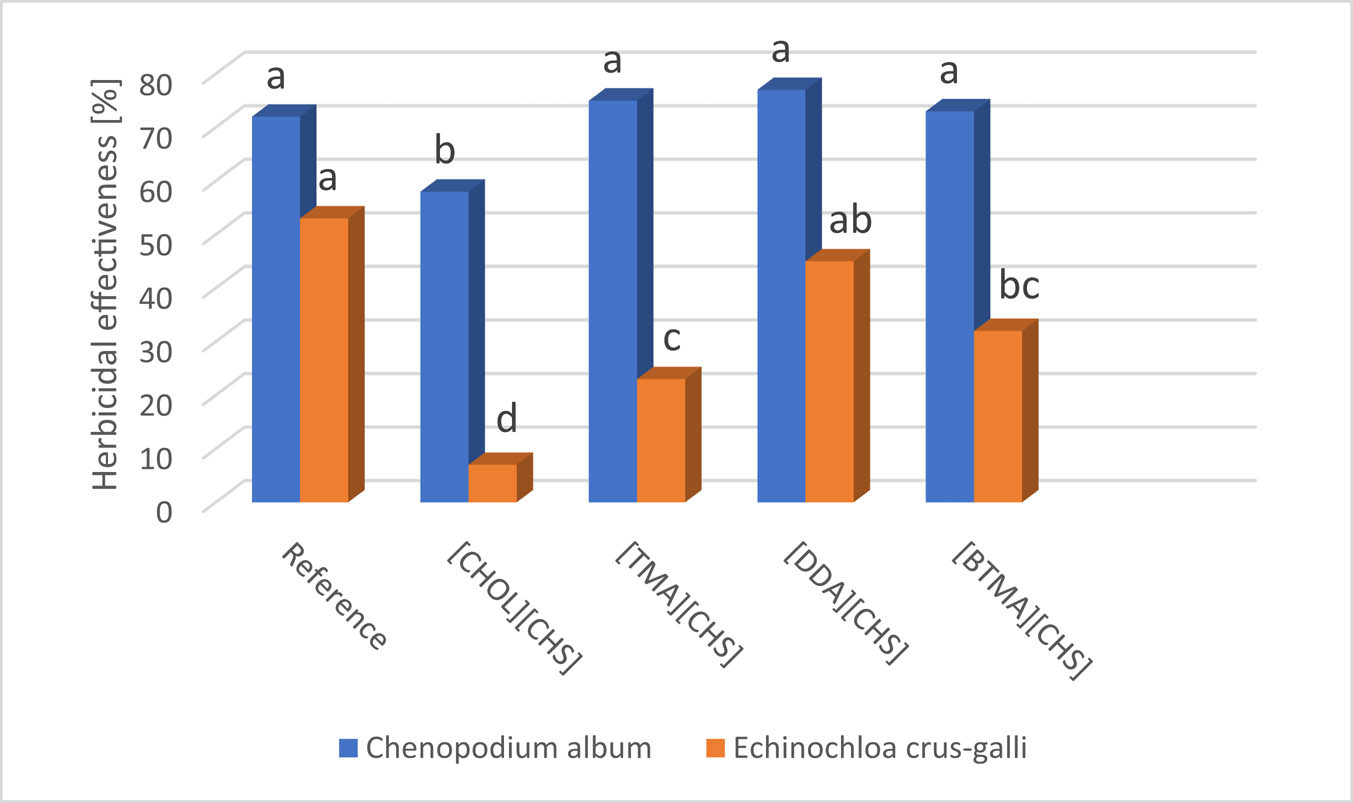
Herbicidal effectiveness of the tested HILs (1–4).
3.3 Effect on crops
All tested HILs were safe for flax plants. No damage symptoms were found throughout the growing season. There were no significant differences between the effects of applied HILs on the yield of fiber flax both in terms of seed yield and straw yield (Table S3). The average yield of straw from the plot (13.5 m2) varied between 6.6 and 8 kg. Among the tested HILs, the highest yield was obtained from plots treated with didecyldimethylammonium (2) and benzyltrimethylammonium (3) (Figure 6). The average yield of seeds from the plot was from 0.76 to 1.03 kg, but the statistical analysis showed that these differences were not significant (Table S3). When comparing the impact of individual HILs on seed yield, it should be stated that HILs benzyltrimethylammonium (3) and tetramethylammonium (1) exhibited the most favorable impact (Figure 7).
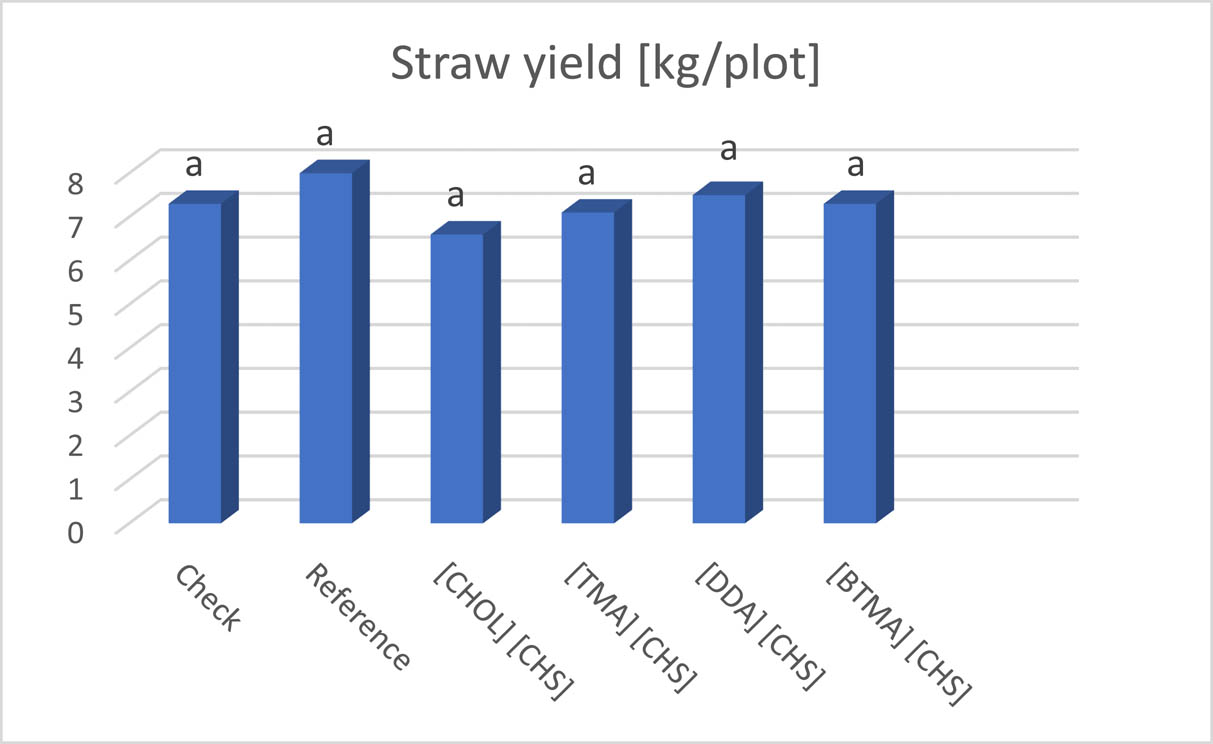
Influence of the tested HILs (1–4) on straw yield (kg/plot) of fiber flax.
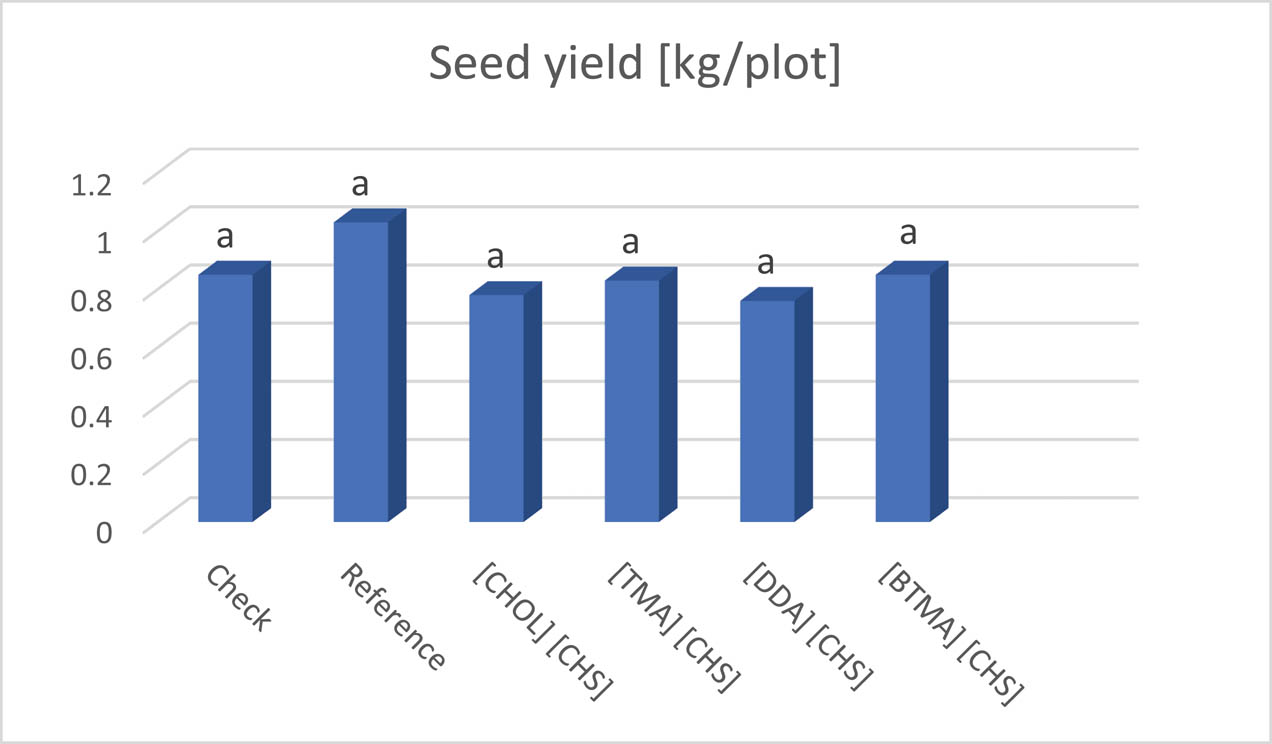
Influence of the tested ILs on seed yield (kg/plot) of fiber flax.
4 Conclusions
In this study, four new ILs with herbicidal anion from the group of sulfonylureas–chlorsulfuron were synthesized. This is the first report describing the use of chlorsulfuron as a source of anion in ILs. Elaborated synthesis methodology allows us to synthesize products with good efficiency, exceeding 90% under mild conditions and using green solvents. Compounds comprising small tetraalkylammonium cations turned out to possess glassy appearance at room temperature, while the presence of two long chains in the cation led to a product that occurred in the form of liquid. All new ILs were identified by using spectroscopic methods, such as infrared spectroscopy (FT-IR) and nuclear magnetic resonance (1H and 13C NMR). Additionally, the obtained products were tested in terms of their thermal properties, solubility and biological activity. All the salts were deprived from melting or crystallization events in the analyzed temperature range; however, they all exhibited a glass transition temperature in a range from −3.7 to 74.8°C, which is in accordance with available literature [16]. Furthermore, all compounds were thermally stable, with lowest decomposition temperature equal to 188°C for salt comprising a cholinium cation. Generally, conversion of chlorsulfuron herbicide into ILs caused an increase in solubility in water and methanol. All tested ILs with chlorsulfuron anion showed herbicidal effects without causing damage to fiber flax plants. The weed control of the tested HILs depended on the weed species. Higher herbicidal effectiveness was found for Chenopodium album L., while the tested HILs were less effective in controlling Echinochloa crus-galli L. Didecyldimethylammonium salt demonstrated by the greatest effectiveness among the examined HILs for both these weed species. The studied HILs had no effect on the yields of flax plants.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education.
Conflict of interest: The authors declare no conflict of interest.
References
[1] Fu YB, Peterson G, Diederichsen A, Richards KW. RAPD analysis of genetic relationships of seven flax species in the genus Linum L. Genet Resour Crop Evol. 2002;49(3):253–9.10.1023/A:1015571700673Suche in Google Scholar
[2] Çopur O, Gür MA, Karakuş M, Demirel U. Determination of correlation and path analysis among yield components and seed yield in oil flax varieties (Linum usitatissimum L.). J Biol Sci. 2006;6(4):738–43.10.3923/jbs.2006.738.743Suche in Google Scholar
[3] Pesticide Management Education Program. Chlorsulfuron – Herbicide Profile 3/85 [Internet]. [cited 2020 May 4]. Available from: http://pmep.cce.cornell.edu/profiles/herb-growthreg/cacodylic-cymoxanil/chlorsulfuron/chlsulf_prf_0385.html.Suche in Google Scholar
[4] Pernak J, Syguda A, Janiszewska D, Materna K, Praczyk T. Ionic liquids with herbicidal anions. Tetrahedron. 2011;67(26):4838–44.10.1016/j.tet.2011.05.016Suche in Google Scholar
[5] Hough WL, Smiglak M, Rodríguez H, Swatloski RP, Spear SK, Daly DT, et al. The third evolution of ionic liquids: active pharmaceutical ingredients. New J Chem. 2007;31(8):1429–36.10.1039/b706677pSuche in Google Scholar
[6] Niu J, Zhangi Z, Tang J, Tang G, Yang J, Wang W, et al. Dicationic ionic liquids of herbicide 2,4-dichlorophenoxyacetic acid with reduced negative effects on environment. J. Agric. Food Chem. 2018;66(40):10362–8.10.1021/acs.jafc.8b02584Suche in Google Scholar PubMed
[7] Niemczak M, Biedziak A, Czerniak K, Marcinkowska K. Preparation and characterization of new ionic liquid forms of 2,4-DP herbicide. Tetrahedron. 2017;73(52):7315–25.10.1016/j.tet.2017.11.032Suche in Google Scholar
[8] Zajac A, Kukawka R, Pawlowska-Zygarowicz A, Stolarska O, Smiglak M. Ionic liquids as bioactive chemical tools for use in agriculture and the preservation of agricultural products. Green Chem. 2018;20:4764–89.10.1039/C8GC01424HSuche in Google Scholar
[9] Tang G, Liu Y, Ding G, Zhang W, Liang Y, Fan C, et al. Ionic liquids based on bromoxynil for reducing adverse impacts on the environment and human health. New J. Chem. 2017;41:8650–5.10.1039/C7NJ01694HSuche in Google Scholar
[10] Tang G, Niu J, Zhang W, Yang J, Tang J, Tang R, et al. Preparation of acifluorfen-based ionic liquids with fluorescent properties for enhancing biological activities and reducing the risk to the aquatic environment. J. Agric. Food Chem. 2020;68(20):6048–57.10.1021/acs.jafc.0c00842Suche in Google Scholar PubMed
[11] Cojocaru OA, Shamshina JL, Gurau G, Syguda A, Praczyk T, Pernak J, et al. Ionic liquid forms of the herbicide dicamba with increased efficacy and reduced volatility. Green Chem. 2013;15(8):2110–20.10.1039/c3gc37143cSuche in Google Scholar
[12] Zhu J, Ding G, Liu Y, Wang B, Zhang W, Guo M, et al. Ionic liquid forms of clopyralid with increased efficacy against weeds and reduced leaching from soils. Chem Eng J. 2015;279:472–7.10.1016/j.cej.2015.05.025Suche in Google Scholar
[13] Ding G, Liu Y, Wang B, Punyapitak D, Guo M, Duan Y, et al. Preparation and characterization of fomesafen ionic liquids for reducing the risk to the aquatic environment. New J Chem. 2014;38(11):5590–6.10.1039/C4NJ01186DSuche in Google Scholar
[14] Syguda A, Marcinkowska K, Materna K. Pyrrolidinium herbicidal ionic liquids. RSC Adv. 2016;6:63136–42.10.1039/C6RA12157HSuche in Google Scholar
[15] Choudhary H, Pernak J, Shamshina JL, Niemczak M, Giszter R, Chrzanowski Ł, et al. Two herbicides in a single compound: double salt herbicidal ionic liquids exemplified with glyphosate, dicamba, and MCPA. ACS Sustainable Chem Eng. 2017;5(7):6261–73.10.1021/acssuschemeng.7b01224Suche in Google Scholar
[16] Wang W, Liang Y, Yang J, Tang G, Zhou Z, Tang R, et al. Ionic liquid forms of mesotrione with enhanced stability and reduced leaching risk. ACS Sustainable Chem. Eng. 2019;7(19):16620–28.10.1021/acssuschemeng.9b03948Suche in Google Scholar
[17] Wang B, Ding G, Zhu J, Zhang W, Guo M, Geng Q, et al. Development of novel ionic liquids based on bentazone. Tetrahedron. 2015;71(41):7860–4.10.1016/j.tet.2015.08.029Suche in Google Scholar
[18] Wang W, Zhu J, Tang G, Huo H, Zhang W, Liang Y, et al. Novel herbicide ionic liquids based on nicosulfuron with increased efficacy. New J Chem. 2019;43(2):827–33.10.1039/C8NJ05903ASuche in Google Scholar
[19] Pernak J, Czerniak K, Niemczak M, Ławniczak Ł, Kaczmarek DK, Borkowski A, et al. Bioherbicidal ionic liquids. ACS Sustainable Chem Eng. 2018;6(2):2741–50.10.1021/acssuschemeng.7b04382Suche in Google Scholar
[20] Tang G, Wang B, Ding G, Zhang W, Liang Y, Fan C, et al. Developing ionic liquid forms of picloram with reduced negative effects on the aquatic environment. Sci Total Environ. 2018;616–617:128–34.10.1016/j.scitotenv.2017.10.288Suche in Google Scholar PubMed
[21] Ławniczak Ł, Materna K, Framski G, Szulc A, Syguda A. Comparative study on the biodegradability of morpholinium herbicidal ionic liquids. Biodegradation. 2015;26:327–40.10.1007/s10532-015-9737-2Suche in Google Scholar PubMed PubMed Central
[22] Niemczak M, Rzemieniecki T, Sobiech Ł, Skrzypczak G, Praczyk T, Pernak J. Influence of the alkyl chain length on the physicochemical properties and biological activity in a homologous series of dichlorprop-based herbicidal ionic liquids. J Mol Liq. 2019;276:431–40.10.1016/j.molliq.2018.12.013Suche in Google Scholar
[23] Czuryszkiewicz D, Maćkowiak A, Marcinkowska K, Borkowski A, Chrzanowski Ł, Pernak J. Herbicidal ionic liquids containing the acetylcholine cation. ChemPlusChem. 2019;84(3):268–76.10.1002/cplu.201800651Suche in Google Scholar PubMed
[24] Sanders LM, Zeisel SH. Choline: dietary requirements and role in brain development. Nutr Today. 2007;42(4):181–6.10.1097/01.NT.0000286155.55343.faSuche in Google Scholar PubMed PubMed Central
[25] Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis. 2011;34(1):3–15.10.1007/s10545-010-9088-4Suche in Google Scholar PubMed
[26] Kaczmarek DK, Czerniak K, Klejdysz T. Dicationic ionic liquids as new feeding deterrents. Chem. Pap. 2018;72:2457–66.10.1007/s11696-018-0495-6Suche in Google Scholar PubMed PubMed Central
[27] Syguda A, Gielnik A, Borkowski A, Woźniak-Karczewska M, Parus A, Piechalak A, et al. Esterquat herbicidal ionic liquids (HILs) with two different herbicides: evaluation of activity and phytotoxicity. New J. Chem. 2018;42:9819–27.10.1039/C8NJ01239CSuche in Google Scholar
[28] Pernak J, Chwala P. Synthesis and antimicrobial activities of choline-like quaternary ammonium chlorides. Eur J Med Chem. 2003;38:1035-42.10.1002/chin.200412078Suche in Google Scholar
[29] Abbott AP, Bell TJ, Handa S, Stoddart B. O-Acetylation of cellulose and monosaccharides using a zinc based ionic liquid. Green Chem. 2005;7(10):705–7.10.1039/b511691kSuche in Google Scholar
[30] Tretyn A, Kendrick RE. Acetylcholine in plants: presence, metabolism and mechanism of action. Bot Rev. 1991;57(1):33–73.10.1007/BF02858764Suche in Google Scholar
[31] Wilson C, Lee MD, McCarron JG. Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J Physiol. 2016;594(24):7267–307.10.1113/JP272927Suche in Google Scholar PubMed PubMed Central
[32] Gowda BT, Jyothi K, D’Souza JD. Infrared and NMR spectra of arylsulphonamides, 4-X-C6H4SO2NH2 and i-X, j-YC6H3SO2NH2 (X = H; CH3; C2H5; F; Cl; Br; I or NO2 and i-X, j-Y = 2,3-(CH3)2; 2,4-(CH3)2; 2,5- (CH3)2; 2-CH3, 4-Cl; 2-CH3, 5-Cl; 3-CH3, 4-Cl; 2,4-Cl2 or 3,4-Cl2). Z Naturforsch Sect A J Phys Sci. 2002;57(12):967–73.10.1515/zna-2002-1210Suche in Google Scholar
[33] Liu G, Zhang R, Li L, Huang X, Li T, Lu M, et al. Anti-agglomeration behavior and sensing assay of chlorsulfuron based on acetamiprid-gold nanoparticles. Nanomaterials. 2018;8(7):499.10.3390/nano8070499Suche in Google Scholar
[34] Modak A, Mondal J, Sasidharan M, Bhaumik A. Triazine functionalized ordered mesoporous polymer: a novel solid support for Pd-mediated C–C cross-coupling reactions in water. Green Chem. 2011;13(5):1317–31.10.1039/c1gc15045fSuche in Google Scholar
[35] Tanaka Y, Tanaka Y. Infrared absorption spectra of organic sulfur compounds. I. Studies on S–N stretching bands of benzenesulfonamide derivatives. Chem Pharm Bull. 1965;13(4):399–405.10.1248/cpb.13.399Suche in Google Scholar
[36] Niemczak M, Rzemieniecki T, Biedziak A, Marcinkowska K, Pernak J. Synthesis and structure–property relationships in herbicidal ionic liquids and their double salts. ChemPlusChem. 2018;83(6):529–41.10.1002/cplu.201800251Suche in Google Scholar
[37] Pernak J, Nawrot J, Kot M, Markiewicz B, Niemczak M. Ionic liquids based stored product insect antifeedants. RSC Adv. 2013;3(47):25019–29.10.1039/c3ra41716fSuche in Google Scholar
[38] Hough-Troutman WL, Smiglak M, Griffin S, Matthew Reichert W, Mirska I, Jodynis-Liebert J, et al. Ionic liquids with dual biological function: sweet and anti-microbial, hydrophobic quaternary ammonium-based salts. New J Chem. 2009;33(1):26–33.10.1039/B813213PSuche in Google Scholar
[39] Vogel AI. In: Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR, editors. Textbook of practical organic chemistry, 5th edn. New York: Wiley, 1989, p. 138.Suche in Google Scholar
[40] Ropel L, Belvèze LS, Aki SNVK, Stadtherr MA, Brennecke JF. Octanol–water partition coefficients of imidazolium-based ionic liquids. Green Chem. 2005;7(2):83–90.10.1039/B410891DSuche in Google Scholar
[41] Snyder LR. Classification of the solvent properties of common liquids. J Chromatogr A. 1974;92(2):223–30.10.1016/S0021-9673(00)85732-5Suche in Google Scholar
© 2020 Marcin Praczyk et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Artikel in diesem Heft
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

