Abstract
Phosphatidylinositol 3-kinase (PI3K)/AKT signaling is a crucial pathway for cell survival and proliferation, which are regulated by several growth factors and activated receptors. Upregulated PI3K/AKT signaling molecules were reported in several cancers and they are associated with altered cellular functions, leading to oncogenesis. Here, we have examined the implications of elevated PI3K/AKT expression in the apoptosis resistance of human hepatocellular carcinoma (HCC) Huh7 cells. We showed that PI3K/AKT signaling is significantly upregulated in Huh7 cells by quantitative polymerase chain reaction and protein expression analysis. Also, perversely upregulated PI3K/AKT signaling Huh7 cells are highly resistant to treatment with chemotherapy drugs (docetaxel and sorafenib) and acquired apoptosis resistance through downregulation of tumor suppressor protein PTEN (phosphatase and tensin homolog deleted on chromosome ten). Hence, we have investigated the effect of PTEN overexpression on apoptosis induction in Huh7 cells. We showed that PTEN overexpressed Huh7 cells became more sensitive toward the aforesaid drugs and induced apoptotic cell death due to intracellular reactive oxygen species (ROS) generation. Concurrently, the overexpression of PTEN leads to the activation of mitochondria facilitated intrinsic apoptosis, evidenced by upregulated cytochrome C, caspase 3, and caspase 9. Collectively, our data suggest that the aberrant expression of PI3K/AKT signaling contributes to apoptosis resistance in HCC.
1 Introduction
Liver cancer or hepatocellular carcinoma (HCC) belongs to the most prevalent malignancies and has become the third underlying cause for cancer-allied deaths worldwide, accounting for more than 700k deaths per year [1,2]. Treatment strategies include surgical resection and chemotherapy. However, surgical resection endures the first line of choice for patients with smaller tumors with conserved liver function without vascular invasion [3]. Besides advancement in HCC treatment regimens, the outcome of the patient’s survival rate remains poor due to tumor relapse and intra- or extrahepatic metastasis, which occurs within 2 years of surgery [4]. Several reports have demonstrated that deregulation of signaling pathways, transcriptional factors, growth factors, genes, and proteins play a critical role in HCC oncogenesis [5,6]. The key signaling pathways involved in HCC tumorigenesis include WNT/β catenin, angiogenic signaling, hepatocyte growth factor/c-MET, ERK, PI3K/AKT, and mTOR [7,8,9,10,11].
The phosphatidylinositol 3-kinase (PI3K)/AKT signaling pathway particularly performs essential cellular functions, like cell growth, proliferation, and survival. Hence they are termed as “cell survival pathways” [12]. Under normal conditions, PI3K activation leads to the production of phosphatidylinositol (3,4)-[PI(3,4)P2]/(3,4,5)-bisphosphate [PI(3,4,5)P3], which in turn stimulates Akt, a serine-threonine protein kinase B. Ultimately, the activated Akt acts as a secondary messenger and by phosphorylation it performs various cellular functions [13]. In this cycle, the PI3K/AKT signaling is negatively regulated by the phosphatase and tensin homolog (PTEN), a lipid phosphatase, which can remove phosphoric acid from PIP3 and is subjected to degradation [13,14].
Studies have shown that PI3K/AKT signaling tends to be aberrantly activated and overexpressed in HCC cells [15,16,17], which is crucial for epithelial–mesenchymal transition (EMT) and thus leads to HCC invasion and metastasis [17,18,19]. Activated PI3K/AKT signaling causes enhanced expression of matrix metalloproteinases and upregulate the snail transcriptional expression for EMT induction. Interestingly, several studies have reported that PI3K/AKT signaling molecules play a key role in sorafenib resistance in HCC cells [15,16,17]. However, the molecular mechanism or the factors involved in the apoptosis and chemoresistance of HCC cells are not well characterized so far. Therefore, elucidating such molecular mechanisms and causative factors involved in the resistance of chemotherapeutic drugs and apoptosis would improve the understanding of pathogenesis as well as the treatment modules of HCC. Considering these facts, this study was first designed to examine the expression pattern of PI3K/AKT signaling molecules in HCC Huh7 cells. Furthermore, we have investigated the factors involved in PI3K/AKT signaling-mediated apoptosis/chemoresistance in Huh7 cells.
2 Materials and methods
2.1 Cell culture
Human HCC Huh7 cells were purchased from the Cell Bank, Chinese Academy of Sciences (Shanghai, China). The normal (non-cancerous) human liver cells (hepatocytes) with normal functionalities were used as a control for all the experiments. Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS (DMEM and FBS from Gibco, Shanghai) and antibiotics were used for cell culturing at 37°C and 5% CO2.
2.2 Huh-7 origin and characteristics
The HuH-7 is an immortal, epithelial-like, well-differentiated hepatocyte cellular carcinoma cell line, which was established in 1982. It is originally derived from a 57-year-old Japanese male liver cancer patient. Huh-7 cells are adherent and able to grow in 2D cultures.
2.3 Reverse transcription polymerase chain reaction (RT-PCR) analysis
cDNA synthesis kit, RT-PCR/PCR kit, polymerases, and restriction enzymes were purchased from Ta Ka Ra Biotechnology, China. Total cellular RNA extraction was performed as per the protocol explained in the RNA extraction kit (Beijing Solar Biotechnology). The RT-PCR (Biorad) was performed as described previously [20]. The primers used for gene amplification were PI3K: F-AACACAGAAGACCAATACTC and R-TTCGCCATCTACCACTAC [21]; AKT: F-GTGGCAAGATGTGTATGAG and R-CTGGCTGAGTAGGAGAAC; PTEN: F-AAGGCACAAGAGGCCTAGATTTCT and R-ACTGAGGATTGCAAGTTCCGCCA [22]; and GAPDH: F-ATGTCGTGGAGT CTACTGGC, and R-TGACCTTGCCCACAGCCTTG [23]. The mRNA expression folds of PI3 and AKT were calculated using Ct values and normalized with GAPDH (house-keeping gene) as per the formula: 2−ΔCt [ΔCt = Ct target gene-Ct-GAPDH]. The quantification graph represents the average value from four individual experiments.
2.4 Transfection
Approximately 2 × 106 cells/well were seeded in 6-well plates. After 24 h of culturing, cells were transfected with plasmids such as 809 pcDNA3-GFP-PTEN (Plasmid #10759) [24] or empty vector pCMV-PTEN (Plasmid #28298) by using 8 µL of the transfection reagent, Lipofectamine (Qiagen). After 24 h of incubation at 37°C in 5% CO2, cells were subsequently subjected to in vitro assays.
2.5 Flow cytometry analysis
After 48 h of transfection with PTEN overexpression Cassette, cells were fixed in ice-cold methanol (70%) and subjected to propidium iodide (PI) staining (PI staining kit from Sigma-Aldrich) at 37°C under dark conditions overnight. The next day, cells were subjected to flow cytometry analysis (BD Biosciences, USA). The rate of apoptosis was evaluated based on the mean fluorescent intensity of PI measured by flow cytometry. The mean intensity values are represented as a quantification graph. The values were taken from three individual experiments.
2.6 Intracellular ROS measurements
Cells were cultured for 48 h incubation, both non-transfected and GFP-PTEN transfected cells were washed by 1XPBS, and subsequently treated with 2′,7′-dichloro fluorescin diacetate (DCFH-DA) under dark conditions for 30 min. Subsequently, cells were subjected to flow cytometry to measure the mean intensity of fluorescence exhibited by DCFH-DA. The mean intensity values are represented as a quantification graph. The values were taken from three individual experiments.
2.7 Western blot analysis
Proteins were extracted and boiled in the 2× Biorad sample buffer, separated by SDS-polyacrylamide (10%) gel electrophoresis, transferred to polyvinylidene difluoride membranes, and subsequently blocked with 5% skim milk in 1× phosphate buffered saline/tween (PBST). Blots were probed with primary antibodies against Akt (1:1,000), PI3 (1:500), PTEN (1:1,500) (from Santa Cruz Biotechnology); caspase 3 (1:500), caspase 9 (1:500), Bcl-2 (1:2,000), cytochrome C (1:1,000), and GAPDH (1:5,000) from Beijing Zhongshan Biotechnology. Horse-radish peroxidase conjugated the secondary antibody (1:10,000) from the Cell Signaling Technology. The protein signal was visualized by 2 mL of ECL chemiluminescence (Biorad). The signal intensities of the protein band were quantified by FIJI image analysis software, and the signal values were normalized with the loading control GAPDH protein.
2.8 Apoptosis resistance assay
Cells were seeded in a range of 104 cells/well and incubated overnight in 96-well plates. Then cells were treated with 10 μM of Docetaxel (Rhone-Poulenc Rorer Pharmaceuticals) and 10 μM of Sorafenib (Jinan Trio Pharmatech) and incubated for 48 h. Apoptosis resistance was evaluated exactly as described previously by using the formula: Cell resistance rate (%) = (experimental group OD450 value/control group OD450 value) × 100 [25].
2.9 Cell proliferation assay
Cell proliferation assay was performed in 6-well plate by seeding approximately 106 cells/well. After 24 h of drug treatment (docetaxel and sorafenib) or transfected cells (PTEN overexpression), the rate of cell proliferation was measured by treating cells with 10 μL of cell counting kit-8 solution for 2–3 h. Then the growth rate was measured at 450 nm and the values (from the three independent experiments) obtained were represented in the quantification graph.
2.10 Statistical analysis
The values denoted in the graphs were mean ± SD. The Student t test was performed for statistical analysis and the one-way analysis of variance was used to compare the two groups. When the P-value was **P < 0.01 and *P < 0.05, they were considered as statistically significant.
3 Results
3.1 Activation of PI3K/AKT signaling pathway is associated with apoptosis resistance in Huh7 cells
We have assessed the transcriptional regulation of PI3K and AKT in HCC Huh7 cells. Our q-PCR analysis revealed that the mRNA expression folds of PI3K and AKT are significantly (P < 0.01) upregulated in Huh7 cells when compared to control cells (Figure 1a and b). Meanwhile, the western blot analysis confirmed the enhanced protein expression of PI3k and Akt in Huh7 cells (Figure 1c and d). Hence, these findings reflect the previous reports that have demonstrated the aberrant activation of the PI3K/AKT signaling pathway in liver cancer cells [15,16,17]. One step further, these PI3K/AKT elevated Huh7 cells were subjected to chemoresistance assay. Upon treatment with docetaxel and sorafenib, the Huh7 cells became more resistant and their cell viability was significantly (P < 0.01) higher in Huh7 cells (Figure 2a). Reports in several cancers explained that activated PI3K/AKT signaling is involved in the downregulation of tumor suppressor PTEN and upregulation of anti-apoptotic factor BCL-2, respectively. Hence, we have examined the expression pattern of PTEN and Bcl-2 by western blot. PTEN expression is decreased dramatically in Huh7 cells, whereas the anti-apoptotic protein Bcl-2 is highly enhanced (Figure 2b and c). Therefore, these data suggest that aberrant upregulation of PI3K/AKT signaling molecules is involved in HCC apoptosis resistance through the decreased expression of tumor suppressor protein PTEN.

Activation of PI3K/AKT in HCC Huh7 cells. (a) Agarose gel electrophoresis and RT-PCR analysis (b) showing enhanced transcriptional regulation of PI3K/AKT signaling in Huh7 cells (>4-fold increased). Western blot (c) and the quantification data (d) showing enhanced protein expression of PI3K and AKT in HC C Huh7cells. The error bar represented in the graph is ±SD, **P < 0.01.
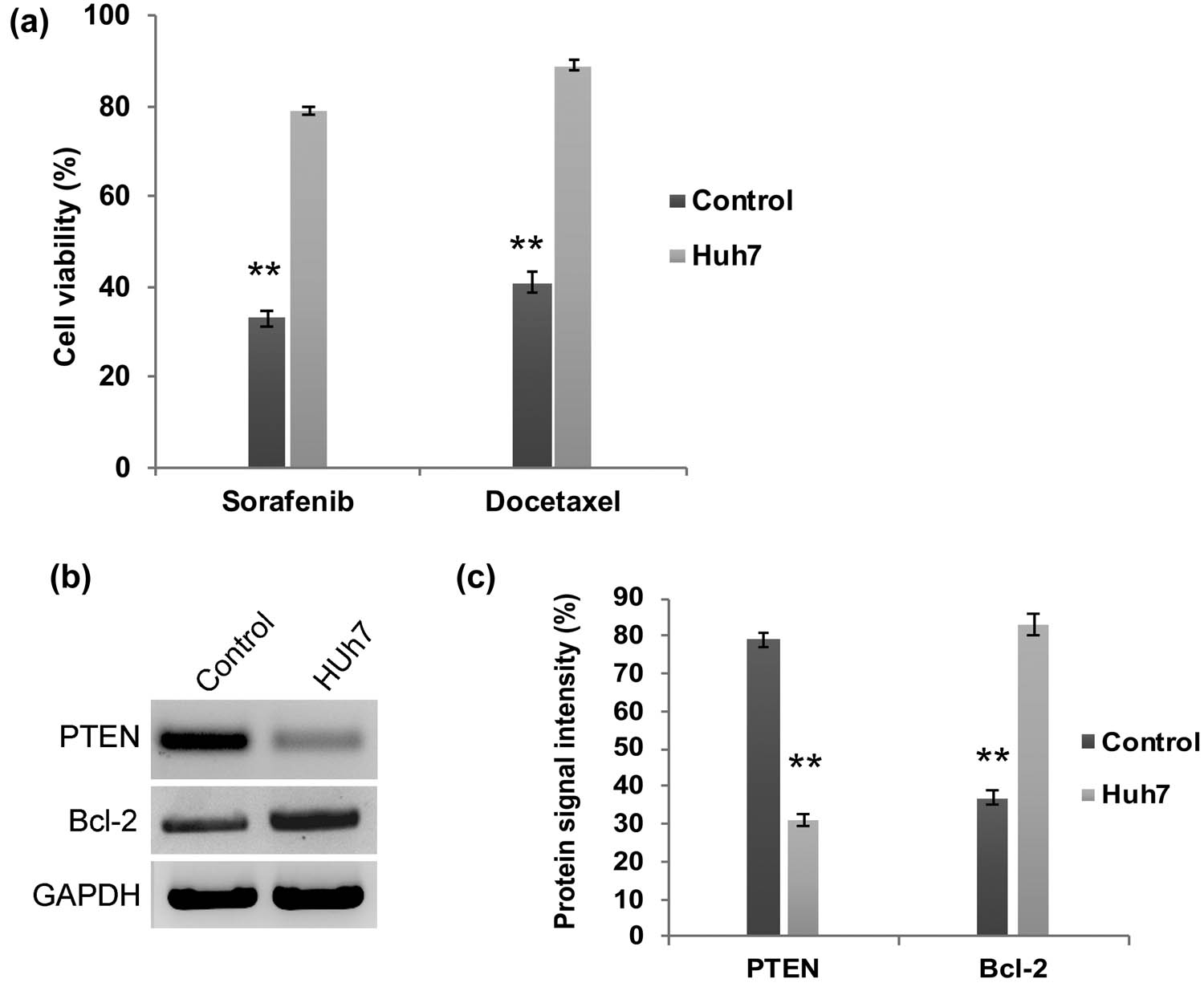
PI3K/AKT activated Huh7 cells are highly resistant to chemotherapy. (a) Quantification graph from chemoresistance assay demonstrating that Huh7 cell viability was not declined upon treatment with docetaxel and sorafenib. Western blot analysis (b) and densitometry bar diagram of blots (c) showing expression of Bcl-2 and PTEN. The error bar represented in the graph is ±SD, **P < 0.01.
3.2 Overexpression of PTEN contributes to improved chemotherapy sensitivity and apoptosis induction in Huh7 cells
The major inferences of the chemotherapy treatment regimens include apoptosis/multidrug resistance and tumor recurrence when the therapy is withdrawn. From the aforesaid findings, we speculate that apoptosis resistance of Huh7 cells might be due to the downregulation of PTEN. Therefore, we have transfected Huh7 cells with GFP-PTEN overexpression cassette plasmid and examined its effect on chemotherapy resistance and apoptosis induction. As we expected, upon PTEN overexpression (Figure 3a and b) Huh7 cells became more sensitive to docetaxel and sorafenib and the cell viability was significantly (P < 0.01) decreased compared to parental Huh7 cells (Figure 3c). In addition, our flow cytometry data revealed that upon PTEN overexpression, the intracellular ROS generated was significantly higher in Huh7 cells (Figure 3d). As a consequence, the rate of apoptosis induction was significantly (P < 0.01) elevated in PTEN overexpressed Huh7 cells (Figure 3e).
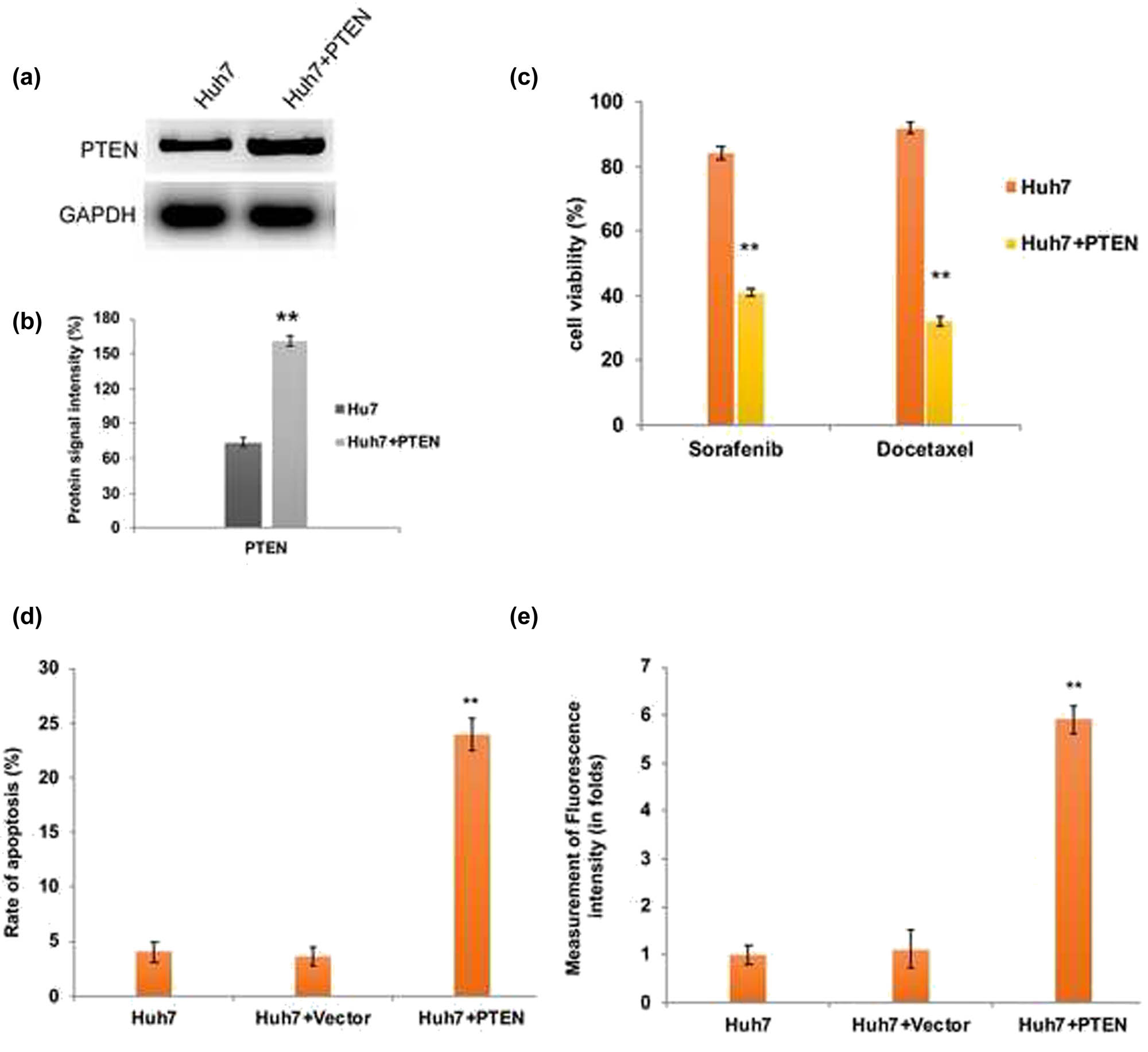
PTEN overexpression induced apoptotic cell death in Huh7 cells. (a) Western blot analysis and densitometry bar diagram of blots (b) showing the overexpression of PTEN in Huh7 cells after transfection with pcDNA3-GFP-PTEN. Quantification graphs from chemoresistance assay (c), assessment of apoptosis by flow cytometry (d), and intracellular ROS measurement by flow cytometry (e) confirm the induction of elevated apoptosis in PTEN overexpressed Huh7 cells due to enhanced ROS generation. The error bar represented in the graph is ±SD, **P < 0.01.
It has been well documented that stimulating cytochrome C leads to activation and release of downstream caspases essential for mitochondria facilitated intrinsic apoptosis [26,27,28]. Thus we performed a western blot to analyze the expression level of caspases in PTEN overexpressed Huh7 cells. We found that the levels of cytochrome C, caspase 3, and caspase 9 are highly elevated in Huh7 cells overexpressing PTEN (Figure 4). Interestingly, the expression level of PI3K and AKT is comparatively reduced in PTEN overexpressed cells (Figure 4a and b). Hence, PTEN overexpression leads to enhanced chemotherapy sensitivity, intracellular ROS generation, and thus ultimately results in apoptotic cell death through caspase activation and downregulation of PI3K/AKT signaling.

(a) Western blot and its quantification graph. (b) Displaying the apoptosis induction through caspase activation and downregulation of PI3K/AKT signaling.
4 Discussion
Dysregulation of PI3K/AKT signaling molecules is frequently associated with different types of cancers such as lung, breast, ovarian, prostate, uterine leiomyomata, and liver cancers [17,18,19,29,30,31]. Abnormal PI3K/AKT activation has been reported in liver cancer invasion, metastasis, EMT, sorafenib resistance, and angiogenesis [15,16,17]. Consequently, we also found abnormally activated and enhanced PI3K/AKT signaling in HCC Huh7 cells. Furthermore, these cells were highly resistant to docetaxel and sorafenib, and hence apoptosis was impeded by deregulated PI3K/AKT signaling. On the cell membrane, activation of PI3K leads to PIP3 production, which leads to phosphorylation and activation of Akt [13]. The tumor suppressor gene PTEN negatively regulates Akt through dephosphorylation, and therefore inactivation of PTEN causes enhanced expression of growth factors, receptors, and cytokines required for Akt phosphorylation, which are crucial for promoting carcinogenesis [32].
In this study, reduced expression of PTEN was observed in Huh7 cells. At the same time, the activated anti-apoptotic mechanism was evident by the enhanced expression of Bcl-2 in Huh7 cells. These results lead to the hypothesis that depleted PTEN expression might contribute to apoptosis inhibition, and therefore, Huh7 cells are highly resistant to chemotherapy drugs. Corroborating our findings, hyperactivation of Akt and downregulated PTEN expression was found associated with poor prognosis, cancer growth or large fibroid, and tumor recurrence and they have been documented [22,33]. Increasing evidence showed that overexpression of PTEN is involved in cell cycle arrest and stimulates apoptosis by P13K/AKT signaling downregulation in liver cancer, breast cancer, renal carcinoma, and glioma cells [34,35]. In our study, when we complemented PTEN function in Huh7 cells, apoptotic cell death was induced which was evident in the chemoresistance assay. The PTEN overexpressed cells were responding well to docetaxel and sorafenib. As a result, enhanced intracellular ROS was produced and this ultimately leads to apoptotic cell death. Concomitantly, studies on liver cancer reported that a combination of docetaxel with PTEN overexpression became an effective adjuvant therapy [36]. In consistence with previous findings, our findings also demonstrated that apoptosis was persuaded upon PTEN overexpression in cancer cells [37].
The possible molecular mechanism behind the apoptosis induction could be that PTEN directly inhibits P13K signaling and interrupts Akt binding to PI3K by dephosphorylation of PIP3. Several studies reported that PI3K and Akt inhibitors worked efficiently, which sensitizes cancer cells to ionizing radiation and induced cell cycle arrest and thus ultimately results in apoptosis [38,39,40,41]. Our findings revealed the downregulation of pro-survival protein Bcl-2 and release of cytochrome C due to altered mitochondrial membrane potential. Furthermore, reports showed that the released cytochrome C causes activation of caspases 9 which stimulates caspase 3 in association with apoptotic protease activating factor-1 [26,27,28]. As a result, apoptotic cell death happened and cell viability significantly declined in PTEN overexpressed Huh7 cells which was evident from the enhanced level of cytochrome C, caspase 9, and caspase 3.
To conclude, our data suggest that an increased level of tumor suppressor protein PTEN contributes to the downregulation of PI3K/AKT signaling, and therefore, tumorigenesis is checked by the stimulation of apoptotic cell death in liver cancer cells. Considering the fact that there is a cross-talk between signaling pathways (PI3K/AKT/mTOR and Wnt/β-catenin/TGF-β) and the influence of ABC transporter proteins in chemoresistance, further detailed studies are required to target multiple pathways simultaneously to disseminate the underlying molecular mechanism of PI3K/AKT signaling-mediated tumor invasion and metastasis.
-
Funding information: The authors state no funding involved.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.10.3322/caac.21551Suche in Google Scholar PubMed
[2] Wu TC, Shen YC, Cheng AL. Evolution of systemic treatment for advanced hepatocellular carcinoma. Kaohsiung J Med Sci. 2021 Jul 2;37(8):643–53.10.1002/kjm2.12401Suche in Google Scholar PubMed
[3] Cho YY, Yu SJ, Lee HW, Kim DY, Kang W, Paik YH, et al. Clinical characteristics of long-term survivors after Sorafenib treatment for unresectable Hepatocellular Carcinoma: a Korean national multicenter retrospective cohort study. J Hepatocell Carcinoma. 2021;8:613.10.2147/JHC.S304439Suche in Google Scholar PubMed PubMed Central
[4] Wei T, Zhang XF, Bagante F, Ratti F, Marques HP, Silva S, et al. Early versus late recurrence of Hepatocellular Carcinoma after surgical resection based on post-recurrence survival: an international multi-institutional analysis. J Gastrointest Surg. 2021;25(1):125–33.10.1007/s11605-020-04553-2Suche in Google Scholar PubMed
[5] Liu X, Liao W, Yuan Q, Ou Y, Huang J. TTK activates Akt and promotes proliferation and migration of hepatocellular carcinoma cells. Oncotarget. 2015;6(33):34309–20.10.18632/oncotarget.5295Suche in Google Scholar PubMed PubMed Central
[6] Dimri M, Satyanarayana A. Molecular signaling pathways and therapeutic targets in Hepatocellular Carcinoma. Cancers (Basel). 2020;12(2):491.10.3390/cancers12020491Suche in Google Scholar PubMed PubMed Central
[7] Li T, Lai Q, Wang S, Cai J, Xiao Z, Deng D, et al. MicroRNA-224 sustains Wnt/β-catenin signaling and promotes aggressive phenotype of colorectal cancer. J Exp Clin Cancer Res. 2016;35:1–11.10.1186/s13046-016-0287-1Suche in Google Scholar PubMed PubMed Central
[8] Jeong D, Ham J, Park S, Lee S, Lee H, Kang HS, et al. MicroRNA-7-5p mediates the signaling of hepatocyte growth factor to suppress oncogenes in the MCF-10A mammary epithelial cell. Sci Rep. 2017;7:1–10.10.1038/s41598-017-15846-zSuche in Google Scholar PubMed PubMed Central
[9] Guo L, Bai Y, Ji S, Ma H. MicroRNA‑98 suppresses cell growth and invasion of retinoblastoma via targeting the IGF1R/k‑Ras/Raf/MEK/ERK signaling pathway. Int J Oncol. 2019;54:807–20.10.3892/ijo.2019.4689Suche in Google Scholar PubMed PubMed Central
[10] Lu G, Wu X, Zhao Z, Ding Y, Wang P, Wu C, et al. MicroRNA-126 regulates the phosphatidylinositol-3 kinase (PI3K)/protein kinase B (AKT) pathway in SLK cells in vitro and the expression of its pathway members in Kaposi’s sarcoma tissue. Med (Baltim). 2018;97(35):e11855.10.1097/MD.0000000000011855Suche in Google Scholar PubMed PubMed Central
[11] Chen S, Wu J, Jiao K, Wu Q, Ma J, Chen D, et al. MicroRNA-495-3p inhibits multidrug resistance by modulating autophagy through GRP78/mTOR axis in gastric cancer. Cell Death Dis. 2018;9(11):1–2.10.1038/s41419-018-0950-xSuche in Google Scholar PubMed PubMed Central
[12] Koundouros N, Poulogiannis G. Phosphoinositide 3-kinase/Akt signaling and redox metabolism in cancer. Front Oncol. 2018;15(8):160.10.3389/fonc.2018.00160Suche in Google Scholar PubMed PubMed Central
[13] Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol cancer. 2019;18(1):1–28.10.1186/s12943-019-0954-xSuche in Google Scholar PubMed PubMed Central
[14] Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–76.10.1023/B:APPT.0000045801.15585.ddSuche in Google Scholar
[15] Dong J, Zhai B, Sun W, Hu F, Cheng H, Xu J. Activation of phosphatidylinositol 3-kinase/AKT/snail signaling pathway contributes to epithelial-mesenchymal transition-induced multidrug resistance to sorafenib in hepatocellular carcinoma cells. PLoS one. 2017;12(9):e0185088.10.1371/journal.pone.0185088Suche in Google Scholar PubMed PubMed Central
[16] Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, et al. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011;337(1):155–61.10.1124/jpet.110.175786Suche in Google Scholar PubMed
[17] Wang H, Xu L, Zhu X, Wang P, Chi H, Meng Z. Activation of phosphatidylinositol 3-kinase/Akt signaling mediates sorafenib-induced invasion and metastasis in hepatocellular carcinoma. Oncol Rep. 2014;32(4):1465–72.10.3892/or.2014.3352Suche in Google Scholar PubMed
[18] Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–10.10.1074/jbc.M005912200Suche in Google Scholar PubMed
[19] Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24:7455–64.10.1038/sj.onc.1209085Suche in Google Scholar PubMed
[20] Li H, Wang P, Gao Y, Zhu X, Liu L, Cohen L, et al. Na+/K+-ATPase α3 mediates sensitivity of hepatocellular carcinoma cells to bufalin. Oncol Rep. 2011;25:825–30.10.3892/or.2010.1120Suche in Google Scholar
[21] Fan B, Yu Y, Zhang Y. PI3K-Akt1 expression and its significance in liver tissues with chronic fluorosis. Int J Clin Exp Pathol. 2015;8(2):1226.Suche in Google Scholar
[22] Makker A, Goel MM, Mahdi AA, Bhatia V, Das V, Agarwal A, et al. PI3K/Akt/mTOR signaling & its regulator tumour suppressor genes PTEN & LKB1 in human uterine leiomyomas. Indian J Med Res. 2016;143(Suppl 1):S112.10.4103/0971-5916.191808Suche in Google Scholar PubMed PubMed Central
[23] Wang M, Wang Y, Zhong J. Side population cells and drug resistance in breast cancer. Mol Med Rep. 2015;11(6):4297–302.10.3892/mmr.2015.3291Suche in Google Scholar PubMed
[24] Liu F, Wagner S, Campbell RB, Nickerson JA, Schiffer CA, Ross AH. PTEN enters the nucleus by diffusion. J Cell Biochem. 2005;96(2):221–34.10.1002/jcb.20525Suche in Google Scholar PubMed
[25] He QZ, Luo XZ, Wang K, Zhou Q, Ao H, Yang Y, et al. Isolation and characterization of cancer stem cells from high-grade serous ovarian carcinomas. Cell Physiol Biochem. 2014;33(1):173–84.10.1159/000356660Suche in Google Scholar PubMed
[26] McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2015;7(4):a026716.10.1101/cshperspect.a026716Suche in Google Scholar PubMed PubMed Central
[27] Elena-Real CA, Díaz-Quintana A, González-Arzola K, Velázquez-Campoy A, Orzáez M, López-Rivas A, et al. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3ε-dependent Apaf-1 inhibition. Cell Death Dis. 2018;9(3):1–2.10.1038/s41419-018-0408-1Suche in Google Scholar PubMed PubMed Central
[28] Parsons MJ, Rehm M, Bouchier-Hayes L. Imaging-based methods for assessing caspase activity in single cells. Cold Spring Harb Protoc. 2015;2015(1):pdb-top070342.10.1101/pdb.top070342Suche in Google Scholar PubMed
[29] Karimi Roshan M, Soltani A, Soleimani A, Rezaie Kahkhaie K, Afshari AR, Soukhtanloo M. Role of AKT and mTOR signaling pathways in the induction of epithelial-mesenchymal transition (EMT) process. Biochimie. 2019;165:229–34.10.1016/j.biochi.2019.08.003Suche in Google Scholar PubMed
[30] Yang Q, Jiang W, Hou P. Emerging role of PI3K/AKT in tumor-related epigenetic regulation. Semin Cancer Biol. 2019;59:112–24.10.1016/j.semcancer.2019.04.001Suche in Google Scholar PubMed
[31] Wu R, Hu TC, Rehemtulla A, Fearon ER, Cho KR. Preclinical testing of PI3K/AKT/mTOR signaling inhibitors in a mouse model of ovarian endometrioid adenocarcinoma. Clin Cancer Res. 2011;17:7359–72.10.1158/1078-0432.CCR-11-1388Suche in Google Scholar PubMed PubMed Central
[32] Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605–35.10.1016/j.cell.2017.07.029Suche in Google Scholar PubMed PubMed Central
[33] Yan SY, Chen MM, Li GM, Wang YQ, Fan JG. MiR-32 induces cell proliferation, migration, and invasion in hepatocellular carcinoma by targeting PTEN. Tumor Biol. 2015;36(6):4747–55.10.1007/s13277-015-3124-9Suche in Google Scholar PubMed
[34] Li MF, Guan H, Zhang DD. Effect of overexpression of PTEN on apoptosis of liver cancer cells. Genet Mol Res. 2016;15(2):10-4238.10.4238/gmr.15028120Suche in Google Scholar PubMed
[35] Lu XX, Cao LY, Chen X, Xiao J, Zou Y, Chen Q. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells. Biomed Res Int. 2016;2016:2476842.10.1155/2016/2476842Suche in Google Scholar
[36] Liu Z, Li J, Li J, Huang J, Ke F, Qi Q, et al. Mannan-modified Ad5-PTEN treatment combined with docetaxel improves the therapeutic effect in H22 tumor-bearing mice. Int J Nanomed. 2012;7:5039–49.10.2147/IJN.S34022Suche in Google Scholar
[37] Carbognin L, Miglietta F, Paris I, Dieci MV. Prognostic and predictive implications of PTEN in breast cancer: unfulfilled promises but intriguing perspectives. Cancers (Basel). 2019;11(9):1401.10.3390/cancers11091401Suche in Google Scholar PubMed PubMed Central
[38] Duarte A, Silveira GG, Soave DF, Costa JPO, Silva AR. The role of the LY294002 – a non-selective inhibitor of phosphatidylinositol 3-kinase (PI3K) pathway – in cell survival and proliferation in cell line SCC-25. Asian Pac J Cancer Prev. 2019;20:3377–83.10.31557/APJCP.2019.20.11.3377Suche in Google Scholar PubMed PubMed Central
[39] Bavelloni A, Focaccia E, Piazzi M, Orsini A, Ramazzotti G, Cocco L, et al. Therapeutic potential of nvp-bkm120 in human osteosarcomas cells. J Cell Physiol. 2019;234:10907–17.10.1002/jcp.27911Suche in Google Scholar PubMed
[40] Weinberg MA. RES-529: a PI3K/AKT/mTOR pathway inhibitor that dissociates the mTORC1 and mTORC2 complexes. Anticancer Drugs. 2016;27:475–87.10.1097/CAD.0000000000000354Suche in Google Scholar PubMed PubMed Central
[41] Kaley TJ, Panageas KS, Mellinghoff IK, Nolan C, Gavrilovic IT, DeAngelis LM, et al. Phase II trial of an AKT inhibitor (perifosine) for recurrent glioblastoma. J Neurooncol. 2019;144:403–7.10.1007/s11060-019-03243-7Suche in Google Scholar PubMed PubMed Central
© 2021 Zhuangqiang Wang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Artikel in diesem Heft
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

