Abstract
The purpose of this study was to investigate whether the Dictyophora echinovolvata spore polysaccharides (DESP) affect the immunity in immunocompromised mice induced by cyclophosphamide (CTX). The healthy female Kunming mice were randomly divided into six groups, including a normal control (NC) group, a positive control group, a model control (MC) group, and three groups treated with low-, intermediate-, and high-dose polysaccharide, respectively. A series of immunoregulatory properties were determined, including humoral and cellular immunity, immune function, and immune factors of mononuclear macrophages. Compared with NC and MC groups, treatment with DESP significantly increased the spleen index and decreased the thymus index; increased the serum concentrations of immunoglobulin (Ig)A, IgG, IgM, hemolysin, IL-1β, and IL-2; delayed the allergic reaction; and improved the splenic lymphocyte transformation ability; and enhanced the phagocytosis of macrophages and the ability to secrete IL-6, TNF-α, caspase-1, and NO with DESP supplementation. These results indicated that DESP might have a good regulatory effect on CTX-induced immunodeficiency in mice, adjust the body’s immune imbalance, and improve the symptoms of low immunity.
1 Introduction
Dictyophora indusiata is a stinkhorn fungus that belongs to Eumycota, Basidiomycotina, Basidiomycetes, Phallales, and Phallaceae families [1,2]. It is edible as a delicacy in China, has a long history [3], and is called “queen of the mushrooms” due to its beautiful appearance and delicious taste [4]. This mushroom is also rich in vitamins and micro minerals, has a lot of health benefits to the eyes, acts as a tonic to cardiovascular systems, and shows partially medicinal effects like mental tranquilization and antitumor activity [5,6,7,8]. Polysaccharides are a kind of natural polymer linked by aldose or ketose through glycosidic bonds. As one of the fundamental substances to maintain the normal function of life, polysaccharides are essential biological macromolecules in vivo [1,9].
The pileus of D. indusiata is covered with olive green mucus, which contains the basidiospore that includes all germplasm of D. indusiata. The polysaccharides from the volva of Dictyophora rubrovolvata were extracted by hot water, and one of them had an inhibitory effect on the tumor cells of S180 to some extent [6]. As in the previous study, D. indusiata polysaccharide could inhibit the immunosuppressive function of cancer-associated fibroblasts [10]. Dictyophora echinovolvata Zang is one of the species of D. indusiata. At present, it has been the most important commercialized species that occupy most of the market shares of D. indusiata in China due to its easy cultivation, high nutritional value, and biological function [11]. The mature fruiting body of D. indusiata can be divided into three parts: the pileus covered with spores, the volva, and the edible part, which consists of the stipe and white net-like veil [2]. The edible part of the fruiting body of D. indusiata is very delicious and expensive. The volva and pileus that account for 65% of the whole mushroom (by fresh weight) are dumped without use and may cause environmental pollution and resource wastage [12]. The new study has reported that water-extractable polysaccharides by D. indusiata play a vital role in the process of antioxidation, hepatic-, and renal protection on obese mice [13]. In addition, previous research also provided the similar evidences associated with the polysaccharide such as Lonicera japonica and Ganoderma lucidum [14,15,16,17,18]. L. japonica polysaccharide was used to analyze the immune regulation function in immunosuppressed mice induced by cyclophosphamide (CTX) in order to better develop this Chinese herbal medicine plant [15]. Other studies have reported that the spores of G. lucidum were confirmed to have various biological functions [16,17,18]. These results indicated that the nutritional and biological functions of this abundant waste needed to be thoroughly evaluated to realize the highest possible economical profit [19,20].
However, the spores of D. indusiata polysaccharides associated with the immunity regulated function are not clean, especially D. echinovolvata. Previous results have shown that the crude polysaccharide fraction of edible D. echinovolvata might be a potential function for preventing neurodegenerative diseases in which oxidative stress and apoptosis were involved [21]. In our lab, we isolated and characterized polysaccharides from D. echinovolvata spore. The objective of this study was to conduct a qualitative analysis of D. echinovolvata spore polysaccharides (DESP) and explore and investigate its immunomodulatory function in CTX-induced immunosuppressed mice models. The ameliorative effects of DESP were estimated by organ index, splenic lymphocyte proliferation, macrophage phagocytosis, and serum cytokines.
2 Materials and methods
2.1 Polysaccharides and reagents
DESP were prepared in the laboratory. The fruiting body of D. echinovolvata was purchased from a local commercial market in Shunchang, Fujian, China. The homogenate of D. echinovolvata gelatin was boiled in distilled water for 2 h at a designed temperature. After centrifugation to remove debris fragments, the supernatant was concentrated by rotary evaporation. Protein was removed and collected as the crude polysaccharide fraction of D. echinovolvata gelatin. The gelatin was obtained through precipitation with four volumes of 95% ethanol, centrifugation, and freeze-drying.
CTX and levamisole hydrochloride were obtained from Baxter International Co., Ltd. and 20% sheep red blood cell (SRBC) was obtained from Nanjing Senbega Biotechnology Co., Ltd. The fetal bovine serum was obtained from Zhejiang Tianhang Biotechnology Co., Ltd. RPMI-1640 medium is the product of GE Medical Life Science Co., Ltd.; NP-40, knife bean globulin (ConA) is a Sigma product; Hank’s solution, MTT kit, and neutral gum are products of Beijing Solebo Technology Co., Ltd., whereas Penicillin (10,000 U/mL) and streptomycin (10,000 μg/L), 1:1 (v/v) acetone–methanol fixing solution, 4% (v/v) Giemsa-phosphate buffer, 0.1% sodium carbonate solution, heparin, and ink were prepared by us. Methanol, acetone, sodium carbonate, Giemsa dye solution, and heparin were obtained from Beijing Dingguo Changsheng Biotechnology Co., Ltd.
2.2 Experimental design and samples
Female, 7-week-old Kunming mice were obtained from SLAC Laboratory Animal Company (Shanghai, China, certificate no.: CXK [Shanghai] 2003-0003). After 1 week of acclimatization to the animal laboratory with drinking and feeding freely, all mice were randomly assigned to six groups, including a normal control (NC) group, a positive control (PC) group, a model control (MC) group, and three groups treated with DESP (low-, intermediate-, and high-dose; 400, 800, and 1,600 mg/kg day, respectively; LP, MP, and HP) for 4 months. The PC group was given levamisole hydrochloric acid with 25 mg/kg day intragastrically. The NC and MC groups were given saline intragastrically. The gavage volume of mice in each group was 0.1 mL (10 g/bw). On the 21st day, except for the NC group, all mice were intraperitoneally injected with 50 mg/kg day CTX for 1 week to develop low immunity. The mice in the NC group were injected with the same amount of saline. The mice were fed under controlled environmental conditions (temperature: 21–23°C; humidity: 40–60%) and a 12 h light/dark cycle.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals and were approved by the Fujian Provincial Animal Care and Use Committee and the Fujian province Zoological Society.
2.3 Organ index
At the end of the experimental period, the mice, fasted without water for 12 h, were weighed and sacrificed after cervical dislocation. The spleen, thymus, and liver samples were collected, and excess fascia and adipose tissue were removed simultaneously. Then, the organs were weighed, and the immune organ index was calculated according to the following formula:
2.4 Delayed allergic reaction
After lavage 24 days in mice, they received intraperitoneal injections of 0.2 mL of 5% (v/v) SRBC. The thickness of the metatarsal part of the left posterior foot of mice was measured by a thickness gauge 4 days after immunization with 5% (v/v) SRBC. The average value was obtained by measuring the thickness of the metatarsal part of the left posterior foot of mice three times. Then, 20% of 20 μL (v/v) SRBC was subcutaneously injected at the measurement site. The thickness of the left posterior metatarsal was measured 24 h after injection. The same measurement was made three times, and the average was taken. Mice vola pedis thickening is equal to the average thickness of the second vola pedis minus the average thickness of the first vola pedis.
2.5 Splenic lymphocyte transformation experiment
The mice were sacrificed through cervical dislocation, and they soaked in 75% alcohol for 5 min. The spleen was exposed through an abdominal incision and then isolated, collected, and put into a petri dish containing 5 mL Hank’s solution. Subsequently, the spleen was placed on a 200-mesh stainless steel net and placed in a small dish containing the proper amount of aseptic Hank’s solution to make a single-cell suspension. After filtration with a 200-mesh screen, the suspension was washed with Hank’s solution three times. The target cell concentration was achieved by diluting to 1 × 106 cell/mL in RPMI-1640 medium containing 10% fetal bovine serum. The cell suspension 200 μL/well was added to the 96-well cell culture plate. Each splenic cell suspension was divided into experimental group and control group. The ConA solution (final concentration 5 μg/mL) was used in the experimental group. They were cultured in an incubator at 37°C with 5% CO2 for 48 h. The 110 μL of DMSO was added into the cell to terminate reaction. The OD value was measured by an enzyme labeling instrument at 570 nm. The proliferation capacity of lymphocyte A is equal to the average OD value of the experimental group minus the average OD value of the control group.
2.6 Serum hemolysin and cytokine determination
The concentration of serum hemolysin, immunoglobulin (Ig) A, G, and M, interleukin-1β, and interleukin-2 was determined by ELISA kit (Langton, Shanghai, China) according to the manufacturer’s instruction.
2.7 Macrophage phagocytosis
The blood was collected from the inferior pterygoid vein of a chicken with an aseptic blood collection needle for obtaining chicken red blood cells (CRBCs). Also, 2% (v/v) CRBC suspension was prepared with normal saline after centrifuging at 1,000 rpm for 10 min. Mice were intraperitoneally injected with 2% CRBC suspension. After 30 min, the mice were sacrificed by cervical dislocation, and the peritoneal macrophages after intraperitoneal injection of 2 mL of normal saline were collected. Peritoneal macrophages were prepared as described in previous studies [22,23]. After dying in Giemsa-phosphate buffer for 3 min, the number of semi-swallowed red blood cells, total phagocytes, and total macrophages were counted using an optical microscope. The phagocytosis index and phagocytosis rate were calculated based on the formula. The percentage of phagocytosis (%) and tosis index were analyzed by the following formula:
2.8 Carbon clearance test
A total of 0.2 mL ink (diluted with saline at 1:4) was injected intravenously into the tail vein of mice. After inoculating ink, blood was sampled and put into a heparin tube from the eyeball of mice at 5 and 18 min, respectively. Then, 20 μL of blood samples was added into 2 mL of 0.1% sodium carbonate solution with a tube for accuracy and analyzed in a spectrophotometer at 600 nm. Finally, the mice were killed by cervical dislocation, and the spleen and liver samples were collected. They were weighed and recorded accurately. The carbon clearance index α was calculated according to the following formula:
where W 1 is the body weight and W 2 is the weight of liver and spleen.
2.9 Data analysis
The experimental data were analyzed by the least significant difference model of ANOVA with SPSS 20.0 software. Each repetition was used as a statistical unit of the experiment, and the results were expressed as mean ± SEM.
3 Results and discussion
3.1 Effects on body weight and organ index in immunocompromised mice
As shown in Figure 1, compared with the NC group, the bodyweight of mice was significantly increased (P < 0.05) in the PC group and the MP group (P < 0.05). The results showed that the treatment of the middle-dose DESP and levamisole hydrochloric acid could achieve the same effect, which further indicated that the treatment of the middle-dose DESP had the effect of promoting immunity similar to that of levamisole hydrochloric acid.
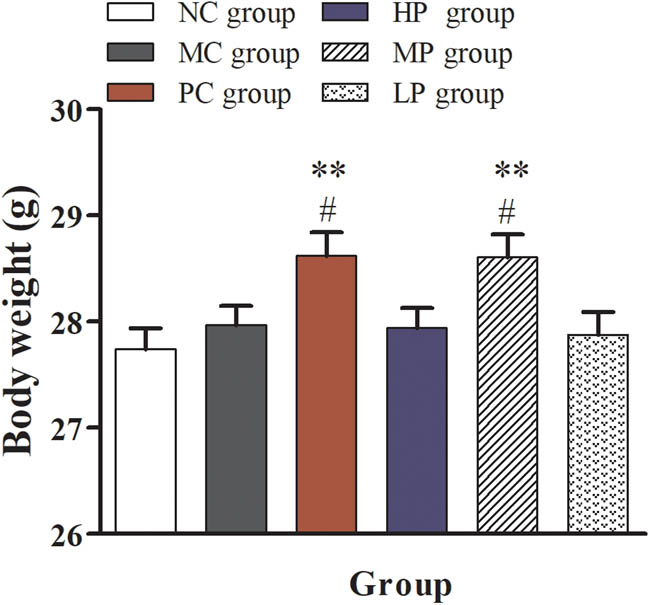
Effect of DESP on the weight of immunosuppressed mice. Compared with the NC group, * P < 0.05 and ** P < 0.01; compared with the MC group, # P < 0.05.
As shown in Figure 2, compared with the NC group, the liver index in the MP group was significantly higher than that of the other groups (P < 0.05); the spleen index was significantly decreased in the MC, PC, LP, MP, and HP groups (P < 0.001 or P < 0.001). These results indicated that the immunocompromised mice model was successfully constructed. Compared with MC and PC groups, the spleen index in the LP, MP, and HP groups was significantly increased (P < 0.05 or P < 0.01). Meanwhile, the thymus index was also significantly increased in the group treated with the middle-dose DESP (P < 0.05). These results suggested that DESP intragastrically could increase the weight of important immune organs like the spleen and thymus gland in mice, thereby affecting the immune function. Although DESP treatment increased the index of the spleen and thymus, the organ atrophy caused by low immunity was irreversible. Thymus and spleen are the main immunization organs. The spleen is the largest peripheral immune organ innervated with sympathetic nerves and controlled by the adrenomedullary system in the body [24]. A bigger immunity index indicates a stronger immune capability [25]. The results of the thymus index and spleen index indicated that DESP could enhance the cell-mediated immunity and stimulate T cell formation [26].
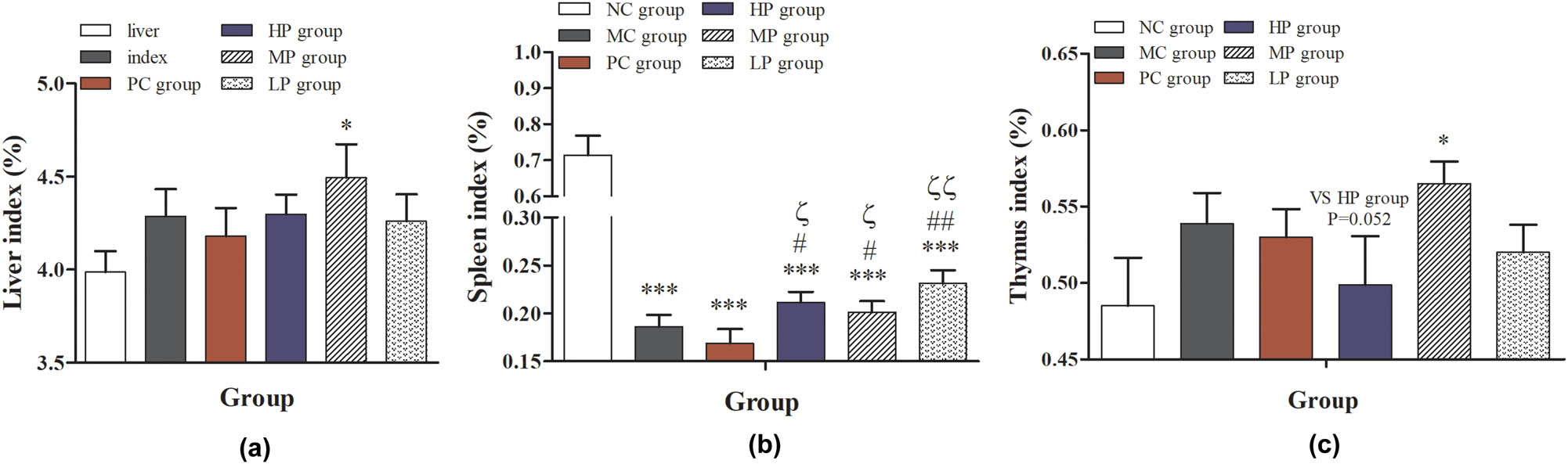
Effect of DESP on organ index in immunocompromised mice: (a) liver index, (b) spleen index, and (c) thymus index. Compared with the NC group, * P < 0.05, ** P < 0.01, and *** P < 0.001; compared with the MC group, # P < 0.05 and ## P < 0.01; compared with the PC group, ζ P < 0.05 and ζζ P < 0.01.
3.2 Effect of DESP on cellular immune function in immunocompromised mice
As shown in Figure 3a, compared with the NC group, the rate of swelling in the hind paw was significantly reduced (P < 0.01 or P < 0.001). Compared with the MC group, the rate of swelling in the hind paw in the PC group and the MP group was significantly increased (P < 0.05), which further indicated that the middle-dose DESP treatment could improve the immunity of immunocompromised mice. Figure 3b shows that the DESP treatment could significantly increase the proliferation and transformation of lymphocytes in mice, especially in the MP group (P < 0.001). These results suggest that an intermediate dose of DESP can improve the delayed allergic reaction and the splenic lymphocyte transformation ability and improve the immunity of immunocompromised mice. Previous studies have shown that splenic lymphocyte has an important role during the process of cell-mediated immunity based on the CD4+, CD8+, and invariant natural killer T (iNKT) cell [27,28,29]. The iNKT cells are a distinct population of innate T lymphocytes selected in the thymus [30,31]. They have an important function of rapidly secreting cytokines and an innate T cell population capable of activating and steering adaptive immune responses [28]. Previous reports have shown that CTX suppressed both humoral and cellular immune responses [32]. The present study showed that treatment with DESP could significantly increase the splenic lymphocyte transformation ability (Figure 3b). This result may suggest that DESP may be capable of improving immunity.
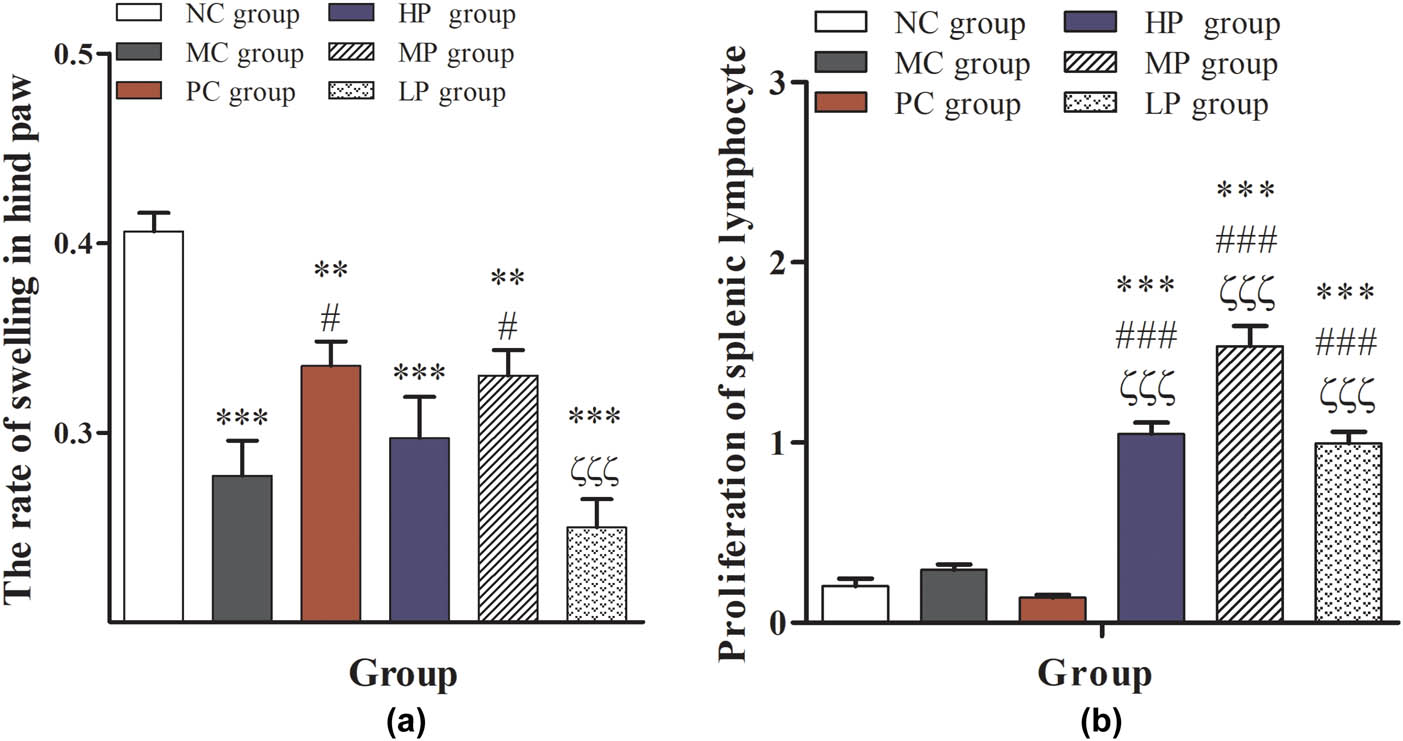
Effects of DESP on delayed allergic reaction and the splenic lymphocyte transformation ability in immunocompromised mice: (a) the rate of swelling in hind paw and (b) proliferation of splenic lymphocyte. Compared with the NC group, * P < 0.05, ** P < 0.01, and *** P < 0.001; compared with the MC group, # P < 0.05, ## P < 0.01, and ### P < 0.001; compared with the PC group, ζ P < 0.05, ζζ P < 0.01, and ζζζ P < 0.001.
3.3 Effects of DESP on the humoral and cellular immune factor
As shown in Figure 4, compared with the NC group, the serum concentrations of IgA, IgG, and IgM in the MC, MP, and LP groups were significantly or immensely significantly increased (P < 0.05 or P < 0.001), but they were significantly decreased (P < 0.001) in the PC and HP groups. Compared with the MC group, the serum contents of IgA, IgG, and IgM in PC, HP, MP, and LP groups were significantly decreased (P < 0.001). However, the serum contents of IgA in MP and LP show a downward trend. Compared with the PC group, the serum contents of IgA, IgG, and IgM in MP and LP groups were significantly increased (P < 0.05 or P < 0.01). Based on the present results, they suggested that the intermediate- and low-dose of DESP can stimulate the body to produce higher concentrations of Ig, especially the intermediate-dose of DESP treatment. Immune deficiencies appear to exhibit antibody production deficiencies [33]. In some cases, Ig replacement treatment is the most effective treatment in primary immunodeficiency diseases [34,35,36]. In the present study, DESP treatment significantly increased IgA, IgG, and IgM concentration, and we speculate that DESP can effectively alleviate the damage of immune deficiency to protect immunocompromised mice.
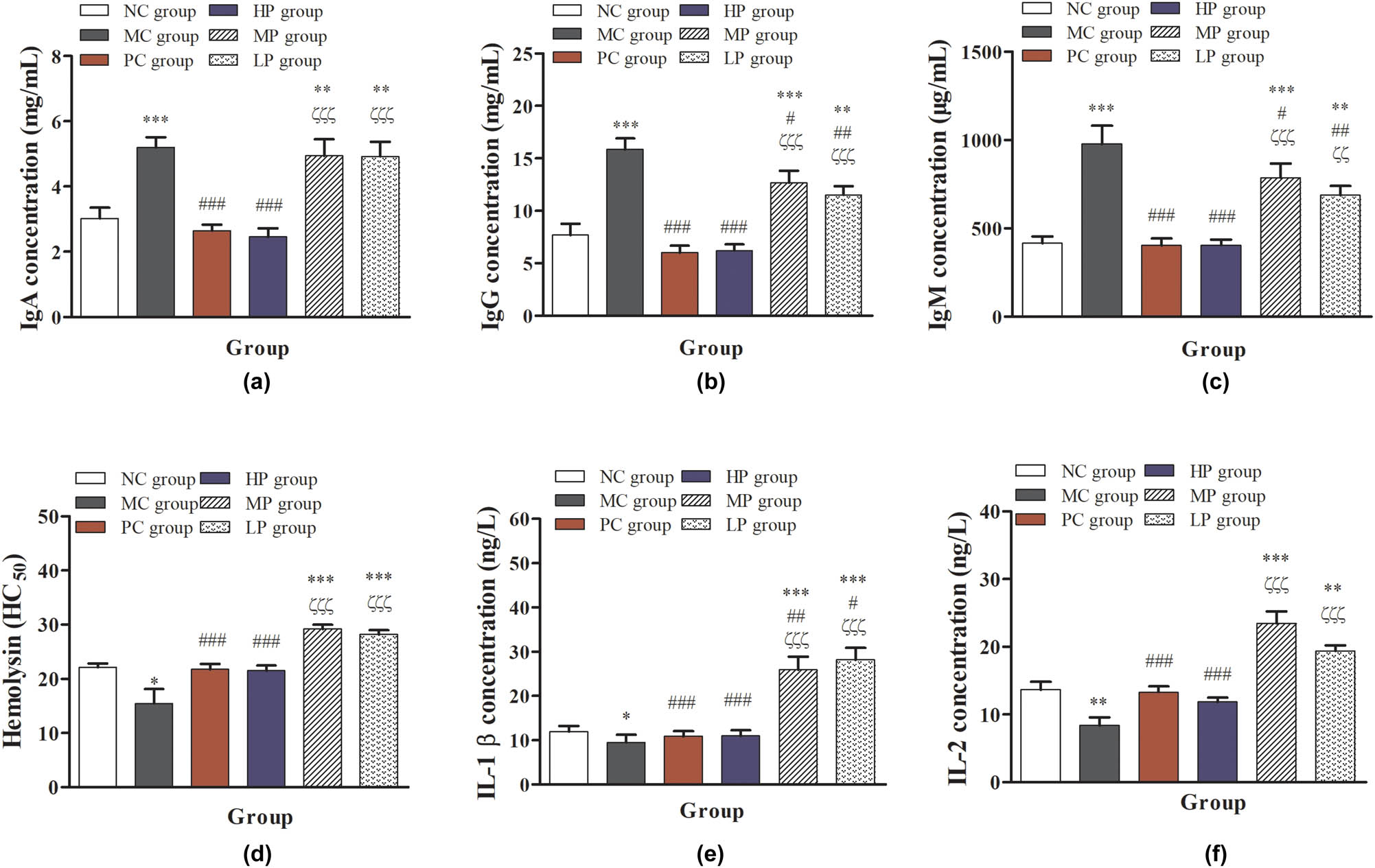
Effects of DESP on the serum concentration of Ig: (a) IgA contents, (b) IgG contents, (c) IgM contents, (d) hemolysin, (e) IL-1β, and (f) IL-2 in immunosuppressed mice. Compared with the NC group, * P < 0.05, ** P < 0.01, and *** P < 0.001; compared with the MC group, # P < 0.05, ## P < 0.01, and ### P < 0.001; compared with the PC group, ζ P < 0.05, ζζ P < 0.01, and ζζζ P < 0.001.
CTX treatment reduced the concentration of hemolysin, IL-1β, and IL-2 significantly in the MC group, compared with the NC group, as shown in Figure 4d–f (P < 0.05 or P < 0.01). Compared with NC and MC groups, the contents of hemolysin, IL-1β, and IL-2 were significantly increased (P < 0.01 or P < 0.001) in the MP and LP groups. Compared with the PC group, the concentrations of hemolysin, IL-1β, and IL-2 were significantly increased (P < 0.001) in the MP and LP groups. Hemolysin as an index was used to evaluate the body’s immune function by the degree of aggregation of SRBC. IL-1β is produced by activated macrophages, belonging to a cytokine type that can stimulate the proliferation and differentiation of immune response cells and improve its function [8,27]. IL-2 can affect the immune system by affecting T cells and promoting the proliferation of activated B cells, participating in antibody response, detecting tumor and hematopoiesis [29,37]. In addition, the previous results have shown that the D. echinovolvata polysaccharide could inhibit the mitochondria-dependent apoptotic pathway to protect against H2O2-induced neurotoxicity in PC12 cells [21]. Apoptosis and oxidative stress are important features of chronic diseases, including neurodegenerative disorders and immunocompromised individuals [38]. The results showed that the hemolysin in serum of immunocompromised mice induced by CTX was significantly decreased after SRBC injection, indicating that the activity of B cells in mice was inhibited. After intermediate- and low-dose DESP treatment, the hemolysin was significantly increased, which effectively increased the proliferation and antibody production of B cells in mice. In addition, intermediate- and low-dose DESP treatment can significantly increase the contents of IL-1β and IL-2 in immunocompromised mice, suggesting that DESP treatment may promote the activity of macrophages and proliferation of B cells in vivo, improve the immune defense ability, and prevent various chronic diseases.
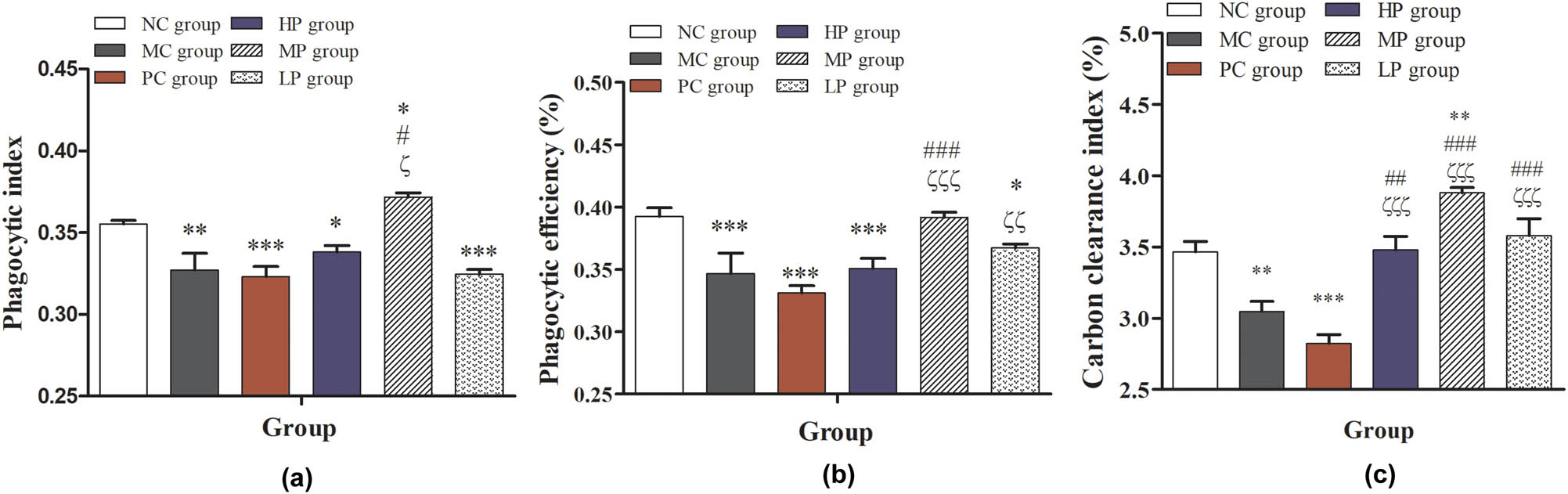
Effects of DESP on phagocytosis of macrophages in immunosuppressed mice: (a) phagocytic index, (b) phagocytic efficiency, and (c) carbon clearance index. Compared with the NC group, * P < 0.05, ** P < 0.01, and *** P < 0.001; compared with the MC group, # P < 0.05, ## P < 0.01, and ### P < 0.001; compared with the PC group, ζ P < 0.05, ζζ P < 0.01, and ζζζ P < 0.001.
3.4 Effects of DESP on function of macrophages
As shown in Figure 5a and b, the phagocytic index and phagocytic efficiency of peritoneal macrophages in MC, PC, HP, and LP groups were significantly decreased (P < 0.05), but the phagocytic index of peritoneal macrophages in the MP group was significantly increased (P < 0.05) compared with the NC group. In the DESP treatment groups, the phagocytic index and phagocytic efficiency of peritoneal macrophages were significantly higher than those in the MC and PC group (P < 0.001 or P < 0.001), only in the MP group. This indicated that middle-dose DESP could significantly increase the phagocytic activity of peritoneal macrophages in immunocompromised mice. As shown in Figure 5c, compared with the NC group, the carbon clearance ability was significantly decreased (P < 0.01, P < 0.001) in the MC and PC groups. DESP treatment can increase the carbon clearance ability of immunocompromised mice as the intermediate-dose DESP treatment significantly increased in particular (P < 0.01). Compared with MC and PC groups, the carbon clearance ability of immunocompromised mice was significantly improved with the three doses of DESP treatment (P < 0.001 or P < 0.001). This result indicated that DESP could significantly improve the phagocytosis of macrophages in immunocompromised mice and suggested that DESP treatment may improve the nonspecific immune ability of immunocompromised mice. Previous immunological assays showed that D. indusiata acid-soluble polysaccharides could improve phagocytosis of monocytes [7]. Thus, based on the present results, DESP could be regulating the auxiliary cell ability in the specific immune system.
As shown in Figure 6, treatment with intermediate- and low-dose DESP significantly increased the contents of IL-6, TNF-α, caspase-1, and NO secreted by peritoneal macrophages compared with NC and PC groups (P < 0.001). Compared with the MC group, the contents of IL-6, TNF-α, and caspase-1 secreted by peritoneal macrophages in the HP, MP, and LP groups were significantly decreased (P < 0.001 or P < 0.001). Cytokines as a signal transduction molecule between cells can regulate immune response and participate in immune cell differentiation development [39]. As shown in Figure 4f, treatment with intermediate- and low-dose DESP recovered the amount of IL-2 compared with the MC group (P < 0.001), rather than high-dose DESP. IL-2 as a cell growth factor promotes cell proliferation and differentiation, which can induce interferon production, which is involved in the process of autoimmune reactions [40]. TNF-α stimulates the expression of immune mediators and plays a significant role in the host defense [41]. As in previous results, the macrophages are broadly classified into M1 macrophages and M2 macrophages, which have selective anti-inflammatory, pro-fibrotic activities, and induced immunotolerance [42]. Usually, the M1 macrophages have an important ability to elevate secrete cytokines, such as IL-1β, TNF, and IL-6, and activate the inducible nitric oxide synthase generating NO [43]. Notably, the lipopolysaccharide stimulation, indeed, can induce the metabolism of arginine to NO [44]. Based on the present results, DESP had a positive regulatory effect on CTX-induced immunodeficiency in mice. The concentration of hemolysin, IL-2, TNF-α, and NO in the MP and LP groups was higher than the levels in the control group. This result shows that DESP can adjust the body’s immune imbalance and improve the symptoms of low immunity.
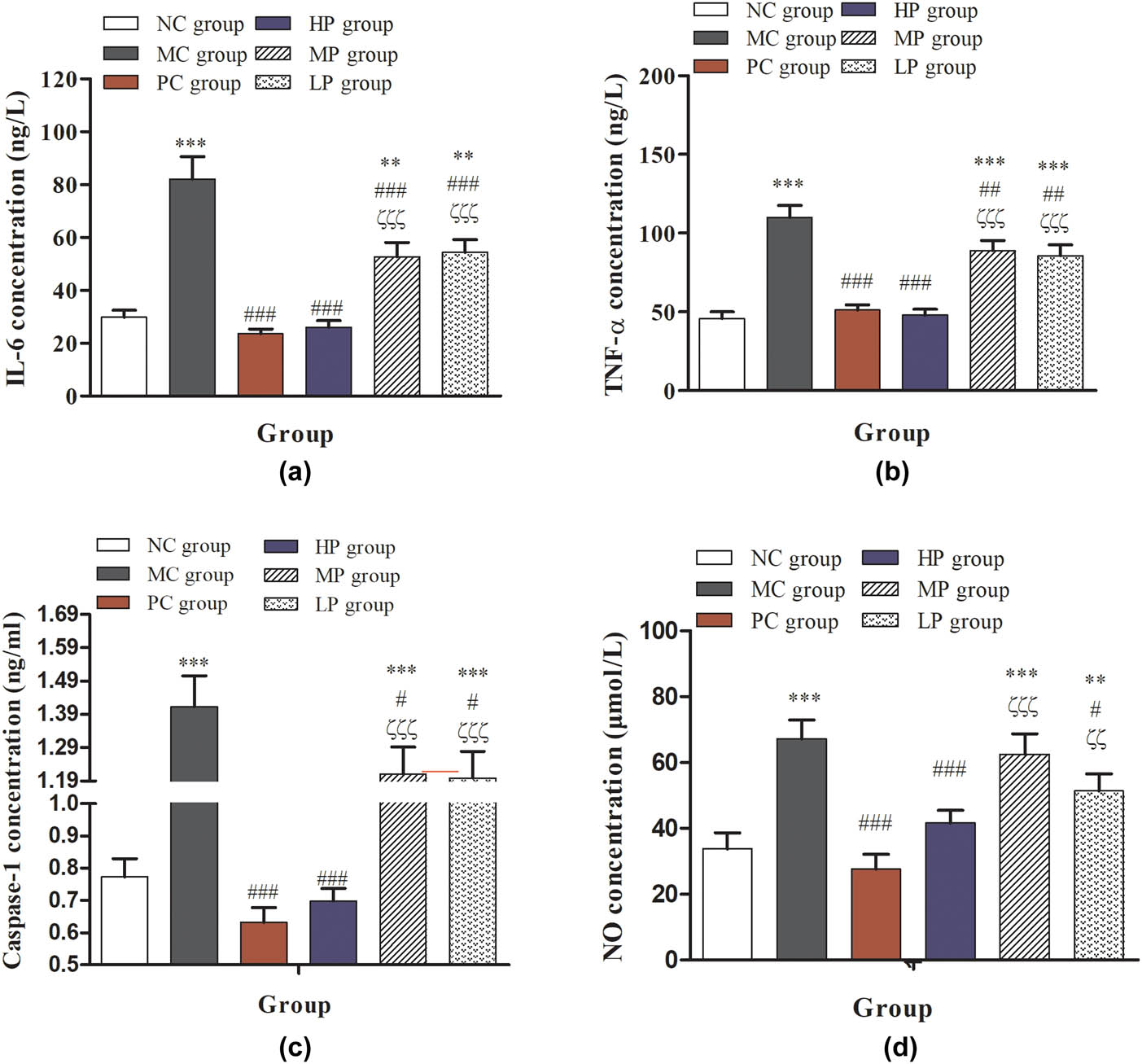
Effects of DESP on cytokine secretion of peritoneal macrophages in immunosuppressed mice: (a) IL-6 concentration, (b) TNF-α concentration, (c) caspase-1 concentration, and (d) NO concentration. Compared with the NC group, * P < 0.05, ** P < 0.01, and *** P < 0.001; compared with the MC group, # P < 0.05, ## P < 0.01, and ### P < 0.001; compared with the PC group, ζ P < 0.05, ζζ P < 0.01, and ζζζ P < 0.001.
4 Discussion
The experimental results showed that different doses of DESP could change humoral immunity, cellular immunity, and nonspecific immune function of immunocompromised mice. Among them, treatment with intermediate-dose DESP significantly improved the function of macrophages. The main points are stated as follows. (1) DESP improves liver and thymus organ indexes in immunocompromised mice. (2) DESP significantly improves the delayed allergic reaction and the ability of proliferation and transformation of lymphocytes in immunocompromised mice, indicating that DESP could significantly improve the cellular immune function. (3) Intermediate-dose DESP treatment could significantly increase the content of IgA, IgG, IgM, hemolysin, IL-1β, and IL-2 in serum and enhance the humoral and cellular immune ability. (4) DESP could significantly increase the phagocytic activity and carbon clearance ability of peritoneal macrophages in immunocompromised mice, increase the speed of blood carbon clearance, and increase the contents of IL-6, TNF- α, caspase-1, and NO secreted by peritoneal macrophages, which indicated that DESP significantly improves the nonspecific immune ability of immunocompromised mice. Overall, the present results suggested that DESP can be developed as a potential health care product that can enhance immunity. However, the specific molecular mechanism of DESP treatment to improve the immune ability of immunocompromised mice needs to be further studied.
-
Funding information: This work was supported by grants from the Special Fund of Scientific Research for Research Institutes in the Public Interest, Fujian Province (2016R1021-5) and the Science and Technology Innovation Team Program of Fujian Academy of Agricultural Sciences (STIT2017-3-11).
-
Author contributions: Conceived and designed the experiments – J.C.; performed the experiments and analyzed the data – C.L.; contributed reagents/materials/analysis tools – H.Z., L.C., and Y.F.; wrote the manuscript – C.L.; revised the manuscript – C.L. and J.C.; read and approved the final manuscript – C.L., H.Z., L.C., Y.F., and J.C.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Ishiyama D, Fukushi Y, Ohnishi-Kameyama M, Nagata T, Mori H, Inakuma T, et al. Monoterpene-alcohols from a mushroom Dictyophora indusiata. Phytochemistry. 1999;50(6):1053–6.10.1016/S0031-9422(98)00630-XSearch in Google Scholar
[2] Lee IK, Yun BS, Han G, Cho DH, Kim YH, Yoo ID. Dictyoquinazols A, B, and C, new neuroprotective compounds from the mushroom Dictyophora indusiata. J Nat Prod. 2002;65(12):1769–72.10.1021/np020163wSearch in Google Scholar PubMed
[3] Li XY, Wang ZY, Wang L, Walid E, Zhang H. In vitro antioxidant and anti-proliferation activities of polysaccharides from various extracts of different mushrooms. Int J Mol Sci. 2012;13(5):5801–17.10.3390/ijms13055801Search in Google Scholar PubMed PubMed Central
[4] Wang J, Xu X, Zheng H, Li J, Deng C, Xu Z, et al. Structural characterization, chain conformation, and morphology of a beta-(1 → 3)-d-glucan isolated from the fruiting body of Dictyophora indusiata. J Agric Food Chem. 2009;57(13):5918–24.10.1021/jf9009872Search in Google Scholar PubMed
[5] Deng C, Fu H, Teng L, Hu Z, Xu X, Chen J, et al. Antitumor activity of the regenerated triple-helical polysaccharide from Dictyophora indusiata. Int J Biol Macromol. 2013;61:453–8.10.1016/j.ijbiomac.2013.08.007Search in Google Scholar PubMed
[6] Zhong B, Ma YS, Fu D, Zhang C. Induction of apoptosis in osteosarcoma s180 cells by polysaccharide from Dictyophora indusiata. Cell Biochem Funct. 2013;31(8):719–23.10.1002/cbf.2961Search in Google Scholar PubMed
[7] Hua Y, Gao Q, Wen L, Yang B, Tang J, You L, et al. Structural characterisation of acid- and alkali-soluble polysaccharides in the fruiting body of Dictyophora indusiata and their immunomodulatory activities. Food Chem. 2012;132(2):739–43.10.1016/j.foodchem.2011.11.010Search in Google Scholar
[8] Wang Y, Lai L, Teng L, Li Y, Cheng J, Chen J, et al. Mechanism of the anti-inflammatory activity by a polysaccharide from Dictyophora indusiata in lipopolysaccharide-stimulated macrophages. Int J Biol Macromol. 2019;126:1158–66.10.1016/j.ijbiomac.2019.01.022Search in Google Scholar PubMed
[9] Deng C, Hu Z, Fu H, Hu M, Xu X, Chen J. Chemical analysis and antioxidant activity in vitro of a beta-d-glucan isolated from Dictyophora indusiata. Int J Biol Macromol. 2012;51(1–2):70–5.10.1016/j.ijbiomac.2012.05.001Search in Google Scholar PubMed
[10] Han S, Ma C, Hu M, Wang Y, Ma F, Tao N, et al. A polysaccharide from Dictyophora indusiata inhibits the immunosuppressive function of cancer-associated fibroblasts. Cell Biochem Funct. 2017;35(7):414–9.10.1002/cbf.3290Search in Google Scholar PubMed
[11] Hang MQ, Zou QQ, Tian HY, Sun BG, Chen HT. Analysis of volatile components from Dictyophora rubrovolota Zang, ji et liou. Procedia Eng. 2012;37:240–9.10.1016/j.proeng.2012.04.234Search in Google Scholar
[12] Zhuang YL, Sun LP. Nutritional characteristics of proteins from the volva and pileus in cultivated mushroom Dictyophora rubrovolvata. Int J Food Sci Nutr. 2011;62(4):392–6.10.3109/09637486.2010.539552Search in Google Scholar PubMed
[13] Wang W, Song X, Zhang J, Li H, Liu M, Gao Z, et al. Antioxidation, hepatic- and renal-protection of water-extractable polysaccharides by Dictyophora indusiata on obese mice. Int J Biol Macromol. 2019;134:290–301.10.1016/j.ijbiomac.2019.05.028Search in Google Scholar PubMed
[14] Su D, Li S, Zhang W, Wang J, Wang J, Lv M. Structural elucidation of a polysaccharide from Lonicera japonica flowers, and its neuroprotective effect on cerebral ischemia-reperfusion injury in rat. Int J Biol Macromol. 2017;99:350–7.10.1016/j.ijbiomac.2017.02.096Search in Google Scholar PubMed
[15] Zhou X, Dong Q, Kan X, Peng L, Xu X, Fang Y, et al. Immunomodulatory activity of a novel polysaccharide from Lonicera japonica in immunosuppressed mice induced by cyclophosphamide. PLoS One. 2018;13:10.10.1371/journal.pone.0204152Search in Google Scholar PubMed PubMed Central
[16] Liang C, Tian D, Liu Y, Li H, Zhu J, Li M, et al. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: ganoderic acids A, C2, D, F, DM, X and Y. Eur J Med Chem. 2019;174:130–41.10.1016/j.ejmech.2019.04.039Search in Google Scholar PubMed
[17] Ahmad MF. Ganoderma lucidum: persuasive biologically active constituents and their health endorsement. Biomed Pharmacother. 2018;107:507–19.10.1016/j.biopha.2018.08.036Search in Google Scholar PubMed
[18] Zeng P, Guo Z, Zeng X, Hao C, Zhang Y, Zhang M, et al. Chemical, biochemical, preclinical and clinical studies of Ganoderma lucidum polysaccharide as an approved drug for treating myopathy and other diseases in China. J Cell Mol Med. 2018;22(7):3278–97.10.1111/jcmm.13613Search in Google Scholar PubMed PubMed Central
[19] Deng C, Fu HT, Xu JJ, Shang JY, Cheng YM. Physiochemical and biological properties of phosphorylated polysaccharides from Dictyophora indusiata. Int J Biol Macromol. 2015;72:894–9.10.1016/j.ijbiomac.2014.09.053Search in Google Scholar PubMed
[20] Liao W, Lu Y, Fu J, Ning Z, Yang J, Ren J. Preparation and characterization of Dictyophora indusiata polysaccharide-zinc complex and its augmented antiproliferative activity on human cancer cells. J Agric Food Chem. 2015;63(29):6525–34.10.1021/acs.jafc.5b00614Search in Google Scholar PubMed
[21] Yu WX, Lin CQ, Zhao Q, Lin XJ, Dong XL. Neuroprotection against hydrogen peroxide-induced toxicity by Dictyophora echinovolvata polysaccharide via inhibiting the mitochondria-dependent apoptotic pathway. Biomed Pharmacother. 2017;88:569–73.10.1016/j.biopha.2017.01.103Search in Google Scholar PubMed
[22] Cohen N, Emilie A, Morisset J. Modulation of glucocorticoid-induced leucine zipper (GILZ) synthesis on macrophages functions. Immunology 2004: cytokine network, regulatory cells, signaling, and apoptosis. Malden, MA, USA: Wiley-Blackwell Publishing Ltd. 2004. p. 81–5.Search in Google Scholar
[23] Berrebi D, Bruscoli S, Cohen N, Foussat A, Migliorati G, Bouchet-Delbos L, et al. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood. 2003;101(2):729–38.10.1182/blood-2002-02-0538Search in Google Scholar PubMed
[24] Li Y, Jiang W, Li ZZ, Zhang C, Huang C, Yang J, et al. Repetitive restraint stress changes spleen immune cell subsets through glucocorticoid receptor or beta-adrenergic receptor in a stage dependent manner. Biochem Biophys Res Commun. 2018;495(1):1108–14.10.1016/j.bbrc.2017.11.148Search in Google Scholar PubMed
[25] Yin YM, Fu W, Fu ML, He GQ, Traore L. The immune effects of edible fungus polysaccharides compounds in mice. Asia Pac J Clin Nutr. 2007;16:258–60.Search in Google Scholar
[26] Chen XY, Zhang LN, Cheung PCK. Immunopotentiation and antitumor activity of carboxymethylated-sulfated beta-(1 → 3)-d-glucan from Poria cocos. Int Immunopharmacol. 2010;10(4):398–405.10.1016/j.intimp.2010.01.002Search in Google Scholar PubMed
[27] Ho NI, Camps MGM, de Haas EFE, Ossendorp F. Sustained cross-presentation capacity of murine splenic dendritic cell subsets in vivo. Eur J Immunol. 2018;48(7):1164–73.10.1002/eji.201747372Search in Google Scholar PubMed PubMed Central
[28] Govindarajan S, Elewaut D, Drennan M. An Optimized Method for Isolating and Expanding Invariant Natural Killer T Cells from Mouse Spleen. J Vis Exp. 2015;105:e53256.10.3791/53256Search in Google Scholar PubMed PubMed Central
[29] Chiba A, Cohen N, Brigl M, Brennan PJ, Besra GS, Brenner MB. Rapid and reliable generation of invariant natural killer T-cell lines in vitro. Immunology. 2009;128(3):324–33.10.1111/j.1365-2567.2009.03130.xSearch in Google Scholar PubMed PubMed Central
[30] Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–62.10.1146/annurev.immunol.15.1.535Search in Google Scholar PubMed
[31] Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2(8):557–68.10.1038/nri854Search in Google Scholar PubMed
[32] Hoover SK, Barrett SK, Turk TM, Lee TC, Bear HD. Cyclophosphamide and abrogation of tumor-induced suppressor T cell activity. Cancer Immunol Immunother. 1990;31(2):121–7.10.1007/BF01742376Search in Google Scholar PubMed
[33] Gathmann B, Grimbacher B, Beauté J, Dudoit Y, Mahlaoui N, Fischer A, et al. The European internet-based patient and research database for primary immunodeficiencies: results 2006–2008. Clin Exp Immunol. 2009;157:3–11.10.1111/j.1365-2249.2009.03954.xSearch in Google Scholar PubMed PubMed Central
[34] Peter JG, Chapel H. Immunoglobulin replacement therapy for primary immunodeficiencies. Immunotherapy (UK). 2014;6(7):853–69.10.2217/imt.14.54Search in Google Scholar PubMed
[35] Sriaroon P, Ballow M. Immunoglobulin replacement therapy for primary immunodeficiency. Immunol Allergy Clin. 2015;35(4):713–30.10.1016/j.iac.2015.07.006Search in Google Scholar PubMed
[36] Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119(7):1650–7.10.1182/blood-2011-09-377945Search in Google Scholar PubMed PubMed Central
[37] Tian J, Che HL, Ha D, Wei YP, Zheng SY. Characterization and anti-allergic effect of a polysaccharide from the flower buds of Lonicera japonica. Carbohydr Polym. 2012;90(4):1642–7.10.1016/j.carbpol.2012.07.044Search in Google Scholar PubMed
[38] Zhao HP, Han ZP, Ji XM, Luo YM. Epigenetic regulation of oxidative stress in ischemic stroke. Aging Dis. 2016;7(3):295–306.10.14336/AD.2015.1009Search in Google Scholar PubMed PubMed Central
[39] Dirchwolf M, Podhorzer A, Marino M, Shulman C, Cartier M, Zunino M, et al. Immune dysfunction in cirrhosis: Distinct cytokines phenotypes according to cirrhosis severity. Cytokine. 2016;77:14–25.10.1016/j.cyto.2015.10.006Search in Google Scholar PubMed
[40] Rajasagi NK, Rouse BT. IL-2 complex treatment amplifies CD8(+) T cell mediated immunity following herpes simplex virus-1 infection. Microbes Infect. 2016;18(12):735–46.10.1016/j.micinf.2016.10.010Search in Google Scholar PubMed PubMed Central
[41] Ozbey G, Gorczynski R, Erin N. Stability of cytokines in supernatants of stimulated mouse immune cells. Eur Cytokine Netw. 2014;25(2):30–4.10.1684/ecn.2014.0353Search in Google Scholar PubMed
[42] Laganà AS, Salmeri FM, Ban Frangež H, Ghezzi F, Vrtačnik-Bokal E, Granese R. Evaluation of M1 and M2 macrophages in ovarian endometriomas from women affected by endometriosis at different stages of the disease. Gynecol Endocrinol. 2020;36(5):441.10.1080/09513590.2019.1683821Search in Google Scholar PubMed
[43] Chavez-Galan L, Olleros ML, Vesin D, Garcia I. Much more than M1 and M2 macrophages, there are also CD169(+) and TCR+ macrophages. Front Immunol. 2015;6:263.10.3389/fimmu.2015.00263Search in Google Scholar
[44] Yeramian A, Martin L, Arpa L, Bertran J, Soler C, McLeod C, et al. Macrophages require distinct arginine catabolism and transport systems for proliferation and for activation. Eur J Immunol. 2006;36(6):1516–26.10.1002/eji.200535694Search in Google Scholar PubMed
© 2021 Chenqiang Lin et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

