Abstract
The role of inflammation has been identified in the pathogenesis of diabetic ketoacidosis (DKA). The neutrophil/lymphocyte ratio (NLR) and white blood cells (WBC) can be used to predict a systemic inflammatory response. Changes in NLR and WBC levels have never been explored in type 1 diabetes mellitus (T1DM) patients with DKA and an uninfected state. This retrospective study included a total of 644 participants. NLR and WBC were measured in the control group (n = 316) and in T1DM patients with mild-DKA (n = 92), severe-DKA (n = 52), and non-DKA (n = 184) in an uninfected state. Then, we assessed the independent predictors of DKA occurrence in T1DM patients in an uninfected state. The diagnostic performance of variables was determined by receiver operating characteristic curve analysis. Serum NLR of T1DM patients is significantly higher than that of normal controls, and if DKA occurs, NLR increases further and increases with the severity of DKA. In addition to diastolic blood pressure, blood urea nitrogen, glycated hemoglobin (HbA1c), and WBC, NLR was also independently associated with DKA in T1DM patients with an uninfected state (OR = 1.386, 95% CI: 1.127–1.705, p = 0.002). Furthermore, the diagnosis analysis showed that except for NLR and WBC, the area under the curve (AUC) of indicators with a statistical difference in patients with and without DKA were 0.747 for DKA diagnosis, and after the addition of NLR and WBC, the AUC was 0.806. The increased NLR level represents a low-cost and highly accessible predictor for DKA in T1DM patients with an uninfected state. The addition of inflammation indicators can play a statistically significant role in the prediction model of the DKA occurrence.
1 Introduction
Diabetic ketoacidosis (DKA) is an acute and severe complication of diabetes, and DKA is more likely to occur in type 1 diabetes mellitus (T1DM). Therefore, early detection and treatment of DKA are beneficial to the prognosis of diabetic patients [1,2]. If the simple and low-cost indicators for predicting the occurrence of DKA can be found from routine clinical examination data, the early diagnosis rate of DKA may be improved and its progress can be blocked, thus greatly reducing the harm of DKA.
The most common cause of DKA is infection. In addition, some patients with T1DM may develop DKA and other acute diabetic complications due to irregular use of insulin or the induction of diseases such as infection [3,4]. These patients may also have systemic oxidative stress or inflammatory response, which exacerbates the disorder of the patient’s internal environment [5]. However, DKA in T1DM patients without infection is an uninfected form of systemic inflammatory response syndrome with significantly increased proinflammatory factors [6]. The release of inflammatory factors will weaken the function of pancreatic β cells, while the deficiency of insulin in the DKA state can promote the obvious increase of inflammatory factors by increasing the acute-phase response, thereby forming a vicious circle [7,8]. Therefore, the identification of the exact relationship between inflammatory response and DKA is significant for the early treatment and later recovery of DKA patients.
The neutrophil/lymphocyte ratio (NLR) and white blood cells (WBC) can be used to predict a systemic inflammatory response. The NLR, a new marker of the systemic inflammatory response, integrates the information of neutrophils and lymphocytes. Furthermore, it is easy to obtain NLR and is less affected by physiological factors and specimen treatment [9]. Recent studies have found that NLR is closely related to the occurrence, severity, and prognoses of many acute diseases, such as acute coronary syndrome [10], acute ischemic stroke (AIS) [11], acute pancreatitis [12], acute respiratory distress syndrome [13], acute appendicitis [14], and acute type A aortic dissection [15]. During the development of systemic inflammatory response, WBCs are the effector cells of inflammatory response and the effector mediator of inflammatory conduction. In addition, WBC can regulate the body’s immune function by regulating the cellular immune function and play a key role in the systemic inflammatory response caused by infection or noninfectious factors [16,17,18].
Despite our knowledge about increased inflammation in the context of DKA, the data about the relationship between DKA and NLR, a simple and economical index, are lacking in this population. Changes in NLR and WBC levels have never been explored in T1DM patients with DKA and an uninfected state. Therefore, the aim of this study was to depict the difference in NLR and WBC levels in T1DM patients with DKA and non-DKA in an uninfected state and determine the DKA diagnostic performance of NLR and WBC.
2 Subjects and methods
2.1 Study subjects
This retrospective study included a total of 644 participants. We first selected 328 T1DM patients hospitalized in Shandong Provincial Hospital affiliated to Shandong University (Jinan, China) and Weihai Municipal Hospital (Weihai, China) from January 2008 to January 2020. With age, sex, and examination time-matched for T1DM participants, we then collected 316 healthy controls from our database of the Health Examination Center. According to the onset characteristics, fasting blood glucose, islet autoantibodies, serum C-peptide, ketone bodies, and blood gas analysis results [19], the individuals were divided into the normal control group (n = 316) and T1DM patients with mild-DKA (n = 92), severe-DKA (n = 52) and non-DKA (n = 184) groups. The normal control inclusion criteria were as follows: a healthy person with no physiological, physical, or psychologic abnormalities; Mild-DKA group: T1DM patients with ketosis and an uninfected state; Severe-DKA group: T1DM patients with ketoacidosis and an uninfected state; Non-DKA group: T1DM patients in an uninfected state without ketosis or ketoacidosis. Subjects with serious cardiovascular diseases, such as acute myocardial infarction, cachexia, immune disease, hepatic dysfunction, renal dysfunction (creatinine-based eGFR <90 mL/min/1.73 m2), infection, inflammation, or treatment with anti-inflammatory drugs, pregnancy, taking aspirin or statins, were excluded from this study.
-
Informed consent: Informed consent from each patient and healthy control was not required due to the retrospective study design and the use of anonymized data.
-
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration, and has been approved by the Ethics Committee of Shandong Provincial Hospital affiliated to Shandong University.
2.2 Measurements
The authors retrieved all data from previous hospital records of patients and healthy controls admitted to Shandong Provincial Hospital affiliated to Shandong University and Weihai Municipal Hospital; physical examination and extraction and analysis of blood were performed by other experts when patients and healthy controls were admitted to the hospital. After fasting overnight, venous blood was collected. An automatic biochemistry analyzer (Beckman Coulter Analyzer AU58 Series, USA) was used to analyze aspartate transaminase (AST), alanine transaminase (ALT), albumin (ALB), blood urea nitrogen (BUN), creatinine (CREA), total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and uric acid (UA). Glycated hemoglobin (HbA1c) was determined using high-performance liquid chromatography (HPLC) with a hemoglobin A1c analyzer (TOSOH Corporation, Japan). WBC and NLR were analyzed on a blood cell analyzer (Sysmex Corporation XN-2100, Japan). NLR = neutrophil count/lymphocyte count.
2.3 Statistical analysis
The Kolmogorov–Smirnov test was used to test the normality of all parameters prior to performing parametric tests. Normally distributed continuous data are represented as the mean ± SD, while nonnormally distributed continuous parameters are represented as the medians with interquartile ranges. Categorical variables are expressed as percentages. To analyze differences between the four groups, data with a normal distribution were tested by one-way ANOVA, and skewed variables were tested by the Kruskal–Wallis H-test. Categorical variables were tested by the Chi-square test. Pearson, Spearman, and partial correlation analyses were used to determine factors that affect NLR and WBC levels. Then, the association between NLR or WBC levels and the occurrence of DKA in T1DM patients were assessed by logistic regression analysis of factors with p < 0.05 in single-factor analysis. Notably, we performed collinearity diagnostics of the variables included in the logistic regression analysis. To address the problem of multicollinearity in the multiple linear regression analysis, we used a stepwise model to exclude variables with a minimal explanation of the dependent variable from the combination of multiple collinearities of independent variables. The independent variables included in the logistic regression model are not significantly collinearity. The diagnostic performance of the variables was determined by receiver operating characteristic (ROC) curve analysis. All p values were two-tailed, and p values less than 0.05 were considered statistically significant. SPSS version 24.0 (SPSS, Chicago, IL, USA) was used for all parametric tests.
3 Results
3.1 General characteristics of the study population with 644 subjects
We first analyzed the differences in clinical indicators of the normal control group (n = 316) and T1DM patients in an uninfected state with non-DKA (n = 184), mild-DKA (n = 92), and severe-DKA (n = 52) groups (Table 1). Non-DKA patients are older than the normal control group. Compared with T1DM patients without DKA, the patients with DKA were younger, and the patients with ketoacidosis were younger than those with ketosis. Patients with DKA have a shorter duration of diabetes than those without DKA. Compared with the non-DKA group, the SBP and DBP of the mild-DKA group decreased. The ALB level of T1DM patients decreased compared with the control group. Among T1DM patients, the ALB level of patients with DKA was lower than those of patients without DKA. The BUN level of patients with ketosis was lower than those of the control group and patients without DKA, and the BUN level of patients with DKA was higher than those of patients with ketosis. Although the BUN level of patients with DKA was also higher than those of the control group and patients without DKA, there was no statistical difference. Compared with the control group, the CREA level of patients with non-DKA and ketosis decreased, while the CREA level of DKA patients was higher than those of patients with non-DKA and ketosis. The level of HbA1c in DKA patients was higher than those of patients without DKA. Compared with the control group, the TC level of patients with non-DKA and ketoacidosis increased, the TGs level of patients with non-DKA decreased, and the LDL-c or HDL-c level of patients with non-DKA increased. The level of UA in mild-DKA patients was lower than those of the control group and patients without DKA, and the level of UA in severe-DKA patients was higher than those of the other three groups. The WBC and NLR levels of severe-DKA patients were higher than those of patients with non-DKA and ketosis (Figure 1). Next, we divided the diabetic patients into two groups based on whether DKA occurred (Table A1). Regarding the difference in NLR and WBC between the two groups, T1DM patients with DKA had significantly higher serum NLR and WBC levels than those without DKA (1.94 (IQR: 1.35–3.21) vs 1.67 (IQR: 1.15–2.50), p = 0.004; 6.23 (IQR: 4.89–7.83) vs 5.66 (IQR: 4.71–6.93), p = 0.002, respectively).
Basic characteristics of the control group and T1DM group
| Parameters | Control (n = 316) | Non-DKA (n = 184) | Mild-DKA (n = 92) | Severe-DKA (n = 52) | p |
|---|---|---|---|---|---|
| Age (years) | 31.90 ± 14.45 | 35.17 ± 15.91a | 31.02 ± 14.70b | 28.71 ± 13.43b | 0.014 |
| Males% (n) | 47.15 (149) | 50.54 (93) | 44.57 (41) | 44.23 (23) | 0.744 |
| Duration of diabetes (years) | — | 5.00 (IQR: 1.00–12.00) | 1.00 (IQR: 0.17–6.00)b | 2.50 (IQR: 0.17–7.00)b | <0.001 |
| SBP (mmHg) | 123.27 ± 10.82 | 126.24 ± 21.34 | 120.20 ± 20.05b | 122.13 ± 19.65 | 0.029 |
| DBP (mmHg) | 81.13 ± 8.96 | 81.21 ± 14.23 | 75.66 ± 15.18a,b | 78.38 ± 12.73 | 0.001 |
| AST (U/L) | 19.0 (IQR: 16.0–23.0) | 19.0 (IQR: 15.0–24.0) | 19.0 (IQR: 15.0–24.0) | 17.5 (IQR: 15.0–25.75) | 0.685 |
| ALT (U/L) | 17.0 (IQR: 12.0–25.0) | 16 (IQR: 12.0–23.0) | 14.0 (IQR: 11.0–23.0) | 16.0 (IQR: 13.0–20.5) | 0.259 |
| ALB (g/L) | 43.30 (IQR: 41.50–45.60) | 41.90 (IQR: 38.88–44.03)a | 39.60 (IQR: 35.88–42.23)a,b | 36.30 (IQR: 33.55–41.15)a,b | <0.001 |
| BUN (mmol/L) | 4.90 (IQR: 4.13–5.98) | 5.00 (IQR: 4.00–6.30) | 4.31 (IQR: 2.70–5.41)a,b | 5.55 (IQR: 3.74–7.04)c | <0.001 |
| CREA (µmol/L) | 68.00 (IQR: 59.38–76.96) | 61.00 (IQR: 48.89–73.50)a | 55.90 (IQR: 47.30–68.00)a | 69.10 (IQR: 58.23–96.93)b,c | <0.001 |
| HbA1c (%) | — | 9.10 (IQR: 7.68–11.43) | 11.70 (IQR: 9.60–13.10)b | 13.20 (IQR: 10.75–15.00)b | <0.001 |
| TC (mmol/L) | 4.64 ± 0.76 | 4.97 ± 1.76a | 4.68 ± 1.45 | 5.13 ± 2.14a | 0.017 |
| TGs (mmol/L) | 1.03 (IQR: 0.82–1.36) | 0.87 (IQR: 0.64–1.30)a | 0.88 (IQR: 0.65–1.37) | 1.07 (IQR: 0.85–1.83) | 0.008 |
| HDL-c (mmol/L) | 1.22 ± 0.25 | 1.42 ± 0.36a | 1.28 ± 0.40b | 1.34 ± 0.87 | <0.001 |
| LDL-c (mmol/L) | 2.54 ± 0.56 | 2.84 ± 1.33a | 2.75 ± 1.07 | 2.82 ± 1.01 | 0.004 |
| UA (mmol/L) | 269.52 ± 62.27 | 260.98 ± 81.38 | 212.61 ± 76.96a,b | 345.89 ± 173.09a,b,c | <0.001 |
One-way ANOVA, Kruskal–Wallis H-test and Chi-square test. Mean ± standard deviation or median (IQR: interquartile range) and n (%). Non-DKA group: T1DM patients in an uninfected state without ketosis or ketoacidosis. Mild-DKA group: T1DM patients with ketosis and in an uninfected state. Severe-DKA group: T1DM patients with ketoacidosis and in an uninfected state. SBP, systolic blood pressure; DBP, diastolic blood pressure; AST, aspartate transaminase; ALT, alanine transaminase; ALB, albumin; BUN, blood urea nitrogen; CREA, creatinine; HbA1c, glycated hemoglobin; TC, total cholesterol; TGs, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; UA, uric acid. Differences with a probability value of p < 0.05 were considered statistically significant and are given in bold. Compared with the control group, a p < 0.05. Compared with the Non-DKA group, b p < 0.05. Compared with the Mild-DKA group, c p < 0.05.
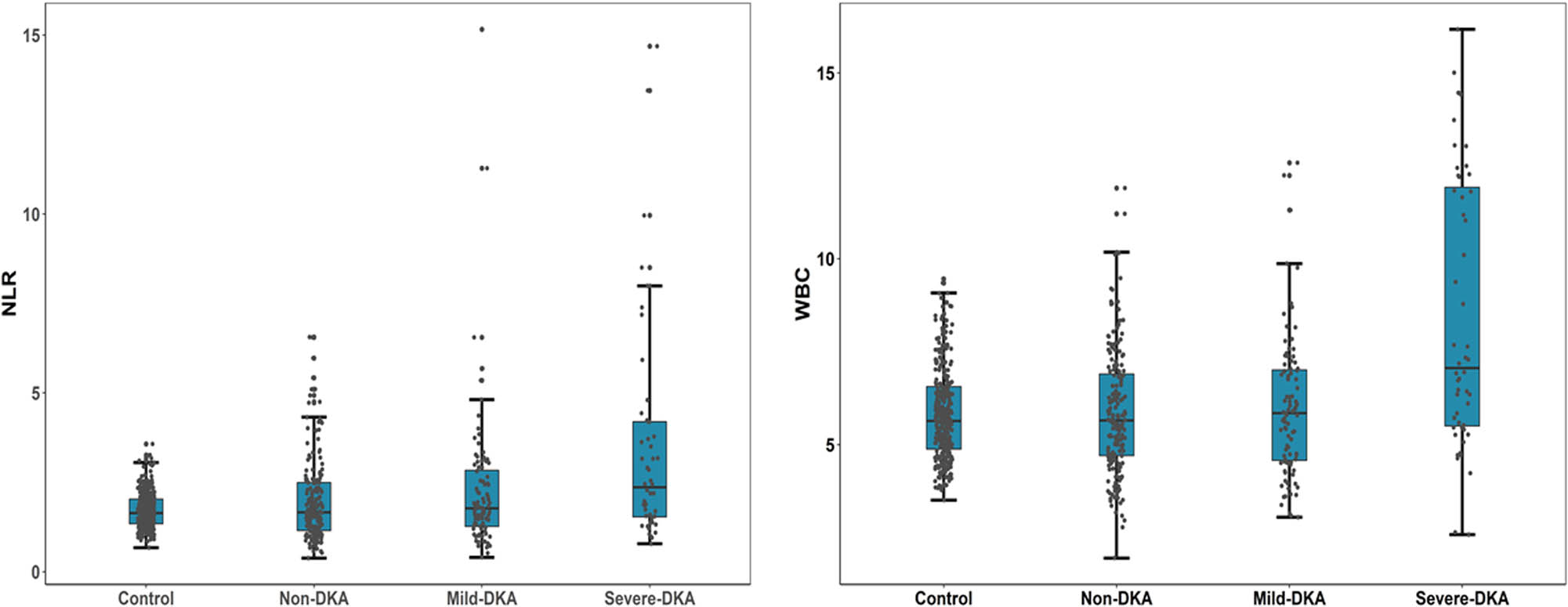
Differences in NLR and WBC levels among the four groups. Serum NLR and WBC levels of T1DM patients are significantly higher than that of normal controls and if DKA occurs, the NLR and WBC levels increase further and increase with the severity of DKA. Abbreviation: NLR, neutrophil/lymphocyte ratio; WBC, white blood cells; Non-DKA group: T1DM patients in an uninfected state without ketosis or ketoacidosis. Mild-DKA group: T1DM patients with ketosis and in an uninfected state. Severe-DKA group: T1DM patients with ketoacidosis and in an uninfected state.
In addition, there were no differences in gender, AST, or ALT levels between the four groups.
Our findings indicated that serum NLR and the WBC level of T1DM patients is significantly higher than that of normal controls, and if DKA occurs, the NLR and the WBC level increase further and increase with the severity of DKA.
3.2 Correlation between NLR or WBC and the clinical variables
The correlations between NLR and clinical parameters in patients with T1DM are shown in Table 2. According to these results, serum NLR was positively correlated with age, duration of diabetes, SBP, BUN, CREA, TC, TGs, LDL-c, UA, and WBC and negatively correlated with AST, ALT, and ALB. As previously reported in patients with type 2 diabetes mellitus [20], Sefil et al. showed that the duration of diabetes was related to NLR levels. In addition, age and duration of diabetes had a significant correlation in T1DM patients (r = 0.367; p <0.001). To avoid the influence of age and duration of diabetes on NLR levels and eliminate potential confounding factors, we adjusted for age and duration of diabetes. After adjustment, CREA, HbA1c, and WBC positively correlated between NLR (r = 0.184, p = 0.003; r = 0.125, p = 0.043; r = 0.554, p <0.001, respectively) and ALB negatively correlated with NLR (r = −0.179, p = 0.004), whereas the other positive or negative correlations above were no longer significant.
Correlation of serum NLR levels with the clinical variables
| Parameters | NLR | Adjusted NLR | ||
|---|---|---|---|---|
| r | p | r | p | |
| Age (years) | 0.114 | 0.038 | — | — |
| Duration of diabetes (years) | 0.217 | <0.001 | — | — |
| Sex | −0.027 | 0.626 | 0.072 | 0.246 |
| SBP (mmHg) | 0.218 | <0.001 | 0.086 | 0.164 |
| DBP (mmHg) | 0.150 | 0.007 | 0.060 | 0.330 |
| AST (U/L) | −0.131 | 0.019 | −0.046 | 0.452 |
| ALT (U/L) | −0.119 | 0.033 | −0.052 | 0.403 |
| ALB (g/L) | −0.127 | 0.022 | −0.179 | 0.004 |
| BUN (mmol/L) | 0.202 | <0.001 | 0.086 | 0.161 |
| CREA (µmol/L) | 0.257 | <0.001 | 0.184 | 0.003 |
| HbA1c (%) | 0.031 | 0.584 | 0.125 | 0.043 |
| TC (mmol/L) | 0.176 | 0.002 | 0.059 | 0.336 |
| TGs (mmol/L) | 0.198 | 0.001 | 0.097 | 0.118 |
| HDL-c (mmol/L) | −0.038 | 0.513 | −0.080 | 0.196 |
| LDL-c (mmol/L) | 0.174 | 0.003 | 0.065 | 0.295 |
| UA (mmol/L) | 0.181 | 0.001 | 0.054 | 0.384 |
| WBC (109/L) | 0.531 | <0.001 | 0.554 | <0.001 |
SBP, Systolic blood pressure; DBP, diastolic blood pressure; AST, aspartate transaminase; ALT, alanine transaminase; ALB, albumin; BUN, blood urea nitrogen; CREA, creatinine; HbA1c, glycated hemoglobin; TC, total cholesterol; TGs, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; UA, uric acid; NLR, neutrophil/lymphocyte ratio; WBC, white blood cells. Differences with a probability value of p < 0.05 were considered statistically significant and are given in bold.
Considering the strong correlation between WBC and NLR, we assessed factors associated with WBC and found that WBC was positively correlated with NLR, HbA1c, TC, TGs, LDL-c, and UA and negatively correlated with ALB after adjustment for age and duration of diabetes (Table 3).
Correlation of serum WBC levels with the clinical variables
| Parameters | WBC | Adjusted WBC | ||
|---|---|---|---|---|
| r | p | r | p | |
| Age (years) | 0.006 | 0.919 | — | — |
| Duration of diabetes (years) | 0.177 | 0.001 | — | — |
| Sex | −0.010 | 0.861 | 0.011 | 0.860 |
| SBP (mmHg) | 0.150 | 0.007 | 0.022 | 0.718 |
| DBP (mmHg) | 0.157 | 0.004 | 0.070 | 0.259 |
| AST (U/L) | −0.130 | 0.020 | −0.058 | 0.345 |
| ALT (U/L) | −0.134 | 0.016 | −0.065 | 0.294 |
| ALB (g/L) | −0.155 | 0.005 | −0.139 | 0.024 |
| BUN (mmol/L) | 0.195 | <0.001 | 0.114 | 0.064 |
| CREA (µmol/L) | 0.267 | <0.001 | 0.116 | 0.059 |
| HbA1c (%) | 0.093 | 0.102 | 0.154 | 0.012 |
| TC (mmol/L) | 0.209 | <0.001 | 0.134 | 0.029 |
| TGs (mmol/L) | 0.285 | <0.001 | 0.203 | 0.001 |
| HDL-c (mmol/L) | −0.058 | 0.321 | −0.047 | 0.447 |
| LDL-c (mmol/L) | 0.208 | <0.001 | 0.126 | 0.040 |
| UA (mmol/L) | 0.253 | <0.001 | 0.253 | <0.001 |
| NLR | 0.531 | <0.001 | 0.554 | <0.001 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; AST, aspartate transaminase; ALT, alanine transaminase; ALB, albumin; BUN, blood urea nitrogen; CREA, creatinine; HbA1c, glycated hemoglobin; TC, total cholesterol; TGs, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; UA, uric acid; NLR, neutrophil/lymphocyte ratio; WBC, white blood cells. Differences with a probability value of p < 0.05 were considered statistically significant and are given in bold.
3.3 Correlation between serum NLR and WBC levels and the occurrence of diabetic ketoacidosis in T1DM patients in an uninfected state
Similarly, due to the strong correlation between WBC and NLR, we included WBC and NLR into the multiple logistic regression model, respectively. Table 4 demonstrates the results of the analysis carried out to identify the correlation between serum NLR levels and the occurrence of DKA in T1DM patients. The independent variables entering the equation included age, duration of diabetes, SBP, DBP, ALB, BUN, HbA1c, HDL-c, and NLR. Consequently, HbA1c and NLR were independent risk factors for the occurrence of DKA in T1DM patients, while DBP and BUN were protective factors. Table 5 demonstrates the results of the analysis carried out to identify the correlation between serum WBC levels and the occurrence of DKA in T1DM patients. The independent variables entering the equation included age, duration of diabetes, SBP, DBP, ALB, BUN, HbA1c, HDL-c, and WBC. HbA1c and WBC were independent risk factors for the occurrence of DKA in T1DM patients, while DBP and BUN were protective factors.
Predictors of DKA in T1DM patients in multivariate logistic regression analysis with DKA as a dependent variable; and age, duration of diabetes, SBP, DBP, ALB, BUN, HbA1c, HDL-c, and NLR as independent variables
| Parameters | OR | p | 95% CI |
|---|---|---|---|
| Age (years) | 0.994 | 0.590 | 0.973–1.016 |
| Duration of diabetes (years) | 0.957 | 0.115 | 0.906–1.011 |
| SBP (mmHg) | 1.011 | 0.339 | 0.989–1.034 |
| DBP (mmHg) | 0.968 | 0.026 | 0.940–0.996 |
| ALB (g/L) | 0.973 | 0.278 | 0.926–1.022 |
| BUN (mmol/L) | 0.879 | 0.028 | 0.783–0.986 |
| HbA1c (%) | 1.401 | <0.001 | 1.237–1.588 |
| HDL-c (mmol/L) | 0.805 | 0.469 | 0.447–1.449 |
| NLR | 1.386 | 0.002 | 1.127–1.705 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; ALB, albumin; BUN, blood urea nitrogen; HbA1c, glycated hemoglobin; HDL-c, high-density lipoprotein cholesterol; NLR, neutrophil/lymphocyte ratio; OR, odds ratio; CI, confidence interval. Differences with a probability value of p < 0.05 were considered statistically significant and are given in bold.
Predictors of DKA in T1DM patients in multivariate logistic regression analysis with DKA as the dependent variable; and age, duration of diabetes, SBP, DBP, ALB, BUN, HbA1c, HDL-c, and WBC as independent variables
| Parameters | OR | p | 95% CI |
|---|---|---|---|
| Age (years) | 0.997 | 0.798 | 0.975–1.019 |
| Duration of diabetes (years) | 0.948 | 0.055 | 0.897–1.001 |
| SBP (mmHg) | 1.016 | 0.184 | 0.993–1.039 |
| DBP (mmHg) | 0.963 | 0.012 | 0.935–0.992 |
| ALB (g/L) | 0.969 | 0.212 | 0.921–1.018 |
| BUN (mmol/L) | 0.855 | 0.015 | 0.754–0.971 |
| HbA1c (%) | 1.406 | <0.001 | 1.238–1.597 |
| HDL-c (mmol/L) | 0.838 | 0.543 | 0.474–1.481 |
| WBC (109/L) | 1.337 | <0.001 | 1.160–1.540 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; ALB, albumin; BUN, blood urea nitrogen; HbA1c, glycated hemoglobin; HDL-c, high-density lipoprotein cholesterol; WBC, white blood cell; OR, odds ratio; CI, confidence interval. Differences with a probability value of p < 0.05 were considered statistically significant and are given in bold.
3.4 ROC curve of the predicted variables associated with the occurrence of DKA in T1DM patients in an uninfected state
Furthermore, the diagnosis analysis showed that except for NLR and WBC, the area under the curve (AUC) of indicators with the statistical difference in patients with and without DKA were 0.747 for DKA diagnosis, and after the addition of NLR and WBC, the AUC was 0.806 (Figure 2). The addition of inflammation indicators can play a statistically significant gain in the prediction model of DKA occurrence.
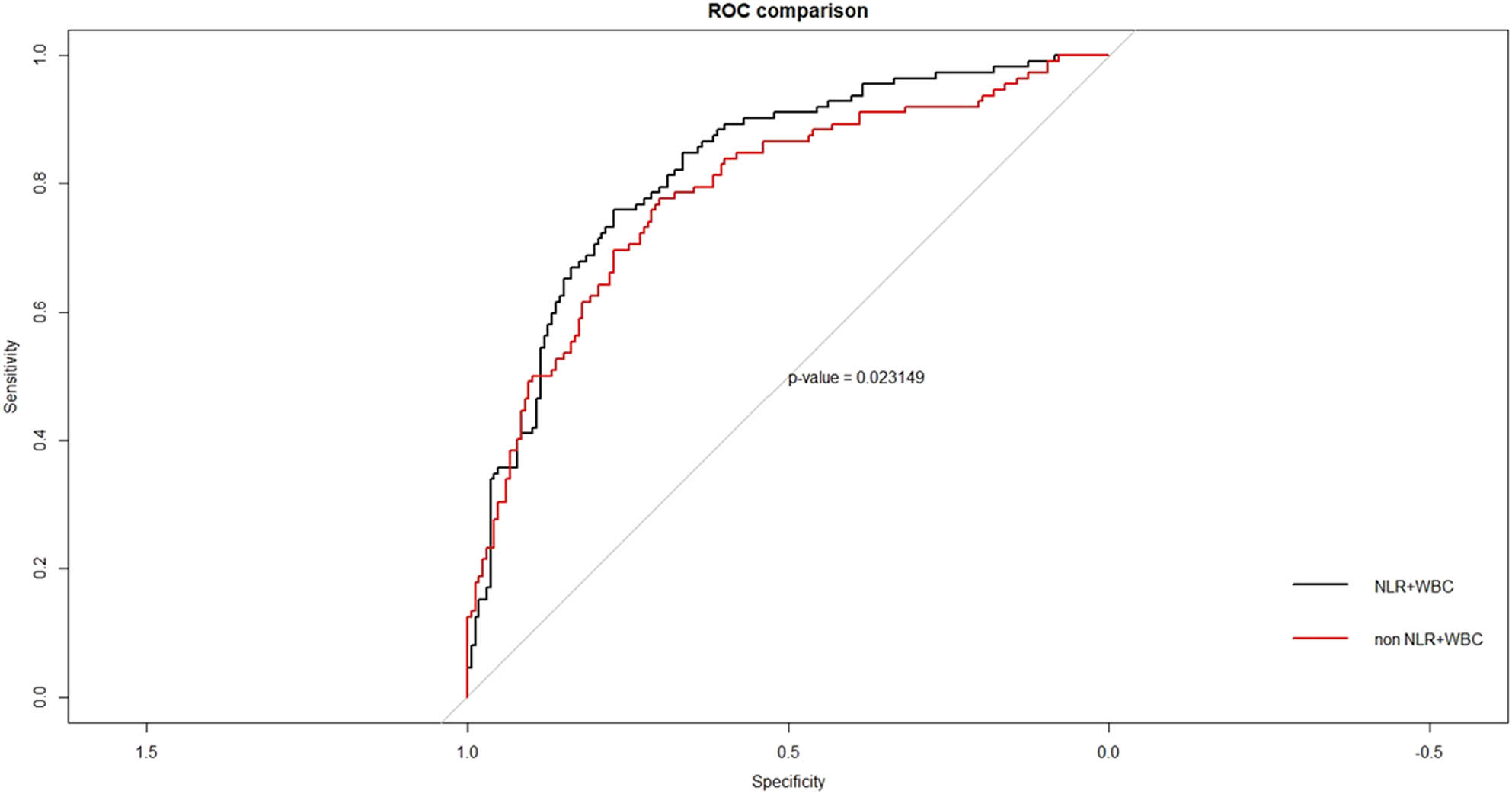
ROC curve of the predicted variables associated with the occurrence of DKA in T1DM patients. The diagnosis analysis showed that except for NLR and WBC, the area under curve (AUC) of indicators with the statistical difference in patients with and without DKA were 0.747 for DKA diagnosis, and after the addition of NLR and WBC, the AUC was 0.806. The addition of inflammation indicators can play a statistically significant gain in the prediction model of the occurrence of DKA. Abbreviation: ROC, receiver operating characteristic curve analysis; NLR, neutrophil/lymphocyte ratio; WBC, white blood cells. Differences with a probability value of p < 0.05 were considered statistically significant.
4 Discussion
The current study investigated NLR and WBC levels in normal people and in T1DM patients with non-DKA, mild-DKA, and severe-DKA in an uninfected state. Intriguingly, we found that serum NLR and the WBC level of T1DM patients is significantly higher than that of normal controls, and if DKA occurs, the NLR and WBC level increase further and increase with the severity of DKA. The increased serum NLR and WBC levels were independently associated with the occurrence of DKA in T1DM patients in an uninfected state, and the addition of NLR and WBC can play a statistically significant role in the prediction model of the occurrence of DKA. To the best of our knowledge, we have not seen any report describing the NLR levels in T1DM patients with DKA and an uninfected state.
There is increasing evidence that easily obtainable laboratory biomarkers can have a predictive role and be used for diagnosis and outcome prediction in various medical conditions. NLR, a relatively inexpensive marker of inflammation, has recently been explored as a predictor of mortality in patients who undergo percutaneous coronary intervention and can aid in the risk stratification and prognosis of patients diagnosed with acute coronary syndromes [21]. Moreover, NLR is an independent predictor of contrast-induced acute nephropathy in patients with acute myocardial infarction [22]. Remarkably, Lattanzi found that higher NLR levels upon admission were independently associated with poor outcomes at 30 days after the acute intracerebral hemorrhage onset [23]. The inflammatory response that occurs after cerebral infarct plays a critical role in stroke pathobiology and can affect the outcome of the recanalization procedure [24]. Furthermore, NLR showed good discriminatory power in predicting symptomatic hemorrhagic transformation, a life-threatening complication of AIS, in patients with AIS undergoing revascularization [25]. In cancer patients, higher pretreatment NLR levels are associated with poor survival outcomes. A study based on a retrospective cohort of 5,363 patients treated at the Moffitt Cancer Center (Tampa, FL) found that NLR demonstrated stronger associations with survival among African-American patients, patients receiving radiation therapy, stage IV patients, and melanoma patients when compared with the overall study population. In other words, for patients with certain demographic and clinical characteristics, NLR may have a greater prognostic value [26]. A retrospective study evaluated 240 patients with newly diagnosed metastatic colorectal cancer (stage IV), who underwent surgical resection, and found a high SII value calculated based on preoperative laboratory data regarding platelet, neutrophil, and lymphocyte counts that independently predicted poor clinical outcomes among patients with metastatic colorectal cancer [27]. Likewise, NLR has been shown as a simple promising method to predict bacteremia in adult patients admitted to the Emergency Department with suspected community-acquired bacteremia [28].
As is well known, both T1DM and type 2 diabetes mellitus (T2DM) are considered to be systemic inflammatory states [29,30]. Studies have shown that the increased release of inflammatory factors (such as TNF-α, IL-6, C-reactive protein) and the decreased release of anti-inflammatory cytokines (such as IL-10) can lead to the increased activation of adipose tissue, increased insulin resistance, and further progression to diabetes [31,32]. In our study, T1DM patients with an uninfected state had significantly higher serum NLR levels than individuals in the normal control group (1.80 [IQR: 1.24–2.67] vs 1.64 [IQR: 1.34–2.03], p <0.001). In addition to the explanation that T1DM is a chronic inflammatory disease and NLR reflects the state of systemic inflammation, we suspected that the increase of NLR in T1DM patients may also be related to poor blood glucose regulation. Sefil et al. [20]. found that NLR was higher in T2DM patients with HbA1c levels >7% than those ≤7%. Similarly, we found a positive correlation between NLR and HbA1c in T1DM patients after adjustment for age and duration of diabetes. A previous study about the relationship of NLR with aortic stiffness found that the NLR levels were significantly higher in T1DM patients than in healthy individuals, which was consistent with our findings. It was reported that the increased blood glucose caused a drop in lymphocyte levels [33], and lymphocytes did not proliferate enough in diabetic patients [34]. In the context of diabetes, inflammatory responses can be intensified by the hyperglycemic state [31]. Nonetheless, the exact mechanism of the increased NLR in T1DM patients in an uninfected state still needs further investigation, as our conjectures cannot completely elucidate this point.
Furthermore, T1DM patients with DKA and an uninfected state had significantly higher serum NLR and WBC levels than those in the non-DKA group, and NLR and WBC levels increased with the severity of DKA. In addition, the increased serum NLR and WBC levels were independent predictors of the occurrence of DKA in T1DM patients with an uninfected state, and the addition of inflammation indicators can play a statistically significant gain in the prediction model of the occurrence of DKA. We have a few possible explanations for these findings. First, neutrophils are involved in the body’s inflammatory response, while lymphocytes as the inflammatory regulator can protect endothelial cells and reduce the inflammatory response. The ratio of neutrophils and lymphocytes reflects the balance between the inflammatory activator and the inflammatory regulator. The higher the NLR, the more severe the inflammatory response [35]. The uninfected state of DKA can be identified as a serious form of systemic inflammatory response syndrome with significantly increased proinflammatory factors [6]. Therefore, our study suggests that the NLR may be an indicator of the underlying severity of acute systemic inflammation in DKA patients with an uninfected state. Second, elaborated cytokines can trigger inflammatory responses during hypoxemic episodes [36]. We suspect that DKA leads to an abnormality in the oxygen system and the occurrence of inflammation. In addition, hypoxia may inhibit the apoptosis of neutrophils in DKA patients, as in the asthma attack [37]. Third, the number of neutrophils and lymphocytes is regulated by the autonomic nervous system. There are adrenergic receptors on the surface of neutrophils, and the neutrophilic number and function are regulated by sympathetic nerves, while there are cholinergic receptors on the surface of lymphocytes that are regulated by parasympathetic nerves [38]. Increased sympathetic nerve activity can produce more neutrophils and proinflammatory substances [39,40]. When DKA occurs, the sympathetic nerve is excited, which stimulates the proliferation of neutrophils in the bone marrow. Fourth, the acute hyperglycemia and blood glucose fluctuations promote the increase of reactive oxygen species (ROS) in the state of DKA [41]. ROS can damage the DNA of peripheral blood lymphocytes, lead to the apoptosis of lymphocytes, and affect their proliferation [42,43]. Fifth, WBC can regulate the body’s immune function by regulating the cellular immune function and play a key role in the systemic inflammatory response caused by infection or noninfectious factors [16,17,18], which reflects the severity of the inflammatory response.
The death of DKA patients due to delayed diagnosis and lack of reasonable treatment is still common. Therefore, analyzing the risk factors for DKA in T1DM patients with an uninfected state is of great significance for early prevention, early diagnosis, and treatment. In addition to NLR, WBC and HbA1c were also independent predictors of DKA occurrence in T1DM patients in an uninfected state. Poor blood glucose control indicates severe metabolic disorders and severe insulin deficiency in the body. Consistent with other studies [44,45], a higher frequency of DKA was strongly associated with poorer glycemic control. This strongly suggests strengthening the monitoring frequency and controlling the level of blood glucose in T1DM patients. In addition, DBP and BUN were protective factors of the occurrence of DKA in T1DM patients in an uninfected state. During DKA, high blood glucose and ketones increase the excretion of glucose and ketones in the kidneys. Osmotic diuresis occurs and a large amount of water is lost, leading to a decreased effective circulating blood volume. Peripheral circulatory failure and hypovolemic shock can occur due to blood volume reduction and microcirculatory disturbances caused by acidosis. Lower blood pressure and renal perfusion can lead to acute renal failure [46]. Therefore, prevention and treatment of shock are very important for DKA patients. The relationship between WBC levels and blood glucose control has been studied by several groups [47,48]. When DKA occurs in a body without infection, the mechanism of increased WBC levels may be similar to that of increased neutrophil levels, but the existence of other causes, except for the activation of the immune system, cannot be still denied.
Interestingly, we found that serum NLR was positively correlated with SBP and DBP in T1DM patients with an uninfected state before adjusting for age and duration of diabetes (Table 2). The relationship between NLR and hypertension has been thoroughly investigated, and NLR is a reliable predictor of hypertension such as resistant hypertension [49,50]. The probable cause is that neutrophils secrete mediators of the inflammatory response, such as elastase, which are associated with plaque rupture and may contribute to an increased risk of hypertension [51,52]. In addition, neutrophils can release ROS that causes oxidative stress, which is an important factor in the pathogenesis of hypertension [53,54]. Additionally, UA is the final product of human purine catabolism and UA crystals deposited in tissues are the strong inflammatory stimulant. Elevated levels of UA show a significant relationship with the increase of certain inflammation markers and oxidative stress indicators [55,56]. In the form of monosodium urate crystals, UA can promote neutrophil recruitment through the complement pathway [57]. Therefore, our findings that UA was significantly positively correlated with serum NLR levels in T1DM patients in an uninfected state may be explained by the above mechanism. When DKA occurs in T1DM patients, the body suffers from endogenous insulin deficiency, glucose metabolism disorder, and increased adipose tissue mobilization. As mentioned earlier, inflammation plays an important role in the pathogenesis of DKA. Studies have found that some patients with severe systemic inflammatory response syndrome may have decreased serum albumin levels, and the degree of change is related to the prognosis of the disease [58]. This study shows that the ALB level of T1DM patients decreased compared with the control group, the ALB level of patients with DKA was lower than that of patients without DKA, and ALB was negatively correlated with NLR. The relationship between NLR and ALB levels needs to be explored in depth. Interestingly, our study showed a positive correlation between NLR and TC, TGs, LDL-c; so, whether the inflammatory response affects blood lipid levels in DKA patients? This may be a fascinating research direction.
It is incontestable that this study has many limitations. First, only one measurement of neutrophil and lymphocyte count and one calculation of NLR were carried out in the analysis. There was no monitoring of the dynamic trend of NLR. Second, selection bias may arise because our data are only from one hospital. Additionally, the sample size was small, and we look forward to future verification in large samples and multicenter studies. Third, our data came from a retrospective study. To fully understand the role of NLR in T1DM patients with DKA and an uninfected state, basic large-scale prospective studies should be conducted.
5 Conclusion
In summary, our study demonstrates that NLR and WBC levels increased in T1DM patients with DKA and an uninfected state and increased with DKA severity. NLR and WBC levels can be used as a simple and economical predictor for DKA occurrence in T1DM patients in an uninfected state. When DKA occurs, there may be a severe inflammatory reaction. In addition to lowering blood glucose, anti-inflammatory drugs are expected to relieve the internal environment disorder to a certain extent.
-
Funding information: This work was supported by grants from the National Natural Science Foundation (No. 81670720), the National Key Research and Development Program of China (No. 2017YFC1309805), special funds for the Taishan Scholar Project (No. tsqn20161071), and the Medical Quality Management Project of Shandong Province (2016).
-
Author contributions: Y.C., Y.Z., and C.X. created the study protocol, contributed to the analysis plan, wrote the first draft of the manuscript, and revised the manuscript to create the final version. H.C. and T.Z. contributed to the design of the study protocol and reviewed the manuscript. W.Y. contributed to the data analysis. C.X. is the guarantor of this work and has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Y.C., W.Y., and Y.Z. contributed equally to this article.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Appendix
Basic characteristics of the DKA group and non-DKA group
| DKA (n = 144) | Non-DKA (n = 184) | p | |
|---|---|---|---|
| Age (years) | 30.19 ± 14.25 | 35.17 ± 15.91 | 0.003 |
| Males% (n) | 44.44 (64) | 50.54 (93) | 0.272 |
| Duration of diabetes (years) | 1.25 (IQR: 0.17–6.75) | 5.00 (IQR: 1.00–12.00) | <0.001 |
| SBP (mmHg) | 120.90 ± 19.86 | 126.24 ± 21.34 | 0.021 |
| DBP (mmHg) | 76.65 ± 14.36 | 81.21 ± 14.23 | 0.004 |
| AST (U/L) | 18.0 (IQR:15.0–25.00) | 19.0 (IQR:15.0–24.0) | 0.466 |
| ALT (U/L) | 15.0 (IQR:11.0–21.0) | 16.0 (IQR:12.0–23.0) | 0.177 |
| ALB (g/L) | 38.20 (IQR:35.30–42.00) | 41.90 (IQR:38.88–44.00) | <0.001 |
| BUN (mmol/L) | 4.63 (IQR:3.20–6.10) | 5.00 (IQR:4.00–6.30) | 0.013 |
| CREA (µmol/L) | 62.00 (IQR:49.57–73.00) | 61.00 (IQR:48.89–73.50) | 0.983 |
| HbA1c (%) | 12.25 (IQR:10.10–13.83) | 9.10 (IQR:7.68–11.43) | <0.001 |
| TC (mmol/L) | 4.83 ± 1.72 | 4.97 ± 1.76 | 0.514 |
| TGs (mmol/L) | 0.94 (IQR:0.68–1.46) | 0.87 (IQR:0.64–1.30) | 0.144 |
| HDL-c (mmol/L) | 1.30 ± 0.60 | 1.42 ± 0.36 | 0.035 |
| LDL-c (mmol/L) | 2.77 ± 1.05 | 2.84 ± 1.33 | 0.647 |
| UA (mmol/L) | 259.41 ± 135.19 | 260.98 ± 81.38 | 0.899 |
| NLR | 1.90 (IQR:1.34–3.16) | 1.66 (IQR:1.14–2.49) | 0.011 |
| WBC (109/L) | 6.14 (IQR:4.80–7.59) | 5.66 (IQR:4.70–6.95) | 0.010 |
SBP, Systolic blood pressure; DBP, diastolic blood pressure; AST, aspartate transaminase; ALT, alanine transaminase; ALB, albumin; BUN, blood urea nitrogen; CREA, creatinine; HbA1c, glycated hemoglobin; TC, total cholesterol; TGs, triglycerides; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; UA, uric acid; NLR, neutrophil/lymphocyte ratio; WBC, white blood cells. Differences with a probability value of p < 0.05 were considered statistically significant and are given in bold.
References
[1] Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Prim. 2020;6(1):40.10.1038/s41572-020-0165-1Search in Google Scholar PubMed
[2] Martinez E, Portillo N, Lizarralde E, Grau G, Vela A, Rodriguez A, et al. For debate: paediatric T1DM: DKA is still a problem. Pediatr Endocrinol Rev. 2018;16(2):233–9.Search in Google Scholar
[3] Musso G, Sircana A, Saba F, Cassader M, Gambino R. Assessing the risk of ketoacidosis due to sodium-glucose cotransporter (SGLT)-2 inhibitors in patients with type 1 diabetes: a meta-analysis and meta-regression. PLoS Med. 2020;17(12):e1003461.10.1371/journal.pmed.1003461Search in Google Scholar PubMed PubMed Central
[4] Casteels K, Mathieu C. Diabetic ketoacidosis. Rev Endocr Metab Disord. 2003;4(2):159–66.10.1023/A:1022942120000Search in Google Scholar
[5] Zhu Z, Hu T, Wang Z, Wang J, Liu R, Yang Q, et al. Anti-inflammatory and organ protective effect of insulin in scalded MODS rats without controlling hyperglycemia. Am J Emerg Med. 2018;36(2):202–7.10.1016/j.ajem.2017.07.070Search in Google Scholar PubMed
[6] Dalton RR, Hoffman WH, Passmore GG, Martin SL. Plasma C-reactive protein levels in severe diabetic ketoacidosis. Ann Clin Lab Sci. 2003;33(4):435–42.Search in Google Scholar
[7] Emamalipour M, Seidi K, Jahanban-Esfahlan A, Jahanban-Esfahlan R. Implications of resistin in type 2 diabetes mellitus and coronary artery disease: impairing insulin function and inducing pro-inflammatory cytokines. J Cell Physiol. 2019;234(12):21758–69.10.1002/jcp.28913Search in Google Scholar PubMed
[8] Kitabchi AE, Umpierrez GE, Fisher JN, Murphy MB, Stentz FB. Thirty years of personal experience in hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab. 2008;93(5):1541–52.10.1210/jc.2007-2577Search in Google Scholar PubMed PubMed Central
[9] Huang Z, Fu Z, Huang W, Huang K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: a meta-analysis. Am J Emerg Med. 2020;38(3):641–7.10.1016/j.ajem.2019.10.023Search in Google Scholar PubMed
[10] Sia CH, Leow AS, Tan BY, Low CJ, Kaur R, Yeo TC, et al. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predict left ventricular thrombus resolution in acute myocardial infarction without percutaneous coronary intervention. Thromb Res. 2020;194:16–20.10.1016/j.thromres.2020.06.003Search in Google Scholar PubMed
[11] Pikija S, Sztriha LK, Killer-Oberpfalzer M, Weymayr F, Hecker C, Ramesmayer C, et al. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J Neuroinflammation. 2018;15(1):319.10.1186/s12974-018-1359-2Search in Google Scholar PubMed PubMed Central
[12] Abaylı B, Gençdal G, Değirmencioğlu Ş. Correlation between neutrophil/lymphocyte ratio and Ranson score in acute pancreatitis. J Clin Lab Anal. 2018;32(6):e22437.10.1002/jcla.22437Search in Google Scholar PubMed PubMed Central
[13] Wang Y, Ju M, Chen C, Yang D, Hou D, Tang X, et al. Neutrophil-to-lymphocyte ratio as a prognostic marker in acute respiratory distress syndrome patients: a retrospective study. J Thorac Dis. 2018;10(1):273–82.10.21037/jtd.2017.12.131Search in Google Scholar
[14] Hajibandeh S, Hajibandeh S, Hobbs N, Mansour M. Neutrophil-to-lymphocyte ratio predicts acute appendicitis and distinguishes between complicated and uncomplicated appendicitis: a systematic review and meta-analysis. Am J Surg. 2020;219(1):154–63.10.1016/j.amjsurg.2019.04.018Search in Google Scholar
[15] Erdolu B, As AK. C-Reactive protein and neutrophil to lymphocyte ratio values in predicting inhospital death in patients with Stanford type A acute aortic dissection. Heart Surg Forum. 2020;23(4):E488–92.10.1532/hsf.3055Search in Google Scholar
[16] Jimenez MF, Watson RW, Parodo J, Evans D, Foster D, Steinberg M, et al. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg. 1997;132(12):1263–9. Discussion 1269–70.10.1001/archsurg.1997.01430360009002Search in Google Scholar
[17] Kavanagh EG, Kelly JL, Lyons A, Soberg CC, Mannick JA, Lederer JA. Burn injury primes naive CD4 + T cells for an augmented T-helper 1 response. Surgery. 1998;124(2):269–76. Discussion 276–67.10.1016/S0039-6060(98)70130-8Search in Google Scholar
[18] Ferguson NR, Galley HF, Webster NR. T helper cell subset ratios in patients with severe sepsis. Intensive Care Med. 1999;25(1):106–9.10.1007/s001340050795Search in Google Scholar PubMed
[19] Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155–77.10.1111/pedi.12701Search in Google Scholar PubMed
[20] Sefil F, Ulutas KT, Dokuyucu R, Sumbul AT, Yengil E, Yagiz AE, et al. Investigation of neutrophil lymphocyte ratio and blood glucose regulation in patients with type 2 diabetes mellitus. J Int Med Res. 2014;42(2):581–8.10.1177/0300060513516944Search in Google Scholar PubMed
[21] Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102(6):653–7.10.1016/j.amjcard.2008.05.006Search in Google Scholar PubMed
[22] Butt K, D’Souza J, Yuan C, Jayakumaran J, Nguyen M, Butt HI, et al. Correlation of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) with contrast-induced nephropathy in patients with acute coronary syndrome undergoing percutaneous coronary interventions. Cureus. 2020;12(12):e11879.10.7759/cureus.11879Search in Google Scholar PubMed PubMed Central
[23] Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. 2018;387:98–102.10.1016/j.jns.2018.01.038Search in Google Scholar PubMed
[24] Lattanzi S, Norata D, Divani AA, Di Napoli M, Broggi S, Rocchi C, et al. Systemic inflammatory response index and futile recanalization in patients with Ischemic stroke undergoing endovascular treatment. Brain Sci. 2021;11(9):1164.10.3390/brainsci11091164Search in Google Scholar PubMed PubMed Central
[25] Świtońska M, Piekuś-Słomka N, Słomka A, Sokal P, Żekanowska E, Lattanzi S. Neutrophil-to-lymphocyte ratio and symptomatic hemorrhagic transformation in Ischemic stroke patients undergoing revascularization. Brain Sci. 2020;10(11):771.10.3390/brainsci10110771Search in Google Scholar PubMed PubMed Central
[26] Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep. 2019;9(1):19673.10.1038/s41598-019-56218-zSearch in Google Scholar PubMed PubMed Central
[27] Xie QK, Chen P, Hu WM, Sun P, He WZ, Jiang C, et al. The systemic immune-inflammation index is an independent predictor of survival for metastatic colorectal cancer and its association with the lymphocytic response to the tumor. J Transl Med. 2018;16(1):273.10.1186/s12967-018-1638-9Search in Google Scholar PubMed PubMed Central
[28] de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14(5):R192.10.1186/cc9309Search in Google Scholar PubMed PubMed Central
[29] Donath MY, Dinarello CA, Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat Rev Immunol. 2019;19(12):734–46.10.1038/s41577-019-0213-9Search in Google Scholar PubMed
[30] Zhou T, Hu Z, Yang S, Sun L, Yu Z, Wang G. Role of adaptive and innate immunity in type 2 diabetes mellitus. J Diabetes Res. 2018;2018:7457269.10.1155/2018/7457269Search in Google Scholar PubMed PubMed Central
[31] Dalmas E. Role of innate immune cells in metabolism: from physiology to type 2 diabetes. Semin Immunopathol. 2019;41(4):531–45.10.1007/s00281-019-00736-5Search in Google Scholar PubMed
[32] Böni-Schnetzler M, Meier DT. Islet inflammation in type 2 diabetes. Semin Immunopathol. 2019;41(4):501–13.10.1007/s00281-019-00745-4Search in Google Scholar PubMed PubMed Central
[33] Passos ME, Borges L, dos Santos-Oliveira LC, Alecrim-Zeza AL, Lobato TB, de Oliveira HH, et al. Recreational dance practice modulates lymphocyte profile and function in diabetic women. Int J Sports Med. 2021;42(8):749–59.10.1055/a-1309-2037Search in Google Scholar
[34] Chang FY, Shaio MF. Decreased cell-mediated immunity in patients with non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract. 1995;28(2):137–46.10.1016/0168-8227(95)00168-8Search in Google Scholar
[35] Zhang B, Zhou X, Zhu C, Song Y, Feng F, Qiu Y, et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020;7:157.10.3389/fmolb.2020.00157Search in Google Scholar PubMed PubMed Central
[36] Arter JL, Chi DS, Girish M, Fitzgerald SM, Guha B, Krishnaswamy G. Obstructive sleep apnea, inflammation, and cardiopulmonary disease. Front Biosci. 2004;9:2892–900.10.2741/1445Search in Google Scholar PubMed
[37] Ventura I, Vega A, Chacon P, Chamorro C, Aroca R, Gomez E, et al. Neutrophils from allergic asthmatic patients produce and release metalloproteinase-9 upon direct exposure to allergens. Allergy. 2014;69(7):898–905.10.1111/all.12414Search in Google Scholar PubMed
[38] Abo T, Kawamura T. Immunomodulation by the autonomic nervous system: therapeutic approach for cancer, collagen diseases, and inflammatory bowel diseases. Ther Apher. 2002;6(5):348–57.10.1046/j.1526-0968.2002.00452.xSearch in Google Scholar PubMed
[39] Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fat Acids. 2000;63(6):351–62.10.1054/plef.2000.0226Search in Google Scholar PubMed
[40] Pavlov VA, Chavan SS, Tracey KJ. Bioelectronic medicine: from preclinical studies on the inflammatory reflex to new approaches in disease diagnosis and treatment. Cold Spring Harb Perspect Med. 2020;10(3):a034140.10.1101/cshperspect.a034140Search in Google Scholar PubMed PubMed Central
[41] Nichols BE, Hook JS, Weng K, Ahn C, Moreland JG. Novel neutrophil phenotypic signature in pediatric patients with type 1 diabetes and diabetic ketoacidosis. J Leukoc Biol. 2021;1–8. 10.1002/JLB.3A1220-826R.Search in Google Scholar PubMed
[42] Hu H, Xu F, Yang W, Ren J, Ge W, Yang P. Apoptosis as an underlying mechanism in lymphocytes induced by riboflavin and ultraviolet light. Transfus Apher Sci. 2020;59(6):102899.10.1016/j.transci.2020.102899Search in Google Scholar PubMed
[43] Mirzaei S, Hadadi Z, Attar F, Mousavi SE, Zargar SS, Tajik A, et al. ROS-mediated heme degradation and cytotoxicity induced by iron nanoparticles: hemoglobin and lymphocyte cells as targets. J Biomol Struct Dyn. 2018;36(16):4235–45.10.1080/07391102.2017.1411832Search in Google Scholar PubMed
[44] Duca LM, Reboussin BA, Pihoker C, Imperatore G, Saydah S, Mayer‐Davis E, et al. Diabetic ketoacidosis at diagnosis of type 1 diabetes and glycemic control over time: the SEARCH for diabetes in youth study. Pediatr Diabetes. 2019;20(2):172–9.10.1111/pedi.12809Search in Google Scholar PubMed PubMed Central
[45] Hassan MM, Arafa N, Abdou M, Hussein O. Characteristics of diabetes diagnosis and control in toddlers and preschoolers from families with limited resources: a single center experience. Diabetes Res Clin Pract. 2020;159:107966.10.1016/j.diabres.2019.107966Search in Google Scholar PubMed
[46] Bugarski M, Ghazi S, Polesel M, Martins JR, Hall AM. Changes in NAD and lipid metabolism drive acidosis-induced acute kidney injury. J Am Soc Nephrol. 2021;32(2):342–56.10.1681/ASN.2020071003Search in Google Scholar PubMed PubMed Central
[47] Kashima S, Inoue K, Matsumoto M, Akimoto K. White blood cell count and C-reactive protein independently predicted incident diabetes: Yuport medical checkup center study. Endocr Res. 2019;44(4):127–37.10.1080/07435800.2019.1589494Search in Google Scholar PubMed
[48] Park JM, Lee HS, Park JY, Jung DH, Lee JW. White blood cell count as a predictor of incident type 2 diabetes mellitus among non-obese adults: a longitudinal 10-year analysis of the Korean genome and epidemiology study. J Inflamm Res. 2021;14:1235–42.10.2147/JIR.S300026Search in Google Scholar PubMed PubMed Central
[49] Bozduman F, Yildirim E, Cicek G. Biomarkers of nondipper hypertension in prehypertensive and hypertensive patients. Biomark Med. 2019;13(5):371–8.10.2217/bmm-2018-0247Search in Google Scholar PubMed
[50] Srinivasagopalane B, Andrew Rajarathinam S, Balasubramaiyan T. Clinical pertinence of neutrophil-to-lymphocyte ratio among hypertensives with different grades and duration of hypertension – an insight. Clin Exp Hypertens. 2019;41(4):394–9.10.1080/10641963.2018.1510942Search in Google Scholar PubMed
[51] Mehta J, Dinerman J, Mehta P, Saldeen TG, Lawson D, Donnelly WH, et al. Neutrophil function in ischemic heart disease. Circulation. 1989;79(3):549–56.10.1161/01.CIR.79.3.549Search in Google Scholar
[52] Small HY, Migliarino S, Czesnikiewicz-Guzik M, Guzik TJ. Hypertension: focus on autoimmunity and oxidative stress. Free Radic Biol Med. 2018;125:104–15.10.1016/j.freeradbiomed.2018.05.085Search in Google Scholar PubMed
[53] Reckelhoff JF, Romero DG, Yanes Cardozo LL. Sex, oxidative stress, and hypertension: insights from animal models. Physiol (Bethesda). 2019;34(3):178–88.10.1152/physiol.00035.2018Search in Google Scholar PubMed PubMed Central
[54] Chaudhary P, Pandey A, Azad CS, Tia N, Singh M, Gambhir IS. Association of oxidative stress and endothelial dysfunction in hypertension. Anal Biochem. 2020;590:113535.10.1016/j.ab.2019.113535Search in Google Scholar PubMed
[55] Ito H. Serum uric acid levels: a surrogate marker of oxidative stress or dehydration? Eur J Intern Med. 2021;86:110.10.1016/j.ejim.2021.01.004Search in Google Scholar PubMed
[56] Li Z, Shen Y, Chen Y, Zhang G, Cheng J, Wang W. High uric acid inhibits cardiomyocyte viability through the ERK/P38 pathway via oxidative stress. Cell Physiol Biochem. 2018;45(3):1156–64.10.1159/000487356Search in Google Scholar PubMed
[57] Sabbione F, Keitelman IA, Iula L, Ferrero M, Giordano MN, Baldi P, et al. Neutrophil extracellular traps stimulate proinflammatory responses in human airway epithelial cells. J Innate Immun. 2017;9(4):387–402.10.1159/000460293Search in Google Scholar PubMed PubMed Central
[58] Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn Jr. H, Kopple JD, et al. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transpl. 2005;20(9):1880–8.10.1093/ndt/gfh941Search in Google Scholar PubMed
© 2021 Yiping Cheng et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

