Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
-
Aamir Rasheed
, Kalsoom Akhter
Abstract
In the present work, a novel continuous flow system (CFS) is developed for the preconcentration and determination of Cr (VI) using Pseudomonas aeruginosa static biomass immobilized onto an effective and low-cost solid support of powdered eggshells. A mini glass column packed with the immobilized biosorbent is incorporated in a CFS for the preconcentration and determination of Cr (VI) from aqueous solutions. The method is based on preconcentration, washing and elution steps followed by colorimetric detection with 1,5-diphenyl carbazide in sulphuric acid. The effects of several variables such as pH, retention time, flow rate, eluent concentration and loaded volume are studied. Under optimal conditions, the CFS method has a linear range between 10 and 100 μg L-1 and a detection limit of 6.25 μg L-1 for the determination of Cr (VI). The sampling frequency is 10 samples per hour with a preconcentration time of 5 mins. Furthermore, after washing with a 0.1 M buffer (pH 3.0), the activity of the biosorbent is regenerated and remained comparable for more than 200 cycles. Scanning electron microscopy reveals a successful immobilization of biomass on eggshells powder and precipitation of Cr (VI) on the bacterial cell surface. The proposed method proves highly sensitive and could be suitable for the determination of Cr (VI) at an ultra-trace level.
1 Introduction
Uncontrolled industrial discharge of toxic metals is continuously contaminating the air and aquatic environment. These toxic metals are non-biodegradable and possess an unfavorable tendency to accumulate in biological systems. Consequently, chemists and environmental engineers are actively engaged in designing simple, sensitive, low cost and eco-friendly processes to remove these toxic metals from waste waters. Different analytical instrumental techniques such as UV-Vis spectrophotometry, high performance liquid chromatography-flame atomic absorption spectrometry (HPLC-FAAS), electro-thermal atomic absorption spectrometry (ETAAS), and HPLC-inductively couple plasma mass spectrometry (HPLC-ICP-MS) have been exploited for the determination of toxic metals in environmental samples including wastewater, drinking water and soils [1]. Trace element determination is analytically challenging at low concentrations of the trace elements and also due to the potential interference of the sample matrix. A common technique to address the challenges is to pre-concentrate the trace elements prior to detection. A variety of sample pre-concentration methods including co-precipitation, liquid-phase extraction, HPLC and solid-phase extraction have been employed for the trace/ultra-trace level determination of toxic metals [2]. Solid-phase extraction is a preferred and the most widely used method, as it works even in the presence of interferences and is also safe from usage of toxic organic solvents. In batch methods these techniques face some limitations such as large reagent consumption, accumulation of waste sludge, being time consuming, loss of results reproducibility, risk of contamination of analyte [3]. These limitations can be restrained by the incorporation of solid-phase extraction technique in flow injection (FI) system.
Various approaches have been made to exploit low-cost agriculture and industrial wastes as sorbents for the removal of toxic metals [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. In the last few years, biosorption/bioaccumulation processes have been studied extensively using microbial biomass as biosorbents for the removal of toxic metals [15, 16, 17, 18, 19, 20, 21, 22]. Comparing to other adsorbents, microbial biomass (microalgae, fungi, bacteria) outperformed because of their strong affinity with the metallic species due to the presence of various functional groups on the microbial cell wall [23, 24], such as phosphates, carboxyl, hydroxyl and amino groups [25]. The mechanism of the metal uptake by these microbes may be due to physicochemical sorption, complexation or bioaccumulation [26]. Both dead and living biomass could be applied for the removal of toxic metals, however the use of powdered biomass has some problems, such as difficulty in the separation and regeneration after use and small particle size makes it difficult to use in column applications [16, 17, 27, 28]. The current state of the art of microbacteria-based sorbents for the preconcentration of metal ions at trace levels and various aspects of the biosorption technology comparing analytical figures of merit are reviewed [29, 30, 31]. Immobilization of the biomass on solid structures gives mechanical strength, rigidity to the biomass and also offers greater potential for its regeneration and reuse.
Cell immobilization has been initiated as an alternative for enzyme immobilization. Selection of the support material plays a crucial role in the process of immobilization and practical applications of microbial biomass [32]. Various support materials, such as calcium aliginate, alumina and nanomaterials like nanofibers are used in biomass immobilization by entrapment or physical adsorption methods [33, 34, 35, 36, 37, 38]. Whereas controlled porosity glass (CPG) has been employed in biomass immobilization by covalent bonding method [35]. Immobilization by entrapment and physical adsorption has practical limitations such as inadequate transference of biomass in the inner part of support matrix, cell leakage, adverse effects on cell sustainability and abrasion of support matrix during use [39]. Covalent bonding is one of the most widely used methods for irreversible immobilization of biomass. Covalent bonding provides powerful links between the microbial cells and the carrier matrix thus preventing leakage of biomass, enhancing stability and viability of biomass for industrial and environmental applications and making them repeatedly useable. Therefore, a covalent bonding method is often the method of choice when other methods of immobilization are abortive. Different popular CPG preparations are commercially available as supports for immobilization, but are costly [40].
In this study, an effective and low-cost solid support of powdered eggshells is used for the immobilization of Pseudomonas aeruginosa. The mini glass column filled with immobilized bacterial biomass is incorporated in a flow injection system (CFS). Flow injection analysis is a versatile technique with multiple options of manifold design and the added benefits of minicolumns incorporated in the system, loaded with different materials for preconcentration and determination of various analytes. Chromium, an effluent of leather, tanning, paints, electroplating and textile industries, exists in two oxidation states: Cr (III) and Cr (VI). At low concentrations, Cr (III) is essential for living systems for the maintenance of various metabolic pathways whereas Cr (VI) is toxic even at low concentrations due to its carcinogenic effects [41, 42, 43]. In Pakistan, industrial waste waters contain Cr (VI) concentrations ranging from 0.002-35 mg L-1 whereas the Environment Protection Agency (EPA) has a drinking water standard of total chromium about 0.1 mg L-1 [44, 45]. The purpose of this work is to develop a cost-effective, sensitive, efficient and green method for on-line enrichment and determination of Cr (VI) at ultra-trace levels. The analytical methodology described here employs eggshells powder for the first time as a solid support to immobilize microbial biomass for the on-line preconcentration and determination of Cr (VI). Various factors are optimized and working performance of the system to determine Cr (VI) was also studied.
2 Materials and Methods
2.1 Solution preparation
HNO3, potassium dichromate, 1,5-diphenyl carbazide (DPC) and H2SO4 were purchased from Merck (Merck, Darmstadt, Germany). All the chemicals and reagents used in this study were of analytical grade. All glassware was soaked overnight in 10% HNO3, rinsed with distilled water and dried in an oven before use. 1000 ppm stock solution of Cr (VI) was prepared by dissolving 0.707 g of K2Cr2O7 in 250 mL of distilled deionized water and working solutions were prepared in the range of 10-100 mg L-1 (ppm) and 10-100 μg L-1 (ppb) immediately before use by a stepwise dilution of stock solution in buffer solution of pH 3. A DPC solution was prepared by first dissolving 250
mg of DPC in 10 mL of ethanol with constant stirring using a magnetic stirrer and then diluted up to 100 mL with sulphuric acid (0.2 mol L-1).
2.2 Treatment of eggshells
Chicken eggshells were collected from the local market of Muzaffarabad, Pakistan and broken into pieces, followed by washing with acetone and being dried in an oven at 60°C. Washed pieces were then ground to powder, passed through sieves of 100 mesh and stored until further use.
2.3 Immobilization of Pseudomonas aeruginosa on eggshells powder
The microbiology laboratory of Zoology Department, University of Azad Jammu and Kashmir, Muzaffarabad, Pakistan provided the pure culture of Pseudomonas aeruginosa. The bacterial strain was isolated from soil samples and identified using Gram’s staining and biochemical tests. Pseudomonas aeruginosa (DSMZ 6195) was grown in a nutrient broth medium (Oxide: CM1) and incubated in a rotary shaker for 24 h at 37°C. The biomass was collected by centrifugation of culture mixture and the collected biomass was thoroughly washed with distilled water to remove the residual growth medium. The procedure was initiated with 10 mg of dried microbial biomass immobilized on 0.5 g of eggshell powder and the immobilized material was stored and only 100 mg was packed in column for flow injection process.
Immobilization of biomass was carried out using the method described by Maquleria et al. [35] with slight modification, here we used powdered eggshells as solid support instead of controlled porosity glass (CPG). First the bacterial solution was prepared by weighing 20 mg of sample of Pseudomonas aeruginosa into a small beaker (25 mL). After that, NaOH solution (0.5 g of NaOH pellets in 25 mL of water) was added with stirring to the beaker containing the bacteria and the pH of mixture was carefully adjusted to pH 7.0 with 1 M HCl (as in basic media, turbidity was observed). Then 10 mL of this bacterial solution was diluted to 25 mL with a phosphate buffer (pH 7) and ground eggshells (0.5 g 100 mesh size) were added into 15 mL of bacterial solution and stirred slowly for 15 mins with the addition of 25% glutraldehyde to bring the concentration to 2.0%. The mixture was stored at 4°C till further use. The successful immobilization of the microbes was observed with a scanning electron microscope (SEM) and infrared (IR) spectral analysis.
2.4 Flow injection manifold and procedure
A spectrophotometer (model UV 1800 Shimadzu, Kyoto, Japan) equipped with flow-through cell (30 μl volume and 10 mm path length) was used for absorbance measurement. A flow injection system consists of a reglo digital peristaltic pump (Ismatec, Wertheim, Germany) and six channel injection valve (Rheodyne RH 5020) with other basic components. Immobilized Pseudomonas aeruginosa (100 mg) packed in a glass minicolumn (5 cm length, 1.5 cm i.d) was incorporated in the system. The whole process consisted of three steps: preconcentration, washing, and elution. During preconcentration, 5 mL standard Cr (VI) solution was passed through the minicolumn using the peristaltic pump at the flow rate of 0.8 mL min-1 for 5 min. In order to remove the unadsorbed Cr (VI) from the column as well as the retained Cr (VI) from the tubes, 2 min washing was done with buffer (Phosphate-citrate), which reduces the risk of interference from unabsorbed Cr (VI) at the elution step. After washing, eluent was injected by the first injection valve followed by the injection of DPC from the second injection valve. The eluted Cr (VI) was mixed with DPC at mixing point and the purple-red colored complex formed, which was detected spectrophotometrically at 545 nm. The overall setup is presented in scheme 1 and the three-step flow injection on-line operation is presented in Figure 1. In order to establish the best chemical and flow conditions for flow injection on-line preconcentration and determination of Cr (VI) with good sensitivity and precision, various parameters such as pH (1-6), flow rate (0.7-1.6 mL min-1) and sample volume of Cr (VI) solution (5-20 mL) were optimized. Further, it is known that the reuse and recycling of the biosorbent for successive removal of metal ions from aqueous medium contributes to the economic feasibility of the biosorption process. A necessary factor is that the desorbing agent used for regeneration of the biomass should not damage the biosorbent. So, keeping this in mind, for regeneration of biomass, the effect of the type of reagents (NaCl, KNO3, HNO3 and HCl) was studied and concentrations of the selected reagents in the range of 0.05-8 mol L-1 were also optimized. The optimized parameters and selected conditions for determination, preconcentration and regeneration of Cr (VI) are given in Table 1.
Optimization of different parameters in Flow injection system.
| Parameters | Studied range | Optimum and selected |
|---|---|---|
| pH | 1.0-6.0 | 3.0 |
| Flow rate (mL min-1) | 0.7-1.6 | 0.8 |
| Sample volume (mL) | 5.0-20 | 5.0 |
| Eluent concentration (mol L-1) | 0.05-8.0 | 7.0 |
| Eluents | HCl, HNO3, KNO3, NaCl | NaCl |
The percentage recovery (R, %) was calculated by the equation given below:
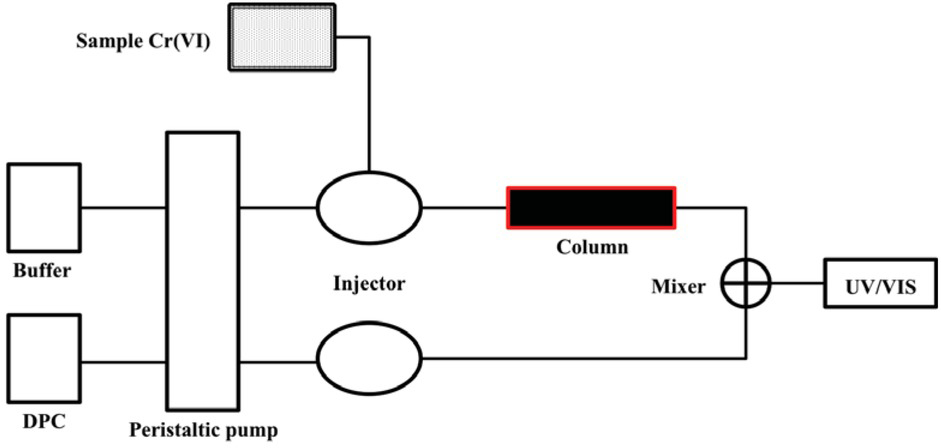
A schematic representation of the over all setup of CFS.
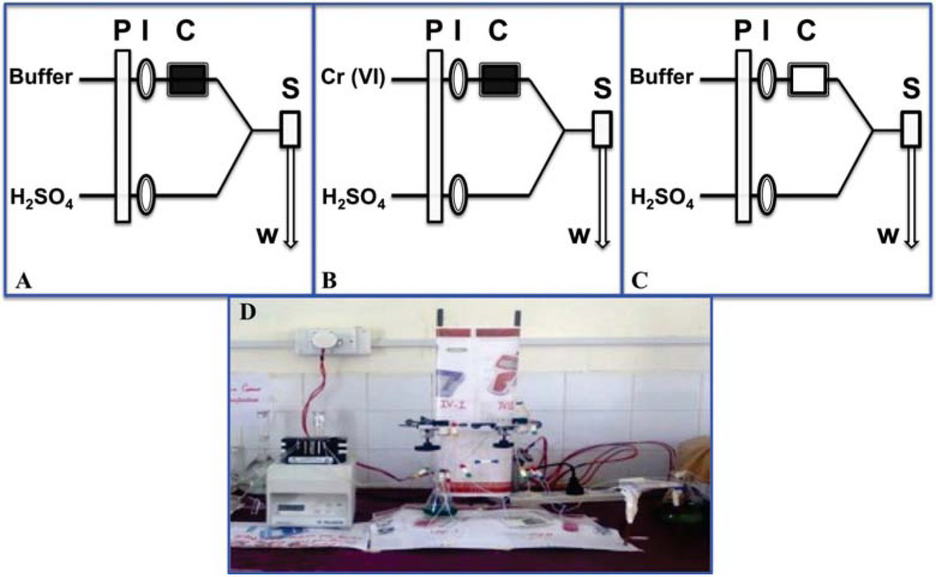
Flow injection manifolds and procedure (A) preconcentration, (B) washing and (C) elution and (D) image of the flow system used in this study (where P, I, C, S and w represents pump, injector, column, spectrophotometer and waste).
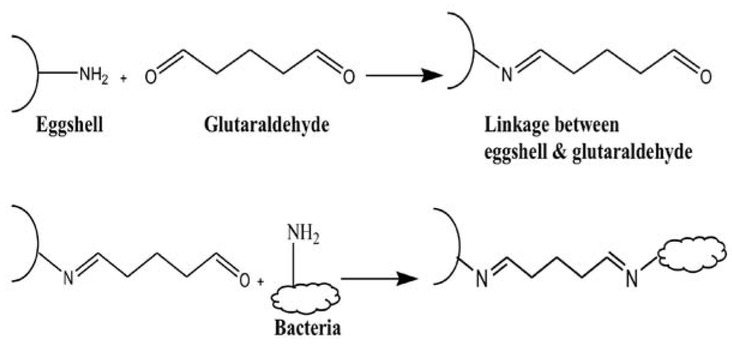
Reaction chemistry for the immobilization process.
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
3.1 Immobilization of Pseudomonas aeruginosa on eggshells powder
In this study it was interesting to use eggshells for the immobilization of bacterial biomass instead of using any expensive solid support like CPG, which has been used for the immobilization of algal biomass [35]. In chicken eggshells, calcium carbonate crystals are stabilized by the Ovocleidin-17 (OC-17) protein matrix and without the protein crystal structure it would be too brittle to keep in original form [46]. Glutraldehyde carrying carbonyl bifunctional group is used in most of the immobilization procedures as a covalent cross-linking agent. Reaction chemistry for the immobilization of bacterial biomass on eggshells is given in scheme 2, which shows the linkage of carbonyl groups of glutraldehyde with -NH2 of protein matrix of eggshell at one end and -NH2 of bacterial cell wall at the other end. Cross-linking between solid support and biomass through glutraldehyde makes maximum surface area of biomass available for the interaction of metal ions on cell surface. Initial treatment of eggshells with acetone lessens the adhesion between egg membrane and eggshell and thus detaches the membrane from the shell and curtails the role of egg membrane in immobilization or biosorption procedures.
3.2 Effect of pH on biosorption and preconcentration of Cr (VI)
The pH is a very important factor and plays a vital role in the biosorption of a specific metal ion to the adsorbent surface. The effect of pH on preconcentration of Cr (VI) in a biosorption column is studied in the range of pH 1-6. Results presented in Figure 2A show that the percent recovery of Cr (VI) has a maximum at pH 3. The metal biosorption depends on the presence of protonated or unprotonated functional groups on the surface of the microbial cell wall and ionic state of the metal ions in solutions, which is correlated by the pH of the solution. Pseudomonas aeruginosa is a gram-negative bacterium and its cell wall is composed of peptidoglycan backbone, rich in phosphate, carboxylate and amino groups. Functional groups containing nitrogen could be quaternized at pH ≤ 4 and the positive charges would magnetize the anions and the isoelectric point (pI) for gram-negative bacteria lies at low pH [47]. The possible explanation of high Cr (VI) adsorption at low pH could be due to the fact that when pH is low then the total charge on cells surface would be converted into positive and the Cr (VI) which is in anionic form binds electrostatically with microbial cells [48]. At acidic pH, negatively charged chromium species (chromate/dichromate) bind through electrostatic attraction to positively charged nitrogen containing functional groups on the surface of the bacterial cell wall. The possible explanation of high Cr (VI) preconcentration at pH 3 could be due to the fact that at this pH the total charge on cells surface would be converted into positive and the Cr (VI) which is in anionic form binds electrostatically with microbial cells. Further decrease in pH has a damaging effect on eggshell powder.
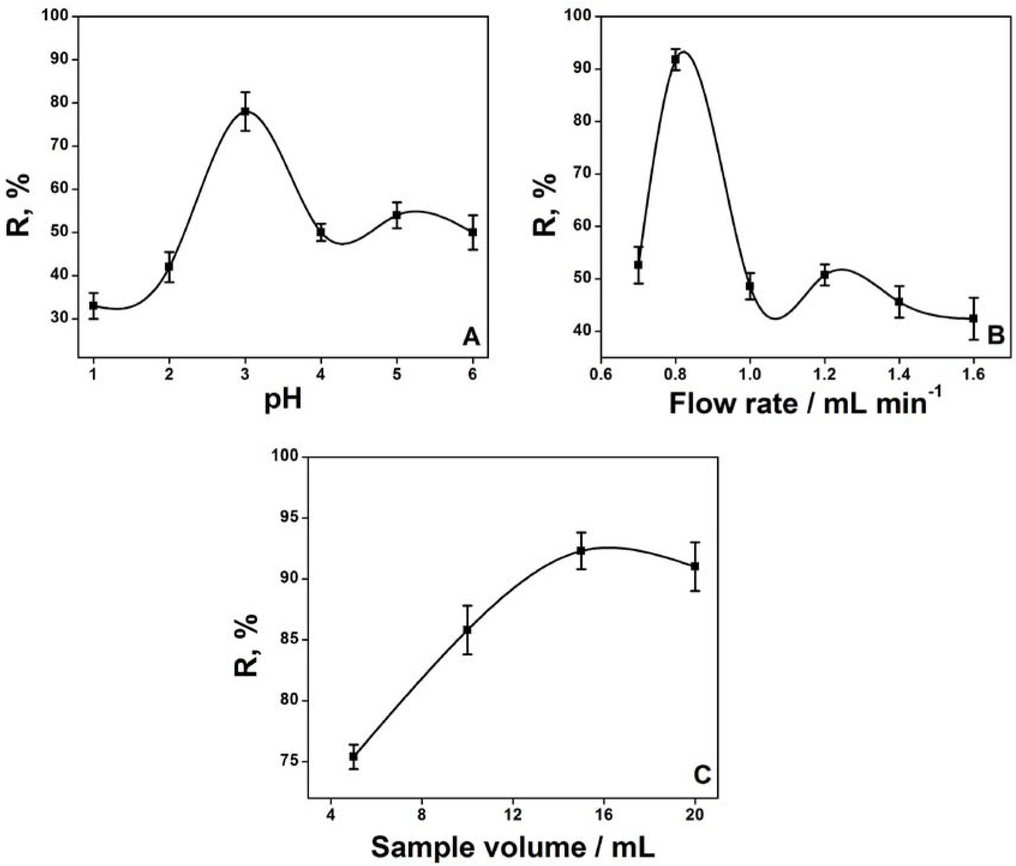
Optimization and effect of different parameters on biosorption, preconcentration of Cr(VI) (A) Effect of pH, (B) Effect of flow rate and (C) Effect of sample loading. The percentage recovery (R, %) is calculated by concentration after preconcentration/concentration before preconcentration*100.
A sharp decrease in % recovery is observed at pH ≤ 3, which is due to the loss of microbial biomass from the damaged supporting material. Increase in pH from 4-6 has a decreased number of protonated functional groups, consequently a decrease in % recovery is observed.
3.3 Effect of sample flow rate on biosorption and preconcentration of Cr (VI)
Flow rate also plays an important role in controlling the sensitivity and sampling frequency in the flow injection system. The increase in flow rate increases sampling frequency but may decrease sensitivity. High flow rate also could exert back pressure, which may cause leakage or rupture of connections. The effect of flow rate on the preconcentration and determination of Cr (VI) is performed in the range of 0.7-1.6 mL min-1. The results show (Figure 2B) that as the flow rate is increased from 0.7 to 0.8 mL min-1, there is an increase in Cr (VI) adsorption and after that a decrease in Cr (VI) adsorption is observed when the flow rate is increased from 1 to 1.6 mL min-1. At the higher flow rate, back pressure and leakage at connection points is observed which may be the reason for low adsorption of Cr (VI). Although lower flow rate decreases sample throughput rate but for stability in flow injection system it is often a better choice. The flow rate of 0.8 mL min-1 is selected for further study.
3.4 Effect of sample volume on biosorption and preconcentration of Cr (VI)
The volume of the loaded metal ion solution is an important factor to be optimized in order to identify the maximum saturation potential of the adsorbant. In the present study, 5-20 mL of the sample is loaded in order to determine maximum uptake of the analyte. Results of the study depicted in Figure 2C reveals that there is a small increase in % recovery with an increase in sample volume with no further increase after 15 mL of loaded volume. This may be attributed to the fact that with an increase in loaded sample volume, the sites available for sorption become fewer as compared to the moles of analyte present and hence preconcentration of the analyte is strongly dependent upon the volume of the loaded sample. Small changes in % recovery with an increase in sample volume may also be linked to the small size of biosorption material in packed column in flow injection system. Although there is a steady increase in % recovery with an increase in loaded volume (5-15 mL) yet 5 mL of the volume is selected for further studies to increase the sample throughput rate with less effect on the sensitivity of the method.
3.5 Effect of various reagents on regeneration of biomass
Appropriate selection of eluents for a successful desorption process depends on the type of biosorbent and mechanism of biosorption. It is important that eluent must be non-damaging to the biomass, cheap, effective and environment friendly. Although some chemical agents performed very well, like acids and alkalies in the case of desorption of cations and anions, they are highly damaging for microbial cell surface [30]. Most of the previous studies were aimed at the binding ability of biomass but less attention has been focused on the regeneration of biomass. This study has vitally focused on the probability of regeneration of biomass to make the process applicable for practical applications. For this purpose, various eluents (NaCl, KNO3, HNO3 and HCl) in the concentration range (0.05-8 mol L-1) are studied. Results of the study presented in Figure 3 clearly demonstrate that NaCl has significant potential for maximum desorption of the analyte from the biosorption column and KNO3 also showed good desorption from the column. Significant desorption potential of the salts with increase in ionic strength is due to destabilization of electrostatic interactions between negatively charged chromium species (chromate/dichromate) and positively charged nitrogen containing functional groups on the surface of the bacterial cell wall.
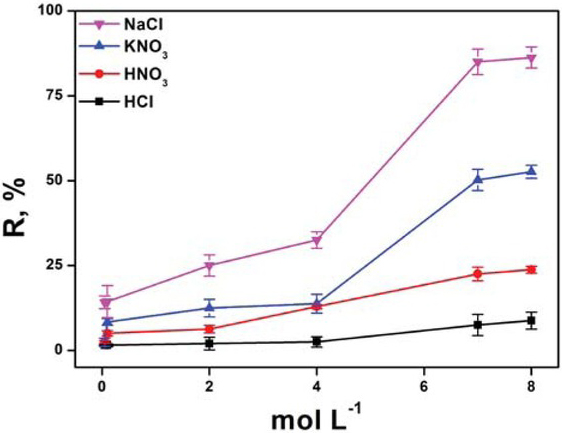
Effect of different concentrations of different reagents on the regeneration of biosorbent. The percentage recovery (R, %) is calculated by concentration after preconcentration/concentration before preconcentration*100.
While HCl and HNO3 show weaker action and also badly damage the efficiency of biosorption column which may be due to the damaging effects of the acids on the microbial cell surface and the solid support (eggshells). Concentration and volume of the eluents also influence the elution process and would be important for complete desorption of the analyte, because a too-low eluent concentration would elute the analyte incompletely from preconcentration column and on the other hand, too high eluent concentration would waste the reagent. As in this study, a minimal fixed volume (100 μL) is used for eluent. The optimum concentration of 7 M for NaCl is selected for complete desorption of the analyte, which is confirmed by successive injections of the eluent and chromogenic reagent.
3.6 Interference studies
In fact, biosorption is a proven technique for having the potential of adsorption of metal ions. However, its application on real water samples is a great concern. Interference in real samples may be anionic or cationic species, which have an affinity for biosorbents and compete with the species of interest. Interference may also be offered from other metal ions, which can make a complex with DPC. Although DPC is very sensitive and almost specific for Cr (VI), there are several chemical species that may interfere either by complexing by DPC or by reducing Cr (VI) into Cr (III). In this study interference of some anions like;
3.7 Examination of immobilized biomass before and after preconcentration
Eggshell, besides being widely available at almost no cost, also has good mechanical strength and resistance to microbial growth and are reported as good carriers for the immobilization of certain enzymes [49, 50, 51]. In current research, immobilization of biomass and biosorption of Cr (VI) is confirmed by scanning electron microscopy (SEM). The SEM images of eggshells powder, P. aeruginosa, immobilized P. aeruginosa onto eggshells powder and adsorbed Cr (VI) on immobilized P. aeruginosa onto eggshells are shown in Figure 4. The SEM images confirm the immobilization of P. aeruginosa onto eggshells (Figure 4B) and the adsorption of Cr (VI) on immobilized P. aeruginosa (Figure 4C). The Figure 4C clearly shows that Cr (VI) has covered the complete surface of the immobilized P. aeruginosa.
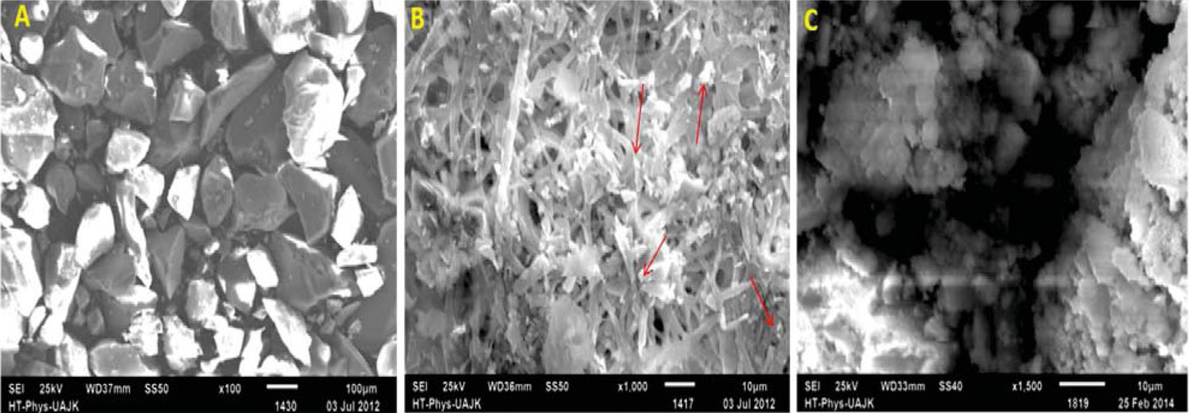
SEM images of immobilization of eggshells powder with P. aeruginosa. (A) egg shells powder, (B) rod like immobilized P. aeruginosa covering eggshells surface and (C) immobilized P. aeruginosa on eggshells powder with adsorbed Cr(VI).
Infrared spectral studies are also carried out to observe the immobilization of biomass and biosorption of Cr (VI). The Figure S1A shows the IR spectra of the 100 mesh eggshells powder and Figure S1B shows the static biomass, P. aeruginosa immobilized on eggshells powder. In both Figures S1A and S1B, the IR spectra reveal the presence of certain functional groups on the surface of eggshells powder before and after immobilization. It can be observed that many peaks are shifted, some are retained, and some have disappeared while new peaks are also identified after immobilization of P. aeruginosa. A peak at 1423 cm-1 appears in Figure S1B and the peaks in the range of 3155-3542 cm-1 disappear in Figure S1C. The peaks at 3442 and 3220 cm-1 were retained in Figure S1B, supporting successful immobilization of biomass. These peaks might be due to -NH2 asymmetric stretching mode of amines showed the overlapping of hydroxyl and amines stretching on the surface of bacterial cell [52]. IR spectral analysis is also carried out after loading of Cr (VI) (Figure S1C). The infrared spectra of the Cr (VI) loaded biomass are almost similar to that of the raw biomass. There is decrease and shift of peak at 890 cm-1 in the Cr (VI) exposed biomass (Figure S1C) compared to unexposed biomass (Figure S1B) indicating -CH=CH of trans-di-substituted alkenes out of plane deformation showing the strong interaction of Cr (VI) with bacterial cell wall [21]. Further SEM images also confirm the adsorption of Cr (VI) and Figure 4C clearly shows that Cr (VI) covered the complete surface of immobilized biomass.
3.8 Analytical and working performance of the system
After optimization and characterization, the performance of the system in the sense of calibration range and detection limit is studied. Under the optimized conditions, various concentrations Cr (VI) are injected into the flow system. The calibration curve is linear over the concentration range of 10-100 μg L-1 with a detection limit of 6.25 μg L-1. Improvement in enhancement factors (EF) and sensitivity of the system are determined by taking ratio of slopes of the curves (0.0034/0.0006) and of detection limits (5.30 ppm/6.25 ppb), obtained from linear calibration curves plotted before and after preconcentration. Results of the study given in Table 2 show that both enhancement factors and sensitivity are remarkably enhanced after preconcentration. Further the column has retained its activity for more than 200 cycles. A comparison of analytical characteristics of Cr on bacteria already reported in literature is presented in Table 3. In our future study, we plan to work on the selectivity of this method towards different cations and also this established method will be extended for the determination of other toxic metals.
Flow injection system performance for online preconcentration and determination of Cr(VI) at optimized conditions.
| Sr. no. | Performance parameters | Values |
|---|---|---|
| 1 | Detection limit (μg L-1) | 6.25 |
| 2 3 | Precision (%RSD) Sensitivity | 1.82% (100 ppb) 848 |
| 4 | Sample throughput ( h-1) | 10.0 |
| 5 | Enhancement Factor (EF) | 5.66 |
| 6 | Sample consumption (mL) | 5.00 |
| 7 | Calibration (10-100 ppb) | R2 = 0.9702 |
Comparison of analytical characteristics of Cr on bacteria.
| Bacteria support material | Preconcentration factor | Column resue | Sorption capacity (mg g-1) | Reference |
|---|---|---|---|---|
| Agrobacterium tumefacients on Amberlite XAD-4 | 25 | 10 | -- | [53] |
| Bacillus thuringiensis var. israelensis on Chromosorb 101 | 31 | 100 | 11.5 | [54] |
| Esherichia coli on Amberlite XAD-4 | 25 | 15 | -- | [55] |
| Pseudomonas aeruginosa on MWCNT | 50 | 50 | 6.2 | [37] |
| Saccharomyces carlsbergensis on Amber- lite XAD-4 | 10 | 10 | -- | [56] |
| Pseudomonas aeruginosa on powdered eggshells | 6 | 200 | -- | This study |
4 Conclusions
A simple analytical procedure with improved physicochemical features as well as enhanced biosorption characteristics has been designed. The methodology described here employs eggshells powder for the first time as a solid support for the immobilization of microbial biomass for the on-line retention of Cr (VI) followed by its elution and spectrophotometric detection. Under optimized conditions, the method has a detection limit of 6.25 ppb, with 848 times increase in sensitivity. The results have revealed excellent reproducibility for Cr (VI). For the better economic value of the biosorption procedure, repeated reuse of the biosorbent with minimum loss of efficiency by desorption of the metal pollutant as well as regeneration of the biosorbent is successfully achieved. The metal burdened biomass packed in a mini-column retained its activity for more than 200 cycles by using salt solution as eluent. In addition, the proposed method is simple, economical, fast, highly sensitive and environment friendly which can be used complimentarily with other traditional metal removal technologies.
Acknowledgments
Tahseen Ghous is grateful to the Higher Education Commission (HEC) of Pakistan for funding this research project (project no. 20-1011/R&D/07-680).
Conflict of interest: Authors declare no conflict of interest.
Supplemental Material: The online version of this article offers supplementary material (https://doi.org/10.1515/chem-2020-0031)
References
[1] Yin J, Jiang Z, Chang G, Hu B. Simultaneous on-line preconcentration and determination of trace metals in environmental samples by flow injection combined with inductively coupled plasma mass spectrometry using a nanometer-sized alumina packed micro-column. Analytica Chimica Acta. 2005;540:333-9.10.1016/j.aca.2005.03.045Search in Google Scholar
[2] Onchoke KK, Sasu SA. Determination of Hexavalent Chromium (Cr (VI)) Concentrations via Ion Chromatography and UV-Vis Spectrophotometry in Samples Collected from Nacogdoches Wastewater Treatment Plant, East Texas (USA). Advances in Environmental Chemistry. 2016;2016:Article ID 3468635.10.1155/2016/3468635Search in Google Scholar
[3] Saxena R, Sharma N, Tiwari S. Chromium Speciation Using Flow-injection Preconcentration on Xylenol Orange Functionalized Amberlite XAD-16 and Determination in Industrial Water Samples by Flame Atomic Absorption Spectrometry. Analytical Sciences. 2015;31:1303-8.10.2116/analsci.31.1303Search in Google Scholar PubMed
[4] Rizzuti AM, Newkirk CR, Wilson KA, Cosme LW, Cohen AD. Biosorption of hexavalent chromium from aqueous solutions using highly characterised peats. Mires and Peats. 2017;19:1-10.Search in Google Scholar
[5] Mondal NK, Roy S. Optimization study of adsorption parameters for removal of phenol on gastropod shell dust using response surface methodology. Clean Technologies and Environmental Policy. 2016;18:429-47.10.1007/s10098-015-1026-6Search in Google Scholar
[6] Niu CH, Volesky B, Cleiman D. Biosorption of arsenic (V) with acid-washed crab shells. Water research. 2007;41:2473-8.10.1016/j.watres.2007.03.013Search in Google Scholar PubMed
[7] Pyrzyńska K, Bystrzejewski M. Comparative study of heavy metal ions sorption onto activated carbon, carbon nanotubes, and carbon-encapsulated magnetic nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2010;362:102-9.10.1016/j.colsurfa.2010.03.047Search in Google Scholar
[8] Wang WX, Qiao Y, Li T, Liu S, Zhou J, Yao H, et al. Improved removal of Cr (VI) from aqueous solution using zeolite synthesized from coal fly ash via mechano-chemical treatment. Asia-Pacific Journal of Chemical Engineering. 2017;12:259-67.10.1002/apj.2069Search in Google Scholar
[9] Setyono D, Valiyaveettil S. Functionalized paper—A readily accessible adsorbent for removal of dissolved heavy metal salts and nanoparticles from water. Journal of hazardous materials. 2016;302:120-8.10.1016/j.jhazmat.2015.09.046Search in Google Scholar PubMed
[10] Naseer A, Jamshaid A, Hamid A, Muhammad N, Ghauri M, Iqbal J, et al. Lignin and Lignin Based Materials for the Removal of Heavy Metals from Waste Water-An Overview. Zeitschrift für Physikalische Chemie. 2019;¨:doi:10.1515/zpch-2018-1209.10.1515/zpch-2018-1209Search in Google Scholar
[11] Naseem K, Huma R, Shahbaz A, Jamal J, Ur Rehman Muhammad Z, Sharif A, et al. Extraction of Heavy Metals from Aqueous Medium by Husk Biomass: Adsorption Isotherm, Kinetic and Thermodynamic study. Zeitschrift für Physikalische Chemie. 2019;233:201-23.10.1515/zpch-2018-1182Search in Google Scholar
[12] Ata S, Tabassum A, Bibi I, Majid F, Sultan M, Ghafoor S, et al. Lead Remediation Using Smart Materials. A Review. Zeitschrift für Physikalische Chemie. 2019;doi:10.1515/zpch-2018-1205.10.1515/zpch-2018-1205Search in Google Scholar
[13] Bethke K, Palantöken S, Andrei V, Roß M, Raghuwanshi VS, Kettemann F, et al. Functionalized Cellulose for Water Purification, Antimicrobial Applications, and Sensors. Advanced Functional Materials. 2018;28:1800409.10.1002/adfm.201800409Search in Google Scholar
[14] Samet C, Valiyaveettil S. Fruit and Vegetable Peels as Efficient Renewable Adsorbents for Removal of Pollutants from Water: A Research Experience for General Chemistry Students. Journal of Chemical Education. 2018;95:1354-8.10.1021/acs.jchemed.8b00240Search in Google Scholar
[15] Krishnani KK, Meng X, Christodoulatos C, Boddu VM. Biosorption mechanism of nine different heavy metals onto biomatrix from rice husk. Journal of hazardous materials. 2008;153:1222-34.10.1016/j.jhazmat.2007.09.113Search in Google Scholar PubMed
[16] Mythili K, Karthikeyan B. Bioremediation of Cr (VI) from tannery effluent using Bacillus spp and Staphylococcus spp. International Multidisciplinary Research Journal. 2011;1:38-41.Search in Google Scholar
[17] Xiao G, Zhang X, Su H, Tan T. Plate column biosorption of Cu(II) on membrane-type biosorbent (MBS) of Penicillium biomass: Optimization using statistical design methods. Bioresource Technology. 2013;143:490-8.10.1016/j.biortech.2013.06.035Search in Google Scholar PubMed
[18] Kang C, Wu P, Li Y, Ruan B, Li L, Tran L, et al. Understanding the role of clay minerals in the chromium(VI) bioremoval by Pseudomonas aeruginosa CCTCC AB93066 under growth condition: microscopic, spectroscopic and kinetic analysis. World Journal of Microbiology and Biotechnology. 2015;31:1765-79.10.1007/s11274-015-1928-9Search in Google Scholar PubMed
[19] Akhter K, Ghous T, Andleeb S, Nasim F-u-H, Ejaz S, Abdin Z-u, et al. Bioaccumulation of heavy metals by metal resistant bacteria isolated from Tagetes minuta Rhizosphere, growing in soil adjoining automobile workshops. Pakistan Journal of Zoology. 2017;49:1841-6.10.17582/journal.pjz/2017.49.5.1841.1846Search in Google Scholar
[20] Vendruscolo F, da Rocha Ferreira GL, Antoniosi Filho NR. Biosorption of hexavalent chromium by microorganisms. International Biodeterioration & Biodegradation. 2017;119:8795.10.1016/j.ibiod.2016.10.008Search in Google Scholar
[21] Bharagava RN, Mishra S. Hexavalent chromium reduction potential of Cellulosimicrobium sp. isolated from common effluent treatment plant of tannery industries. Ecotoxicology and Environmental Safety. 2018;147:102-9.10.1016/j.ecoenv.2017.08.040Search in Google Scholar PubMed
[22] Shanmugalingam A, Murugesan A. Removal of Hexavalent Chromium by Adsorption on Microwave Assisted Activated Carbon Prepared from Stems of Leucas Aspera. Zeitschrift für Physikalische Chemie. 2018;232:489-506.10.1515/zpch-2017-0998Search in Google Scholar
[23] Daboor SM, Haroon AM, Esmael N, Hanona S. Heavy metal adsorption of Streptomyces chromofuscus K101. Journal of Coastal Life Medicine. 2014;2:431-7.Search in Google Scholar
[24] Mondal NK, Samanta A, Dutta S, Chattoraj S. Optimization of Cr (VI) biosorption onto Aspergillus niger using 3-level Box-Behnken design: Equilibrium, kinetic, thermodynamic and regeneration studies. Journal of Genetic Engineering and Biotechnology. 2017;15:151-60.10.1016/j.jgeb.2017.01.006Search in Google Scholar PubMed PubMed Central
[25] Bahafid W, Joutey NT, Sayel H, Iraqui-Houssaini M, El Ghachtouli N. Chromium adsorption by three yeast strains isolated from sediments in Morocco. Geomicrobiology Journal. 2013;30:422-9.10.1080/01490451.2012.705228Search in Google Scholar
[26] Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH. Biosorption of heavy metals: A review. Journal of Chemical Science and Technology. 2014;3:74-102.Search in Google Scholar
[27] Spinti M, Zhuang H, Trujillo EM. Evaluation of immobilized biomass beads for removing heavy metals from wastewaters. Water Environment Research. 1995;67:943-52.10.2175/106143095X133176Search in Google Scholar
[28] Tsezos MH, Engineering aspects of metal binding by biomass, in: Ehrlich L., Brierley C.L. (Eds.) Microbial Mineral Recovery, McGraw-Hill, New York, USA, 1990, pp. 325-9.Search in Google Scholar
[29] Özdemir S, Okumuş V, Dündar A, Kılınç E. Preconcentration of metal ions using microbacteria. Microchimica Acta. 2013;180:719-39.10.1007/s00604-013-0991-xSearch in Google Scholar
[30] Gupta VK, Nayak A, Agarwal S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environmental Engineering Research. 2015;20:1-18.10.4491/eer.2015.018Search in Google Scholar
[31] Abdulaziz M, Musayev S. Multicomponent Biosorption of Heavy Metals from Aqueous Solutions: A Review. Polish Journal of Environmental Studies. 2017;26:1433-41.10.15244/pjoes/67975Search in Google Scholar
[32] Martins SCS, Martins CM, Fiúza LMCG, Santaella ST. Immobilization of microbial cells: A promising tool for treatment of toxic pollutants in industrial wastewater. African Journal of Biotechnology. 2013;12:4412-8.10.5897/AJB12.2677Search in Google Scholar
[33] Norouzian D, Akbarzadeh A, Atyabi SM, Farhangi A. Immobilization of mushroom Tyrosinase by different methods in order to transform L-Tyrosine to L-3, 4 Dihydroxyphenylalanine (L-dopa). Biotechnology. 2007;6:4369.10.3923/biotech.2007.436.439Search in Google Scholar
[34] Ezoddin M, Shemirani F, Abdi K, Saghezchi MK, Jamali MR. Application of modified nano-alumina as a solid phase extraction sorbent for the preconcentration of Cd and Pb in water and herbal samples prior to flame atomic absorption spectrometry determination. Journal of hazardous materials. 2010;178:900-5.10.1016/j.jhazmat.2010.02.023Search in Google Scholar PubMed
[35] Maquieira A, Elmahadi HAM, Puchades R. Immobilized Cyanobacteria for online trace metal enrichment by flow injection atomic absorption spectrometry. Analytical Chemistry. 1994;66:3632-8.10.1021/ac00093a016Search in Google Scholar
[36] Nath A, Mondal S, Chakraborty S, Bhattacharjee C, Chowdhury R. Production, purification, characterization, immobilization, and application of β-galactosidase: a review. Asia-Pacific Journal of Chemical Engineering. 2014;9:330-48.10.1002/apj.1801Search in Google Scholar
[37] Tuzen M, Saygi KO, Usta C, Soylak M. Pseudomonas aeruginosa immobilized multiwalled carbon nanotubes as biosorbent for heavy metal ions. Bioresource Technology. 2008;99:1563-70.10.1016/j.biortech.2007.04.013Search in Google Scholar PubMed
[38] Jang Y, Shapiro A, Horani F, Kauffmann Y, Lifshitz E. Towards Low-Toxic Colloidal Quantum Dots. Zeitschrift für Physikalische Chemie. 2018;232:1443-55.10.1515/zpch-2018-1148Search in Google Scholar
[39] Zdarta J, Meyer AS, Jesionowski T, Pinelo M. A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties, Practical Utility. Catalysts. 2018;8:92 (1-27).10.3390/catal8020092Search in Google Scholar
[40] Zucca P, Sanjust E. Inorganic materials as supports for covalent enzyme immobilization: methods and mechanisms. Molecules (Basel, Switzerland). 2014;19:14139-94.10.3390/molecules190914139Search in Google Scholar PubMed PubMed Central
[41] Sobol Z, Schiestl RH. Intracellular and extracellular factors influencing Cr (VI) and Cr (III) genotoxicity. Environmental and molecular mutagenesis. 2012;53:94-100.10.1002/em.20679Search in Google Scholar PubMed
[42] Duan S, Ma W, Pan Y, Meng F, Yu S, Wu L. Synthesis of magnetic biochar from iron sludge for the enhancement of Cr (VI) removal from solution. Journal of the Taiwan Institute of Chemical Engineers. 2017;80:835-41.10.1016/j.jtice.2017.07.002Search in Google Scholar
[43] Li R, An Q-D, Mao B-Q, Xiao Z-Y, Zhai S-R, Shi Z. PDA-meditated green synthesis of amino-modified, multifunctional magnetic hollow composites for Cr (VI) efficient removal. Journal of the Taiwan Institute of Chemical Engineers. 2017;80:596-606.10.1016/j.jtice.2017.08.036Search in Google Scholar
[44] Chromium in Drinking Water, in, United States Environmental Protection Agency, USA.Search in Google Scholar
[45] Waseem A, Arshad J, Iqbal F, Sajjad A, Mehmood Z, Murtaza G. Pollution Status of Pakistan: A Retrospective Review on Heavy Metal Contamination of Water, Soil, and Vegetables. BioMed Research International. 2014;2014:Article ID 813206.10.1155/2014/813206Search in Google Scholar PubMed PubMed Central
[46] Hincke MT, Nys Y, Gautron J, Mann K, Rodriguez-Navarro AB, McKee MD. The eggshell: structure, composition and mineralization. Frontiers in Bioscience. 2012;17:1266-80.10.2741/3985Search in Google Scholar PubMed
[47] Yan G, Viraraghavan T. Effect of pretreatment on the bioadsorption of heavy metals on Mucor rouxii. Water SA. 2000;26:119-23.Search in Google Scholar
[48] Park D, Yun Y-S, Park JM. Use of dead fungal biomass for the detoxification of hexavalent chromium: screening and kinetics. Process Biochemistry. 2005;40:2559-65.10.1016/j.procbio.2004.12.002Search in Google Scholar
[49] Zhou M, Liu Y, Zeng G, Li X, Xu W, Fan T. Kinetic and equilibrium studies of Cr (VI) biosorption by dead Bacillus licheniformis biomass. World Journal of Microbiology and Biotechnology. 2007;23:43-8.10.1007/s11274-006-9191-8Search in Google Scholar
[50] Ozdemir G, Ceyhan N, Ozturk T, Akirmak F, Cosar T. Biosorption of chromium(VI), cadmium(II) and copper(II) by Pantoea sp. TEM18. Chemical Engineering Journal. 2004;102:249-53.10.1016/j.cej.2004.01.032Search in Google Scholar
[51] Chattopadhyay S, Sen R. A comparative performance evaluation of jute and eggshell matrices to immobilize pancreatic lipase. Process Biochemistry. 2012;47:749-57.10.1016/j.procbio.2012.02.003Search in Google Scholar
[52] Mungasavalli DP, Viraraghavan T, Jin Y-C. Biosorption of chromium from aqueous solutions by pretreated Aspergillus niger: Batch and column studies. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2007;301:214-23.10.1016/j.colsurfa.2006.12.060Search in Google Scholar
[53] Baytak S, Türker AR. The use of Agrobacterium tumefacients immobilized on Amberlite XAD-4 as a new biosorbent for the column preconcentration of iron(III), cobalt(II), manganese(II) and chromium(III). Talanta. 2005;65:938-45.10.1016/j.talanta.2004.08.021Search in Google Scholar PubMed
[54] Mendil D, Tuzen M, Usta C, Soylak M. Bacillus thuringiensis var. israelensis immobilized on Chromosorb 101: A new solid phase extractant for preconcentration of heavy metal ions in environmental samples. Journal of hazardous materials. 2008;150:357-63.10.1016/j.jhazmat.2007.04.116Search in Google Scholar PubMed
[55] Turker AR, Baytak S. Use of Escherichia coli immobilized on amberlite XAD-4 as a solid-phase extractor for metal preconcentration and determination by atomic absorption spectrometry. Analytical Science. 2004;20:329-34.10.2116/analsci.20.329Search in Google Scholar PubMed
[56] Baytak S, Türker AR. Determination of Iron(III), Cobalt(II) and Chromium(III) in Various Water Samples by Flame Atomic Absorption Spectrometry After Preconcentration by Means of Saccharomyces Carlsbergensis Immobilized on Amberlite XAD-4. Microchimica Acta. 2005;149:109-16.10.1007/s00604-004-0294-3Search in Google Scholar
© 2020 Aamir Rasheed et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

