A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
-
Majed Alshamaileh
Abstract
In the current study, the metabolism of two novel psychoactive substances (NPSs), mephedrone and methoxetamine (MXE), was studied in vitro in pig liver microsomes to determine potential metabolites by liquid chromatography-mass spectrometry (LC-MS). Later, in vitro studies were performed using HepaRG™ cells to determine the human metabolites of these drugs using gas chromatography-mass spectrometry (GC-MS). The aim of the study was to detect metabolites from the metabolic mixture in the human cell lines using GC-MS, since this is a more readily available technique within forensic laboratories. Microsomes were prepared through a conventional ultracentrifugation method and incubated under optimized conditions with the drugs for 3 h. Subsequently, the samples were investigated using LC-MS. A similar methodology was then applied in the HepaRG™ cells, and the GC-MS conditions were optimized using N,O-bis(trimethylsilyl)trifluoroacetamide as a derivatization agent. The analysis showed two molecules from a successful in vitro metabolism, namely, hydroxytoly-mephedrone and nor-dihydro mephedrone. For MXE, two metabolites are presented produced by the O-demethylation and reduction of the ketone moiety to the corresponding alcohol, respectively. Using the human HepaRG™ cells, only nor-dihydro mephedrone could be identified by GC-MS. Since hydroxytoly-mephedrone and the MXE metabolites are more polar, it is suggested that GC-MS even with derivatization may not be suitable. In addition, cytotoxicity was studied utilizing HepaRG™ cell lines. The drugs show cytotoxic effects causing in vitro cell death, within the specified range of EC50 0.3211 mM (79 μg/mL) and 0.6297 mM (111 μg/mL) for mephedrone and MXE, respectively. These drugs were able to cause 73–84% cell death.
1 Introduction
In recent years, many designer drugs have appeared on the recreational drug market, where most of them are sold as “legal highs” by street suppliers or via the Internet [1]. “Designer drugs” are those produced by performing minor alterations to one or more functional groups of a known chemical with specific pharmacological activity to avoid the legal regulations and to produce more effective substances [2].
The therapeutic and toxicological profiles of the designer drugs are not systemically studied by pharmaceutical companies and regulatory authorities as in the case of pharmaceutical drugs. Therefore, the analysis of designer drugs and their metabolites has recently received increasing interest from academic and governmental researchers [3]. Metabolic studies with regard to the toxicological profiles of designer drugs are one of the most important research areas. The limited opportunities to obtain reference standards for the metabolites of novel psychoactive substances (NPSs) make in vitro production of these metabolites a valuable tool to develop analytical methods for the detection of NPS and their metabolites. However, caution needs to be added as the conclusions from these studies might not be directly applicable to humans. As Tice et al. suggested [4] the following reasons to observe caution: (i) problems to include xenobiotic metabolism into in vitro assays, (ii) problems to follow interactions between different cell types, (iii) difficulties in extrapolating the results from in vivo doses to in vitro concentrations, and (iv) problems to track long-term exposures in vitro.
Since mephedrone was described as one of the first legal highs in 2007, it has been detected in many toxicological samples [5,6,7,8]. Due to the fatalities reported after the consumption of mephedrone, it was added to the UK Misuse of Drugs Act 1971 in 2010 before the Psychoactive Substance Bill was passed in the UK in 2015 [9]. No much data are available about the clinical or toxic effects of mephedrone, and it has never been licensed as a medicine since its first appearance in the illicit drug market in 2007. Other countries have followed a similar approach; and it is now banned, but in others it still remains as a legal substance. Although being a popular drug, limited data are still found on the metabolism of mephedrone in the literature [10,11,12]. More recent work from M. Farré et al. has opened the door to a greater understanding of the pharmacokinetics [13,14], in vivo human metabolism [15], pharmacology [16,17] and clinical studies [18].
Methoxetamine (MXE) 2-(ethylamino)-2-(3-methoxyphenyl)cyclohexanone is a newer, synthetic, psychoactive drug derived from ketamine [12,19]. It is believed that MXE is being used as a ketamine substitute, owing to its ability to produce comparable hallucinogenic and dissociative effects [20]. Initially, MXE was designed in part to prevent the urotoxicity associated with ketamine and to be tested as an antidepressant [19,20]. However, since its debut on the Internet in 2010, it has become a popular recreational drug especially among adolescents. Indeed, there has been an increase in the number of reports regarding the abuse of MXE by humans, which resulted in serious or even fatal outcomes [21,22]. Accordingly, MXE was included in the list of new psychoactive substances of the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), which was categorized as a ketamine derivative [20]. In April 2012, MXE was placed under temporary class drug control in the UK and is now banned through the Psychoactive Substance Bill (2015). Some information regarding the toxicological and clinical data can be found in literature for MXE, with limited metabolic studies being available [19,23,24].
Even though these compounds are now banned in the UK and other countries, the limited information on their metabolism may still make it hard for investigations and emergency services in the UK or other countries without a blanket ban. The increasing reported abuse of NPSs including mephedrone and MXE, combined with restricted information about their toxicological profile, has created a requirement for the production and identification of their metabolites.
Therefore, this research will focus on the initial screening of the metabolites. Considering the limitations, in vitro approaches are preferred methods for studying the metabolism of designer drugs when ethical considerations are in place. Additionally, in vitro drug metabolism methods are generally easier and more rapid to apply compared to in vivo approaches. In vitro metabolism using microsomes, an approach to study the metabolic profile of new emerging drugs, is widely used in the pharmaceutical industry. Microsomes are vesicle-like artefacts that are made from portions of endoplasmic reticulum after disintegrating eukaryotic cells; they can be concentrated and separated from other cellular debris by differential centrifugation [25]. The pig liver has been used as an animal model for drug metabolic studies because of its similarity in size and physiology with that of humans and the similar distribution of different families of CYP450 enzymes between pigs and humans [26]. It is therefore a good screening tool to identify potential metabolites.
Also, the use of in vitro human cell lines, such as HepaRG™ cells, to identify any metabolites needs to be considered in studies as animal models may not predict the exact human metabolism. The aim of the study was to detect metabolites from the metabolic mixture in the human cell lines using a gas chromatography–mass spectrometry (GC-MS), since this is a more readily available technique within forensic laboratories. Also, a comparison with an animal model using liquid chromatography–mass spectrometry (LC-MS) is included. Consequently, this study tries to identify potential metabolites of MXE and mephedrone, which could be easily analysed in commercial laboratories. To the authors’ knowledge, human HepaRG™ cells have not been used to identify any metabolites for MXE or mephedrone.
2 Materials and methods
Mephedrone and MXE were purchased legally from web-based companies before they were declared illegal and stored under the appropriate drug license after the ban. The structure and purity of the purchased drugs were confirmed by Fourier transform infrared spectroscopy (FTIR) and MS.
Tris–Cl 1 M stock solution, sodium pyrophosphate and EDTA, nicotinamide adenine dinucleotide phosphate (NADP), glucose-6-phosphate dehydrogenase enzyme (G6PD) and glucose-6-phosphate (G6P), trypan blue, and phosphate buffer (pH = 7) solution were all of analytical grade (Sigma, UK). Potassium chloride, monobasic sodium phosphate, dibasic sodium phosphate, magnesium chloride, methanol, and formic acid (99%) were all of analytical grade (Fisher, UK). Deionized water (resistance = 15 MΩ) was used in all preparations and, where needed, filtered, and degassed prior to use. For derivatization, N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA was purchased from Supelco (UK).
Select NPS were studied using pooled human suspension hepatocytes, namely, primary human hepatocytes, cryopreserved hepatocytes recovery medium (catalogue number: CM7000), and primary human hepatocytes maintenance supplement pack (catalogue number: CM4000) containing dexamethasone and COCKTAIL-B solution were all from Gibco (UK).
For the study of selected NPS using HepaRG™: HepaRG™ cryopreserved cell line (catalogue number: HPRGC10), William’s E Medium-no phenol red (catalogue number: A12176-01), HepaRG™ Thaw, Plate, & General Purpose Medium Supplement (catalogue number: HPRG770), HepaRG Tox Medium Supplement (catalogue number: HPRG730), HepaRG Maintenance/Metabolism Medium Supplement (catalogue number: HPRG720), and GlutaMAX™-I Supplement (catalogue number: A12860-01) were all from Gibco (UK).
Fresh pig liver was obtained from a local abattoir.
2.1 Preparation of buffers and solutions
The NADPH-regenerating system (30 mM G6P, 5 U/mL G6PD, 10 mM NADP, and 30 mM MgCl2) was freshly prepared every day before incubation. The homogenizing buffer consisted of 0.1 M Tris HCl, 10 mM EDTA, and 150 mM KCl; the microsome-preservation buffer of 0.05 M Tris HCl, 10 mM EDTA and 20% (v/v) glycerol, and the pyrophosphate buffer of 0.1 M Na-pyrophosphate and 10 mM EDTA, with the final pH values adjusted to 7.4 using sodium chloride or hydrochloric acid.
HepaRG™ Thaw, Plate, & General Purpose Medium and HepaRG Tox Medium were freshly and aseptically prepared by adding 1 mL of GlutaMAX and 14 mL of HepaRG™ Thaw, Plate, & General Purpose Medium or HepaRG™ Tox Medium Supplement to 100 mL of William’s Medium E.
HepaRG™ Maintenance/Metabolism Medium was freshly and aseptically prepared by adding 1 mL of GlutaMAX and 16 mL of HepaRG™ Maintenance/Metabolism Medium Supplement to 100 mL of William’s Medium E.
Master stock solutions of mephedrone and MXE were prepared in HepaRG™ Thaw, Plate, & General Purpose Working Medium and were at least 10 times more concentrated than the highest concentration tested (i.e. 1.6 × 102 mM). All stock solutions were preserved at −20°C for later use.
Pooled human hepatocyte stocks were stored and maintained under liquid nitrogen (−196°C) until needed. The cryopreserved pooled hepatocyte vial was thawed and re-suspended into 10 mL media to a final concentration of 1 × 106 cells/mL (10 × 106 total cells count). Cell viability was estimated to be greater than 85% using trypan blue exclusion test. Trypan blue test solution test was prepared at 0.4% in phosphate-buffered solution and 0.1 mL added to 1 mL cell solution and examined under microscope.
2.2 Isolation and incubation of liver microsomes
Liver microsomes were isolated following previously published protocols with minor modifications [23,27,28]. Briefly, liver was freshly obtained, washed with homogenizing buffer at pH 7, and sliced into 4 g portions. The sliced portions were further cut into small pieces using a surgical blade, broken apart with 3 volumes of ice-cold homogenizing buffer using a handheld bio-homogenizer, and homogenized using Teflon mortar and pestle. The homogenate was centrifuged for 15 min at 12,500 × g at 4°C, in an Allegra X-22 from Beckman Coulter™ (UK), and the supernatant was divided into two portions, of which one was stored as S9 fraction and the other was further ultracentrifuged for 45 min at 76,800 × g at 4°C in a Mikro 20 Hettich Zentrefugen Microcentrifuge (USA). The pellet was re-suspended with 2 volumes of ice-cold pyrophosphate buffer, further homogenized, and ultracentrifuged for 70 min at 76,800 × g at 4°C; the supernatant was discarded and the formed pellet was suspended with 3 volumes of microsome-preservation buffer, further homogenized, and stored at −80°C in a Binder UF V 500 ULT Freezer until needed.
Five 1.5-mL microcentrifuge tubes for each test compound were prepared; five 1.5-mL microcentrifuge tubes included duplicate tubes for both 0 time and 3 h, and one tube for the negative control. Each tube contained 375 µL 0.1 M phosphate buffer (pH 7.4), pre-warmed to 37°C, 50 µL NADPH-regeneration system, and 50 µL of 10-fold test compound stock solution (100 µg/mL). The mixture was then incubated in a water bath for 5 min at 37°C. For the negative control, 50 µL NADPH-regeneration systems were replaced by 50 µL 0.1 M phosphate buffer. Prepared microsomes or S9 fractions were thawed and diluted to 10 mg/L and 25 µL added to each tube. Samples were mixed and incubated at 37°C in a shaking water bath. The process was terminated by adding 250 µL ice-cold methanol at 0 time for two of the positive test tubes and at 3 h for the other two positive tubes and the negative tube sample. Sample tubes were centrifuged for 10 min at 13,000 × g. The supernatant was then filtered through 0.45 µm filters (Sartorius, Germany) and 5 µL injected onto the LC-MS system (Agilent, US).
LC-MS analyses were performed with an Agilent 6310 LC-MS ion trap system equipped with electrospray ionization. Data acquisition was performed using DataAnalysis for 6300 Series Ion Trap LC-MS version 4.0 © software, and the scans were performed at a scan range between 40 and 500 amu. The analytes were detected in positive ionization mode (MS+). The instrument was tuned and calibrated according to manufacturer’s specifications. Chromatographic separations were carried out at room temperature on Agilent Eclipse plus C18, 3.5 µm, 4.6 × 150 mm. The mobile phase consisted of methanol/water/formic acid (50/50/0.1, v/v/v), isocratic flow rate of 0.30 mL/min for 10 min. The MS detector was tuned according to the following conditions: capillary voltage: 3,500 V, capillary intensity: 1 nA, maximum acquisition time: 200 ms, nebulizer pressure: 30 psi, dry gas flow: 11 L/min, and dry gas temperature: 325°C.
2.3 Preparation and culture of pooled human hepatocytes
Pooled human hepatocyte stocks were stored and maintained under liquid nitrogen (−196°C) until needed. The cryopreserved pooled hepatocyte vial was thawed and re-suspended in 10 mL media to a final concentration of 1 × 106 cells/mL (10 × 106 total cells count). Cell viability was estimated to be greater than 85% using trypan blue exclusion test. Trypan blue test solution test was prepared at 0.4% in phosphate-buffered solution and 0.1 mL added to 1 mL cell solution and examined under microscope.
The final concentration of 10, 20, 40, 60, 80, and 100 μM of mephedrone and MXE was used for metabolism studies using pooled human hepatocytes. For each concentration, the metabolic profile was studied at 0, 30, 60, 90, and 120 min. The metabolic reaction was initiated by adding 60 μL of the prepared cell suspension and 15 μL of the 10× stock drug onto 1.5 mL Eppendorf tube and topped up with media into 150 μL (final drug concentration = 1×, final cell count > 50 × 103 viable cells). At the specified time, the reaction was terminated by adding 50 μL 10% DMSO.
2.4 Preparation and culture of cryopreserved HepaRG™ cells
HepaRG™ cell stocks were stored and maintained under liquid nitrogen (−196°C) until needed. HepaRG™ vial was semi-thawed in a water bath at 37°C and the cell suspension was aseptically transferred into pre-warmed 9 mL of HepaRG™ Thaw, Plate, & General Purpose Working Medium. After centrifugation of cells at room temperature (20°C) at 357 g for 2 min, the supernatant was aspirated and the pellet re-suspended in 5 mL of the HepaRG™ Thaw, Plate, & General Purpose Working Medium. The viability of the HepaRG™ was estimated to be greater than 85% using trypan blue exclusion test.
Concentrations of 10, 20, 40, 60, 80, and 100 μM of mephedrone and MXE were used for metabolism studies using HepaRG™. For each concentration, the metabolic profile was studied at 0 time, 90 min and 24 h. Previously prepared HepaRG™ cells were seeded onto central wells of collagen-coated 96-well plates at a density of 50 × 103 cells/well in HepaRG™ Thaw, Plate, & General Purpose Working Medium (50 μL cell solution topped up to 200 μL media). After 6 h, the media were renewed with 20 μL 10× master solution of each drug concentration and topped up to 200 μL HepaRG™ Tox Medium Supplement. Two wells were spared as blank which were topped up to 200 μL HepaRG™ Tox Medium Supplement media; and for zero times sample, 20 μL of each drug concentration was topped up to 200 μL HepaRG™ Tox Medium Supplement media.
The final 200 μL resultant aqueous mixture was extracted using 400 μL ethyl acetate and then centrifuged at 3,500 rpm for 10 min. Organic layer was moved into a clean glass vial via a pipette and then evaporated to dryness using a sample concentrator under streaming N2, reconstituted with 50 μL acetonitrile, and BSTFA was added in excess to the mixture warmed in water bath at 50°C for 10 min to enhance the derivatization reaction before the samples were then injected into GC-MS (Perkin Elmer, USA).
The GC-MS components were from Perkin Elmer (USA). GC model Clarus 600 equipped with an auto sampler and MS model Clarus 600 operated with TurboMass 6.4.2 (Perkin Elmer, USA, 2008). Standards and samples were run on an Agilent Technologies DB-1 MS column (30 m × 0.25 mm × 0.25 μm). Oven temperature programme was as following: 150°C for 4 min increasing at 25°C/min to a final temperature of 350°C. Injector port temperature was held at 250°C. The carrier gas was helium at 1 mL/min and the injection volume was 1 μL. The transfer line temperature was held at 280°C. Positive ionization was achieved using an electron impact source at 200°C with electron energy of 70 eV and the multiplier was set to 300 V. The peaks were observed in total ion count after a 3-min solvent delay. The scan range was 45–320 m/z with a scan time of 0.5 s and an inter-scan delay of 0.01 s.
Electronic balance was from Sartorius (Germany), GPE-scientific Teflon mortar & pestle (UK), ultrasonic bath was from Kerry (UK). Shaking water bath was from Grant Instruments (UK), and a sample concentrator model was DRI-BLOCK®, DB.3A from TECHNE (UK), which were used for sample preparation.
2.5 Using HepaRG™ for MTT reduction studies – Cytotoxicity
Previously prepared HepaRG™ cells were seeded onto central wells of collagen-coated 96-well plates at a density of 100 × 103 cells/well in HepaRG™ Thaw, Plate, & General Purpose Working Medium (50 μL cell solution topped up to 200 μL media). Two wells were spared as blank control, and the peripheral wells were filled with sterile water to prevent media evaporation. The 96-well plate was incubated at 37°C in a humidified atmosphere with 5%/95% CO2/ambient atmosphere and 100% relative humidity for cell adhesion. After 6 h, the medium was renewed; and on day 1 and day 4, the medium was replaced with HepaRG™ Tox medium and incubated under the same conditions, while observing the cell morphology at the time of replacing the media, the methylthiazolyldiphenyl-tetrazolium bromide test (MTT) reduction assay experiments were carried on day 7 with freshly prepared test drugs. On day 7 after cell seeding, the HepaRG™ Tox medium was renewed and the cells were incubated for 48 h with different concentrations of mephedrone and MXE at 37°C, in a humidified atmosphere with 5%/95% CO2/ambient atmosphere and 100% relative humidity. Those drug concentrations were freshly prepared from the master stock concentration in HepaRG™ Tox medium. Each individual plate included two replicates of blank controls (just media) and two replicates of negative control (full cell and media with no test agents). After the 48-h incubation period, the incubation medium was aspirated, and the attached cells were washed one time, followed by the addition of fresh HepaRG™ Tox medium containing 0.5 mg/L MTT. The cells were re-incubated at 37°C in a humidified atmosphere with 5%/95% CO2/ambient atmosphere and 100% relative humidity for 3 h. After aspirating the medium, the formed insoluble crystals were dissolved in 100% dimethyl sulfoxide (DMSO). A multiwell plate reader was used to run the spectrophotometric analysis at 570 nm wavelength.
For each drug, a range of concentrations from 4 × 10−2 to 16 mM was tested, each in three independent experiments. The mean absorbance of the triplicate experiments for each drug concentration on each occasion was expressed as a percentage of the mean absorbance of the control wells.
2.6 Ethical standards
We declare that the presented work complies with the current British law and ethical standards. This article does not contain any studies with human participants or live animals performed by any of the authors. The pigs were slaughtered in the abattoir and sold for food consumption. Consequently, the results presented in this work are not linked to animal testing. All procedures were ethically approved and risks assessed by the ethics committee of the University of Lincoln.
3 Results
3.1 Study with pig liver microsomes using LC-MS
Initially, LC-MS was used for all studies of pig liver microsomes to determine the possible metabolites. Mephedrone was incubated with an in-house prepared pig liver microsomes under the specified conditions and after termination of the metabolic process samples were centrifuged, filtered, and injected onto the LC-MS system. In this study, two metabolites, namely, hydroxytoly-mephedrone produced by hydroxylation of the methyl group attached to the aromatic ring, and nor-dihydro mephedrone produced by a two-step N-demethylation and reduction of the ketone moiety were monitored as illustrated in Figure 1. The proposed structures of the detected metabolites were determined from the fragments present in the respective mass spectra and according to the general known routes (shown in Figure 1 top). Negative controls were used to verify the absence of these metabolites in the absence of these drugs. As seen in Figure 1 (bottom), the suggested metabolic pathway provides different routes to detoxification by making the molecules more soluble: using hydroxylation to produce hydroxytoly-mephedrone, by reducing the carbonyl group to an alcohol and N-demethylation to modify or reduce the hydrophobicity by eliminating methyl groups from the molecule.

Suggested phase I metabolic pathway of mephedrone adapted from published data for the metabolism of mephedrone and similar drugs (top). A proposed present metabolic pathway through the identified metabolites of mephedrone utilizing LC-MS (bottom): (1) mephedrone, (2) hydroxytoly-mephedrone, (3) nor-dihydro mephedrone, and (*) undetected intermediates.
Spectra of the identified metabolites of mephedrone are illustrated in Figure 2. Figure 2a shows the characteristic masses for mephedrone (m/z 177, 160) confirming the presence in the metabolic pool. As seen in Figure 1, one suggested metabolite is hydroxytoly-mephedrone, in which two characteristic ions (m/z 160, 177) can be seen in the mass spectra of Figure 2b, confirming its presence. Figure 2c also shows the characteristic mass (m/z 166) for the N-demethylation to produce nor-dihydro mephedrone. Many of the masses and processes imply a loss of water as seen in Figure 2.
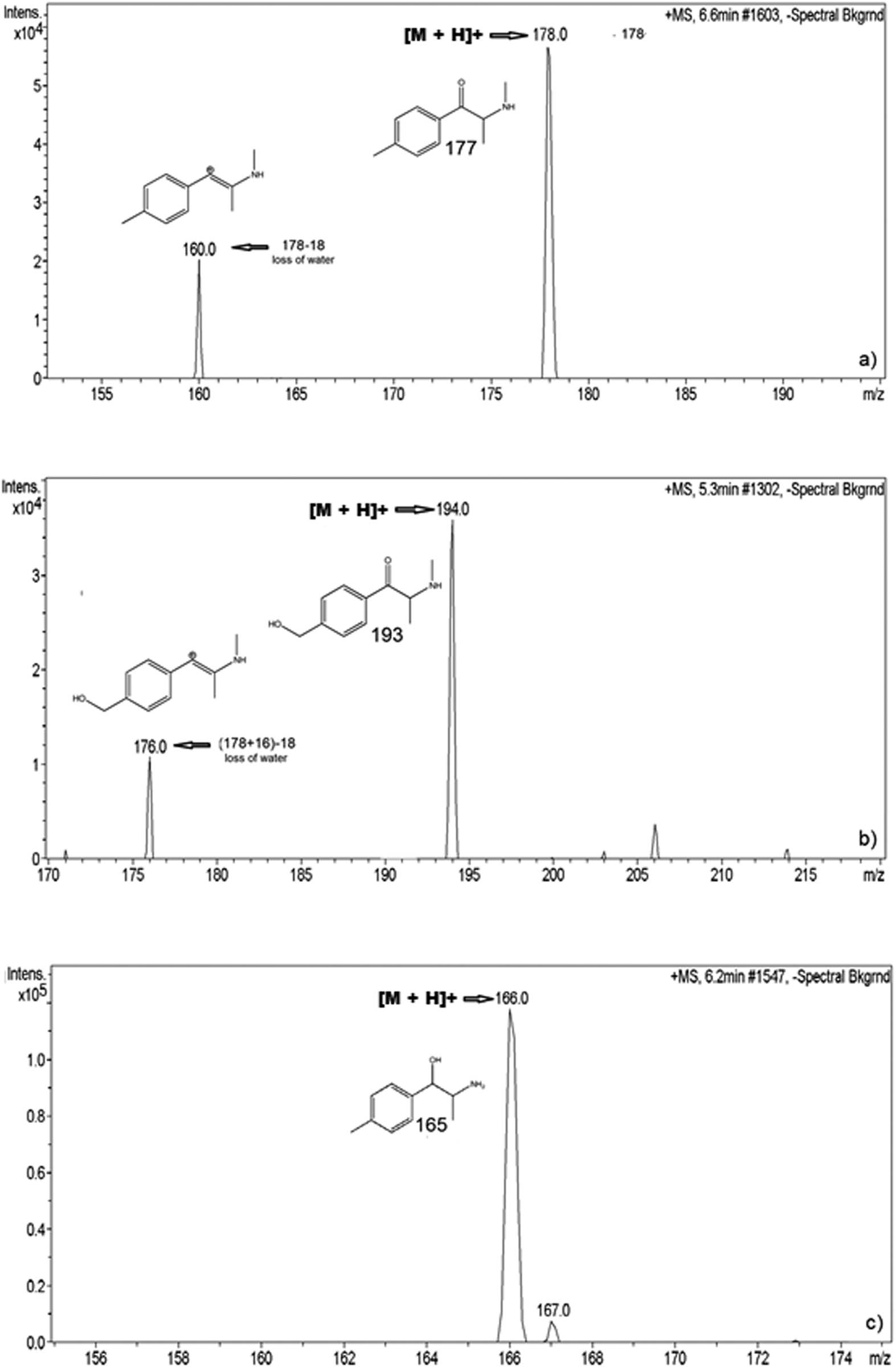
LC-MS data of (a) mephedrone and (b) a metabolite produced by in vitro metabolism of mephedrone, in the hydroxylation step: hydroxytoly-mephedrone. It also presents (c) a metabolite produced by a two-step process of reduction and demethylation: nor-dihydromephedrone.
In a similar way to that performed for mephedrone, two metabolites were identified for MXE using the animal model: one metabolite formed by O-demethylation and the other metabolite formed by the reduction of the ketone moiety to the corresponding alcohol, confirming the metabolic routes for MXE already found in the literature for some of the metabolites (Figure 3a). The reported metabolic pathway of ketamine (for comparison and reference) and the proposed one for MXE are illustrated in Figure 3a and b, respectively. Apart from the differences in chemical structure because of the presence of Cl and a shorter alkyl group in the amine group in ketamine, the rest of the molecule is very similar. A metabolic pathway can be expected with similar processes (but not identical) for both drugs. In our study, dihydro-MXE (Figure 3c, compound 2) and nor-MXE (Figure 3c, compound 3) confirmed our hypothesis (note the loss of the methyl group occurs differently through O-demethylation and N-demethylation).

(a) Suggested phase I metabolic pathway for MXE (extrapolated from published data), (b) metabolic pathway of ketamine, and (c) the present proposed metabolic pathway of MXE, where (1) represents MXE, (2) dihydro-MXE, and (3) nor-MXE.
The mass spectra of MXE and the produced metabolites are illustrated in Figure 4. Figure 4a shows the characteristic masses for MXE (m/z 247, 203 and 175) with some typical fragmentation indicated in the figure. As for Figure 3, one of the suggested metabolites was nor-MXE, produced by O-methylation, which had three characteristic ions (m/z 233, 189 and 161) in the metabolic pool as shown in Figure 4b. Figure 4c also shows the characteristic masses (m/z 249, 205) for the reduction of MXE to produce dihydro-MXE as suggested in Figure 3c.

LC-MS data. (a) Mass spectra information for MXE with characteristic m/z ratios for the molecule at 247, 203, and 175; (b) mass spectra information for nor-MXE produced by O-demethylation with characteristic m/z ratios at 161, 189, and 233; and (c) dihydro-MXE produced by reduction of the ketone moiety with characteristic m/z ratios at 205 and 249.
3.2 Study of HepaRG™ human cell line using GC-MS
It is necessary to compare the metabolism obtained through animal studies with in vitro human studies to confirm whether the metabolic routes are similar. A new peak was identified by studying the chromatograms of mephedrone after incubation with human HepaRG™ cell lines (Figure 5). The fragmentation pattern may suggest that the new peak is related to the parent drug and related to methcathinones, with structural change affecting the amine group. N-demethylation is a likely pathway for metabolism of mephedrone to produce this metabolite, which was found to be nor-dihydro mephedrone in the previous pig liver microsome study using LC-MS (Figure 2). The fragmentation pattern is shown in Figure 5b and shows the fragments from the peak observed at 6.24 min are compatible with a cathinone. However, due to the higher fragmentation achieved in the GC-MS it was not possible to identify either the molecular mass (m/z 165) or the MH+ for nor-dihydro mephedrone. However, given the fact that the mephedrone molecule was identified at a different retention time, as shown in Figure 5a, we need to speculate this new peak is one of the expected metabolites.

Sample chromatogram (a) and mass spectra (b) peak appearing at 6.24 min from samples of mephedrone after incubation with HepaRG™ compared to mephedrone.
In comparison, MXE showed no reliable peaks corresponding to the reported metabolites after incubation with HepaRG™ and GC-MS analysis.
3.3 Study with pooled human hepatocytes
In another study, mephedrone showed a continuous decrease in drug concentration with HepaRG™ cell lines over time, while MXE showed a sharp decrease in drug concentration followed by an increase.
For mephedrone, the drug concentrations relative to the primary drug concentration dropped to an average of 37% (32–44%) after 90 min of incubation with HepaRG™ and then there was another decrease down to an average of 12% (9–18%) after 24 h. In comparison, drug concentrations relative to the primary drug concentration for MXE dropped to an average of 52% (45−58%) after 90 min of incubation, but then it increased to an average of 74% (64–84%) after 24 h (Figure 6). This sudden increase might be related to cellular apoptosis and the breaking of the cellular membrane releasing all the internal contents of unchanged drug.

Relative drug concentrations of selected NPS to the primary drug concentration after 90 min and 24 h of incubation with HepaRG™. Top: mephedrone. Bottom: methoxetamine.
3.4 Cell death studies for mephedrone and MXE
For cytotoxicity study using HepaRG™ cells, the data were collected from three independent experiments using 11 different concentrations of each drug in the range of 4 × 10−2 to 16 mM. The collected data, i.e. about the percentage of cell death, were normalized, where the blanks were considered as having zero percentage cell death. The potency of the drug to cause the effect is evaluated by EC50 value, which is defined as “the concentration of the agonist required to provoke a response halfway between the baseline and maximal response” [29]. In the current part of the work, the agonist is one of the selected NPSs and the provoked response is the percentage of cell death.
The collected data were plotted as percentage of cell death normalized to maximum response versus concentration, and the resultant normalized data were analysed using curve fitting with non-linear regression best-fit approach using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla California USA. The graph for all drugs showed increase in the response with increasing concentration (Figure 6).
All tested and selected NPSs showed reproducible response that is dependent on concentration, where increasing the concentration increases the percentage of cell death. Within the concentration range used, mephedrone and MXE showed nearly similar average maximal percentage cell death of about 84% and 78%, respectively. Under the specified condition (see materials and methods) and within the specified concentration range (4 × 10−2 to 16 mM), MXE showed the most potent effect with an EC50 value of 0.3211 mM (79 μg/mL). For 4-methyl methcathinone, the EC50 value was 0.6297 mM (111 μg/mL; Figure 7).

Normalized cell death percentage of HepaRG cells induced by selected NPS 48 h after incubation under specified conditions. Top: mephedrone. Bottom: methoxetamine.
The calculated molarity values were converted into the equivalent SI to compare with the available reported toxic values through in vitro, post-mortem, or clinical samples. Equivalent concentrations were 111 μg/mL and 79 μg/mL for mephedrone and MXE, respectively (Table 1).
MTT of selected NPS summary: mephedrone and MXE
| Drug | Max cell deatha (%) | EC50b (mM) | Equivalent (μg/mL) | Reported toxic (μg/mL)c |
|---|---|---|---|---|
| Mephedrone | 84 | 0.6297 | 111.0 | 0.5–22 |
| MXE | 78 | 0.3211 | 79.00 | NA |
- a
Max death cell induced under the specified condition and within the specified range (4 × 10−2 to 1.6 × 101 mM).
- b
EC50 is the concentration required to cause cell death a halfway between the baseline and maximal response.
- c
Reported toxic values through in vivo, post-mortem, or clinical samples.
4 Discussion
4.1 Study of pig liver microsomes
For mephedrone, a previous study of the in vivo metabolism suggested a phase I metabolic pathway, whereby the drug is metabolized by one or more steps of hydroxylation, demethylation, and reduction [12]. This study identified two main metabolites, hydroxytoly-mephedrone and nor-dihydro mephedrone, being consistent with the previously identified metabolites for mephedrone in urine [10], and the general metabolic pathways of cathinone derivatives [30]. Meyer et al. [12] concluded that phase II metabolic pathways are also involved in the metabolism of mephedrone through sulphonation or glucuronidation of the hydroxytoly-mephedrone and its demethylated form. The results presented in Figure 1 confirmed these findings and reaffirmed the previously reported metabolic pathway but also confirmed the validity of our metabolic system for the production of metabolites.
Similarly, the metabolites observed for MXE in our study is similar to other published data and metabolic studies previously published for ketamine [24]. As MXE can be structurally related to ketamine, a similar metabolic strategy for demethylation can be suggested to explain the way the body may eliminate these drugs. Ketamine is metabolized through N-dealkylation to norketamine followed by the hydroxylation of norketamine at different locations and the formation of 5,6-dehydronorketamine. In the case of ketamine, the same metabolites are observed in vitro as well as in vivo in humans and animals [31,32]. This highly suggests that chemically related drugs might use the same enzymatic systems in humans and animals. Meyer et al. [12] utilized GC-MS for in vivo studies and detection of MXE metabolites in human and rat urine. They concluded that MXE may be metabolized through phase I and phase II enzymatic systems. Phase I enzymatic reactions involve N-demethylation, O-demethylation, and hydroxylation or a combination of these metabolic steps. It was concluded through their study also that phase II metabolic pathways are involved in the metabolism of MXE through sulphonation or glucuronidation of most of these metabolites [19]. In Menzies et al. [24], different phase I and II metabolites were identified, including the O-demethylation of MXE described here. However, in this study, not all metabolites could be detected in vitro and the metabolite formed by reduction, dihydro-MXE, was not described. This metabolite, to our knowledge, has not yet been described in the literature.
In our study, LC-MS analysis was used to determine which metabolite may be present in the metabolic studies and to determine whether any of these could be identified using GC-MS analysis. For both drugs, metabolites of the demethylation pathway were identified by LC-MS; and due to their chemical nature, they are more likely to be also identified by GC-MS. As for the hydroxylation pathway, these metabolites are likely to be not too volatile and polar and less likely to be identified by GC-MS analysis, even with the aid of derivatization. Therefore, the HepaRG™ cell study focused on the demethylation pathway for GC-MS analysis.
4.2 Study with HepaRG™ human cell line
Since the previous study of mephedrone using LC-MS showed the presence of both hydroxytoly-mephedrone and nor-dihydro mephedrone as metabolites, it was logical to assume that only nor-dihydro mephedrone or other metabolites produced by demethylation is likely to be detected in the HepaRG™ study using GC-MS, as these compounds are the result of at least one step of N-demethylation and may therefore be volatile enough with the help of derivatization to be visible in GC-MS. Hydroxytoly-mephedrone is the result of hydroxylation and therefore is too polar to be analysed in a GC-MS even with the aid of derivatization. GC-MS was chosen as the analytical technique as it is more common in forensic laboratories, and we aimed to demonstrate the potential versatility of this technique for the identification of novel metabolites in human cell lines. The demethylated metabolite was detected for mephedrone using HepaRG™ human cell line and GC-MS after derivatization as shown in Figure 5.
The absence of these demethylated compounds, not detected for MXE in GC-MS, should not be interpreted as they were not produced in the metabolic system. Instead, it should be interpreted that these metabolites were not volatile enough for detection using GC-MS even with BSTFA derivatization as MXE is already a fairly polar compound, even before metabolism takes place. This may be of interest to forensic laboratories, where sometimes the presence of metabolites is used to confirm the intake of a specific drug. For MXE, this information may not be applicable when using only GC-MS of biological samples, whereas mephedrone’s metabolite is detectable.
4.3 Pooled human hepatocytes
The structural difference in mephedrone and MXE may explain their differences in physicochemical properties, which explains why these drugs behaved differently in biological systems in terms of, for example, transcellular transport, protein affinity, and metabolism. Protein binding is one of the major components that may affect drug’s efficacy, as it is only the free unbound fraction of the drug that is available to be transported to the intracellular space for further biological action, e.g. metabolism.
For both drugs, mephedrone and MXE, a decrease in the concentration of the parent drug in the incubation media is observed with time. This can be associated with the intake by the cell. In both cases, the intake after 90 min is around 70% for mephedrone (leaving 30% in the media) and 55% for MXE (leaving 45% in the media). In the case of mephedrone, this intake continues up to 24 h and later with an absorption of nearly 90%. However, the observed increase in MXE concentration after 24 h of incubation following the sharp decrease after 90 min could be due to cell death and some lysis, which may cause some of the intracellular content of MXE to pool into the extracellular space. This general trend of lower available extracellular drug concentration is a strong evidence that drugs are being used or consumed by the cells, most likely through metabolism. Nearly 60–80% (depending on the concentration) is found back in the media, which approximates well to the percentage of cell death produced by MXE. This would suggest that most of the cells dying suffered a process of lyses when treated with MXE in contrast with the behaviour observed in mephedrone where the cellular wall remained intact after death (in the period of our study: 24 h).
4.4 Cell death
Mephedrone is an amphetamine-related drug with little data available about its cytotoxic effects. However, amphetamines, cathinones, and related drugs have been studied, both in vitro and in vivo. Cytotoxicity caused by amphetamines mainly affects the liver, the organ most at risk in general for drug-related toxicity and specifically amphetamines [33]. No similar data are available for MXE, the ketamine analogue belonging to the arylcyclohexylamine, or other drugs of the same class, for comparison.
Compared to the published data about cell death cytotoxic effect and other cytotoxic effects, the reported EC50 values in our study for mephedrone, 0.3211 mM, are lower than the reported EC50 for amphetamines, cathinones, and related drugs in in vitro studies, where the EC50 values ranged between 0.74 and 5.26 mM [28]. The values for EC50 obtained in the case of mexothetamine were calculated as 0.6297 mM. In this case, no literature data on MXE or related drugs for in vitro EC50 values are available for comparison.
The obtained EC50 values in this part of the work are many folds higher than the reported toxic peak concentration in either clinical-intoxicated patients’ samples or post-mortem samples. The reported concentration of mephedrone, as an example, in post-mortem biological samples in four fatalities in Scotland, ranged between 0.50 and 22 μg/mL [29]. The obtained EC50 value of 0.6297 mM for mephedrone, which is equivalent to 111 μg/mL, is about 5–22 times more than the reported values in biological samples. In fact, it has been reported that the tissue levels of amphetamine-related drugs can be up to 18–30 times higher than the blood concentrations. This discrepancy between in vitro and in vivo data, in case of amphetamines and related drugs, is partially due to their low protein-binding affinity, which makes their diffusion from plasma into tissues more favourable. In addition, post-mortem samples are often from victims who have received emergency care in their pre-mortem intoxicated interval [34].
None of the selected NPS yielded 100% cell death. However, mephedrone and MXE caused at least the death of 73–84% of the cells. The limitations to this study are based on the response of the cells under these specific stress conditions. Other cytotoxic effects need to be explored to elaborate about the toxic effects of NPS in general.
5 Conclusion
The laboratory prepared pig liver microsomes were used as the animal model, and the results of the metabolism were tracked using an LC-MS as separation and identification technique. In the case of mephedrone and MXE, it would suggest that these drugs have similar (not identical) metabolic pathways to transfer them to more soluble compounds. Two metabolites, namely, dihydro-MXE and nor-MXE, for MXE are presented in this study, produced by O-demethylation and by the reduction of the ketone moiety, where, to the best of our knowledge, the presence of none of these metabolites has been previously suggested in literature.
When looking at in vitro studies using the human HepaRG™ cells, only the demethylation pathway for mephedrone could be confirmed using GC-MS. GC-MS was employed to illustrate the facilities that may be available at commercial laboratories. However, due to this, it should be noted that the other metabolites previously described may not be detectable. Yet, GC-MS was able to identify a metabolite for mephedrone, which could in turn help law enforcement and emergency services with the identification of suspected ingestion of this NPS. This point has also been proven by other authors as commented in the introduction.
In terms of cytotoxicity in vitro EC50 values for mephedrone and MXE were reported for the first time. These values for mephedrone and MXE were 0.3211 and 0.6297 mM, respectively.
Considering the limitations, the in vitro study of the metabolism of the designer drugs is a promising approach for the prediction of the toxicological and clinical profile of the newly emerging drugs in the early stages of their appearance in the market and could be used as a preliminary step before developing analytical methods for the study of designer drugs and their metabolites.
Funding: The authors appreciate the financial support to the main author by Mutah University (Jordan) for the current research work as a part of a funded PhD scholarship.
Author contributions: M. S. was involved in data curation, formal analysis, funding acquisition, investigation, methodology, software, validation, visualization, and writing original draft; I. M. contributed to conceptualization, data curation, formal analysis, investigation, methodology, resources, software, validation, visualization, writing review, and editing; M. B. was in charge of project administration, supervision, writing review, and editing; R. C. was involved in project administration, supervision, writing review, and editing; M. V. contributed to formal analysis, software, visualization, writing original draft, writing review, and editing; and J. G. R. was in charge of conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, resources, software, supervision, visualization, writing original draft, writing review, and editing.
Data availability statement: All data generated or analysed during this study are included in this published article.
Conflict of interest: Authors declare no conflict of interest.
References
[1] Gibbons S, Zloh M. An analysis of the “legal high” mephedrone. Bioorg Med Chem Lett. 2010;20(14):4135–9.10.1016/j.bmcl.2010.05.065Search in Google Scholar PubMed
[2] Camilleri A, Johnston MR, Brennan M, Davis S, Caldicott DGE. Chemical analysis of four capsules containing the controlled substance analogues 4-methylmethcathinone, 2-fluoromethamphetamine, alpha-phthalimidopropiophenone and N-ethylcathinone. Forensic Sci Int. 2010;197(1–3):59–66.10.1016/j.forsciint.2009.12.048Search in Google Scholar PubMed
[3] Peters FT, Meyer MR. In vitro approaches to studying the metabolism of new psychoactive compounds. Drug Test Anal. 2011;3(7–8):483–95.10.1002/dta.295Search in Google Scholar PubMed
[4] Tice RR, Austin CP, Kavlock RJ, Bucher JR. Improving the human hazard characterization of chemicals: A Tox21 update. Env Health Perspect. 2013;121:756–65.10.1289/ehp.1205784Search in Google Scholar PubMed PubMed Central
[5] Ammann D, McLaren JM, Gerostamoulos D, Beyer J. Detection and quantification of new designer drugs in human blood: Part 2 – designer cathinones. J Anal Toxicol. 2012;36(6):381–9;Cosbey SH, Peters KL, Quinn A, Bentley A. Mephedrone (methylmethcathinone) in toxicology casework: A northern ireland perspective. J Anal Toxicol. 2013;37(2):74–82.10.1093/jat/bks049Search in Google Scholar PubMed
[6] Lusthof KJ, Oosting R, Maes A, Verschraagen M, Dijkhuizen A, Sprong AGA. A case of extreme agitation and death after the use of mephedrone in The Netherlands. Forensic Sci Int. 2011;206(1–3):e93–5.10.1016/j.forsciint.2010.12.014Search in Google Scholar PubMed
[7] Maskell PD, De Paoli G, Seneviratne C, Pounder DJ. Mephedrone (4-methylmethcathinone)-related deaths. J Anal Toxicol. 2011;35(3):188–91.10.1093/anatox/35.3.188Search in Google Scholar PubMed
[8] Home Office. The psychoactive substances bill; 2015.Search in Google Scholar
[9] Meyer MR, Wilhelm J, Peters FT, Maurer HH. Beta-keto amphetamines: Studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography – Mass spectrometry. Anal Bioanal Chem. 2010;397(3):1225–33.10.1007/s00216-010-3636-5Search in Google Scholar PubMed
[10] Pedersen AJ, Reitzel LA, Johansen SS, Linnet K. In vitro metabolism studies on mephedrone and analysis of forensic cases. Drug Test Anal. 2013;5(6):430–8.10.1002/dta.1369Search in Google Scholar PubMed
[11] Corazza O, Schifano F, Simonato P, Fergus S, Assi S, Stair J, et al. Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine. Hum Psychopharmacol. 2012;27:145–9.10.1002/hup.1242Search in Google Scholar PubMed
[12] Meyer MR, Bach M, Welter J, Bovens M, Turcant A, Maurer HH. Ketamine-derived designer drug methoxetamine: Metabolism including isoenzyme kinetics and toxicological detectability using GC-MS and LC-(HR-)MSn. Anal Bioanal Chem. 2013;405(19):6307–21.10.1007/s00216-013-7051-6Search in Google Scholar PubMed
[13] Olesti E, Farré M, Papaseit E, Krotonoulas A, Pujadas M, de la Torre R, et al. Pharmacokinetics of mephedrone and its metabolites in human by LC-MS/MS. AAPS J. 2017;19(6):1767–78.10.1208/s12248-017-0132-2Search in Google Scholar PubMed
[14] Olesti E, Pujadas M, Papaseit E, Pérez-Mañá C, Pozo ÓJ, Farré M, et al. GC-MS quantification method for mephedrone in plasma and urine: Application to human pharmacokinetics. J Anal Toxicol. 2017;41(2):100–6.10.1093/jat/bkw120Search in Google Scholar PubMed
[15] Pozo OJ, Ibáñez M, Sancho JV, Lahoz-Beneytez J, Farré M, Papaseit E, et al. Mass spectrometric evaluation of mephedrone in vivo human metabolism: Identification of phase I and phase II metabolites, including a novel succinyl conjugate. Drug Metab Dispos. 2015;43(2):248–57.10.1124/dmd.114.061416Search in Google Scholar PubMed
[16] Olesti E, Farré M, Carbó ML, Papaseit E, Perez-Mañá C, Torrens M, et al. Dose-response pharmacological study of mephedrone and its metabolites: Pharmacokinetics, serotoninergic effects, and impact of CYP2D6 genetic variation. Clin Pharmacol Ther. 2019;106(3):596–604.10.1002/cpt.1417Search in Google Scholar PubMed
[17] Papaseit E, Pérez-Mañá C, Mateus JA, Pujadas M, Fonseca F, Torrens M, et al. Human pharmacology of mephedrone in comparison with MDMA. Neuropsychopharmacology. 2016;41(11):2704–13.10.1038/npp.2016.75Search in Google Scholar PubMed PubMed Central
[18] Papaseit E, Olesti E, de la Torre R, Torrens M, Farré M. Mephedrone concentrations in cases of clinical intoxication. Curr Pharm Des. 2017;23(36):5511–22.10.2174/1381612823666170704130213Search in Google Scholar PubMed
[19] EMCDDA. Report on the risk assessment of MT-45 in the framework of the council decision on new psychoactive substances; 2014. Available from http://emcdda.europa.eu/system/files/publications/1865/TDAK14006ENN.pdfSearch in Google Scholar
[20] Zawilska JB. Methoxetamine – A novel recreational drug with potent hallucinogenic properties. Toxicol Lett. 2014;230(3):402–7.10.1016/j.toxlet.2014.08.011Search in Google Scholar PubMed
[21] Morris H, Wallach J. From PCP to MXE: A comprehensive review of the non-medical use of dissociative drugs. Drug Test Anal. 2014;6(7–8):614–32.10.1002/dta.1620Search in Google Scholar PubMed
[22] Hofer KE, Grager B, Müller DM, Rauber-Lüthy C, Kupferschmidt H, Rentsch KM, et al. Ketamine-like effects after recreational use of methoxetamine. Ann Emerg Med. 2012;60(1):97–9.10.1016/j.annemergmed.2011.11.018Search in Google Scholar PubMed
[23] Menzies EL, Hudson SC, Dargan PI, Parkin MC, Wood DM, Kicman AT. Characterizing metabolites and potential metabolic pathways for the novel psychoactive substance methoxetamine. Drug Test Anal. 2014;6(6):506–15.10.1002/dta.1541Search in Google Scholar PubMed
[24] Nakamura Y, Sugihara K, Sone T, Isobe M, Ohta S, Kitamura S. The in vitro metabolism of a pyrethroid insecticide, permethrin, and its hydrolysis products in rats. Toxicology. 2007;235(3):176–84.10.1016/j.tox.2007.03.016Search in Google Scholar PubMed
[25] Achour B, Barber J, Rostami-Hodjegan A. Cytochrome P450 pig liver pie: Determination of individual cytochrome P450 isoform contents in microsomes from two pig livers using liquid chromatography in conjunction with mass spectroscopy. Drug Metab Dispos. 2011;39(11):2130–4.10.1124/dmd.111.040618Search in Google Scholar PubMed
[26] Pigatto MC, Alves De Lima MDC, Galdino SL, Pitta IDR, Vessecchi R, Assis MDD, et al. Metabolism evaluation of the anticancer candidate AC04 by biomimetic oxidative model and rat liver microsomes. Eur J Med Chem. 2011;46(9):4245–51.10.1016/j.ejmech.2011.06.029Search in Google Scholar PubMed
[27] Hill JR. In vitro drug metabolism using liver microsomes. Curr Protoc Pharmacol [Internet]. 2003 Dec [cited 2004 February 15];23(1):7.8.1–11. Available from http://www.ncbi.nlm.nih.gov/pubmed/2468773710.1002/0471141755.ph0708s23Search in Google Scholar PubMed
[28] Motulsky, H, Christopoulos, A. Fitting dose-response curves. Fitting models to biological data using linear and nonlinear regression: A practical guide to curve fitting. Oxford: Oxford University Press; 2004. p. 256–95Search in Google Scholar
[29] Kelly JP. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test Anal. 2011;3(7–8):439–53.10.1002/dta.313Search in Google Scholar PubMed
[30] Portmann S, Kwan HY, Theurillat R, Schmitz A, Mevissen M, Thormann W. Enantioselective capillary electrophoresis for identification and characterization of human cytochrome P450 enzymes which metabolize ketamine and norketamine in vitro. J Chromatogr A. 2010;1217(51):7942–8.10.1016/j.chroma.2010.06.028Search in Google Scholar PubMed
[31] Schmitz A, Thormann W, Moessner L, Theurillat R, Helmja K, Mevissen M. Enantioselective CE analysis of hepatic ketamine metabolism in different species in vitro. Electrophoresis. 2010;31(9):1506–16.Search in Google Scholar
[32] Da Silva DD, Carmo H, Lynch A, Silva E. An insight into the hepatocellular death induced by amphetamines, individually and in combination: The involvement of necrosis and apoptosis. Arch Toxicol. 2013;87(12):2165–85.10.1007/s00204-013-1082-9Search in Google Scholar PubMed
[33] Da Silva DD, Carmo H, Silva E. The risky cocktail: What combination effects can we expect between ecstasy and other amphetamines? Arch Toxicol. 2013;87(1):111–22.10.1007/s00204-012-0929-9Search in Google Scholar PubMed
[34] Torrance H, Cooper G. The detection of mephedrone (4-methylmethcathinone) in 4 fatalities in Scotland. Forensic Sci Int. 2010;202(1–3):e62–3.10.1016/j.forsciint.2010.07.014Search in Google Scholar PubMed
© 2020 Majed Alshamaileh et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

