Abstract
In this study, two ethanolic extracts, from Stokesia aster (Slae26) and Geranium pratense (Gpre36) respectively, were evaluated in order to assess the cytotoxic activity and potential antiproliferative activity upon the nontumorigenic human epithelial cell line derived from the mammary gland (MCF-12A) and the human breast tumor cell line (BT-20). The selection of the plant species was done on the basis of their chemical composition, specifically combinations of luteolin derivatives with caffeic and gallic acid derivatives. Therefore, the S. laevis ethanolic extract proved its capacity to inhibit the viability of both normal and tumor breast cell lines (i.e., up to 90% cell viability inhibition, IC50 = 42 µg/mL). On the contrary, the G. pratense ethanolic extract proved weak stimulatory effects on the viability of the two human breast cell lines studied. The obtained results were discussed in the contexts of computational studies and drug-likeness bioactivity of seven common luteolin derivatives: luteolin, luteolin-7-O-glucoside/cynaroside, luteolin-5-O-glucoside/galuteolin, luteolin-6-C-glucoside/isoorientin, luteolin-8-C-glucoside/orientin, luteolin-3′,4′-di-O-glucoside and luteolin-7,3′-di-O-glucoside. Computational studies have revealed that the hydrophilic behavior of luteolin derivatives (log P values) does not follow other tested parameters (e.g., polar surface area values), possibly explaining different efficacy concerning the biological properties in vitro. These predictions could be a starting point for studies on the biochemical mechanism by which luteolin derivatives induce biological effects.
Graphical Abstract
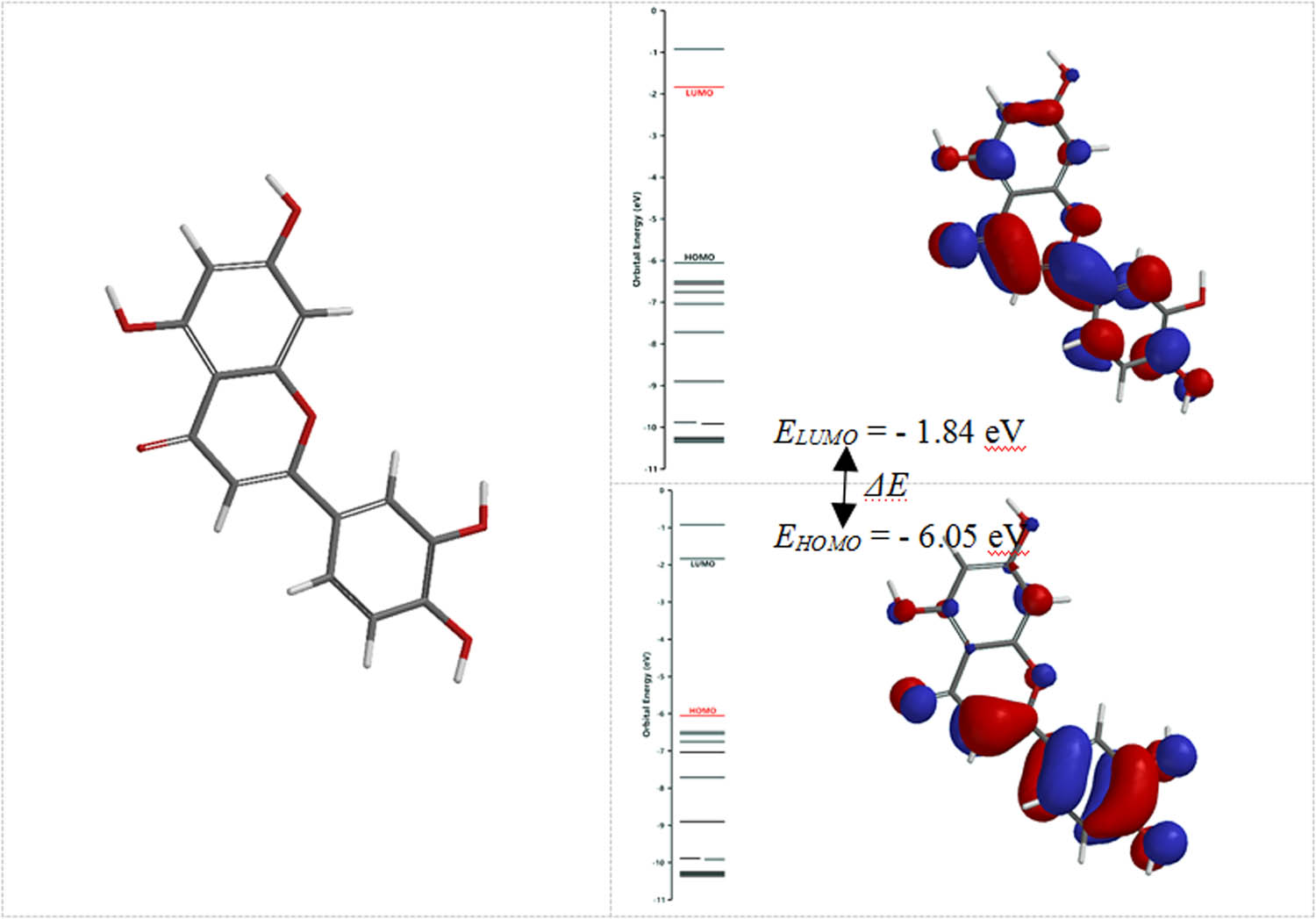
The localization of frontier molecular orbitals (FMOs) for LUTEOLIN: HOMO (the highest occupied molecular orbital) and LUMO (the lowest unoccupied molecular orbital).
1 Introduction
Scientific data of recent years indicate a greater attention to luteolin derivatives, especially as a result of in vitro evidence attesting their inhibitory potential upon breast (cancer) cell line development.
In this context, two ethanolic extracts found to contain luteolin derivatives aside caffeic and gallic/ellagic acid derivatives, from Stokesia laevis and Geranium pratense plant species, were studied concerning their in vitro cytotoxic activity and potential antiproliferative activity of two cell lines: a nontumorigenic epithelial cell line derived from mammary gland (MCF-12A) and a human breast tumor cell line (BT-20). The results were discussed in the context of computational studies and drug-likeness bioactivity of some of the most common luteolin derivatives usually found in plant species.
Regarding the two plant species selected, S. laevis (J. Hill; family Asteraceae), commonly known as “Stokes’ aster”, is a perennial species native to the United States (southeastern region) and mainly used as a decorative plant [1]. In the 1980s, there was an increased economical interest on this plant species as a result of Stokes’ aster achenes content in vernolic acid, which is used in the chemical industry [2,3]; vernolic acid is an epoxide-type structured compound acting as a nonvolatile solvent useful for manufacturing oil-based paints, varnishes, adhesives and other industrial coatings. Data on the phytochemical composition and potential biological effects of S. laevis are scarce; the only data found argue the way in which the copigment luteolin and the pigments petunidin, pelargonidin and cyanidin result in the final coloration of Stokesia aster flower heads [1]. Aster species are reported to be nontoxic to dogs, except for the woody aster, Xylorhiza orcuttii [4].
Geranium species (over 280 species are found worldwide) are particularly known for their geraniol (a volatile oil) and geraniin (a gallic/ellagic acid derivative) contents. The former has been shown to have antimicrobial [5,6,7,8] and antitumor potencies [9,10,11], while the latter shows augmented antioxidant and anti-inflammatory activities [12,13] and antitumor potency [14]. Geraniin compound also demonstrated antiviral properties on herpes simplex virus types 1 and 2 [15] and human enterovirus 71 [16] in vitro and the capacity to reduce the development of Alzheimer’s disease by inhibiting β-secretase activity [17]. Geraniol has been proved to be an effective insecticidal and antimosquito compound [18]. Owing to their specific phytochemical content, the extracts from Geranium species proved to have numerous biological effects and health benefits on human. For example, G. schiedeanum extracts demonstrated hepatoprotective properties against ethanol-induced hepatic injuries [19]; G. robertianum and G. molle extracts showed toxicity against breast, lung, cervical and hepatocellular carcinomas [20,21]; G. robertianum demonstrated strong antioxidant and anti-inflammatory properties [22]; and G. ayavacense indicated antidiabetic activity [23]; while G. carolinianum demonstrated antiviral properties on hepatitis B virus [24].
G. pratense L. plant species (commonly known as “meadow cranesbill”) are proved to be effective against free radical-induced impairment of endothelium-dependent relaxation in isolated rat aorta [25]. The methanol extract from the roots of G. pratense decreased the area with scab lesions on potato tubers; its impact against the pathogen Streptomyces scabies has been attributed to the antimicrobial activity of the geraniin compound [26]. The aqueous extract from the aerial part of G. pratense subsp. finitimum (Woronow) Knuth significantly inhibited the formation of carrageenan-induced hind paw edema (dose of 100 mg/kg) and also proved to have antinociceptive activity [27]. Finally, the Encyclopedia of Traditional Chinese Medicine recommends the utilization of the aerial parts of G. pratense in order to dispel wind damp, free channels and network vessels and treat dysentery and diarrhea [28].
2 Experimental procedure
2.1 Materials
2.1.1 Plant material description
S. laevis plant material was purchased from an authorized distributor in Romania, while G. pratense plant material was collected from Romanian Moldavian Carpathians. Taxonomic identification of the two plant species was done by the botanist’s team at the National Institute of Chemical-Pharmaceutical R&D (ICCF), Bucharest, Romania. Voucher specimens (codified Sla26 and Gpr36) were deposited in the ICCF Plant Material Storing Room.
The two plant materials (the aerial part, herba et flores) were shade dried, ground to medium-size powder and then used in technological studies.
2.1.2 Vegetal extract preparation
S. laevis plant powder of 50 g was extracted with 1,000 mL of 70% (v/v) ethanol, after 1 h at the reflux temperature. After paper filtering, 750 mL of the whole ethanolic extract with a content of 0.70 mg gallic acid equivalent (GAE) per 1 mL extract was obtained. Furthermore, 300 mL of the whole ethanolic extract was concentrated at the residue, which was dissolved into 42 mL of 40% (v/v) ethanol, and then filtered on a glass fiber filter. The resultant extract, codified as Slae26, presents as a homogeneous brown liquid having a content of 5 mg GAE per 1 mL sample.
Similarly, 50 g of G. pratense plant powder was extracted with 500 mL of 70% (v/v) ethanol solvent after 1 h at reflux temperature. The whole ethanolic extract of 330 mL with a content of 3.50 mg GAE per 1 mL extract was obtained. The whole ethanolic extract of 100 mL was further concentrated as the residue, which was dissolved into 70 mL of 40% (v/v) ethanol and then filtered on the glass fiber filter. The resultant extract, codified as Gpre36, presents as a homogeneous light brown liquid with the exact content of 5 mg GAE per 1 mL sample.
The standardized extracts, Slae26 and Gpre36, were used in pharmacological studies.
Figure 1 shows the general method for obtaining the two standardized extracts.
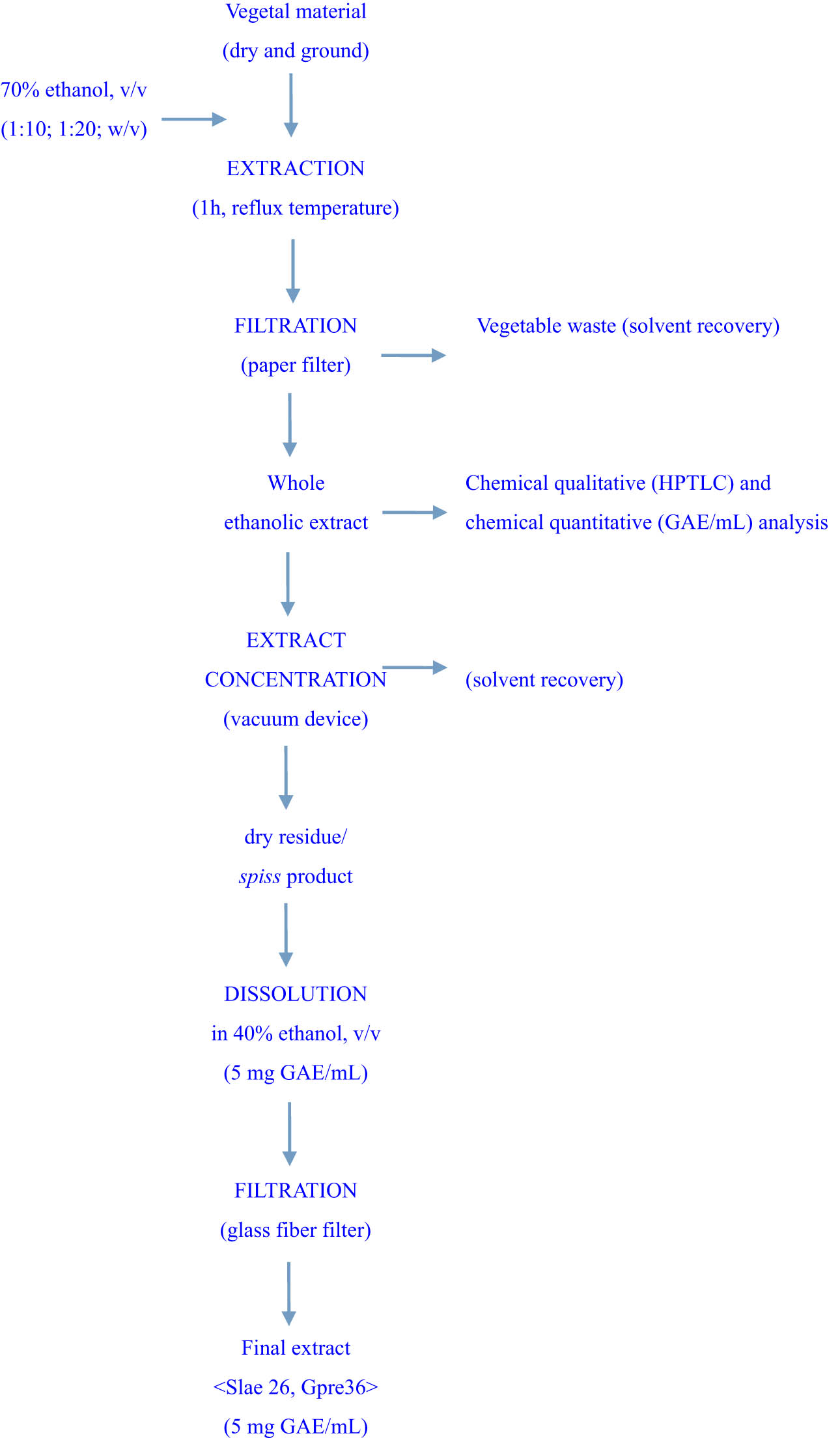
General method for the preparation of vegetal extracts.
2.1.3 Chemicals, reagents and references
Chemicals (aluminum chloride, sodium carbonate and sodium acetate), reagents (Folin–Ciocalteu, Natural Product-NP/PEG), solvents (ethanol, ethyl acetate, formic acid and glacial acetic acid) as well as the reference (ref.) compounds (rutin of min. 95%, quercitrin-3-O-rhamnoside >90%, luteolin >98%, luteolin-7-O-glucoside >98%, luteolin-8-C-glucoside >97%, caffeic acid 99%, chlorogenic acid >95% and gallic acid 95%) were purchased from Sigma-Aldrich, Romania. Cell culture reagents, Dulbecco’s Modified Essential Medium, fetal bovine serum and antibiotics were also purchased from Sigma-Aldrich, Romania.
2.2 Experimental design
2.2.1 Estimation of total phenol content in the test vegetal extracts
The total phenol content was estimated by the Folin–Ciocalteu reagent, the standard method described in Romanian Pharmacopoeia [29], and the results were expressed as milligram GAEs per 1 mL sample (R2 = 0.970).
2.2.2 Qualitative high-performance thin-layer chromatography [(HP)TLC] analysis of test vegetal extracts
Chemical qualitative analyses were performed according to Wagner and Bladt [30] and Reich and Schibli [31] chromatography atlases using two solvent systems: system A (ethyl acetate–glacial acetic acid–formic acid–water/100:12:12:26) recommended for polyphenol assessment and system B (chloroform–glacial acetic acid–methanol–water/64:32:12:8) used for saponin assessment, as described in the work of Pirvu et al. (first author of this study) [32].
2.2.3 In vitro pharmacological studies
The cell lines selected for in vitro pharmacological studies consisted of the nontumorigenic epithelial cell line derived from mammary gland MCF-12A (ATCC CRL-10782) and the human breast tumor cell line BT-20 (ATCC HTB-19).
The evaluation of in vitro cell cytotoxicity and antiproliferative effects was done by the MTS test (also known as the viability test), according to the Technical Bulletin of Promega Corporation CellTiter 96 AQueous One Solution Cell Proliferation Assay [33], as described in the work of Pirvu et al. (first author of this study) [34]. The MTS test allows evaluation of either the cytotoxic effect or the antiproliferative effect. For the (anti)proliferation assay, the application of the modulating factor (in this case the two vegetal extracts, Slae26 and Gpre36) is done on “sub-confluent” cell cultures (about 30%), and the determination of the cell population is performed after at least one division cycle (population doubling interval); for the cytotoxicity assay, the cells are exposed to “semi-confluent” cultures (about 70%) and the activity is measured for a shorter time period, rather than a doubling time (usually 6–12 h). Briefly, each test vegetal extract and dilution point series were prepared as triplicates and compared to a control sample with identical concentration of test vegetal solvent sample, at 40% (v/v) ethanol solution. After 20 h (cytotoxicity test) and 20 and 44 h (antiproliferative test) of exposure, the culture medium was removed. After another 2 h of incubation with the MTS solution, the viability of the adherent cells was determined via CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega, USA). The absorbance of the treated and control samples was measured at 490 nm with a Microplate Reader (Chameleon V Plate Reader; LKB Instruments, Australia) and the recorded values were used for cell viability estimation (see formula below). The results are calculated as mean ± SD, n = 3:
2.2.4 Computational studies
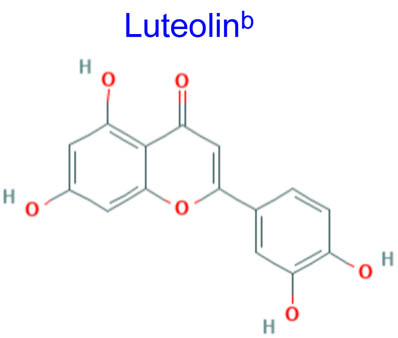
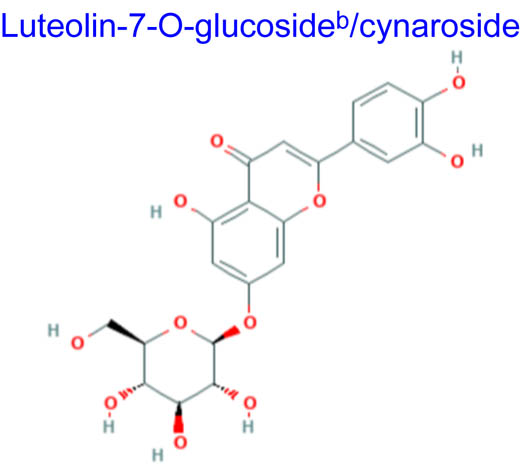
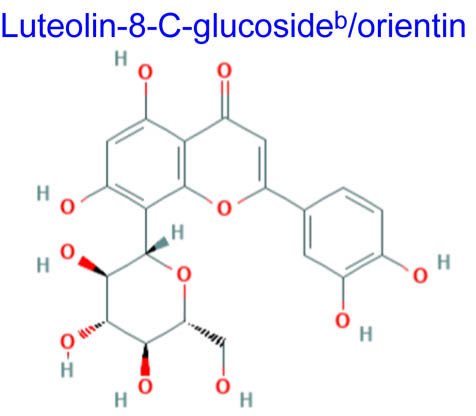
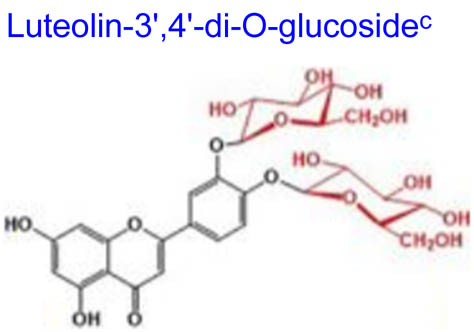
Computational studies have been done on luteolin aglycone and several common luteolin derivatives/glucosides, namely, luteolin-7-O-glucoside/cynaroside, luteolin-5-O-glucoside/galuteolin, luteolin-6-C-glucoside/isoorientin, luteolin-8-C-glucoside/orientin, luteolin-3′,4′-di-O-glucoside and luteolin-7,3′-di-O-glucoside. Computational calculations have been carried out using Spartan ’18 software [35,36], based on the density functional theory (DFT) using Becke’s three-parameter hybrid functional with Lee, Yang, and Parr parameter (LYP) correlation functional (B3LYP) and 6.31G(d,p) basis sets [36,37]. The aims of DFT computations were to reveal site-selective and global chemical reactivity descriptors of the studied luteolin derivatives and to achieve a quantitative structure property relationship/quantitative structure-activity relationship (QSPR/QSAR) analysis of their calculated properties. The calculated properties were based on the optimized structures of molecules, thus presenting the configuration of minimum energy and an optimized geometry, in vacuum conditions; no solvent corrections were done. From their energy values, several descriptors important to assess the global chemical molecules reactivity were calculated by applying the Koopmans theorem [38,39], with the following relations: ionization potential (I = −EHOMO), electron affinity (A = −ELUMO), electronegativity (χ = (I + A)/2), global hardness (η = (I − A)/2), local softness (σ = l/η) [40,41], chemical potential (μ = (EHOMO + ELUMO)/2) and global electrophilicity index (ω = μ2/2η). Also, a drug-likeness analysis was done based on the structural features correlated with Lipinski’s rule of five [42]. The molecular descriptors included in this approach were calculated, for comparison, using the Molinspiration online platform (https://www.molinspiration.com/). In addition, the bioactivity scores toward G protein-coupled receptor (GPCR) ligand, ion channel modulators, kinase inhibitors, nuclear receptor ligands, protease inhibitors and other enzyme targets were predicted online through the Molinspiration virtual screening toolkit miscreen. The results of the seven luteolin derivatives were compared and discussed.
2.2.5 Instruments used
The instruments used for chemical analyses included the UV/Vis spectrophotometer (Hélios γ; Thermo Electron Corporation, UK) and Linomat5 TLC visualizer (CAMAG, Muttenz, Switzerland). Pharmacological in vitro cell proliferation tests were done by xCELLigence®DP Real-Time Cell Analysis (ACEA Biosciences, USA).
Ethical approval: The conducted research is not related to either human or animal use.
3 Results and discussion
3.1 Polyphenol profile of the two-test vegetal extracts
In Figure 2, chromatograms A and B present the polyphenol profiles of S. aster ethanolic extract, i.e., Slae26 test sample. System A setting study of Slae26 test sample (chromatogram A, tracks T2) face to eight reference compounds (tracks T1, T3, T4, T5, T6 and T7), which indicated the occurrence of two main polyphenol classes: luteolin derivatives (yellow fluorescent/fl. spots s2, s4 and s8) and caffeic acid derivatives (blue fl. spots s1, s3, s5 and s6), mainly chlorogenic acid and several chlorogenic acid isomers, respectively. Proved to be very useful for separation and assessment of polyphenol aglycones[32]. Subsequently system B setting studies of the Slae26 test sample, before hydrolysis in 4 N HCl medium (chromatogram B, tracks T2) and after hydrolysis in 4 N HCl medium (chromatogram B, tracks T2H), both confirmed the presence of luteolin-7-O-glucoside/cynaroside (A/s4/Rf ∼ 0.68 and B/s1/Rf ∼ 0.36), luteolin (A/s8/Rf ∼ 0.94 and B/s2/Rf ∼ 0.72) and caffeic acid (A/S7Rf ∼ 0.92/and B/s3, Rf ∼ 0.76).
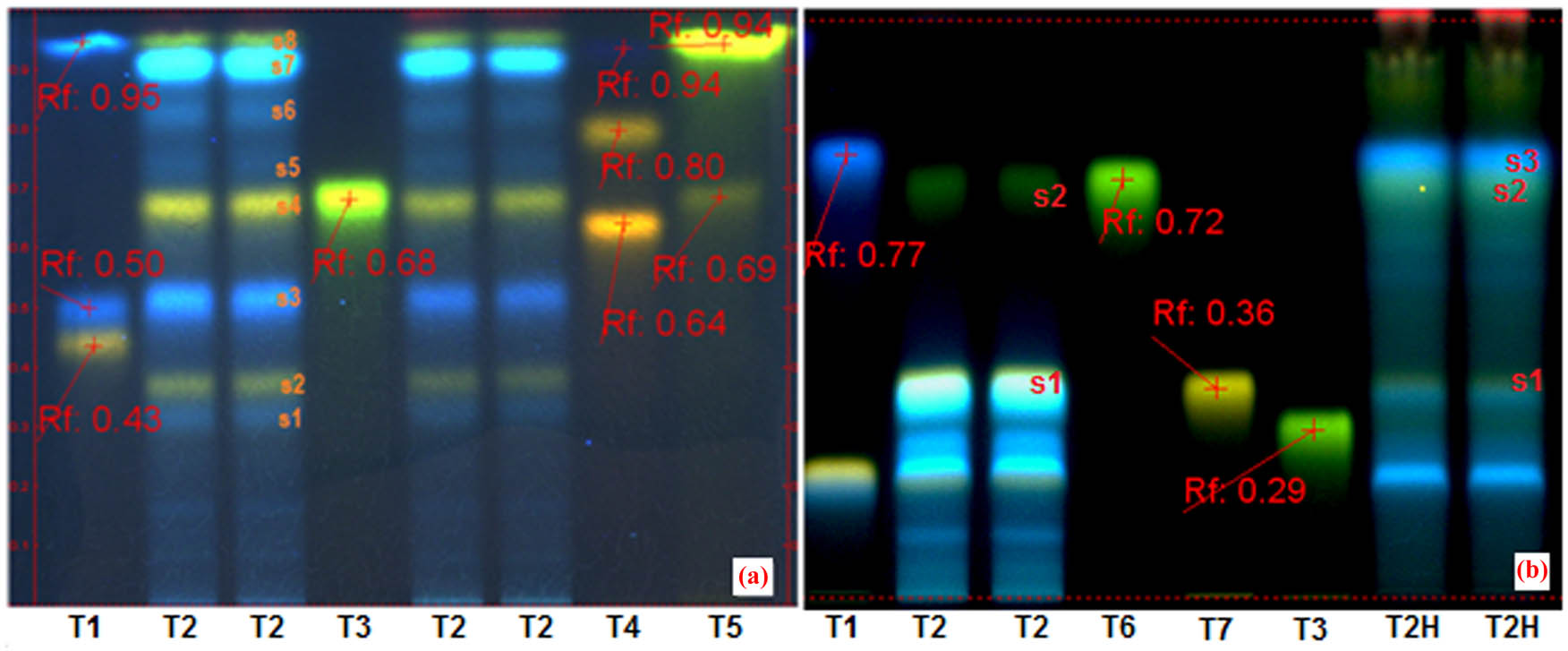
(HP)TLC studies of Stokesia laevis ethanolic extract (Slae26). Chromatogram A (system a) – track T1, rutin, chlorogenic acid and caffeic acid (ref.); tracks T2 – ethanolic extract from S. laevis (Slae26) – two series; track T3, luteolin-8-C-glucoside/orientin (ref.); track T4, quercitrin-3-O-galactoside/hyperoside, quercitrin-3-O-rhamnoside/quercitrin and gallic acid (ref.); track T5, luteolin-7-O-glucoside/cynaroside and luteolin (ref.). Chromatogram B (system b) – track T1, rutin, chlorogenic acid and caffeic acid (ref.); tracks T2 – ethanolic extract from S. laevis (Slae26); track T6, luteolin (ref); track T7, luteolin-7-O-glucoside/cynaroside (ref.); track T3, luteolin-8-C-glucoside/orientin (ref.); track T2H, the hydrolyzed extract from ethanolic extract Slae26.
In Figure 3, chromatograms A, B and C present the polyphenol profile of the G. pratense ethanolic extract i.e., Gpre36 test sample, compared to several reference compounds (tracks T1, T3, T4 and T5). System A setting study of the Gpre36 extract (chromatogram A, tracks T2) reveals an important number of polyphenol compounds, namely, luteolin (yellow fl., spots s5, s8), ellagic (light blue fl., spots s1, s2 and s4) and caffeic acid (blue fl., spots s7 and s10) derivatives and at least one kaempferol derivative (blue-green fl., spot s11).
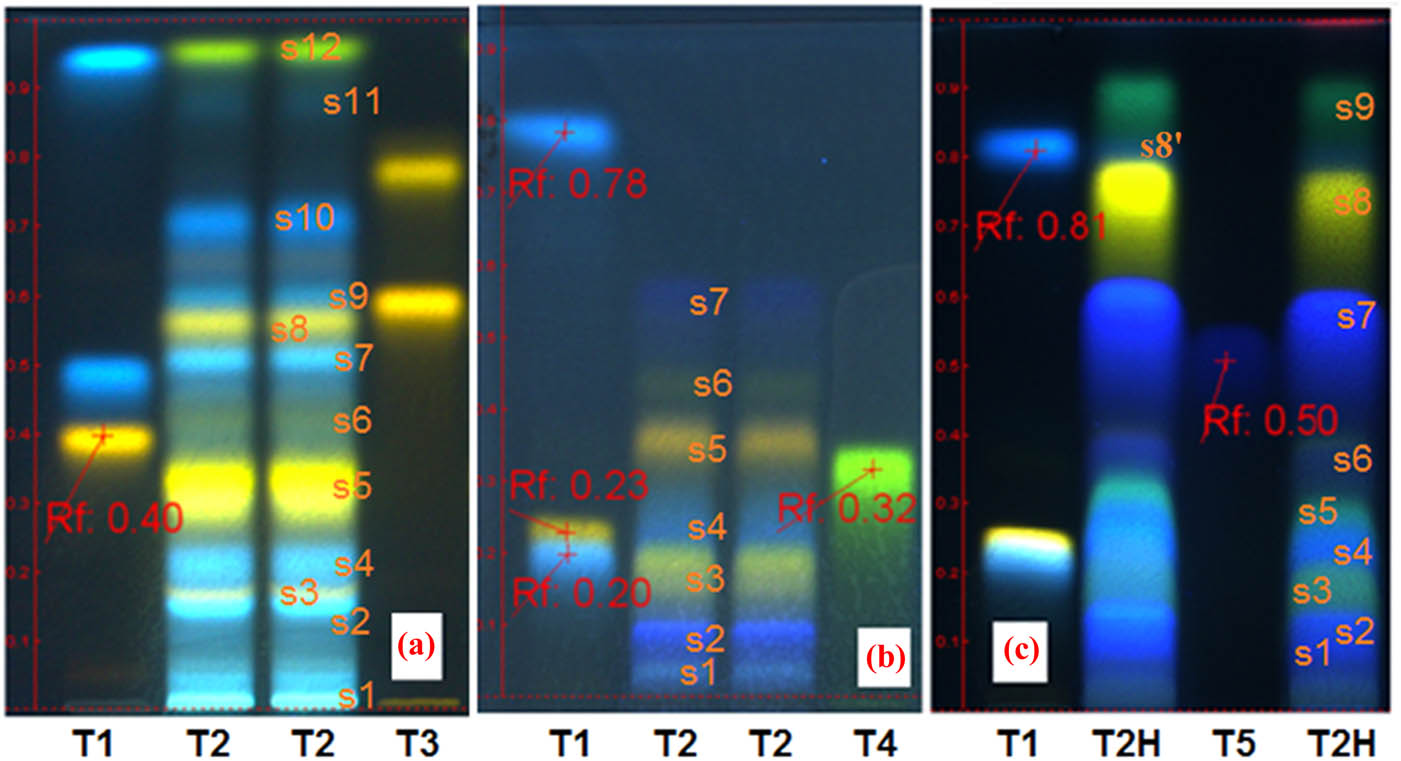
(HP)TLC studies of the G. pratense ethanolic extract (Gpre36). Chromatogram A (system a) – track T1, rutin, chlorogenic acid and caffeic acid (ref.); tracks T2, ethanolic extract from G. pratense (Gpre36); track T3, quercitrin-3-O-glucoside/isoquercitrin and quercitrin-3-O-rhamnoside/quercitrin (ref.); chromatogram B (system b) – track T1, rutin, chlorogenic acid and caffeic acid (ref.); tracks T2 – ethanolic extract from G. pratense (Gpre36); track T4, luteolin-8-C-glucoside/orientin (ref.); chromatogram C (system c) – track T1, rutin, chlorogenic acid and caffeic acid (ref.); track T2H, the hydrolyzed extract from ethanolic extract Gpre36; track T5, gallic acid (ref.).
System B setting study of the Gpre36 test sample, before and after hydrolysis in 4 N HCl medium (chromatogram B, tracks T2 and chromatogram C, tracks T2H) confirmed the presence of gallic acid aglycone (intense blue-indigo fl. spot s7, Rf ∼ 0.55) and condensed derivatives, ellagic acids (intense blue-indigo fl. spot s1, s2 and s4); chromatogram C also revealed augmented quantities of luteolin (yellow fl. spot s8, Rf ∼ 0.75) and kaempferol (green-blue fl. spot s9, Rf ∼ 86) aglycones as well as low quantities of caffeic acid aglycone (blue fl. spot s8′ covered by luteolin s8). Other high-performance liquid chromatography studies [25] of extracts of G. pratense indicated the presence of quercitrin-3-O-α-arabinopyranoside, quercitrin-3-O-β-glucopyranoside, quercitrin-3-O-β-galactopyranoside, quercitrin-3-O-(2-O-galloyl)-β-glucopyranoside, quercitrin-3-O-(2-O-galloyl)-β-galactopyranoside, kaempferol-3-O-β-galactopyranoside, kaempferol-3-O-β-glucopyranoside and also myricetin-3-O-(2-O-galloyl)-β-glucopyranoside and (−)-6-chloro-epicatechin polyphenols.
By comparison, qualitative (HP)TLC studies revealed that the ethanolic extract from S. aster plant species, caffeic acid/s7, chlorogenic acid/s3 and one luteolin monoglycoside (likely luteolin-7-O-glucoside/s4) were found, and the ethanolic extract from G. pratense plant species confirmed numerous gallic/ellagic acid derivatives/s1, s2, s4 and one augmented luteolin polyglycoside/s5. Concerning the quantitative aspects, the two ethanolic extracts, Slae26 and Gpra36, were made in a manner to assure the exact content of 5 mg total phenol content, GAEs per 1 mL sample.
3.2 Cytotoxic and antiproliferative potential assays in vitro
In vitro cytotoxicity and antiproliferative assessments were done on Slae26 and Gpre36 test vegetal extracts (characterized by 5 mg GAE/mL sample) by using six dilution series, that is, 1, 5, 10, 25, 50 and 100 µg GAE/mL samples. The two test vegetal dilution series and the corresponding control sample dilution series (40% ethanol solvent series) were applied on “semi-confluent” and “sub-confluent” mammary gland cell culture MCF-12A and human breast tumor cell culture BT-20, as described in Section 2.2.3. As described, the cytotoxic and antiproliferative activities essentially consist in a colorimetric test based on the selective ability of the viable cells to reduce the tetrazolium component of MTS into a purple-colored formazan crystal; the quantity of the formazan product, as measured by absorbance at 490 nm, is directly proportional to the number of living cells in culture and thus the effect of the two-test vegetal series on the cytotoxic and proliferation tests can be computed as the cell viability percentage (%).
Figures 4 and 5 show the results on the S. laevis and G. pratense test vegetal extracts, Slae26 and Gpre36 dilution series, respectively.
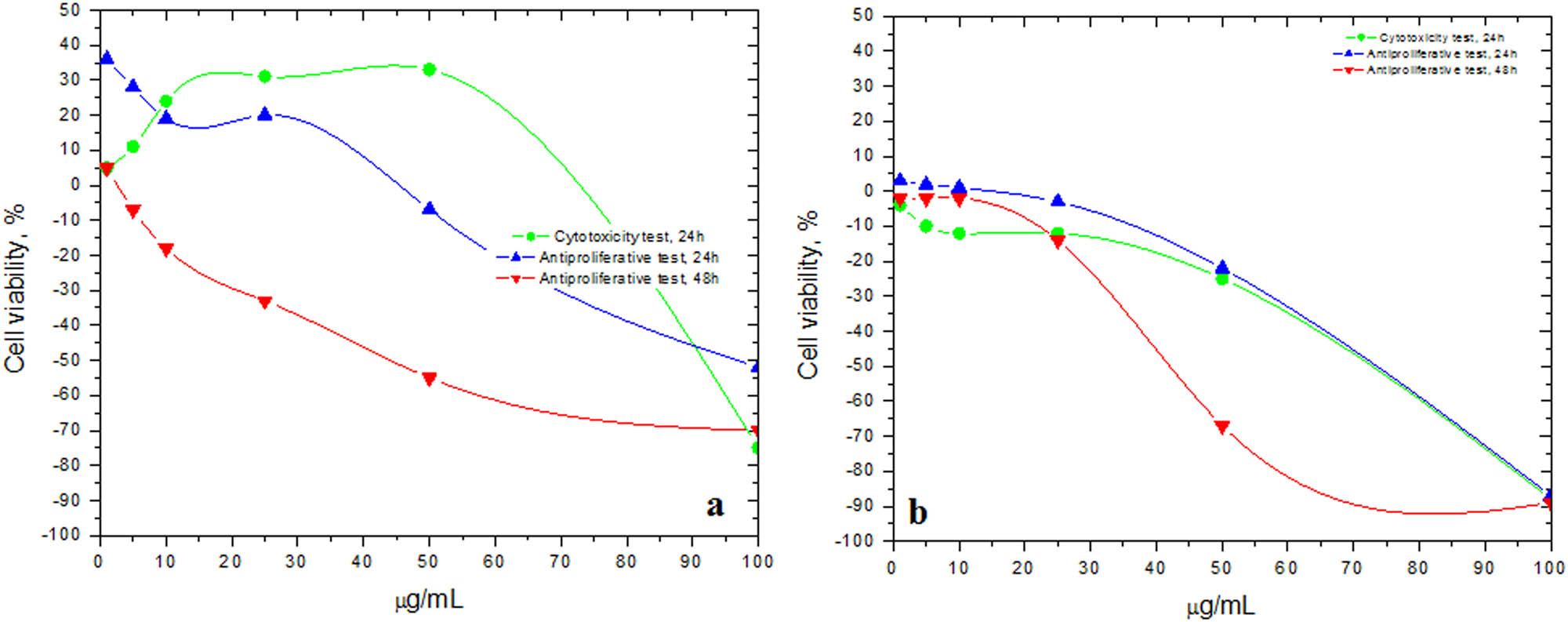
Cytotoxic and antiproliferative effects (cell viability, %) of Slae26 tested on (a) mammary gland cell line MCF-12A and (b) human breast tumor cell line BT-20, compared to the control negative cells; n = 3, ± SD(%).
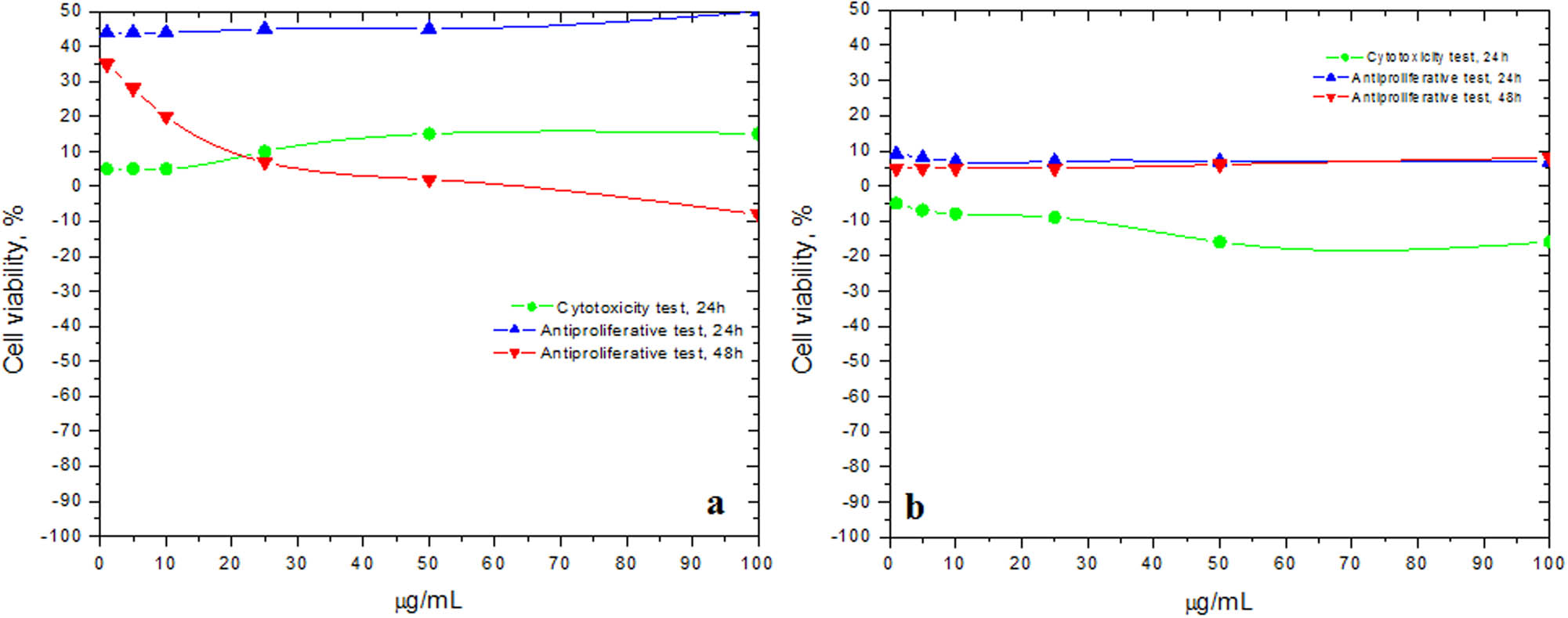
Cytotoxic and antiproliferative effects (cell viability, %) of Gpre36 tested on (a) mammary gland cell line MCF-12A and (b) human breast tumor cell line BT-20, compared to the control negative cells; n = 3, ± SD(%).
Comparison with the control negative cell series indicated the following results: during cytotoxicity test, Slae26 test sample applied on the normal epithelial mammary gland cell line MCF-12A (Figure 3a) led to the stimulation of cell viability up to 50 µg/mL concentration level (up to 33% cell viability stimulation), followed by a severe decline in the cell viability at higher concentrations (up to 75% cell viability inhibition at 100 µg/mL); during the antiproliferative test, Slae 26 induced important inhibitory effects on MCF-12A viability (up to 52% and 70% cell viability inhibition at 24 and 48 h, respectively); Slae26 tested on human breast tumor cell line BT-20 (Figure 3b) revealed either cytotoxic effects (88% cell viability inhibition at 24 h) or antiproliferative activity (87% and 89% cell viability inhibition at 24 and 48 h, respectively), concluding that Stokesia aster ethanolic extract has inhibitory potential on the growth and proliferation of normal and tumor human breast cells. The antiproliferative effect (IC50) was estimated at around values of 68 µg GAE/mL sample at 24 h and 42 µg GAE/mL sample at 48 h.
Due to the combination of luteolin derivatives with gallic or ellagic acid derivatives, which were shown to have strong antitumor, antimetastatic and antiangiogenesis activities [43,44], it is expected that the G. pratense ethanolic extract (Gpre36) has a strong antiproliferative potential. However, when applied on the normal mammary gland cell line MCF-12A (Figure 4a), it indicated a weak stimulatory activity in the cytotoxicity test (up to 15% cell viability stimulation); during the antiproliferative test, at first stimulatory effects were noticed (up to 50% cell viability stimulation at 24 h), followed by inhibitory effects (up to 8% cell viability inhibition at 48 h). Gpre36 test sample tested on human breast tumor cell line BT-20 (Figure 4b) indicated weak cytotoxic effects (up to 16% cell viability inhibition at 24 h), and no antiproliferative activity; in fact, 7% and 8% cell viability stimulatory effects were measured at 24 and 48 h, respectively. The results are available in Supplementary Tables 1s, 2s, 3s and 4s.
3.3 Computational results
Computational study of the seven luteolin derivatives, molecular descriptors and physicochemical properties was planned in order to find a possible explanation for the differences in pharmacological activity of the two-test vegetal extracts. Although the G. pratense ethanolic extract has a theoretically more efficient chemical composition, in vitro assessments indicated the lack of antiproliferative activity of Gpre36 versus certain antiproliferative activity of S. aster ethanolic extract, Slae26. Therefore, it appears that the specific chemical structure of luteolin derivatives (the position and the number of substituents) and less combination with caffeic or gallic/ellagic acid derivatives lead to the final antiproliferative potential of the two vegetal extracts.
Table 1 summarizes the quantum chemical parameters for luteolin and the six most common luteolin derivatives: luteolin-7-O-glucoside/cynaroside, luteolin-5-O-glucoside/galuteolin, luteolin-6-C-glucoside/isoorientin, luteolin-8-C-glucoside/orientin, luteolin-3′,4′-di-O-glucoside and luteolin-7,3′-di-O-glucoside.
Calculated quantum chemical parameters of the studied compounds
| Studied parameter | Luteolin | Luteolin-7-O-glucoside/cynaroside | Luteolin-5-O-glucoside/galuteolin | Luteolin-6-C-glucoside/isoorientin | Luteolin-8-C-glucoside/orientin | Luteolin-3′,4′-di-O-glucoside | Luteolin-7,3′-di-O-glucoside |
|---|---|---|---|---|---|---|---|
| EHOMO | −6.05 | −6.00 | −6.46 | −6.09 | −6.11 | −6.20 | −6.56 |
| ELUMO | −1.84 | −1.82 | −2.29 | −1.83 | −1.89 | −1.89 | −2.42 |
| ΔEgap | 4.21 | 4.18 | 4.17 | 4.26 | 4.22 | 4.31 | 4.14 |
| I | 6.05 | 6.00 | 6.46 | 6.09 | 6.11 | 6.20 | 6.56 |
| A | 1.84 | 1.82 | 2.29 | 1.83 | 1.89 | 1.89 | 2.42 |
| χ | 3.94 | 3.91 | 4.37 | 3.96 | 4.00 | 4.04 | 4.49 |
| η | 2.105 | 2.09 | 2.08 | 2.13 | 2.11 | 2.15 | 2.07 |
| σ | 0.47 | 0.48 | 0.48 | 0.47 | 0.47 | 0.46 | 0.48 |
| μ | −3.94 | −3.91 | −4.37 | −3.96 | −4.00 | −4.04 | −4.49 |
| ω | 3.68 | 3.66 | 4.59 | 3.68 | 3.79 | 3.79 | 4.87 |
EHOMO, the energy of the HOMO orbital; ELUMO, the energy of the LUMO orbital; ΔEgap, difference between frontier molecular orbitals; I(ionization potential), (−EHOMO); A (electron affinity), (−ELUMO); χ (electronegativity), (I + A)/2; H (global hardness), (I − A)/2; σ (local softness), l/η; μ (chemical potential), (EHOMO + ELUMO)/2 and ω (global electrophilicity index), μ2/2 η, expressed in eV.
First of all, it is obvious that the energy of the highest occupied molecular orbital (HOMO) orbital (EHOMO) of all studied compounds is significantly smaller than that of the energy of the lowest unoccupied molecular orbital (LUMO) orbital (ELUMO). Energetically, from the HOMO orbital, it is easiest to donate electrons to form new bonds to be involved in oxidation. The LUMO is energetically more able to receive electrons, possibly involved in reduction reactions.
Second, the ΔEgap parameter (the energy difference between HOMO and LUMO) providing information about chemical reactivity and kinetic stability of the molecules indicated close values, ranging between 4.14 and 4.26 eV.
Table 2 presents the values obtained for physicochemical properties and molecular descriptors involved when discussing the drug-likeness and matching with the statements of Lipinski’s rule of five concerning oral bioavailability. For each structure, the first line of values corresponds to Spartan computations, and on the second line are the values obtained from the Molinspiration platform. A small difference is due to the methods used for computation. Molinspiration employed the semiempirical AM1 method for the optimized 3D molecular geometries, wherein volumes and polar surface area (PSA) are approximated based on the fragment contributions.
Drug-likeness predicted parameters for luteolin and its derivativesa
| Name | Molecular weight | Molecular volume | Nrotb | log P | PSA | HBD | HBA | No. of Lipinski violations |
|---|---|---|---|---|---|---|---|---|
 | 286.239 | 261.11 | 1 | −3.46 | 97.416 | 4 | 6 | 0 |
| 232.07 | 1.97 | 111.12 | ||||||
 | 448.380 | 399.02 | 4 | −5.45 | 168.657 | 7 | 11 | 2 |
| 364.19 | 0.19 | 190.28 | ||||||
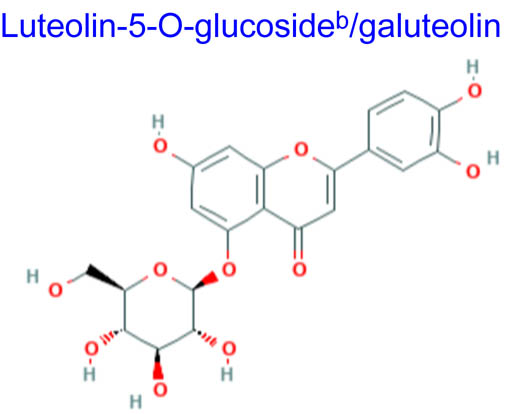 | 448.380 | 398.71 | 4 | −0.83 | 163.463 | 7 | 11 | 2 |
| 364.19 | −0.07 | 190.28 | ||||||
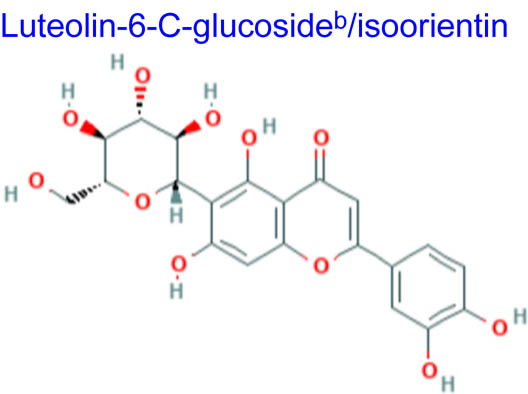 | 448.380 | 394.22 | 3 | −1.61 | 164.602 | 8 | 11 | 2 |
| 363.22 | 0.03 | 201.27 | ||||||
 | 448.380 | 397.09 | 3 | −6.39 | 178.988 | 8 | 11 | 2 |
| 363.22 | 0.03 | 201.27 | ||||||
 | 610.521 | 533.18 | 7 | −2.67 | 214.904 | 10 | 16 | 3 |
 | 610.521 | 535.08 | 7 | −7.45 | 221.728 | 10 | 16 | 3 |
| 496.31 | −1.83 | 269.43 |
Nrotb, number of rotatable bonds; HBD, number of hydrogen bond donors; HBA, number of hydrogen bond acceptors; PSA, polar surface area; log P, water–octanol partition coefficient.
- a
Values on the first line were calculated using Spartan software and values listed on the second line were obtained using Molinspiration tools.
- b
- c
Table 2 clearly demonstrates luteolin aglycone as the most feasible drug candidate due to zero violation of Lipinski’s rule [42]. Although not sufficient and mandatory to send a potential drug compound forward for pharmaceutical development, these limits of physicochemical parameters are used, rather than excluding from screening the compound with poor success rate. As observed from the results of computations, the correlation between the molecular weight and the PSA indicated that, generally, by increasing the molecular weight, from luteolin to luteolin diglucoside, the number of added hydroxyl groups and PSA increases. The biggest structures with the highest hydrophilicity, luteolin-3′,4′-diglucoside and luteolin-7,3′-di-glucoside, showed the most deviations from drug-likeness criteria (Lipinski’s rule), also presenting the highest number of flexible bonds (Nrotb).
Yet, even if luteolin derivatives present a more augmented lipophilic character due to the lack of one hydroxyl group in position 3, the comparison of log P values (the calculated values of octanol–water partition coefficient log P by Spartan computations) with those computed for quercitrin derivatives [34] indicates that (1) luteolin derivatives are more hydrophilic compounds capable of exhibiting greater oral bioavailability and (2) their log P values do not follow PSA values, possibly explaining their different efficacies concerning the biological properties and function of test conditions.
The correlation between PSA, molecular volume (vol) and water–octanol partition coefficient (log P) for the seven luteolin derivatives studied (Figure 6) indicated that luteolin, luteolin-5-O-glucoside, luteolin-6-C-glucoside and luteolin-3′,4′-O-diglucoside have significantly different log P values in comparison with luteolin-7-O-glucoside, luteolin-8-C-glucoside and luteolin-7,3′-O-diglucoside, which could support the differences between the pharmacological activity of the vegetal extracts.
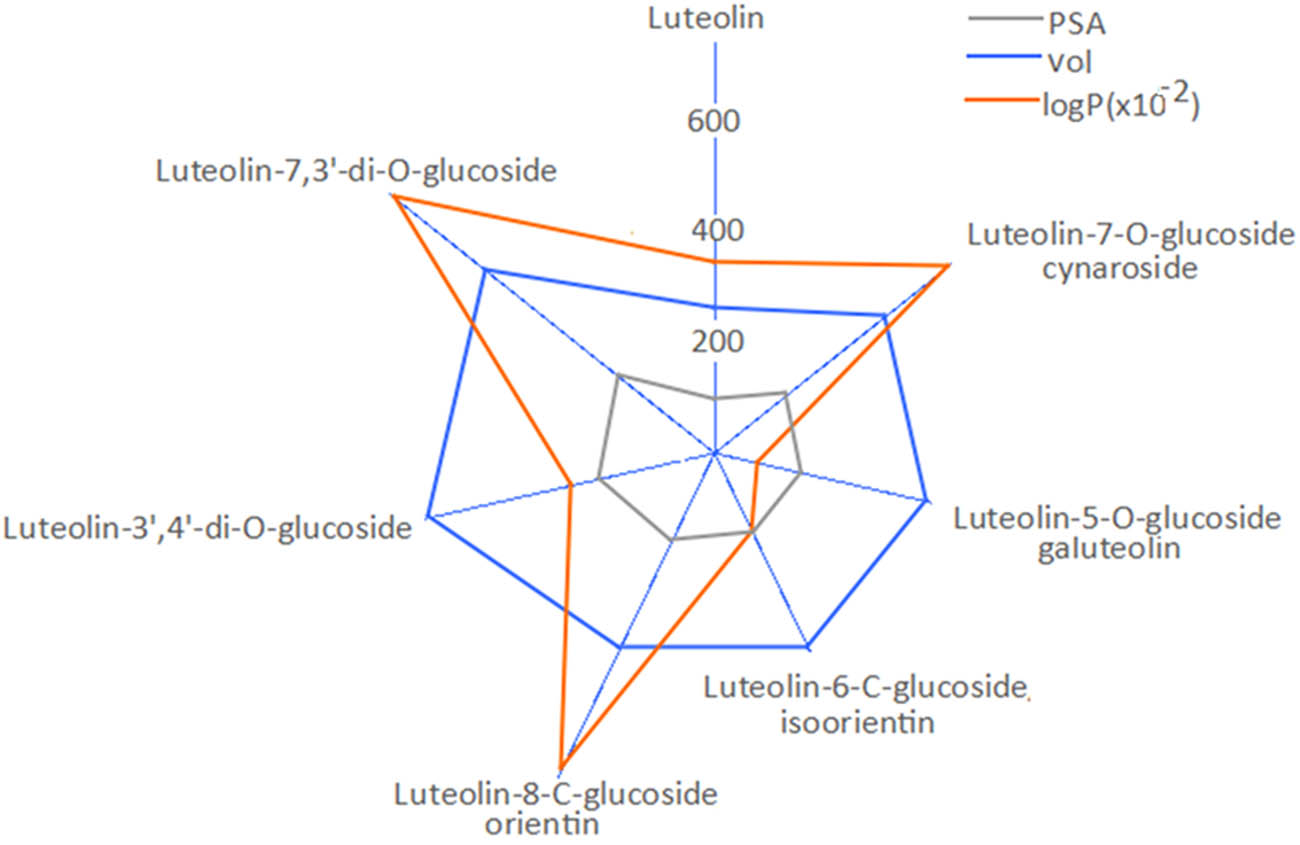
The correlation between molecular volume (vol), PSA and water–octanol partition coefficient (log P) for several common luteolin derivatives.
In Table 3 are listed the bioactivity scores predicted using the Molinspiration software toward GPCR ligands, ion channel modulators, kinase inhibitors, nuclear receptor ligands, protease inhibitors and enzyme targets. It is known that if the bioactivity score is larger than 0.0, the drug candidate is classified as active; if score range is between −5 and 0, then the structure is moderately active and if the score is less than −5, then inactive. Therefore, luteolin exhibits larger score values for kinase inhibitor (0.26 – the largest of all the tested structures), nuclear receptor ligand (0.39, also the highest score, compared with its derivatives) and enzyme inhibitor (0.28), thus being considered as the most promising structure, the lead compound.
Bioactivity scores for luteolin and its derivatives predicted with Molinspiration toolkit
| Name/ChemSpider ID | GPCR ligand | Ion channel modulator | Kinase inhibitor | Nuclear receptor ligand bind | Protease inhibitor | Enzyme inhibitor |
|---|---|---|---|---|---|---|
| Luteolin ID 4444102 | −0.02 | −0.07 | 0.26 | 0.39 | −0.22 | 0.28 |
| Luteolin 7-O-glucoside/Cynaroside 4444241 | 0.09 | −0.02 | 0.15 | 0.27 | −0.01 | 0.42 |
| Luteolin 5-O-glucoside/Galuteolin 10306091 | 0.12 | 0.00 | 0.18 | 0.29 | −0.01 | 0.41 |
| Luteolin 8-C-glucoside/Orientin 4444994 | 0.12 | −0.14 | 0.20 | 0.20 | 0.01 | 0.45 |
| Luteolin 6-C-glucoside/Isoorientin 102753 | 0.11 | 0.01 | 0.16 | 0.20 | 0.01 | 0.46 |
| Luteolin-7,3′-di-O-glucoside 4590322 | −0.03 | −0.50 | −0.11 | −0.06 | −0.04 | 0.07 |
This conclusion is also supported by the results presented in Table 2, showing no deviation from the limiting criteria postulated by Lipinski’s rule of five. On the contrary, luteolin-7,3′-di-O-glucoside, the most flexible molecule presenting seven rotatable bonds and three violations from Lipinski’s rule (molecular weight larger than 500 Da; more than five hydrogen bond donors, more than ten hydrogen bond acceptors), shows the lowest scores for all targets. Thus, it is classified as a moderately active candidate. None of the luteolin derivatives can be considered inactive, as they all show scores larger than −5.0.
Table 3 also indicates that all compounds show greater scores for enzyme inhibition, all values being positives, acting as active drug candidates. Based on this, we can declare that the most active ligand is luteolin 6-C-glucoside with 0.46 score for enzyme inhibition. Concerning protease inhibition, the most active ones are luteolin 6-C-glucoside and luteolin 8-C-glucoside (score = 0.01). Luteolin aglycone is the most active when compared with its derivatives, because it is the ligand that is capable of binding to nuclear receptor (score = 0.39) and kinase inhibitor (score = 0.26). Luteolin 5-O-glucoside and luteolin 8-C-glucoside were found to be highly bioactive toward GPCR ligand (score = 0.12). Regarding ion channel modulator bioactivity, luteolin 6-C-glucoside shows a promising score (0.01).
Considering all the above observations of the drug-likeness parameters (Table 2) and bioactivities (Table 3), it can be concluded that all compounds show great potential in terms of bioactivity as ligands for the tested targets, starting with their lead compound, luteolin. Therefore, the interest in luteolin and luteolin derivatives in the recent years is argued. The advantages offered by luteolin derivatives as opposed to other flavonoid subclasses are drawn from their chemical structures: they are more resistant to the (auto)oxidation process explained by the lack of a hydroxyl group in position 3 of the flavan ring. They are resistant to acidic hydrolysis [32], at the same time more stable and safe and able to pass through the cell membranes more easily, while also showing great bioactivity potential.
Proving these, scientific data indicate that luteolin stimulates breast cancer cell apoptosis [45] and inhibits fatty acid synthase (FASN) activity [46,47,48,49], essential for normal and tumor breast cell growth and function. FASN is the only enzyme capable of inhibiting the novo synthesis of fatty acids with long chains (starting from acetyl-CoA, malonyl-CoA and nicotinamide adenine dinucleotide phosphate). At the same time, it is significantly activated in many types of malignant tumors, especially in the case of human mammary tumor growth and metastasis.
Moreover, due to the complex actions that compete for the final effect, luteolin aglycone was proved to be an effective inhibitor of mammary and prostate cancer metastases [50]. For instance, luteolin inhibits the angiogenesis process induced by the vascular endothelial growth factor, which reduces the chance of colonization of metastatic targets [51]; luteolin inhibits the expression of specific matrix metalloproteinases involved in basement membrane degradation, a key step in the neovascularization and tumor invasion process [52]; luteolin inhibits the secretion of interleukin (IL)-8 [53] and IL-6, acting as an amplifying signal of cancer cell growth and invasion [54]; luteolin inhibits epithelial–mesenchymal transition considered as a pinnacle step in the process of cancer cell invasion and metastasis [55]; luteolin acts as a p90 ribosomal S6 kinase inhibitor that suppresses Notch4 signaling [56]; luteolin promotes cell cycle arrest in breast cancer and inhibits the expression of human epidermal growth factor receptors HER2 [57] and EGFR/aka HER1 [58], related to the new breast cancer cases; luteolin acts as an insulin-like growth factor-mediated proliferation antagonist [59] and stimulates breast cancer cell apoptosis through extrinsic (via caspase 9) [60] and intrinsic (via caspases 8 and 10) [61] pathways, thus being the subject of numerous review articles [62,63,64].
Regarding aspects of bioavailability, luteolin derivatives appear to be generally degraded to smaller metabolites by the intestinal bacterium Eubacterium cellulosolvens within 24 h; in contrast, luteolin C-polyglycosides appear unchanged when they are absorbed in the intestine, before being distributed to the body tissues [65].
Another aspect to be mentioned is the capacity of polyphenols, especially of flavonoid subclasses, to bind iron from the medium; flavonoid C-glycosides fulfil the role of siderophores in plant tissues [66,67]. The sequestration of iron from the intestine by polyphenol-based supplements leads to several shortcomings in humans: the inhibition of heme iron absorption possibly promotes anemia [68], the intestinal inflammation and colon tumor development. Data suggest that good intestinal bacteria are significantly affected by iron depletion. This is because their metabolism is dependent on numerous iron enzymes, while pathogenic bacteria necessitate low or no levels of iron to survive and proliferate [69]. A possible way to oppose iron sequestration by polyphenol-based supplements is by the addition of Fe and EDTA in tea in a molar ratio of 1:2, to avoid iron sequestration by green tea catechins [70].
4 Conclusion
The present study aims to evaluate the antiproliferative potencies of two vegetal extracts combining luteolin derivatives with caffeic and, respectively, gallic/ellagic acid derivatives isolated from S. laevis and G. pratense plant species.
In vitro pharmacological studies indicated that the ethanolic extracts of S. laevis have the ability to inhibit the viability of both sub-confluent and semi-confluent nontumorigenic epithelial mammary gland cell line MCF-12A and sub-confluent and semi-confluent human breast tumor cell line BT-20. This suggests both potential toxic effects and potential antiproliferative activity of human breast (cancer) cell development. Also, the fact that Stokes’ aster ethanolic extract inhibited either normal or tumor breast cell line sustains the involvement of luteolin derivatives, which proved to inhibit the activity of FASN involved in both normal and tumor mammary cell metabolism.
G. pratense ethanolic extract yielded in vitro results that rather seem to suggest stimulatory effects on normal and tumor human breast cell development.
Computational studies made on luteolin and several common luteolin derivatives conducted to a reactivity analysis and to drug-likeness and bioactivity prediction approaches. For example, it was shown that luteolin, the structure that respects all limitations imposed by Lipinski’s drug-likeness criteria, is a promising drug molecule, with a potential for higher activity against several biological targets. The predictions also indicate that all of its studied derivatives could exhibit good or moderate activity toward used receptors. The fact that log P values computed for luteolin derivatives do not follow the PSA values also explains to some extent the results of in vitro pharmacological studies, and antiproliferative effectiveness of Slae26 and Gpre36.
In summary, prediction studies are key factors for developing new drugs, giving a preview of the hydrophilic–lipophilic character, absorption, transport and distribution through the physiological media of the modeled compounds; it must be noticed that the computational scores are based on experimental data collected from the medicinal chemistry literature (about 10,000 data points for every target class) [71].
Overall, for the first time in the literature, the potential antiproliferative activity of the S. aster ethanolic extract was proved on tumor human breast cell line MT-20, but further studies are required to assess the exact chemical composition and to separate more active fractions to decrease the IC50 value. This way, the S. laevis plant species turns out to be a good source of luteolin derivatives for pharmaceutical purposes, but not for alimentary or phytomedicine purposes, since in vitro studies on the normal human epithelial mammary cell line MCT-12A indicate potential toxic effects (up to 88% cell viability inhibition) of the polar extracts from the aerial part of Stokes’ aster.
Acknowledgments
This work was supported by the ANCSI program POC-A1-A1.2.3-G-2015, Project title <New technologies and natural derived products for human health use>, Contract no.60/05.09.2016, ID P_40_406, SMIS 105542.
Conflict of interest: The authors declare that this paper content has no conflict of interest.
References
[1] Barb JG, Werner DJ, Griesbach RJ. Genetics and biochemistry of flower color in Stokes aster. JASHS J Am Soc Horticult Sci. 2008;133:569–78.10.21273/JASHS.133.4.569Search in Google Scholar
[2] Campbell TA. Agronomic potential of Stokes aster. In: Pryde EH, Princen LH, Mukherjee KD, editors. New Sources of Fats and Oils. vol. 20, 1981. p. 287–96.Search in Google Scholar
[3] Princen LH. New oilseed crops on the horizon. Econ Bot. 1983;37:478–92.10.1007/BF02904214Search in Google Scholar
[4] https://www.ehow.com/info_12318214_asters-poisonous-dogs.htmlSearch in Google Scholar
[5] Pattnaik S, Subramanyam VR, Bapaji M, Kole CR. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios. 1997;89:39–46.Search in Google Scholar
[6] Edwards-Jones V, Buck R, Shawcross SG, Dawson MM, Dunn K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns. 2004;30:772–7.10.1016/j.burns.2004.06.006Search in Google Scholar PubMed
[7] Lorenzi V, Muselli A, Bernardini AF, Berti L, Pages J-M, Amaral L, et al. Geraniol restores antibiotic activities against multidrug-resistant isolates from Gram-negative species. Antimicrob Agents Chemother. 2009;53:2209–11.10.1128/AAC.00919-08Search in Google Scholar PubMed PubMed Central
[8] Manu DK Graduate Theses and Dissertations, Iowa State University Capstones, Antimicrobial Activity of Cinnamaldehyde or Geraniol alone or Combined with High Pressure Processing to Destroy Escherichia coli O157:H7 and Salmonella enterica in Juice, 2016, https://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=6976&context=etdSearch in Google Scholar
[9] Kim SH, Bae HC, Park EJ, Lee CR, Kim BJ, Lee S, et al. Geraniol inhibits prostate cancer growth by targeting cell cycle and apoptosis pathways. Biochem Biophys Res Commun. 2011;407:129–34.10.1016/j.bbrc.2011.02.124Search in Google Scholar PubMed
[10] Chaudhary SC, Siddiqui MS, Athar M, Alam MS. Geraniol inhibits murine skin tumorigenesis by modulating COX-2 expression, Ras-ERK1/2 signaling pathway and apoptosis. J Appl Toxicol. 2013;33:828–37.10.1002/jat.2739Search in Google Scholar PubMed
[11] Cho M, So I, Chun JH, Jeon JH. The antitumor effects of geraniol: modulation of cancer hallmark pathways (review). Int J Oncol. 2016;48:1772–82.10.3892/ijo.2016.3427Search in Google Scholar PubMed PubMed Central
[12] Ito H, Iguchi A, Hatano T. Identification of urinary and intestinal bacterial metabolites of ellagitannin geraniin in rats. J Agric Food Chem. 2008;56:393–400.10.1021/jf0726942Search in Google Scholar PubMed
[13] Ito H. Metabolites of the ellagitannin geraniin and their antioxidant activities. Planta Med. 2011;77:1110–5.10.1055/s-0030-1270749Search in Google Scholar PubMed
[14] OkaOkabe S, Suganuma M, Imayoshi Y, Taniguchi T, Fujiki H. New TNF-alpha releasing inhibitors, geraniin and corilagin, in leaves of Acer nikoense, Megusurino-ki. Biol Pharm Bull. 2001;24:1145–8.10.1248/bpb.24.1145Search in Google Scholar PubMed
[15] Yang CM, Cheng HY, Lin TC, Chiang LC, Lin CC. Ethnopharmacological communication. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J Ethnopharmacol. 2007;10:555–8.10.1016/j.jep.2006.09.039Search in Google Scholar
[16] Yang Y, Zhang L, Fan X, Qin C, Liu J. Antiviral effect of geraniin on human enterovirus 71 in vitro and in vivo. Bioorg Med Chem Lett. 2012;22:2209–11.10.1016/j.bmcl.2012.01.102Search in Google Scholar
[17] Youn K, Jun M. In vitro BACE1 inhibitory activity of geraniin and corilagin from Geranium thunbergii. Planta Med. 2013;9:1038–42.10.1055/s-0032-1328769Search in Google Scholar
[18] Tabanca N, Wang M, Avonto C, Chittiboyina AG, Parcher JF, Carroll JF, et al. Bioactivity-guided investigation of geranium essential oils as natural tick repellents. J Agric Food Chem. 2013;61:4101–7.10.1021/jf400246aSearch in Google Scholar
[19] Madrigal-Santillán E, Bautista M, Gayosso-De-Lucio JA, Reyes-Rosales Y, Posadas-Mondragón A, Morales-González A, et al. Hepatoprotective effect of Geranium schiedeanum against ethanol toxicity during liver regeneration. World J Gastroenterol. 2015;21:7718–29.10.3748/wjg.v21.i25.7718Search in Google Scholar
[20] Graca VC, Barros L, Calhelha RC, Dias MI, Carvalho AM, Santos-Buelga C, et al. Chemical characterization and bioactive properties of aqueous and organic extracts of Geranium robertianum L. Food Funct. 2016;7:3807–14.10.1039/C6FO01075JSearch in Google Scholar
[21] Graca VC, Dias MI, Barros L, Calhelha RC, Santos PF, Ferreira IC. Fractionation of the more active extracts of Geranium molle L.: a relationship between their phenolic profile and biological activity. Food Funct. 2018;9:2032–42.10.1039/C7FO01994GSearch in Google Scholar
[22] Catarino MD, Silva AMS, Cruz MT, Cardoso SM. Antioxidant and anti-inflammatory activities of Geranium robertianum L. decoctions. Food Funct. 2017;8:3355–65.10.1039/C7FO00881CSearch in Google Scholar
[23] Aranda-Ventura J, Villacres J, Mego R, Delgado H. Effect of extracts of Geranium ayavacense W. (Pasuchaca) on glycemia on rats with experimental diabetes mellitus. Rev Peru Med Exp Salud Publica. 2014;31:261–6.Search in Google Scholar
[24] Li J, Huang H, Zhou W, Feng M, Zhou P. Anti-hepatitis B virus activities of Geranium carolinianum L. extracts and identification of the active components. Biol Pharm Bull. 2008;31:743–7.10.1248/bpb.31.743Search in Google Scholar
[25] Akdemir ZS, Tatli II, Saracoglu I, Ismailoglu UB, Sahin-Erdemli I, Calis I. Polyphenolic compounds from Geranium pratense and their free radical scavenging activities. Phytochemistry. 2001;56:189–93.10.1016/S0031-9422(00)00367-8Search in Google Scholar
[26] Ushiki J, Tahara S, Hayakawa Y, Tadano T. Medicinal plants for suppressing soil-borne plant diseases. Soil Sci Plant Nutr. 1998;44:157–65.10.1080/00380768.1998.10414436Search in Google Scholar
[27] Kupeli E, Tatli II, Akdemir ZS, Yesilada E. Estimation of antinociceptive and anti-inflammatory activity on Geranium pratense subsp. finitimum and its phenolic compounds. J Ethnopharmacol. 2007;114:234–40.10.1016/j.jep.2007.08.005Search in Google Scholar PubMed
[28] Zhou J, Xie G, Yan X. Encyclopedia of Traditional Chinese Medicines – molecular structures, pharmacological activities, natural sources and applications. Berlin Heidelberg: Springer-Verlag; 2011. vol. 5, 454 p.10.1007/978-3-642-16741-6Search in Google Scholar
[29] Romanian Pharmacopoeia, Xth ed., Medicala, Bucharest, 1993.Search in Google Scholar
[30] Wagner H, Bladt S. Plant drug analysis. A thin layer chromatography atlas. 2nd ed. Berlin Heidelberg: Springer-Verlag; 1996.10.1007/978-3-642-00574-9Search in Google Scholar
[31] Reikh E, Schibli A. HPTLC for the analysis of Medicinal Plants. Thieme NY: Stuttgart; 2008.10.1055/b-0034-65242Search in Google Scholar
[32] Pirvu L, Hlevca C, Nicu I, Bubueanu C. Comparative analytical, antioxidant and antimicrobial activity studies on a series of vegetal extracts prepared from eight plant species growing in Romania, JPC J Planar Chromatogr. 2014;27:346–56.10.1556/JPC.27.2014.5.4Search in Google Scholar
[33] https://www.promega.ro.pdfSearch in Google Scholar
[34] Pirvu L, Stefaniu A, Neagu G, Albu B, Pintilie L. In vitro cytotoxic and antiproliferative activity of Cydonia oblonga flower petals, leaves and fruits pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2. Open Chem. 2018;16:1–14.10.1515/chem-2018-0062Search in Google Scholar
[35] Shao Y, Molnar LF, Jung Y, Kussmann J, Ochsenfeld C, Brown ST, et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys Chem Chem Phys. 2006;8:3172–91.10.1039/B517914ASearch in Google Scholar
[36] Hehre WJ. A guide to molecular mechanics and quantum chemical calculations. Irvine, CA: Wavefunction Inc.; 2003.Search in Google Scholar
[37] Lee C, Yang W, Parr RG. Development of the Colle–Salvetti correlation–energy formula into a functional of the electron density. Phys Rev B. 1988;37:785–9.10.1103/PhysRevB.37.785Search in Google Scholar
[38] Sastri VS, Perumareddi JR. Molecular orbital theoretical studies of some organic corrosion inhibitors. Corros Sci. 1997;53:617–22.10.5006/1.3290294Search in Google Scholar
[39] Yankova R, Genieva S, Halachev N, Dimitrova G. Molecular structure, vibrational spectra, MEP, HOMO–LUMO and NBO analysis of Hf(SeO3)(SeO4)(H2O)4. J Mol Struct. 2016;1106:82–88.10.1016/j.molstruc.2015.10.091Search in Google Scholar
[40] Zarrouk A, Zarrok H, Salghi R, Hammouti B, Al-Dey ab SS, Touzani R, et al. A theoretical investigation on the corrosion inhibition of copper by quinoxaline derivatives in nitric acid solution. Int J Electrochem Sci. 2012;7:6353–64.Search in Google Scholar
[41] Wang H, Wang X, Wang H, Wang L, Liu A. DFT study of new bipyrazole derivatives and their potential activity as corrosion inhibitors. J Mol Model. 2007;13:147–53.10.1007/s00894-006-0135-xSearch in Google Scholar
[42] Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Delivery Rev. 2001;46:3–26.10.1016/S0169-409X(00)00129-0Search in Google Scholar
[43] Moghtaderi H, Sepehri H, Delphi L, Attari F. Gallic acid and curcumin induce cytotoxicity and apoptosis in human breast cancer cell MDA-MB-231. Bioimpacts. 2018;8:185–94.10.15171/bi.2018.21Search in Google Scholar
[44] Ceci C, Lacal PM, Tentori L, De Martino MG, Miano R, Graziani G. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutriets. 2018;10(11):E1756, 10.3390/nu10111756Search in Google Scholar
[45] Lin CH, Chang CY, Lee KR, Lin HJ, Chen TH, Wan L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer. 2015;15:958.10.1186/s12885-015-1965-7Search in Google Scholar
[46] Brusselmans K, Vrolix R, Swinnen JV. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J Biol Chem. 2005;280:5636–45.10.1074/jbc.M408177200Search in Google Scholar
[47] Günter S, Merfort I, Wölfle U, Schempp CM. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13:2628–51.10.3390/molecules13102628Search in Google Scholar
[48] Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–8.10.1016/S0899-9007(99)00266-XSearch in Google Scholar
[49] Lupu R, Menendez JA. Pharmacological inhibitors of fatty acid synthase (FASN)-catalyzed endogenous fatty acid biogenesis: a new family of anti-cancer agents? Curr Pharm Biotechnol. 2006;7:483–49.10.2174/138920106779116928Search in Google Scholar PubMed
[50] Cook MT. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer Targets Ther. 2018;10:89–100.10.2147/BCTT.S144202Search in Google Scholar PubMed PubMed Central
[51] Cook MT, Liang Y, Besch-Williford C, Hyder SM. Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer. 2017;9:9–19.10.2147/BCTT.S124860Search in Google Scholar PubMed PubMed Central
[52] Sun DW, Zhang HD, Mao L, Mao CF, Chen W, Cui M, et al. Luteolin inhibits breast cancer development and progression in vitro and in vivo by suppressing notch signaling and regulating miRNAs. Cell Physiol Biochem. 2015;37:1693–711.10.1159/000438535Search in Google Scholar PubMed
[53] Park SH, Kim JH, Lee DH, Kang JW, Song HH, Oh SR, et al. Luteolin 8-C-β-fucopyranoside inhibits invasion and suppresses TPA-induced MMP-9 and IL-8 via ERK/AP-1 and ERK/NF-κB signaling in MCF-7 breast cancer cells. Biochimie. 2013;95:2082–90.10.1016/j.biochi.2013.07.021Search in Google Scholar PubMed
[54] Tu DG, Lin WT, Yu CC, Lee SS, Peng CY, Lin T, et al. Chemotherapeutic effects of luteolin on radio-sensitivity enhancement and interleukin-6/signal transducer and activator of transcription 3 signaling repression of oral cancer stem cells. J Formos Med Assoc. 2016;115:1032–8.10.1016/j.jfma.2016.08.009Search in Google Scholar PubMed
[55] Lin D, Kuang G, Wan J, Zhang X, Li H, Gong X, et al. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol Rep. 2017;37:895–902.10.3892/or.2016.5311Search in Google Scholar PubMed
[56] Reipas KM, Law JH, Couto N, Islam S, Li Y, Li H, et al. Luteolin is a novel p90 ribosomal S6 kinase (RSK) inhibitor that suppresses Notch4 signaling by blocking the activation of Y-box binding protein-1 (YB-1). Oncotarget. 2013;4:329–45.10.18632/oncotarget.834Search in Google Scholar PubMed PubMed Central
[57] Lee EJ, Oh SY, Sung MK. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Toxicol. 2012;50:4136–43.10.1016/j.fct.2012.08.025Search in Google Scholar PubMed
[58] Chiang CT, Way TD, Lin JK. Sensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21WAF1/CIP1 expression with rapamycin. Mol Cancer Ther. 2007;6:2127–38.10.1158/1535-7163.MCT-07-0107Search in Google Scholar PubMed
[59] Wang LM, Xie KP, Huo HN, Shang F, Zou W, Xie MJ. Luteolin inhibits proliferation induced by IGF-1 pathway dependent ERα in human breast cancer MCF-7 cells. Asian Pac J Cancer Prev. 2012;13:1431–7.10.7314/APJCP.2012.13.4.1431Search in Google Scholar
[60] Park SH, Ham S, Kwon TH, Kim MS, Lee DH, Kang JW, et al. Luteolin induces cell cycle arrest and apoptosis through extrinsic and intrinsic signaling pathways in MCF-7 breast cancer cells. J Env Pathol Toxicol Oncol. 2014;33:219–31.10.1615/JEnvironPatholToxicolOncol.2014010923Search in Google Scholar
[61] Yang MY, Wang CJ, Chen NF, Ho WH, Lu FJ, Tseng TH. Luteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3. Chem Biol Interact. 2014;213:60–68.10.1016/j.cbi.2014.02.002Search in Google Scholar PubMed
[62] Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–80.10.1158/0008-5472.CAN-05-4673Search in Google Scholar PubMed
[63] Pandey PR, Liu W, Xing F, Fukuda K, Watabe K. Anti-cancer drugs targeting fatty acid synthase (FAS). Recent Pat Anti-Cancer Drug Discovery. 2012;7:185–97.10.2174/157489212799972891Search in Google Scholar PubMed
[64] Zhang JS, Lei JP, Wei GQ, Chen H, Ma CY, Jiang HZ. Natural fatty acid synthase inhibitors as potent therapeutic agents for cancers: a review. Pharm Biol. 2016;54:1919–25.10.3109/13880209.2015.1113995Search in Google Scholar PubMed
[65] Xiao J, Capanoglu E, Jassbi AR, Miron A. Advance on the flavonoid C-glycosides and health benefits. Crit Rev food Sci Nutr. 2016;56:S29–45.10.1080/10408398.2015.1067595Search in Google Scholar PubMed
[66] Ma Q, Kim EY, Lindsay EA, Han O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J Food Sci. 2011;76:H143–50.10.1111/j.1750-3841.2011.02184.xSearch in Google Scholar PubMed PubMed Central
[67] Hultin PG. Bioactive C-glycosides from bacterial secondary metabolism. Curr Top Med Chem. 2005;5:1299–331.10.2174/156802605774643015Search in Google Scholar PubMed
[68] Brazier-Hicks M, Evans KM, Gershater MC, Puschmann H, Steel PG, Edwards R. The C-glycosylation of flavonoids in cereals. J Biol Chem. 2009;284:17926–34.10.1074/jbc.M109.009258Search in Google Scholar PubMed PubMed Central
[69] Ylmaz B, Li H. Gut microbiota and iron: the crucial actors in health and disease. Pharmaceuticals. 2018;11:98.10.3390/ph11040098Search in Google Scholar PubMed PubMed Central
[70] Dueik V, Chen BK, Diosady LL. Iron–polyphenol interaction reduces iron bioavailability in fortified tea: competing complexation to ensure iron bioavailability. J Food Qual. 2017;2017:7.10.1155/2017/1805047Search in Google Scholar
[71] https://molinspiration.com/docu/miscreen/virtualscreening.htmlSearch in Google Scholar
© 2020 Lucia Pirvu et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene
Articles in the same Issue
- Regular Articles
- Electrochemical antioxidant screening and evaluation based on guanine and chitosan immobilized MoS2 nanosheet modified glassy carbon electrode (guanine/CS/MoS2/GCE)
- Kinetic models of the extraction of vanillic acid from pumpkin seeds
- On the maximum ABC index of bipartite graphs without pendent vertices
- Estimation of the total antioxidant potential in the meat samples using thin-layer chromatography
- Molecular dynamics simulation of sI methane hydrate under compression and tension
- Spatial distribution and potential ecological risk assessment of some trace elements in sediments and grey mangrove (Avicennia marina) along the Arabian Gulf coast, Saudi Arabia
- Amino-functionalized graphene oxide for Cr(VI), Cu(II), Pb(II) and Cd(II) removal from industrial wastewater
- Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata
- Effect of excess Fluoride consumption on Urine-Serum Fluorides, Dental state and Thyroid Hormones among children in “Talab Sarai” Punjab Pakistan
- Design, Synthesis and Characterization of Novel Isoxazole Tagged Indole Hybrid Compounds
- Comparison of kinetic and enzymatic properties of intracellular phosphoserine aminotransferases from alkaliphilic and neutralophilic bacteria
- Green Organic Solvent-Free Oxidation of Alkylarenes with tert-Butyl Hydroperoxide Catalyzed by Water-Soluble Copper Complex
- Ducrosia ismaelis Asch. essential oil: chemical composition profile and anticancer, antimicrobial and antioxidant potential assessment
- DFT calculations as an efficient tool for prediction of Raman and infra-red spectra and activities of newly synthesized cathinones
- Influence of Chemical Osmosis on Solute Transport and Fluid Velocity in Clay Soils
- A New fatty acid and some triterpenoids from propolis of Nkambe (North-West Region, Cameroon) and evaluation of the antiradical scavenging activity of their extracts
- Antiplasmodial Activity of Stigmastane Steroids from Dryobalanops oblongifolia Stem Bark
- Rapid identification of direct-acting pancreatic protectants from Cyclocarya paliurus leaves tea by the method of serum pharmacochemistry combined with target cell extraction
- Immobilization of Pseudomonas aeruginosa static biomass on eggshell powder for on-line preconcentration and determination of Cr (VI)
- Assessment of methyl 2-({[(4,6-dimethoxypyrimidin-2-yl)carbamoyl] sulfamoyl}methyl)benzoate through biotic and abiotic degradation modes
- Stability of natural polyphenol fisetin in eye drops Stability of fisetin in eye drops
- Production of a bioflocculant by using activated sludge and its application in Pb(II) removal from aqueous solution
- Molecular Properties of Carbon Crystal Cubic Structures
- Synthesis and characterization of calcium carbonate whisker from yellow phosphorus slag
- Study on the interaction between catechin and cholesterol by the density functional theory
- Analysis of some pharmaceuticals in the presence of their synthetic impurities by applying hybrid micelle liquid chromatography
- Two mixed-ligand coordination polymers based on 2,5-thiophenedicarboxylic acid and flexible N-donor ligands: the protective effect on periodontitis via reducing the release of IL-1β and TNF-α
- Incorporation of silver stearate nanoparticles in methacrylate polymeric monoliths for hemeprotein isolation
- Development of ultrasound-assisted dispersive solid-phase microextraction based on mesoporous carbon coated with silica@iron oxide nanocomposite for preconcentration of Te and Tl in natural water systems
- N,N′-Bis[2-hydroxynaphthylidene]/[2-methoxybenzylidene]amino]oxamides and their divalent manganese complexes: Isolation, spectral characterization, morphology, antibacterial and cytotoxicity against leukemia cells
- Determination of the content of selected trace elements in Polish commercial fruit juices and health risk assessment
- Diorganotin(iv) benzyldithiocarbamate complexes: synthesis, characterization, and thermal and cytotoxicity study
- Keratin 17 is induced in prurigo nodularis lesions
- Anticancer, antioxidant, and acute toxicity studies of a Saudi polyherbal formulation, PHF5
- LaCoO3 perovskite-type catalysts in syngas conversion
- Comparative studies of two vegetal extracts from Stokesia laevis and Geranium pratense: polyphenol profile, cytotoxic effect and antiproliferative activity
- Fragmentation pattern of certain isatin–indole antiproliferative conjugates with application to identify their in vitro metabolic profiles in rat liver microsomes by liquid chromatography tandem mass spectrometry
- Investigation of polyphenol profile, antioxidant activity and hepatoprotective potential of Aconogonon alpinum (All.) Schur roots
- Lead discovery of a guanidinyl tryptophan derivative on amyloid cascade inhibition
- Physicochemical evaluation of the fruit pulp of Opuntia spp growing in the Mediterranean area under hard climate conditions
- Electronic structural properties of amino/hydroxyl functionalized imidazolium-based bromide ionic liquids
- New Schiff bases of 2-(quinolin-8-yloxy)acetohydrazide and their Cu(ii), and Zn(ii) metal complexes: their in vitro antimicrobial potentials and in silico physicochemical and pharmacokinetics properties
- Treatment of adhesions after Achilles tendon injury using focused ultrasound with targeted bFGF plasmid-loaded cationic microbubbles
- Synthesis of orotic acid derivatives and their effects on stem cell proliferation
- Chirality of β2-agonists. An overview of pharmacological activity, stereoselective analysis, and synthesis
- Fe3O4@urea/HITh-SO3H as an efficient and reusable catalyst for the solvent-free synthesis of 7-aryl-8H-benzo[h]indeno[1,2-b]quinoline-8-one and indeno[2′,1′:5,6]pyrido[2,3-d]pyrimidine derivatives
- Adsorption kinetic characteristics of molybdenum in yellow-brown soil in response to pH and phosphate
- Enhancement of thermal properties of bio-based microcapsules intended for textile applications
- Exploring the effect of khat (Catha edulis) chewing on the pharmacokinetics of the antiplatelet drug clopidogrel in rats using the newly developed LC-MS/MS technique
- A green strategy for obtaining anthraquinones from Rheum tanguticum by subcritical water
- Cadmium (Cd) chloride affects the nutrient uptake and Cd-resistant bacterium reduces the adsorption of Cd in muskmelon plants
- Removal of H2S by vermicompost biofilter and analysis on bacterial community
- Structural cytotoxicity relationship of 2-phenoxy(thiomethyl)pyridotriazolopyrimidines: Quantum chemical calculations and statistical analysis
- A self-breaking supramolecular plugging system as lost circulation material in oilfield
- Synthesis, characterization, and pharmacological evaluation of thiourea derivatives
- Application of drug–metal ion interaction principle in conductometric determination of imatinib, sorafenib, gefitinib and bosutinib
- Synthesis and characterization of a novel chitosan-grafted-polyorthoethylaniline biocomposite and utilization for dye removal from water
- Optimisation of urine sample preparation for shotgun proteomics
- DFT investigations on arylsulphonyl pyrazole derivatives as potential ligands of selected kinases
- Treatment of Parkinson’s disease using focused ultrasound with GDNF retrovirus-loaded microbubbles to open the blood–brain barrier
- New derivatives of a natural nordentatin
- Fluorescence biomarkers of malignant melanoma detectable in urine
- Study of the remediation effects of passivation materials on Pb-contaminated soil
- Saliva proteomic analysis reveals possible biomarkers of renal cell carcinoma
- Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities
- Design, synthesis and pharmacological profile of (−)-verbenone hydrazones
- Synthesis of magnesium carbonate hydrate from natural talc
- Stability-indicating HPLC-DAD assay for simultaneous quantification of hydrocortisone 21 acetate, dexamethasone, and fluocinolone acetonide in cosmetics
- A novel lactose biosensor based on electrochemically synthesized 3,4-ethylenedioxythiophene/thiophene (EDOT/Th) copolymer
- Citrullus colocynthis (L.) Schrad: Chemical characterization, scavenging and cytotoxic activities
- Development and validation of a high performance liquid chromatography/diode array detection method for estrogen determination: Application to residual analysis in meat products
- PCSK9 concentrations in different stages of subclinical atherosclerosis and their relationship with inflammation
- Development of trace analysis for alkyl methanesulfonates in the delgocitinib drug substance using GC-FID and liquid–liquid extraction with ionic liquid
- Electrochemical evaluation of the antioxidant capacity of natural compounds on glassy carbon electrode modified with guanine-, polythionine-, and nitrogen-doped graphene
- A Dy(iii)–organic framework as a fluorescent probe for highly selective detection of picric acid and treatment activity on human lung cancer cells
- A Zn(ii)–organic cage with semirigid ligand for solvent-free cyanosilylation and inhibitory effect on ovarian cancer cell migration and invasion ability via regulating mi-RNA16 expression
- Polyphenol content and antioxidant activities of Prunus padus L. and Prunus serotina L. leaves: Electrochemical and spectrophotometric approach and their antimicrobial properties
- The combined use of GC, PDSC and FT-IR techniques to characterize fat extracted from commercial complete dry pet food for adult cats
- MALDI-TOF MS profiling in the discovery and identification of salivary proteomic patterns of temporomandibular joint disorders
- Concentrations of dioxins, furans and dioxin-like PCBs in natural animal feed additives
- Structure and some physicochemical and functional properties of water treated under ammonia with low-temperature low-pressure glow plasma of low frequency
- Mesoscale nanoparticles encapsulated with emodin for targeting antifibrosis in animal models
- Amine-functionalized magnetic activated carbon as an adsorbent for preconcentration and determination of acidic drugs in environmental water samples using HPLC-DAD
- Antioxidant activity as a response to cadmium pollution in three durum wheat genotypes differing in salt-tolerance
- A promising naphthoquinone [8-hydroxy-2-(2-thienylcarbonyl)naphtho[2,3-b]thiophene-4,9-dione] exerts anti-colorectal cancer activity through ferroptosis and inhibition of MAPK signaling pathway based on RNA sequencing
- Synthesis and efficacy of herbicidal ionic liquids with chlorsulfuron as the anion
- Effect of isovalent substitution on the crystal structure and properties of two-slab indates BaLa2−xSmxIn2O7
- Synthesis, spectral and thermo-kinetics explorations of Schiff-base derived metal complexes
- An improved reduction method for phase stability testing in the single-phase region
- Comparative analysis of chemical composition of some commercially important fishes with an emphasis on various Malaysian diets
- Development of a solventless stir bar sorptive extraction/thermal desorption large volume injection capillary gas chromatographic-mass spectrometric method for ultra-trace determination of pyrethroids pesticides in river and tap water samples
- A turbidity sensor development based on NL-PI observers: Experimental application to the control of a Sinaloa’s River Spirulina maxima cultivation
- Deep desulfurization of sintering flue gas in iron and steel works based on low-temperature oxidation
- Investigations of metallic elements and phenolics in Chinese medicinal plants
- Influence of site-classification approach on geochemical background values
- Effects of ageing on the surface characteristics and Cu(ii) adsorption behaviour of rice husk biochar in soil
- Adsorption and sugarcane-bagasse-derived activated carbon-based mitigation of 1-[2-(2-chloroethoxy)phenyl]sulfonyl-3-(4-methoxy-6-methyl-1,3,5-triazin-2-yl) urea-contaminated soils
- Antimicrobial and antifungal activities of bifunctional cooper(ii) complexes with non-steroidal anti-inflammatory drugs, flufenamic, mefenamic and tolfenamic acids and 1,10-phenanthroline
- Application of selenium and silicon to alleviate short-term drought stress in French marigold (Tagetes patula L.) as a model plant species
- Screening and analysis of xanthine oxidase inhibitors in jute leaves and their protective effects against hydrogen peroxide-induced oxidative stress in cells
- Synthesis and physicochemical studies of a series of mixed-ligand transition metal complexes and their molecular docking investigations against Coronavirus main protease
- A study of in vitro metabolism and cytotoxicity of mephedrone and methoxetamine in human and pig liver models using GC/MS and LC/MS analyses
- A new phenyl alkyl ester and a new combretin triterpene derivative from Combretum fragrans F. Hoffm (Combretaceae) and antiproliferative activity
- Erratum
- Erratum to: A one-step incubation ELISA kit for rapid determination of dibutyl phthalate in water, beverage and liquor
- Review Articles
- Sinoporphyrin sodium, a novel sensitizer for photodynamic and sonodynamic therapy
- Natural products isolated from Casimiroa
- Plant description, phytochemical constituents and bioactivities of Syzygium genus: A review
- Evaluation of elastomeric heat shielding materials as insulators for solid propellant rocket motors: A short review
- Special Issue on Applied Biochemistry and Biotechnology 2019
- An overview of Monascus fermentation processes for monacolin K production
- Study on online soft sensor method of total sugar content in chlorotetracycline fermentation tank
- Studies on the Anti-Gouty Arthritis and Anti-hyperuricemia Properties of Astilbin in Animal Models
- Effects of organic fertilizer on water use, photosynthetic characteristics, and fruit quality of pear jujube in northern Shaanxi
- Characteristics of the root exudate release system of typical plants in plateau lakeside wetland under phosphorus stress conditions
- Characterization of soil water by the means of hydrogen and oxygen isotope ratio at dry-wet season under different soil layers in the dry-hot valley of Jinsha River
- Composition and diurnal variation of floral scent emission in Rosa rugosa Thunb. and Tulipa gesneriana L.
- Preparation of a novel ginkgolide B niosomal composite drug
- The degradation, biodegradability and toxicity evaluation of sulfamethazine antibiotics by gamma radiation
- Special issue on Monitoring, Risk Assessment and Sustainable Management for the Exposure to Environmental Toxins
- Insight into the cadmium and zinc binding potential of humic acids derived from composts by EEM spectra combined with PARAFAC analysis
- Source apportionment of soil contamination based on multivariate receptor and robust geostatistics in a typical rural–urban area, Wuhan city, middle China
- Special Issue on 13th JCC 2018
- The Role of H2C2O4 and Na2CO3 as Precipitating Agents on The Physichochemical Properties and Photocatalytic Activity of Bismuth Oxide
- Preparation of magnetite-silica–cetyltrimethylammonium for phenol removal based on adsolubilization
- Topical Issue on Agriculture
- Size-dependent growth kinetics of struvite crystals in wastewater with calcium ions
- The effect of silica-calcite sedimentary rock contained in the chicken broiler diet on the overall quality of chicken muscles
- Physicochemical properties of selected herbicidal products containing nicosulfuron as an active ingredient
- Lycopene in tomatoes and tomato products
- Fluorescence in the assessment of the share of a key component in the mixing of feed
- Sulfur application alleviates chromium stress in maize and wheat
- Effectiveness of removal of sulphur compounds from the air after 3 years of biofiltration with a mixture of compost soil, peat, coconut fibre and oak bark
- Special Issue on the 4th Green Chemistry 2018
- Study and fire test of banana fibre reinforced composites with flame retardance properties
- Special Issue on the International conference CosCI 2018
- Disintegration, In vitro Dissolution, and Drug Release Kinetics Profiles of k-Carrageenan-based Nutraceutical Hard-shell Capsules Containing Salicylamide
- Synthesis of amorphous aluminosilicate from impure Indonesian kaolin
- Special Issue on the International Conf on Science, Applied Science, Teaching and Education 2019
- Functionalization of Congo red dye as a light harvester on solar cell
- The effect of nitrite food preservatives added to se’i meat on the expression of wild-type p53 protein
- Biocompatibility and osteoconductivity of scaffold porous composite collagen–hydroxyapatite based coral for bone regeneration
- Special Issue on the Joint Science Congress of Materials and Polymers (ISCMP 2019)
- Effect of natural boron mineral use on the essential oil ratio and components of Musk Sage (Salvia sclarea L.)
- A theoretical and experimental study of the adsorptive removal of hexavalent chromium ions using graphene oxide as an adsorbent
- A study on the bacterial adhesion of Streptococcus mutans in various dental ceramics: In vitro study
- Corrosion study of copper in aqueous sulfuric acid solution in the presence of (2E,5E)-2,5-dibenzylidenecyclopentanone and (2E,5E)-bis[(4-dimethylamino)benzylidene]cyclopentanone: Experimental and theoretical study
- Special Issue on Chemistry Today for Tomorrow 2019
- Diabetes mellitus type 2: Exploratory data analysis based on clinical reading
- Multivariate analysis for the classification of copper–lead and copper–zinc glasses
- Special Issue on Advances in Chemistry and Polymers
- The spatial and temporal distribution of cationic and anionic radicals in early embryo implantation
- Special Issue on 3rd IC3PE 2020
- Magnetic iron oxide/clay nanocomposites for adsorption and catalytic oxidation in water treatment applications
- Special Issue on IC3PE 2018/2019 Conference
- Exergy analysis of conventional and hydrothermal liquefaction–esterification processes of microalgae for biodiesel production
- Advancing biodiesel production from microalgae Spirulina sp. by a simultaneous extraction–transesterification process using palm oil as a co-solvent of methanol
- Topical Issue on Applications of Mathematics in Chemistry
- Omega and the related counting polynomials of some chemical structures
- M-polynomial and topological indices of zigzag edge coronoid fused by starphene

