Abstract
The main purpose of this research was the identification and characterization of low-molecular-weight glutenin subunit (LMW-GS) composition in common wheat and the determination of the effect of these proteins on the rheological properties of dough. The use of capillary zone electrophoresis and reverse-phase high-performance liquid chromatography has made it possible to identify four alleles in the Glu-A3 and Glu-D3 loci and seven alleles in the Glu-B3 locus, encoding LMW-GSs in 70 varieties and breeding lines of wheat tested. To determine the technological quality of dough, analyses were performed at the microscale using a TA.XT Plus Texture Analyzer. Wheat varieties containing the Glu-3 loci scheme (Glu-A3b, Glu-A3f at the Glu-A3 locus; Glu-B3a, Glu-B3b, Glu-B3d, Glu-B3h at the Glu-B3 locus; Glu-D3a, Glu-D3c at the Glu-D3 locus) determined the most beneficial quality parameters.
1 Introduction
Wheat (Triticum aestivum L.) is a cereal species belonging to the family of Poaceae. Owing to its very good nutritional values (a rich source of starch, proteins, vitamins, minerals) and technological properties essential in the food industry, wheat is of great economic importance and is one of the most commonly grown cereals worldwide [1]. Analyses of the qualitative–quantitative composition of wheat storage proteins are a rich source of information regarding the technological properties of flour and are also used to select varieties in terms of desirable traits. The gluten complex, consisting of gliadins and glutenins, plays an important role during plant development and determines the technological use of wheat [2]. Glutenins are polymeric proteins that are divided into high-molecular-weight (HMW) glutenins with a molecular mass of 75–120 kDa and low-molecular-weight (LMW) glutenins with a mass of 20–55 kDa [3]. HMW glutenin subunits (HMW-GSs) account for nearly 10% of gluten proteins and determine 50–70% of the technological quality of the wheat grain, while LMW glutenin subunits (LMW-GSs) account for about 50% of gluten proteins and determine 30% of the technological quality [3]. With the separation of glutenin proteins on a polyacrylamide gel, it is possible to distinguish four regions of protein bands: A, B, C, and D [4]. The region A consists of HMW-GSs, while the LMW-GSs are located in regions B, C, and D. Additionally, in the C and D regions, there are α-, γ-, and ω-gliadins. The synthesis of proteins belonging to the LMW-GS group is mainly controlled by Glu-3 loci located on the short arms of the first group of chromosomes, in the vicinity of the Gli-1 loci complex responsible for coding of both γ- and ω-gliadins [3]. In recent years, based on the qualitative analysis, increased protein content in grains of wheat, which resulted in the presence of certain allelic variants encoding LMW-GSs, was observed [5]. In addition, biochemical and rheological analyses enabled the demonstration of both positive and negative effects of individual LMW subunit encoded by the Glu-3 loci on the technological parameters of wheat flour, dough, and finished bread [5,6,7]. It was also shown that the presence of LMW-GSs encoded by the Glu-A3 loci positively affects the viscoelastic properties, while the subunits encoded by the Glu-B3 loci are important in the creation of mechanical parameters of dough (sodium dodecyl sulfate [SDS] sedimentation index, dough mixing time, dough resistance, and the ratio of dough work to resistance) [8,9]. So far, capillary zone electrophoresis (CZE) and reverse-phase high-performance liquid chromatography (RP-HPLC) methods were used to identify LMW-GSs in a very limited range. In previous studies, scientists only distinguished a group of proteins without detailed identification or identified individual LMW-GS [10,11,12,13,14].
The objective of this study was to identify HMW-GSs using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and LMW-GSs in common wheat (Triticum aestivum L.) using CZE and RP-HPLC and to investigate their effect on the technological properties of wheat dough. The compositions of HMW-GSs and LMW-GSs were characterized in the analyzed plant material. Moreover, the technological quality of wheat and rheological analyses using a TA.XT Plus Texture Analyzer with Kieffer Rig (Stable MicroSystem) was determined at the microscale.
2 Materials and methods
2.1 Plant material
The plant material consisted of 57 varieties and 13 breeding lines of winter wheat (Triticum aestivum L.). The experimental material was cultivated at Smolice Plant Breeding Station in the years 2010–2012. In the first year, plant material was tested for the identification of HMW-GSs and LMW-GSs. In the last year, plant material was tested for technological quality. Plants were grown on 10 m2 in two replications, each on podzolic soil with clayey soil class IIIa. Peas were used as forecrop, and the fertilization dose was, respectively, 110 N, 60 P, and 90 K (kg ha−1) each year. Standard plant protection products against fungal diseases and pests were used during the experiment. The material was harvested to avoid inaccuracies.
Additionally, 22 reference varieties were used in this study (Table 1). This material was obtained from the Australia Winter Cereals Collection and is recommended as a standard for LMW-GSs [15]. These varieties were homozygous, with a strictly defined composition of LMW-GSs.
List of the 22 foreign reference varieties with a strictly defined composition of LMW-GSs
| Varieties | Alleles | Country of origin | ||
|---|---|---|---|---|
| Glu-A3 | Glu-B3 | Glu-D3 | ||
| Alva | a | d | a | Portugal |
| Arcane | c | c | a | France |
| Bastian | a | i | a | France |
| Chara | b | b | b | Australia |
| Cheyenne | c | e | f | USA |
| Chinese Spring | a | a | a | China |
| Democrat | a | h | a | France |
| Gabo | b | b | b | Australia |
| Gluclub | e | d | a | Australia |
| Insygnia | f | c | c | Australia |
| Isis | e | f | a | Australia |
| Jufy-1 | e | i | d | Belgium |
| Kharkov | e | g | a | Russia |
| Kukri | d | h | b | Australia |
| Newbury | c | c | c | UK |
| Norin-61 | d | i | c | Japan |
| Norstar | c | b | b | Canada |
| Orca | d | d | e | France |
| Pato Argentino | d | i | e | Argentina |
| Radja | e | f | b | France |
| Rescue | f | h | a | Canada |
| Thatcher | e | h | e | Canada |
2.2 Extraction of HMW glutenins and SDS-PAGE separation
The characterization of HMW-GSs was performed according to the methods of Tohver [16]. Material for protein extraction was wheat flour obtained from milling a single grain. HMW-GSs were extracted according to the procedure described by Salmanowicz [17] and Dai et al. [18]. The supernatants were transferred to clean microcentrifuge tubes (1.5 mL) and stored at 4°C until separation. A total of 7 µL of the supernatants were loaded onto stacking gel, including 4.5% (w/v) acrylamide, and HMW-GS proteins were separated on resolving gel containing 11.5% (w/v) acrylamide. SDS-PAGE was carried out using Protean II xi gel apparatus (Bio-Rad, Hercules, CA, USA) at 240 V for 4.5 h. Gels were stained overnight with Coomassie Brilliant Blue G-250.
2.3 Extraction of LMW glutenins and separation
LMW-GS proteins were extracted and analyzed using CZE and RP-HPLC techniques as three replicates. Material for protein extraction was obtained from flour that was obtained from milling a single grain. The LMW-GS fraction for the CZE analyses was extracted according to the method described by Salmanowicz et al. [19]. To carry out analyses with RP-HPLC, the LMW-GSs were extracted according to Salmanowicz [20] with some modifications [21]. Capillary electrophoretic separations of LMW-GSs were carried out on the P/ACE apparatus with the Beckman Coulter MDQ system. Silica capillaries with an internal diameter of 50 μm and a total length of 30.2 cm were used for the separation of proteins (the detector length was 21 cm). A Beckman Coulter absorbance UV detector was used for detection. For camera operation, parameter control, and initial analysis of results, the computer software GOLD System version 8.11 (Beckman Coulter) was used. The separation was run at a constant temperature of 38°C and 10 kV. The duration of the separation was 18 min. Detection of proteins occurred at a wavelength of 200 nm, in accordance with Di Luccia et al. [10]. Before each injection, the capillary was washed with 0.1 N hydrochloric acid (0.3 MPa for 4 min) and water (0.3 MPa for 1 min). The buffers and solutions used for the analyses were filtered through membranes and then sonicated. A buffer consisting of 20% acetonitrile (AcN), 0.2% polyvinylpyrrolidone (PVP-360), 0.05% hydroxypropyl methylcellulose, 0.05 M iminodiacetic acid, and lauryl sulfobetaine (SB-12) was used to fill the capillary. For the partition buffer, a mixture of 20% AcN, 0.15% poly(ethylene oxide), 0.05% IDA, and 26 mM SB-12 was used. Testing was carried out at the anode end of the capillary, for 3 s at 0.5 psi (3.447 × 10−3 MPa).
The chromatographic separation was carried out according to Li Vigni et al. [22] with some modifications [13]. Chromatographic separations of proteins isolated from glutenin extracts were carried out using a Beckman Coulter RP-HPLC apparatus equipped with two pumps (126 solvent module) and a UV spectral detector. For the separation of LMW-GSs, a chromatographic Phenomenex 250 C18 column (size 4.6 × 250 mm) was used. All solvents and reagents were filtered through a 0.5 µm Millipore (Bedford, MA, USA) membrane filter and sonicated before each analysis. A gradient of two solvents was used to separate the proteins on the chromatographic column: solvent (A) – ultrapure water with TFA (trifluoroacetic acid) (99.9/0.1%, v/v) and solvent (B) – ultrapure AcN by the addition of TFA (99.9/0.1%, v/v). Extracts were separated with increasing concentration of solvent B from 20 to 60% for 50 min and then to 80% for 5 min. Each time, prior to the analysis, the chromatography column was purged for 3 min under an increased flow of 80% AcN. Camera operation, parameter control, and initial analysis of the results were carried out using the GOLD Nouveau Chromatography Workstation version 1.7 software (Beckman Coulter).
2.4 Preparing the flour for rheological analysis
At the first step of the experiment, an initial assessment and determination of the basic physicochemical parameters of wheat grain were made using the standard near-infrared (NIR) technique [23]. The percentages of protein (%) and moisture (%) were determined for each wheat grain simple. About 300 g of grain samples was adjusted to 14% moisture content with water and stored for 72 h at 18oC prior to milling with a quad-roller mill (Quadrumat Junior). A drum sieve with a mesh size of 250 μm was used to separate the flour from the bran. The milling capacity was 300 g in 4 min, while the maximum extract was about 70%. The obtained flour was packed in paper bags, sealed, and stored for 14 days at 18oC. After that time, the percentages of protein and moisture of wheat flour were made using the standard NIR technique.
2.5 Rheological analysis
At the later step of the experiment, analyses were performed at the microscale using a TA.XT Plus Texture Analyzer. About 10 g flour and 2% brine were added into the mixer chamber. The required volume of brine for each sample was calculated by the Remix 32 program, by the following equation: water absorption (%, 14%mb) = protein (14%mb) × 1.5 × 43.6, based on previously determined protein content. The dough was prepared by mixing for 10 min. The dough obtained was formed into balls, covered tightly with foil, and rested in a heat chamber at 30°C for 30 min. After that time, the dough ball was placed in a mold with five grooves (53 mm × 5 mm × 3 mm). The mold was placed in a clamp, squeezed, excess dough was removed, and kept in a heat chamber for another 10 min. After that time, the formed dough strips were analyzed in extension at a crosshead speed of 3.3 mm s−1 and a trigger force of 5 g [24]. Parameters obtained from the Kieffer force–distance curves were maximum resistance (R max, in grams), maximum extensibility (L max, in millimeters), and area under the force versus distance curve (P max, in grams × millimeters). Each sample was analyzed in triplicate, of which the average was calculated. Due to the small sample amounts available, reference varieties were not used in the rheological analyses.
-
Ethical approval: The conducted research is not related to either human or animal use.
3 Results
3.1 Identification of HMW-GS
The use of the SDS-PAGE method allowed the identification of 10 HMW subunits that occurred in the plant material. Based on the analyses of the obtained images separated by electrophoresis of the subunits, eight HMW-GS schemas were distinguished. Figure 1 presents examples of electrophoretic images obtained for selected wheat. The electrophoretic mobility of individual subunits was referenced to the reference variety Tonacja containing Ax2*/Bx7+By9/Dx2+Dy12 subunits. HMW-GS patterns identified in the studied plant material are listed in Table 2. The HMW-GS scheme N/7+9/5+10 was the most frequent, occurring in 19 samples, constituting 27.14% of the analyzed plant material. Schemes 2*/7+9/5+10 and 1/6+8/5+10 were, jointly, the least common, with each occurring in only three samples (4.29%).
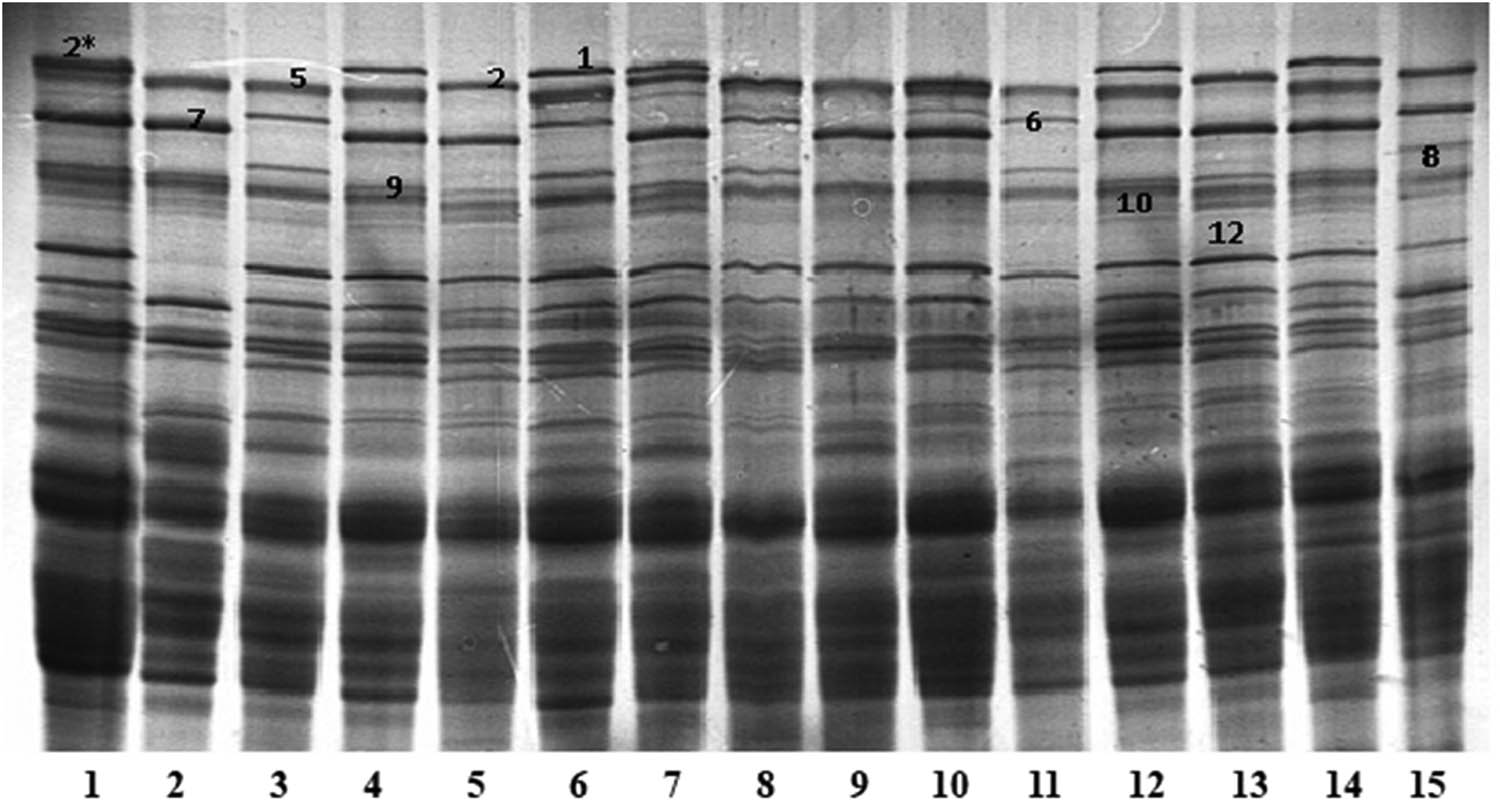
Electrophoretic images of dissociated HMW wheat glutenin subunits separated by SDS-PAGE. Lines: 1. Tonacja (2*/7+9/2+12), 2. Bamberka (N/7+9/5+10), 3. Ludwig (N/6+8/5+10), 4. Figura (1/7+9/5+10), 5. BZ 210801 (N/7+9/2+12), 6. Look (1/6+8/5+10), 7. Opus (1/7+9/2+12), 8. Batuta (N/6+8/5+10), 9. SZD 96 (N/7+9/5+10), 10. SMH 8063 (2*/7+9/5+10), 11. Discus (N/6+8/5+10), 12. Akteur (1/7+9/5+10), 13. Skagen (N/7+9/2+12), 14. KWS Ozon (1/7+9/5+10), 15. Bagou (N/6+8/2+12).
Percentage of HMW-GS patterns in 70 tested varieties and lines of wheat
| HMW-GS scheme | Number of varieties | Percentage | ||
|---|---|---|---|---|
| Glu-A1 | Glu-B1 | Glu-D1 | ||
| N | 7+9 | 5+10 | 19 | 27.14 |
| N | 7+9 | 2+12 | 11 | 15.71 |
| 1 | 7+9 | 5+10 | 9 | 12.86 |
| 1 | 7+9 | 2+12 | 4 | 5.71 |
| 2* | 7+9 | 5+10 | 3 | 4.29 |
| N | 6+8 | 5+10 | 11 | 15.71 |
| N | 6+8 | 2+12 | 10 | 14.29 |
| 1 | 6+8 | 5+10 | 3 | 4.29 |
3.2 Identification of LMW-GSs
Reference varieties of wheat representing the LMW subunit encoding alleles were used to determine the migration times for 52 major protein peaks via CZE analyses. The migration times of all protein peaks encoded by the Glu-A3, Glu-B3, and Glu-D3 loci that were identified in the reference material are listed in Table 3. These results served to identify the LMW-GS alleles in the tested varieties.
Migration time of protein peaks juxtaposed in blocks for the Glu-3 alleles determined based on CZE profiles of wheat reference varieties
| Genome A | Genome B | Genome D | |||||
|---|---|---|---|---|---|---|---|
| Allele | Migration time (min) | Allele | Migration time (min) | Allele | Migration time (min) | Allele | Migration time (min) |
| Glu-A3a | 9.10 | Glu-B3a | 10.50 | Glu-B3e | 9.70 | Glu-D3a | 9.71 |
| 12.14 | 10.70 | 10.45 | 13.38 | ||||
| 11.45 | 11.15 | Glu-D3b | 9.61 | ||||
| 11.78 | 11.60 | 13.57 | |||||
| Glu-A3b | 9.44 | Glu-B3b | 10.47 | Glu-B3f | 10.41 | Glu-D3c | 9.64 |
| 14.79 | 10.73 | 10.64 | 13.39 | ||||
| 11.39 | 11.36 | ||||||
| Glu-A3c | 14.53 | Glu-B3c | 10.22 | Glu-B3g | 10.28 | Glu-D3d | 9.40 |
| 10.49 | 11.23 | 13.50 | |||||
| 10.69 | 12.13 | ||||||
| 11.31 | Glu-B3h | 10.35 | Glu-D3e | 9.70 | |||
| Glu-A3d | 10.16 | Glu-B3d | 9.35 | 10.69 | 13.43 | ||
| 13.24 | 10.38 | 11.40 | |||||
| 14.85 | 11.33 | Glu-B3i | 9.93 | Glu-D3f | 9.85 | ||
| Glu-A3e | — | 11.65 | 10.37 | 13.87 | |||
| Glu-A3f | 12.83 | 11.33 | |||||
Figure 2 presents the identification of LMW-GS samples using capillary electrophoresis in reference wheat Gabo (Glu-A3b, Glu-B3b, Glu-D3b) and previously uncharacterized wheat variety Jantarka (Glu-A3f, Glu-B3b, Glu-D3a).
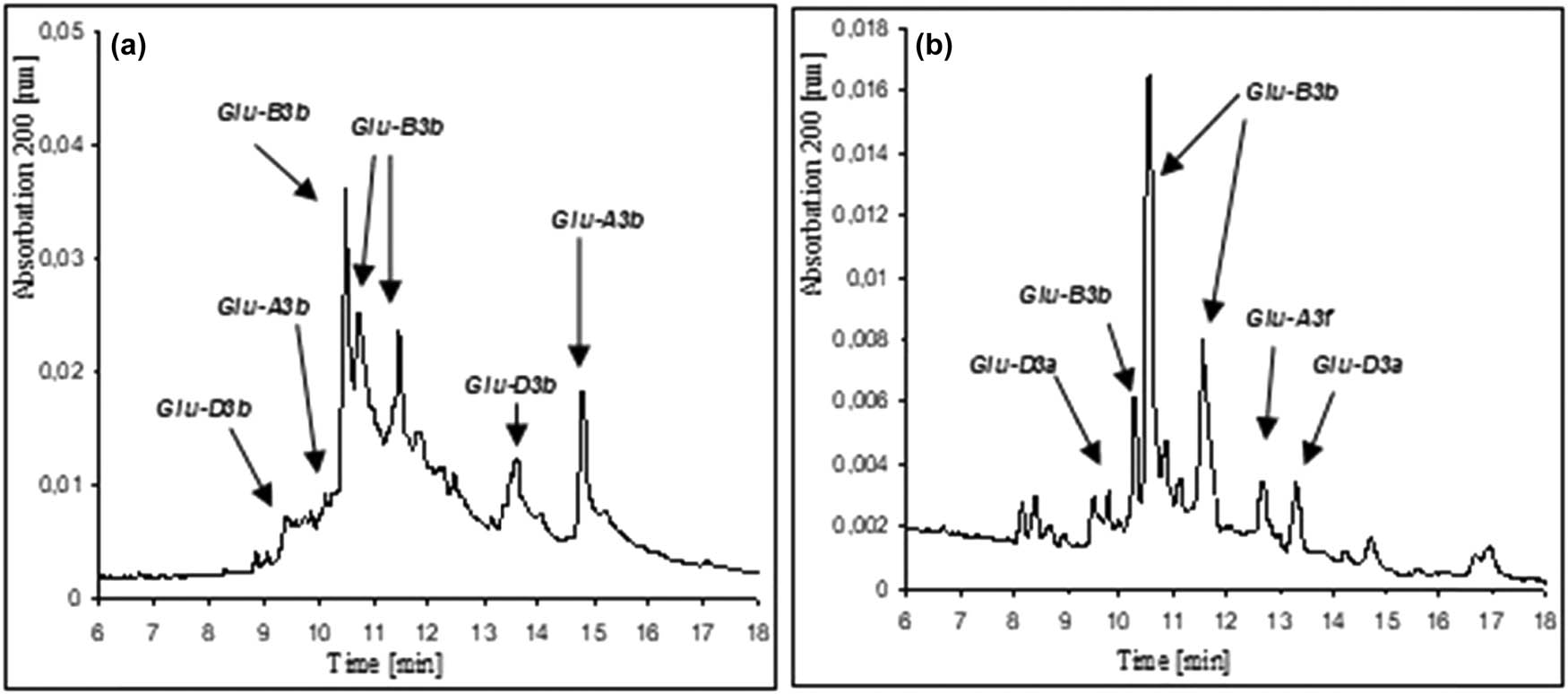
Identification of LMW-GSs using capillary electrophoresis in (a) Gabo and (b) Jantarka.
Sixteen allelic combinations of the Glu-3 loci, based on separation visualized on CZE electropherograms, were distinguished in the 70 tested kinds of wheat. The exact distribution of plant material according to HMW-GSs and LMW-GSs is presented in Appendix Table 1 (Table A1). Four alleles were detected for the Glu-A3 and Glu-D3 loci (Glu-A3b, Glu-A3d, Glu-A3e, Glu-A3f and Glu-D3a, Glu-D3b, Glu-D3c, Glu-D3e, respectively), whereas for the Glu-B3 locus, seven alleles were identified (Glu-B3a, Glu-B3b, Glu-B3c, Glu-B3d, Glu-B3e, Glu-B3h, and Glu-B3i). Across the studied plant material, the most common allele was Glu-D3c present in 52 samples, followed by Glu-A3e and Glu-A3f which were found in 33 samples, and Glu-B3b present in 27 samples. Infrequent variants included Glu-B3i present only in Bogatka, SZD 96, and SZD 205, and Glu-B3a was found in Banderola, Brilliant, Look, and Turkis; while the Glu-A3d allele was found only in Bystra, and the Glu-D3b allele only in line SZD 205.
Verification of the identified LMW-GS schemes in the studied plant material was achieved by chromatographic analyses. First, 22 reference varieties were scored against the LMW-GS fraction (Table 1). As a result, for the Glu-A3 locus (Glu-A3a-d and Glu-A3f), three to five protein peaks were observed on the chromatograms of the reference samples, which elute from 39.53 to 44.97 min. No peaks were observed for the Glu-A3e allele due to the lack of expression of this allele. For the reference varieties containing the Glu-B3h allele, four peaks were observed that eluted from 41.22 to 48.92 min. For the Glu-B3 locus (Glu-3a – Glu-B3g and Glu-B3i), five protein peaks were observed on the chromatograms, which eluted from 40.69 to 48.92 min. Sets of LMW-GS protein peaks encoded by the Glu-D3 locus alleles consisted of three peaks with retention times ranging from 40.51 to 50.46 min. The elution times of all protein peaks encoded by the Glu-A3, Glu-B3, and Glu-D3 loci that were identified in the reference material are listed in Table 4.
Elution time of protein peaks juxtaposed in blocks for the Glu-3 alleles determined based on RP-HPLC profiles of wheat reference varieties
| Genome A | Genome B | Genome D | |||||
|---|---|---|---|---|---|---|---|
| Allele | Elution time (min) | Allele | Elution time (min) | Allele | Elution time (min) | Allele | Elution time (min) |
| Glu-A3a | 40.55 | Glu-B3a | 41.57 | Glu-B3e | 41.77 | Glu-D3a | 43.07 |
| 41.56 | 42.60 | 42.15 | 46.55 | ||||
| 45.05 | 42.67 | 50.33 | |||||
| Glu-D3b | 42.85 | ||||||
| Glu-A3b | 39.53 | 48.27 | 45.00 | 46.64 | |||
| 43.28 | 48.92 | 48.05 | 50.27 | ||||
| Glu-B3b | 40.96 | Glu-B3f | 40.69 | Glu-D3c | 43.20 | ||
| 41.82 | 41.63 | 46.69 | |||||
| 42.35 | 42.63 | 50.29 | |||||
| 45.08 | 45.15 | ||||||
| Glu-A3c | 40.69 | 48.33 | 48.24 | ||||
| Glu-B3c | 41.72 | Glu-B3g | 41.37 | Glu-D3d | 41.92 | ||
| 42.57 | 41.95 | 46.62 | |||||
| Glu-A3d | 39.89 | 43.73 | 42.55 | 50.32 | |||
| 40.41 | 45.14 | 45.18 | |||||
| 43.16 | 48.22 | 48.05 | |||||
| Glu-B3h | 41.22 | Glu-D3e | 42.81 | ||||
| Glu-B3d | 40.88 | 42.27 | 46.58 | ||||
| 45.22 | 50.19 | ||||||
| Glu-A3e | — | 41.20 | 48.15 | ||||
| 41.63 | Glu-B3i | 41.44 | Glu-D3f | 40.51 | |||
| 45.20 | 42.27 | 46.75 | |||||
| Glu-A3f | 44.97 | 48.70 | 43.55 | 50.46 | |||
| 45.08 | |||||||
| 48.09 | |||||||
The use of RP-HPLC made it possible to identify the same amount of LMW-GSs, both in the reference material and in the studied plant material, as in the case of applied CE. As was the case for capillary electrophoresis, alleles of the Glu-3 loci constituting 16 allelic variants were found in the examined material.
3.3 Rheological analysis of wheat dough
The average values of dough parameters determined by the Kieffer method for allelic variations of the Glu-3 loci are presented in Table 6, and an example of a graph obtained using the Kieffer texture analyzer is shown in Figure 3.
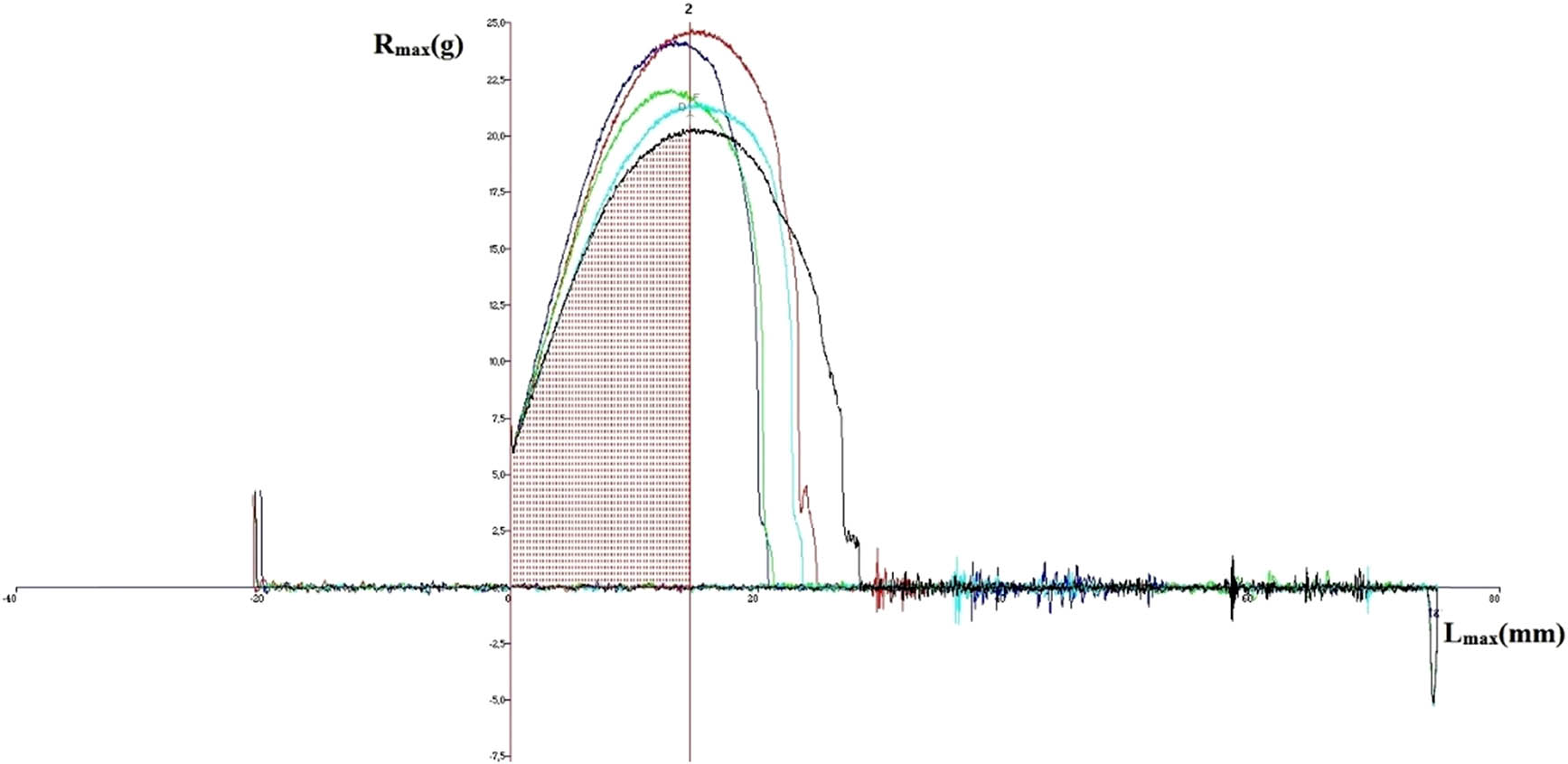
Graph obtained on the texture analyzer for variant containing Glu-A3f, Glu-B3b, and Glu-D3e (R max – resistance, L max – extension).
The results obtained were subjected to an analysis of variance to determine the effect of the LMW-GS variant on the rheological features of the dough (Table 5).
Analysis of variance for the qualitative determinants of the dough of the tested wheat varieties and breeding lines
| Parameter | Sources of variation | D. F. | Mean square | F statistics | F 0.05 | F 0.01 |
|---|---|---|---|---|---|---|
| R max | Variant LMW | 15 | 0.3313 | 2.747 | 1.72 | 2.13 |
| L max | Variant LMW | 15 | 0.7014 | 3.091 | 1.72 | 2.13 |
| P max | Variant LMW | 15 | 0.1308 | 3.765 | 1.72 | 2.13 |
Analyzing the tested samples in terms of average resistance values (R max), significant differences were found between the allelic variants of LMW-GSs (P < 0.01). With an average dough resistance value of R max = 25.89 g for the first group (number1 in Table 6) (variants: Glu-A3f, Glu-B3b, and Glu-D3e; Glu-A3f, Glu-B3e, and Glu-D3e; Glu-A3f, Glu-B3b, and Glu-D3a; Glu-A3f, Glu-B3c, and Glu-D3c; and Glu-A3e, Glu-B3h, and Glu-D3e) and an average resistance of R max = 34.71 g for the second group (number2 in Table 6). Similarly, two groups of allelic combinations were discriminated with respect to the mean values of elongation (L max). The first group (number3 in Table 6) with an average extension of 17.89 mm was wheat doughs characterized by the alleles: Glu-A3f, Glu-B3b, and Glu-D3e; Glu-A3d, Glu-B3b, and Glu-D3c; Glu-A3e, Glu-B3a, and Glu-D3c; Glu-A3f, Glu-B3c, and Glu-D3c; Glu-A3f, Glu-B3b, and Glu-D3a; Glu-A3e, Glu-B3d, and Glu-D3c; Glu-A3e, Glu-B3i, and Glu-D3c; and Glu-A3e, Glu-B3b, and Glu-D3c. The second group (number4 in Table 6) with an average extension of 21.71 mm was the remaining eight variants of the Glu-3 loci. In the case of surface area, P max significant differences were found between the allelic variants of LMW-GSs (P < 0.01). Two groups were distinguished, the first (number5 in Table 6) with P max = 335,86 g mm and variants: Glu-A3f, Glu-B3b, and Glu-D3e; Glu-A3f, Glu-B3b, and Glu-D3a; Glu-A3f, Glu-B3c, and Glu-D3c; Glu-A3d, Glu-B3b, and Glu-D3c; Glu-A3e, Glu-B3a, and Glu-D3c; and the second (number6 in Table 6) with an average P max = 505.92 g mm for the 11 remaining analyzed variants.
Average values of rheological parameters determined by the Kieffer method for allelic variations of the Glu-3 loci
| Glu-A3 | Glu-B3 | Glu-D3 | R max (g) | L max (mm) | P max (g mm) |
|---|---|---|---|---|---|
| b | h | c | 35.452 | 22.404 | 544.736 |
| d | b | c | 34.862 | 16.903 | 389.595 |
| e | a | c | 33.142 | 17.323 | 401.475 |
| e | b | c | 32.682 | 19.353 | 462.946 |
| e | d | c | 34.312 | 18.833 | 449.256 |
| e | h | c | 36.972 | 21.954 | 583.986 |
| e | h | e | 29.291 | 22.154 | 463.716 |
| e | i | a | 38.392 | 20.734 | 536.356 |
| e | i | b | 36.202 | 23.034 | 578.986 |
| e | i | c | 35.142 | 18.853 | 443.266 |
| f | b | a | 27.601 | 17.703 | 348.425 |
| f | b | e | 18.651 | 14.563 | 221.985 |
| f | c | c | 28.651 | 17.333 | 361.335 |
| f | d | c | 36.782 | 20.954 | 532.966 |
| f | e | c | 35.392 | 21.224 | 533.426 |
| f | e | e | 25.091 | 24.194 | 450.766 |
Note: In the table, 1–6 are groups of allelic combinations of LMW-GS which were discriminated with respect to mean values of resistance (R max), elongation (L max) and surface area (P max).
The smallest total mean resistance (R max = 18.65 g) came from a wheat dough with the allelic variant Glu-A3f, Glu-B3b, and Glu-D3e. In turn, the highest total average resistance, and thus the greatest resistance (R max = 38.39 g), was in a dough with an allelic variant of Glu-A3e, Glu-B3i, and Glu-D3a. The weakest dough in terms of extensibility (L max = 14.56 mm) was observed for allelic variants: Glu-A3f, Glu-B3b, and Glu-D3e. The most flexible and stretchable (L max = 24.19 mm) was from dough with the allele variant Glu-A3f, Glu-B3e, and Glu-D3e. Taking into account the area of P max, the smallest cumulative mean values (221.98 g mm) were for the Glu-A3f, Glu-B3b, and Glu-D3e variants, while the largest total mean values (583.98 g mm) were wheat doughs characterized by the allele variant Glu-A3e, Glu-B3a, and Glu-D3c.
4 Discussion
Literature reports show that the technological properties of wheat are largely determined by the composition and the amount of gluten, which includes glutenins and gliadins. A thorough understanding of the polymorphism of glutenin proteins makes it possible to determine the extent to which the rheological properties of wheat dough are determined by a complex of gluten proteins formed by both high- and low-molecular subunits [25,26]. HMW-GSs account for up to 10% of gluten proteins, and it has been shown that they determine up to 70% of the quality characteristics of wheat grain. Otherwise, the impact of LMW-GSs on rheological parameters is still poorly characterized. It seems that the specific rheological properties of wheat dough can also be significantly affected by LMW-GSs, which account for up to 50% of gluten and generate up to 30% variation in technological features of wheat [1,4].
Protein electrophoresis in polyacrylamide gel with the addition of sodium dodecyl sulfate (SDS-PAGE) is a commonly used method for assessing the variability of the qualitative composition of wheat storage proteins. The comparative analyses of protein profiles obtained on SDS-electropherograms allowed us to distinguish 11 HMW-GSs in the examined plant material, coded by the allelic variants of the genes in the Glu-1 locus: Glu-A1-1a (Ax1), Glu-A1-1b (Ax2*), Glu-A1-1c (Null variant), Glu-B1-1a (Bx7), Glu-B1-1d (Bx6), Glu-B1-2a (By8), Glu-B1-2d (By9), Glu-D1-1a (Dx5), Glu-D1-2a (Dx2), Glu-D1-1d (Dy5), and Glu-D1-2b (Dy12). Featured HMW subunits are usually identified in wheat technological studies [27,28].
In the presented work, the identification of the LMW-GS qualitative composition in the tested material was carried out using electrophoretic (CZE) and chromatographic (RP-HPLC) methods. In recent years, CZE has been used to perform qualitative and quantitative determinations of the majority of distinguished classes of wheat storage proteins [17,19,29]. The literature data show that LMW-GSs migrate in the silica capillaries in the time range similar to the HMW-GS fraction, which requires prior accurate separation of these fractions prior to conducting the separation [30]. Li et al. [11], based on identified protein peaks, distinguished two alleles at the Glu-A3 locus, four alleles at the Glu-B3 locus, and three alleles at the Glu-D3 locus. In the presented study, a wide range of migration times (9.10–14.85 min) was found for individual LMW subunits, which enabled their full identification in the tested genotypes. In contrast to the studies of Li et al. [11], we observed one to four protein peaks corresponding to LMW-GSs based on the CZE electropherograms with the exception of the Glu-A3e allele, which was not expressed. Multiple migration times of the individual LMW-GSs result from the presence of multiple genes at a particular locus [11].
At the same time, the RP-HPLC method was used to determine the qualitative composition of LMW-GSs in tested wheat varieties with the previously determined HMW-GS composition. LMW subunits have so far been characterized using this method by several research teams, but the subject of research has been a very small number of trials in individual studies [12,31]. For the separation of LMW subunits, researchers applied various fillings in the chromatographic columns, which makes it more difficult to compare the retention times of protein peaks on the presented chromatograms. The main disadvantage of this method when compared with free capillary electrophoresis is a long time of separation of individual samples (up to 60 min) and high costs of columns and solvents used for protein separation. The LMW subunit separation carried out as part of this study, refining the methodology and using the most modern columns, enabled full identification of all (from one to five) subunits encoded by particular Glu-3 loci. The reports presented previously revealed the presence of only a few subunits but confirm that multiple elution times of LMW-GS proteins are due to the presence of multiple genes at a particular locus [12,31]. In our study, full concordance was obtained in the number of subunits in separation performed using the CZE and RP-HPLC methods. In recent years, along with the refinement of the RP-HPLC method, i.e., the use of ultra-dry liquid chromatography (UPLC), the use of columns with smaller fillings has provided a comparable resolution of proteins for a number of chemical compounds with three times shorter subunit separation time. Yu et al. [13] using the UPLC method to separate the LMW subunits shortened the time of separation (up to 18 min) but did not obtain such a good resolution for individual subunits (only 1–2 subunits were distinguished) as in the case of the methodology used in the presented study.
To determine the performance traits of common wheat, technological research studies on grain or flour are carried out [32,33,34,35]. In recent years, the impact of LMW-GSs on rheological parameters has mainly been determined using a texture analyzer with the Kieffer method [36]. The rheological analyses carried out in this research confirmed the increase in dough resistance in the LMW-GS containing samples encoded by the Glu-A3d, Glu-B3d, Glu-B3h, Glu-B3i, Glu-D3a, and Glu-D3b alleles. The lowest dough resistance was linked with the presence of Glu-A3e and Glu-A3f alleles. The increase in dough resistance in wheat genotypes containing LMW-GSs encoded by the Glu-B3i allele has not previously been reported in the literature. Based on previous studies by the teams of Branlard et al. [37] and Eagles et al. [38], it can be concluded that the increased extensibility and elongation of dough also depends on the presence of LMW subunits coded at the Glu-A3 locus (Glu-A3a, Glu-A3d), the Glu-B3 locus (Glu-B3b and Glu-B3d), and the Glu-D3 locus (Glu-D3b, Glu-D3c). Rai et al. [32] showed that LMW-GSs coded at the Glu-A3 locus (Glu-A3b, Glu-A3c) and the Glu-B3 (Glu-B3b) locus are responsible for the low rheological values. In subsequent studies, the beneficial effects of Glu-A3d, Glu-B3d, Glu-B3b, Glu-B3f alleles, and Glu-D3c [39] on the elongation of dough were demonstrated. Maucher et al. [40] found that the improvement of the dough elongation parameter is influenced by the presence of the Glu-A3b, Glu-B3d, Glu-D3d allelic combination. Oury et al. [41] have shown that LMW-GSs encoded by the Glu-A3a, Glu-B3g, Glu-D3a, and Glu-D3b alleles increase the extensibility of the dough. Park’s team [42] demonstrated the positive effects of the Glu-A3b, Glu-A3d, Glu-B3b, Glu-B3d, GluD3b, and Glu-D3a alleles on the dough extensibility. The rheological data obtained in this study indicated that particularly beneficial effects on the extensionality of the dough came from the Glu-B3b, Glu-D3b, Glu-D3c alleles, which confirms the results obtained by other researchers [38,39,41]. The presence of the Glu-A3b, Glu-A3f, Glu-B3h, and Glu-B3e alleles in samples also had a significant influence on the shaping of this parameter. In-depth studies on the area under the force versus distance curve (P max) were conducted by Maucher’s team [40]. They arranged the LMW-GS coding alleles favorably affecting this parameter in the following order: Glu-A3d > Glu-A3c > Glu-A3b > Glu-A3e at the Glu-A3 locus; Glu-B3d > Glu-B3g > Glu-B3h > Glu-B3f > Glu-B3i at the Glu-B3 locus and Glu-D3d > Glu-D3b > Glu-D3a > Glu-D3c at the Glu-D3 locus. Oury et al. [41] observed an increase in tear resistance in LMW-GS-tested samples encoded by the Glu-A3d, Glu-B3b’, Glu-B3g and Glu-D3b alleles. In a recent study, Zhang’s team [43] ranked the Glu-3 loci alleles contribution to the increase in tear dough resistance within the Glu-A3 locus as Glu-A3c > Glu-A3d > Glu-A3f > Glu-A3b > Glu-A3e; within the Glu-B3 locus as Glu-B3i > Glu-B3b = Glu-B3a > Glu-B3f = Glu-B3g > Glu-B3h > Glu-B3c > Glu-B3d; and within the Glu-D3 locus as GluD3a = Glu-D3b = Glu-D3c > Glu-D3d > Glu-D3f. Based on comparative analyses carried out in this work, it was shown that the presence of Glu-A3f, Glu-A3e, Glu-B3a, Glu-B3e, Glu-B3h, Glu-D3a, Glu-D3b, and Glu-D3c alleles in the tested samples has a significant effect on increasing the breaking strength of the dough. The data obtained on the beneficial effects of LMW-GSs encoded by the Glu-B3h, Glu-D3a and Glu-D3b alleles on breaking strength are in agreement with the literature data [40,43]. In the case of the remaining alleles of Glu-3 loci in the tested samples, the presence of which significantly enhanced the tear strength of the dough, discrepancies were found with the literature data, which may be due to the smaller number of samples tested by other authors.
5 Conclusion
The use of modern analytical methods such as capillary electrophoresis and RP-HPLC enabled the full identification of LMW-GSs encoded by the Glu-3 loci alleles. Our research clearly showed that LMW-GSs play an important role in creating the rheological quality of wheat. Obtained results enabled the selection of wheat varieties containing the Glu-3 loci scheme (Glu-A3b, Glu-A3f at the Glu-A3 locus; Glu-B3a, Glu-B3b, Glu-B3d, Glu-B3h at the Glu-B3 locus; Glu-D3a, Glu-D3c at the Glu-D3 locus) determining the most beneficial quality parameters, namely, Operetka, Smaragd, SMH 90, Ludwig, Brilliant, Natula, Akteur, and Bamberka. These varieties may be used in wheat breeding for crossing and developing new plants with favorable technological parameters. This research can be integrated with a molecular marker approach in an expansion of the knowledge about the genetic background of wheat quality giving an effective marker-assisted selection in the future.
Acknowledgments
The authors thank the Plant Breeding Smolice, which raised and collected the basic research material in the form of 70 wheat varieties and lines, and the Australia Winter Cereals Collection for providing 22 reference wheat varieties.
-
Funding information: The authors state no funding involved.
-
Author contributions: Conceptualization, S.F. and B.S.; methodology, B.S. and S.F.; validation, B.S.; formal analysis, S.F.; investigation, S.F.; resources, B.S.; data curation, S.F.; writing – original draft preparation, S.F.; writing – review and editing, S.F. and B. S.; supervision, B.S.; project administration, B.S.; funding acquisition, B.S. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Appendix
List of wheat genotypes according to HMW-GS and LMW-GS schemes
| Sample number | Genotypes | HMW-GS scheme | LMW-GS scheme | ||
|---|---|---|---|---|---|
| Glu-A3 | Glu-B3 | Glu-D3 | |||
| 1 | AREZZO | N/7+9/5+10 | f | e | c |
| 2 | BALETKA | e | h | c | |
| 3 | BAMBERKA | e | d | c | |
| 4 | BOCKRIS | e | b | c | |
| 5 | BOGATKA | e | i | c | |
| 6 | BRILLIANT | e | a | c | |
| 7 | CUBUS | e | b | c | |
| 8 | DOROTA | f | d | c | |
| 9 | KOHELIA | e | h | e | |
| 10 | KRANICH | f | e | c | |
| 11 | PAMIER | f | c | c | |
| 12 | SKAGEN | f | e | c | |
| 13 | SMARAGD | f | d | c | |
| 14 | SZD 205 | e | i | b | |
| 15 | SZD 87 | e | d | c | |
| 16 | SZD 96 | e | i | a | |
| 17 | TORAS | f | e | c | |
| 18 | TORRILD | f | e | c | |
| 19 | TURKIS | e | a | c | |
| 20 | ANTHUS | N/7+9/2+12 | f | e | e |
| 21 | BB 742206 DH | e | b | c | |
| 22 | BZ 210801 | f | c | c | |
| 23 | FIDELIUS | e | b | c | |
| 24 | GARANTUS | e | d | c | |
| 25 | KREDO | e | b | c | |
| 26 | POB 779 05 | e | h | e | |
| 27 | RUMBA | e | h | c | |
| 28 | RYSA | e | h | c | |
| 29 | SMH 8134 | e | h | c | |
| 30 | VISCOUNT | e | b | c | |
| 31 | ATTLAS | N/6+8/5+10 | f | b | a |
| 32 | BUTEO | f | c | c | |
| 33 | BYSTRA | d | b | c | |
| 34 | DISCUS | f | b | e | |
| 35 | GALVANO | b | h | c | |
| 36 | GECKO | f | b | a | |
| 37 | JANTARKA | f | b | a | |
| 38 | LP 227 1 03 | f | b | a | |
| 39 | LUDWIG | b | h | c | |
| 40 | PREMIO | f | c | c | |
| 41 | ACONEL | N/6+8/2+12 | e | b | c |
| 42 | ADONIS | f | e | c | |
| 43 | AND 3509 | e | b | c | |
| 44 | AUGUSTUS | f | e | c | |
| 45 | BAGOU | f | b | e | |
| 46 | BISCAY | f | b | e | |
| 47 | CENTENAIR | e | b | c | |
| 48 | HENRIK | f | b | a | |
| 49 | KATART | f | b | e | |
| 50 | MULAN | e | d | c | |
| 51 | AKTEUR | 1/7+9/5+10 | e | h | c |
| 52 | ARISTOS | e | b | c | |
| 53 | BANDEROLA | e | a | c | |
| 54 | FIGURA | f | e | c | |
| 55 | KWS OZON | e | b | c | |
| 56 | NATULA | e | h | c | |
| 57 | QUEBON | f | e | c | |
| 58 | SMH 92 | e | b | c | |
| 59 | STETANUS | e | b | c | |
| 60 | KEPLER | 1/7+9/2+12 | f | b | e |
| 61 | OPERETKA | f | d | c | |
| 62 | OPUS | f | b | a | |
| 63 | TUAREG | f | b | a | |
| 64 | LOOK | 1/6+8/5+10 | e | a | c |
| 65 | POTENTIAL | f | e | c | |
| 66 | SZD 11 | e | d | c | |
| 67 | SMH 8063 | 2*/7+9/5+10 | f | c | c |
| 68 | SMH 90 | b | h | c | |
| 69 | SMUGA | 2*/7+9/2+12 | f | c | c |
| 70 | GLOBAL | 2*/6+8/5+10 | f | b | a |
References
[1] Rasheed A, Xia X, Yan Y, Appels R, Mahmood T, He Z. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J Cereal Sci. 2014;60(1):11–24. 10.1016/J.JCS.2014.01.020.Search in Google Scholar
[2] Podolska G, Aleksandrowicz E, Szafrańska A. Bread making potential of Triticum aestivum and Triticum spelta species. Open Life Sci. 2020;15(1):30–40. 10.1515/biol-2020-0004.Search in Google Scholar
[3] D’Ovidio R, Masci S. The low-molecular-weight glutenin subunits of wheat gluten. J Cereal Sci. 2004;39(3):321–39. 10.1016/j.jcs.2003.12.002.Search in Google Scholar
[4] Gale KR. Diagnostic DNA markers for quality traits in wheat. J Cereal Sci. 2005;41(2):181–92. 10.1016/J.JCS.2004.09.002.Search in Google Scholar
[5] Li Y, Zhou R, Branlard G, Jia J. Development of introgression lines with 18 alleles of glutenin subunits and evaluation of the effects of various alleles on quality related traits in wheat (Triticum aestivum L.). J Cereal Sci. 2010;51(1):127–33. 10.1016/j.jcs.2009.10.008.Search in Google Scholar
[6] Jin H, Zhang Y, Li G, Mu P, Fan Z, Xia X, et al. Effects of allelic variation of HMW-GS and LMW-GS on mixograph properties and Chinese noodle and steamed bread qualities in a set of Aroona near-isogenic wheat lines. J Cereal Sci. 2013;57(1):146–52. 10.1016/J.JCS.2012.10.011.Search in Google Scholar
[7] Liang D, Tang J, Peña RJ, Singh R, He X, Shen X, et al. Characterization of CIMMYT bread wheats for high- and low-molecular weight glutenin subunits and other quality-related genes with SDS-PAGE, RP-HPLC and molecular markers. Euphytica. 2010;172(2):235–50. 10.1007/s10681-009-0054-x.Search in Google Scholar
[8] Barak S, Mudgil D, Khatkar BS. Relationship of gliadin and glutenin proteins with dough rheology, flour pasting and bread making performance of wheat varieties. LWT Food Sci Technol. 2013;51(1):211–7. 10.1016/j.lwt.2012.09.011.Search in Google Scholar
[9] Hernández ZJE, Figueroa JDC, Rayas-Duarte P, Martínez-Flores HE, Arámbula GV, Luna GB, et al. Influence of high and low molecular weight glutenins on stress relaxation of wheat kernels and the relation to sedimentation and rheological properties. J Cereal Sci. 2012;55(3):344–50. 10.1016/J.JCS.2012.01.009.Search in Google Scholar
[10] Di Luccia A, Lamacchia C, Mamone G, Picariello G, Trani A, Masi P, et al. Application of capillary electrophoresis to determine the technological properties of wheat flours by a glutenin index. J Food Sci. 2009;74(4):C307–11. 10.1111/j.1750-3841.2009.01117.x.Search in Google Scholar PubMed
[11] Li J, Wang S, Yu Z, Li X, Guo G, Feng S, et al. Optimization and development of capillary electrophoresis for separating and identifying wheat low molecular weight glutenin subunits. J Cereal Sci. 2012;55:254–6. 10.1016/j.jcs.2011.12.005.Search in Google Scholar
[12] Liu W, Zhang Y, Gao X, Wang K, Wang S, Zhang Y, et al. Comparative proteome analysis of glutenin synthesis and accumulation in developing grains between superior and poor quality bread wheat cultivars. J Sci Food Agric. 2012;92(1):106–15. 10.1002/jsfa.4548.Search in Google Scholar PubMed
[13] Yu Z, Han C, Yan X, Li X, Jiang G, Yan Y. Rapid characterization of wheat low molecular weight glutenin subunits by ultraperformance liquid chromatography (UPLC). J Agric Food Chem. 2013;61(17):4026–34. 10.1021/jf400472s.Search in Google Scholar PubMed
[14] Dangi P, Khatkar BS. Extraction and purification of low molecular weight glutenin subunits using size exclusion chromatography. J Food Sci Technol. 2019;56(2):951–6. 10.1007/s13197-018-03560-1.Search in Google Scholar PubMed PubMed Central
[15] Gupta RB, Shepherd KW. Two-step one-dimensional SDS-PAGE analysis of LMW subunits of glutelin – 1. Variation and genetic control of the subunits in hexaploid wheats. Theor Appl Genet. 1990;80(1):65–74. 10.1007/BF00224017.Search in Google Scholar PubMed
[16] Tohver M. High molecular weight (HMW) glutenin subunit composition of some Nordic and Middle European wheats. Genet Resour Crop Evolution. 2007;54(1):67–81. 10.1007/s10722-005-1885-5.Search in Google Scholar
[17] Salmanowicz BP. Detection of high molecular weight glutenin subunits in triticale (×Triticosecale Wittm.) cultivars by capillary zone electrophoresis. J Agric Food Chem. 2008;56(20):9355–61. 10.1021/jf8016546.Search in Google Scholar PubMed
[18] Dai S, Xu D, Yan Y, Wen Z, Zhang J, Chen H, et al. Characterization of high- and low-molecular-weight glutenin subunits from Chinese Xinjiang wheat landraces and historical varieties. J Food Sci Technol. 2020;57(10):3823–35. 10.1007/s13197-020-04414-5.Search in Google Scholar PubMed PubMed Central
[19] Salmanowicz BP, Langner M, Mrugalsk B, Ratajczak D, Górny AG. Grain quality characteristics and dough rheological properties in Langdon durum-wild emmer wheat chromosome substitution lines under nitrogen and water deficits. J Sci Food Agric. 2016;97(7):2030–41. 10.1002/jsfa.8006.Search in Google Scholar PubMed
[20] Salmanowicz BP. Primary structure and polymorphism of 2S albumins from seeds of Andean lupin (Lupinus mutabilis Sweet). Eur Food Res Technol. 1999;209(6):416–22. 10.1007/s002170050519.Search in Google Scholar
[21] Langner M, Franaszek S, Salmanowicz B. Detection of LMW glutenin genes of the Glu-3 locus in some polish wheat cultivars by capillary electrophoresis and RP-HPLC. 10th Symposium on High-Performance Separation Methods. Siofok: Hungarian Society for Separation Sciences; 2015. p. 98.Search in Google Scholar
[22] Li Vigni M, Baschieri C, Marchetti A, Cocchi M. RP-HPLC and chemometrics for wheat flour protein characterisation in an industrial bread-making process monitoring context. Food Chem. 2013;139(1–4):553–62. 10.1016/j.foodchem.2013.01.085.Search in Google Scholar
[23] Demichelis M, Vanzetti LS, Crescente JM, Nisi MM, Pflüger L, Bainotti CT, et al. Significant effects in bread-making quality associated with the gene cluster glu-D3/Gli-D1 from the bread wheat cultivar prointa Guazú. Cereal Res Commun. 2019;47(1):111–22. 10.1556/0806.46.2018.055.Search in Google Scholar
[24] Dobraszczyk BJ, Salmanowicz BP, Ługowska B, Chełkowski J. Rapid quality assessment of wheat cultivars registered in Poland using the 2-g mixograph and multivariate statistical analysis. Cereal Chem. 2005;82(2):182–6. 10.1094/CC-82-0182.Search in Google Scholar
[25] León E, Marín S, Giménez MJ, Piston F, Rodríguez-Quijano M, Shewry PR, et al. Mixing properties and dough functionality of transgenic lines of a commercial wheat cultivar expressing the 1Ax1, 1Dx5 and 1Dy10 HMW glutenin subunit genes. J Cereal Sci. 2009;49(1):148–56. 10.1016/J.JCS.2008.08.002.Search in Google Scholar
[26] Yang FP, Wang LH, Wang JW, He XY, Zhang XK, Shang XW, et al. Characterisation of high- and low-molecular-weight glutenin subunit genes in Chinese winter wheat cultivars and advanced lines using allele-specific markers and SDS-PAGE. Crop Pasture Sci. 2009;61(1):84–91. 10.1071/CP09164.Search in Google Scholar
[27] Gianibelli MC, Gupta RB, Lafiandra D, Margiotta B, MacRitchie F. Polymorphism of high Mr glutenin subunits in triticum tauschii: characterisation by chromatography and electrophoretic methods. J Cereal Sci. 2001;33(1):39–52. 10.1006/jcrs.2000.0328.Search in Google Scholar
[28] Shewry PR, Popineau Y, Lafiandra D, Belton P. Wheat glutenin subunits and dough elasticity: findings of the EUROWHEAT project. Trends Food Sci Technol. 2000;11(12):433–41. 10.1016/S0924-2244(01)00035-8.Search in Google Scholar
[29] Salmanowicz BP, Langner M, Franaszek S. Charge-based characterisation of high-molecular-weight glutenin subunits from common wheat by capillary isoelectric focusing. Talanta. 2014;129:9–14. 10.1016/j.talanta.2014.04.055.Search in Google Scholar PubMed
[30] Herrero M, García-Cañas V, Simo C, Cifuentes A. Recent advances in the application of capillary electromigration methods for food analysis and foodomics. Electrophoresis. 2010;31(1):205–28. 10.1002/elps.200900365.Search in Google Scholar PubMed
[31] Peña E, Bernardo A, Soler C, Jouve N. Relationship between common wheat (Triticum aestivum L.) gluten proteins and dough rheological properties: gluten proteins and rheological properties in wheat. Euphytica. 2005;143(1–2):169–77. 10.1007/s10681-005-3157-z.Search in Google Scholar
[32] Rai A, Singh AM, Ganjewala D, Kumar RR, Ahlawat AK, Singh SK, et al. Rheological evaluations and molecular marker analysis of cultivated bread wheat varieties of India. J Food Sci Technol. 2019;56(4):1696–707. 10.1007/s13197-019-03593-0.Search in Google Scholar PubMed PubMed Central
[33] Dangi P, Chaudhary N, Khatkar BS. Rheological and microstructural characteristics of low molecular weight glutenin subunits of commercial wheats. Food Chem. 2019;297:124989. 10.1016/j.foodchem.2019.124989.Search in Google Scholar PubMed
[34] Bonilla JC, Erturk MY, Kokini JL. Understanding the role of gluten subunits (LMW, HMW glutenins and gliadin) in the networking behavior of a weak soft wheat dough and a strong semolina wheat flour dough and the relationship with linear and non-linear rheology. Food Hydrocoll. 2020;108:106002. 10.1016/j.foodhyd.2020.106002.Search in Google Scholar
[35] Du X, Wei J, Luo X, Liu Z, Qian Y, Zhu B, et al. Low-molecular-weight glutenin subunit LMW-N13 improves dough quality of transgenic wheat. Food Chem. 2020;327:127048. 10.1016/j.foodchem.2020.127048.Search in Google Scholar PubMed
[36] Langner M, Krystkowiak K, Salmanowicz BP, Adamski T, Krajewski P, Kaczmarek Z, et al. The influence of Glu-1 and Glu-3 loci on dough rheology and bread-making properties in wheat (Triticum aestivum L.) doubled haploid lines. J Sci Food Agric. 2017;97(15):5083–91. 10.1002/jsfa.8385.Search in Google Scholar PubMed
[37] Branlard G, Dardevet M, Saccomano R, Lagoutte F, Gourdon J. Genetic diversity of wheat storage proteins and bread wheat quality. Euphytica. 2001;119(1–2):59–67. 10.1023/A:1017586220359.Search in Google Scholar
[38] Eagles HA, Eastwood RF, Hollamby GJ, Martin EM, Cornish GB. Revision of the estimates of glutenin gene effects at the Glu-B1 locus from southern Australian wheat breeding programs. Austr J Agric Res. 2004;55(10):1093–6. 10.1071/AR04113.Search in Google Scholar
[39] Ma W, Appels R, Bekes F, Larroque O, Morell MK, Gale KR. Genetic characterisation of dough rheological properties in a wheat doubled haploid population: additive genetic effects and epistatic interactions. Theor Appl Genet. 2005;111(3):410–22. 10.1007/s00122-005-2001-0.Search in Google Scholar PubMed
[40] Maucher T, Figueroa JDC, Reule W, Peņa RJ. Influence of low molecular weight glutenins on viscoelastic properties of intact wheat kernels and their relation to functional properties of wheat dough. Cereal Chem. 2009;86(4):372–5. 10.1094/CCHEM-86-4-0372.Search in Google Scholar
[41] Oury FX, Chiron H, Faye A, Gardet O, Giraud A, Heumez E, et al. The prediction of bread wheat quality: Joint use of the phenotypic information brought by technological tests and the genetic information brought by HMW and LMW glutenin subunits. Euphytica. 2009;171(1):87–109. 10.1007/s10681-009-9997-1.Search in Google Scholar
[42] Park CS, Kang CS, Jeung JU, Woo SH. Influence of allelic variations in glutenin on the quality of pan bread and white salted noodles made from Korean wheat cultivars. Euphytica. 2011;180(2):235–50. 10.1007/s10681-011-0385-2.Search in Google Scholar
[43] Zhang X, Jin H, Zhang Y, Liu D, Li G, Xia X, et al. Composition and functional analysis of low-molecular-weight glutenin alleles with Aroona near-isogenic lines of bread wheat. BMC Plant Biol. 2012;12:243. 10.1186/1471-2229-12-243.Search in Google Scholar PubMed PubMed Central
© 2021 Sławomir Franaszek and Bolesław Salmanowicz, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

