Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
-
Mei Zhang
Abstract
Background
Hyperhomocysteinemia (HHcy) plays an important role in the progression of many kidney diseases; however, the relationship between HHcy and ischemia-reperfusion injury (IRI)-induced acute kidney injury (IRI-induced AKI) is far from clear. In this study, we try to investigate the effect and possible mechanisms of HHcy on IRI-induced AKI.
Methods
Twenty C57/BL6 mice were reared with a regular diet or high methionine diet for 2 weeks (to generate HHcy mice); after that, mice were subgrouped to receive sham operation or ischemia-reperfusion surgery. Twenty four hour after reperfusion, serum creatinine, blood urea nitrogen, and Malondialdehyde (MDA) were measured. H&E staining for tubular injury, western blot for γH2AX, JNK, p-JNK, and cleaved caspase 3, and TUNEL assay for tubular cell apoptosis were also performed.
Results
Our results showed that HHcy did not influence the renal function and histological structure, as well as the levels of MDA, γH2AX, JNK, p-JNK, and tubular cell apoptosis in control mice. However, in IRI-induced AKI mice, HHcy caused severer renal dysfunction and tubular injury, higher levels of oxidative stress, DNA damage, JNK pathway activation, and tubular cell apoptosis.
Conclusion
Our results demonstrated that HHcy could exacerbate IRI-induced AKI, which may be achieved through promoting oxidative stress, DNA damage, JNK pathway activation, and consequent apoptosis.
1 Introduction
Acute kidney injury (AKI) is a multiphasic clinical syndrome characterized by a rapid decline in renal function. Many factors such as ischemia/reperfusion [1], sepsis [2], trauma [3], and contrast [4] can induce the development of AKI. AKI is a common problem affecting hospitalized patients, with 25–40% mortality rates in severe cases [5]. A multicenter retrospective cohort study of 659,945 hospitalized adults from a wide range of clinical settings in nine regional central hospitals across China has reported that the incidence of community-acquired AKI and hospital-acquired AKI was 2.5 and 9.1%, respectively, giving rise to an overall incidence of 11.6% [6]. AKI is associated with poor clinical outcomes and long-term health and economic consequences; therefore, research about AKI has always been one of the focuses in kidney disease.
Homocysteine (Hcy) is an intermediate product of methionine metabolism. Hyperhomocysteinemia (HHcy), defined as blood Hcy concentration >15 µmol/L, is mainly developed by dysfunction of enzymes and cofactors associated with the biosynthesis and metabolism of Hcy. Other factors such as excessive methionine intake and certain diseases can also induce the development of HHcy [7]. Evidence has demonstrated that HHcy not only has a close relationship with the development of atherosclerosis, congestive heart failure, age-related macular degeneration, Alzheimer’s disease, and cancers, but also plays important roles in the progression of many kidney diseases such as chronic kidney disease (CKD) and diabetic nephropathy (DN) [7]. For instance, in a clinical study performed by Kong et al., the authors found HHcy increases CKD risk in a middle-aged and elderly Chinese population [8]. Xu et al. found serum Hcy was significantly higher in DN patients than simple diabetic patients and concluded that serum Hcy might serve as a biomarker for DN progression [9].
Except for CKD and DN, recent evidence also indicated that HHcy has a close relationship with AKI. For instance, Prathapasinghe and colleagues found that Hcy levels were significantly elevated after ischemia-reperfusion and neutralization of Hcy with anti-Hcy antibodies not only abolished ischemia-reperfusion-induced oxidative stress and cell death, but also transiently restored renal function [10]. In our previous studies, we have demonstrated that HHcy can exacerbate Cisplatin-induced AKI [11] and accelerate AKI to CKD progression by downregulating heme oxygenase-1 expression [12]; however, the relationship between HHcy and AKI, especially ischemia-reperfusion injury (IRI)-induced AKI, is far from clear.
In the present study, we used a high methionine diet (containing 2% methionine) to feed mice for 2 weeks to generate HHcy mice. After that, the IRI-induced AKI model was established to determine whether preexisted HHcy condition can exacerbate IRI-induced AKI through mediating oxidative stress, DNA damage, c-Jun N-terminal kinase (JNK) pathway, and apoptosis.
2 Materials and methods
2.1 Animals experiment
Twenty 6–7 weeks male C57BL/6 mice weighing 18.0–19.2 g were purchased from Liaoning Changsheng Biotechnology Co., Ltd (Benxi, Liaoning, China). Mice were bred and maintained in the Guizhou Medical University (Guiyang, Guizhou, China). All mice were reared under the temperature of 22 ± 2°C with a humidity of 55 ± 2% and a 12/12 h light cycle. After 1 week of habituation, all mice were randomly divided into two groups: the control diet group (n = 10) and the high methionine diet group (H-Met diet, n = 10). Diets were provided ad libitum. Two weeks after grouping, blood was collected through the caudal vein, and serum Hcy level was measured by a Hcy Assay Kit (Ausa, Shenzhen, China) and point-of-care testing device provided by the Shenzhen AoSA Company (Shenzhen, China). After that, the animals of each group were randomly divided into two groups again, including the control group and IRI group (n = 5). Mice in the IRI group were anesthetized with 1.5% pentobarbital sodium (45 mg/kg, i.p.) and placed on a homeothermic station to maintain body temperature at 37.5°C. The kidneys were exposed through bilateral incision and the renal pedicles were clamped for 30 min; the clamps were then released for reperfusion. After surgery, one milliliter of warm saline (37.5°C) was intraperitoneally injected for the purpose of volume supplement. Identical procedures except for clamping of the renal pedicle were done in the mice of the control group. Twenty four hour after reperfusion, blood samples were collected from the eyeball to test serum creatinine and blood urea nitrogen (BUN). Kidney tissues were fixed in 10% neutral-buffered formalin or snap-frozen for later use.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals and was approved by the ethics committee of Guizhou Provincial People’s Hospital (ethics approval number: 2017057).
2.2 Evaluation of renal function
Serum creatinine and BUN, determined by creatinine and BUN assay kits purchased from the Bioassay system (USA), were used according to the manufacturer’s instructions to evaluate the renal function of animals.
2.3 Measurement of lipid peroxidation
Snap-frozen tissues were used to determine the lipid peroxidation levels in the kidney by measuring malondialdehyde (MDA). Briefly, 1 mL of kidney homogenate was mixed with 2 mL of trichloroacetic acid–thiobarbituric acid−HCl reagent (15% trichloroacetic acid, 0.67% thiobarbituric acid, and 0.25 N HCl) and boiled at 100°C for 15 min. After cooling, the mixture was centrifuged at 3,000 rpm for 10 min. The supernatant was collected, and the absorbance was measured at 535 nm wavelength. MDA concentration was calculated using a molar absorption coefficient of 1.56 × 105/M cm and expressed as nmol/mg protein [13].
2.4 Histopathological examination of renal tissue
4 μm paraffin-embedded sections were subjected to routine H&E staining for assessment of the histopathological changes of the kidney. The degree of tubular injury was scored according to the previously described method [11]. Briefly, under the light microscope (Leica, Wetzlar, Germany), at least ten fields in the cortex for each mouse were randomly selected to count the number of injured renal tubular including dilation, necrosis, and tubular formation by trained personnel who was blinded to the interventions. After then, a score was given to each mouse based on the percentage of damaged renal tubules to the total renal tubules: 0, less than 5%; 1, 5–25%; 2, 25–50%; 3, 50–75%; 4, over 75%.
2.5 Western blot analysis
Frozen renal cortex was lysed in the cell lysis buffer containing I and II inhibitor cocktails (Sigma, MO) for 20 min on ice. Samples were centrifuged twice, and the supernatants were obtained to measure the total protein concentration by Bradford’s method. The supernatant was then heated to 100°C with loading buffer for 5 min and separated on 8–15% SDS-PAGE gels and transferred onto PVDF membranes (Millipore, USA) following standard protocol. The PVDF membranes were incubated with primary antibodies against cleaved caspase-3 (1:1,000, Cell Signaling Technology, UK), Phospho-JNK (Thr183/Tyr185) (1:1,000, Cell Signaling Technology, UK), JNK (1:1,000, Cell Signaling Technology, UK), γH2AX (1:1,000, Abcam, USA), and GAPDH (1:1,000, Cell Signaling Technology, UK) overnight at 4°C. The membranes were washed by TBST buffer and incubated with secondary antibody for 1 h at room temperature. Target proteins were then visualized using an ECL Plus kit (Amersham, IL, USA) and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.6 Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay
The paraffin-embedded kidney sections were exposed to the TUNEL reaction mixture (in situ cell death detection kit, POD) according to the manufacturer’s instructions (Roche Diagnostics, Basel, Switzerland) to detect the level of renal tubular epithelial cell apoptosis. The number of apoptotic cells in 10 fields per section and five sections per kidney was counted by identifying cells with TUNEL-positive nuclei under fluorescence microscopy.
2.7 Statistical analysis
Statistical analysis was performed using SPSS19.0 (SPSS, Inc., IL, USA). Data were expressed as mean ± SD and analyzed with independent samples t-test or one-way ANOVA. P < 0.05 was considered statistically significant.
3 Results
3.1 HHcy exacerbates IRI-induced renal dysfunction and oxidative stress
After 2 weeks of diet treatment, the level of serum Hcy in the H-Met diet group was significantly higher than that of the regular diet group (35.01 ± 7.41 vs 9.15 ± 0.68, P < 0.05). Twenty four hour after reperfusion, serum creatinine and BUN levels were significantly higher in IRI mice than that of the control mice under both regular diet and H-Met diet pretreated conditions. Notably, compared with regular diet pretreatment, the H-Met diet didn’t change serum creatinine and BUN levels in control mice, but significantly increased both of them in IRI mice (Figure 1a and b).
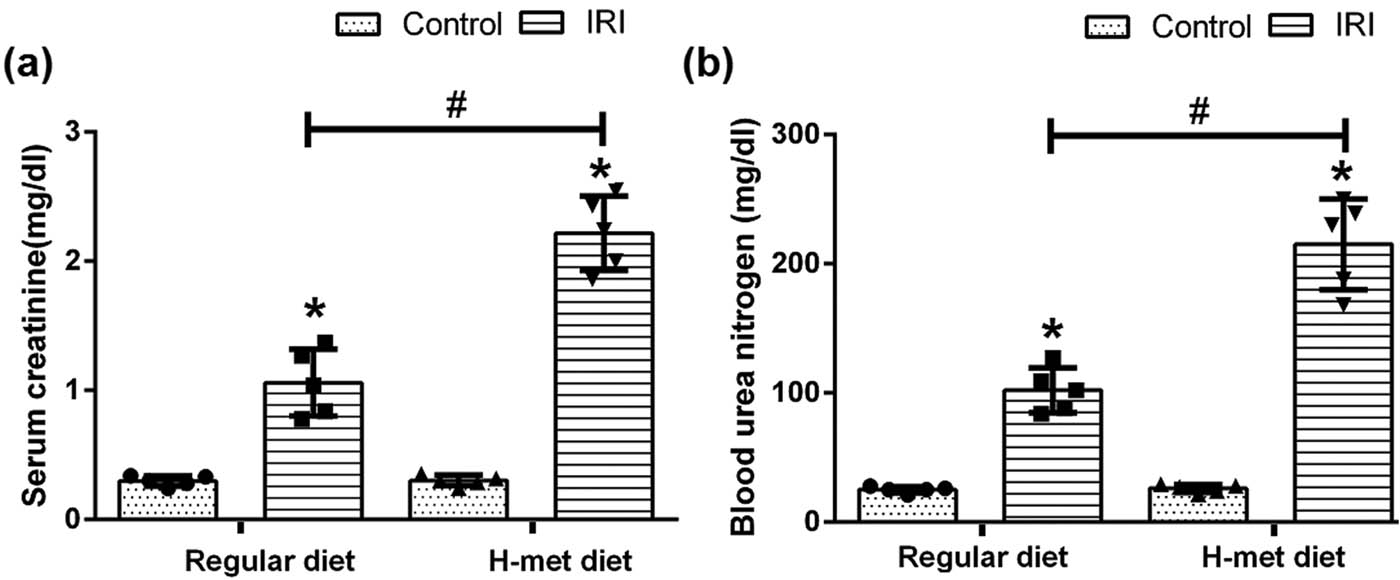
HHcy exacerbates IRI-induced renal dysfunction. (a) The level of serum creatinine in mice. (b) The level of blood urea nitrogen (BUN) in mice. Data are expressed as mean ± SD, n = 5. *P < 0.05 vs the control group under same diet; # P < 0.05 vs the IRI group pretreated with regular diet. H-met diet: High methionine diet.
To determine the influence of HHcy on oxidative stress, the level of MDA (an indicator of lipid peroxidation) was measured. As shown in Figure 2, the MDA level was significantly increased in IRI mice compared with the control mice; particularly, H-Met diet-pretreated IRI mice had the highest MDA level among mice. Our results indicate that preexisted HHcy condition can exacerbate IRI-induced renal dysfunction and oxidative stress.
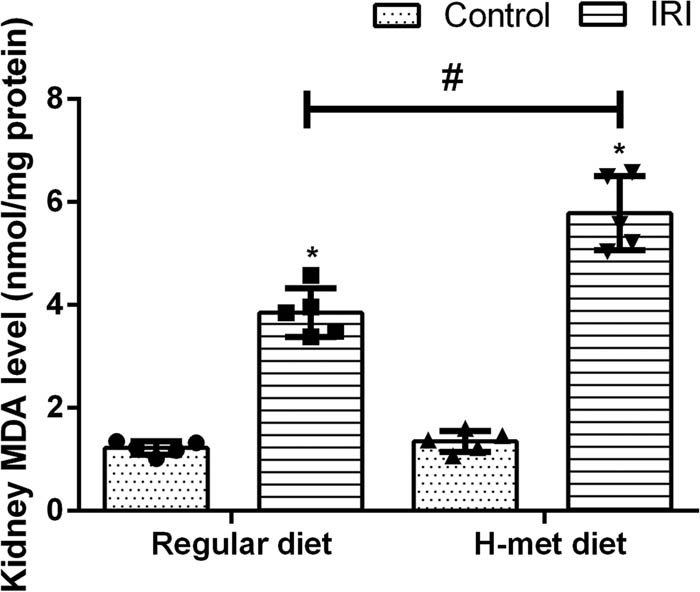
HHcy exacerbates IRI-induced oxidative stress. Data are expressed as mean ± SD, n = 5. *P < 0.05 vs the control group under same diet. # P < 0.05 vs the IRI group pretreated with regular diet. MDA: Malondialdehyde; H-met diet: High methionine diet.
3.2 HHcy exacerbates IRI-induced tubular injury and DNA damage
Twenty four hours after reperfusion, tubular injuries such as necrosis, dilatation, and cell swelling were observed in IRI mice under both regular diet and H-Met diet pretreated conditions. Notably, the degree of renal tubular injury was significantly severer in the H-Met diet pretreated IRI mice than regular diet pretreated IRI mice (Figure 3a and b), suggesting that preexisted HHcy condition can exacerbate IRI-induced tubular injury.
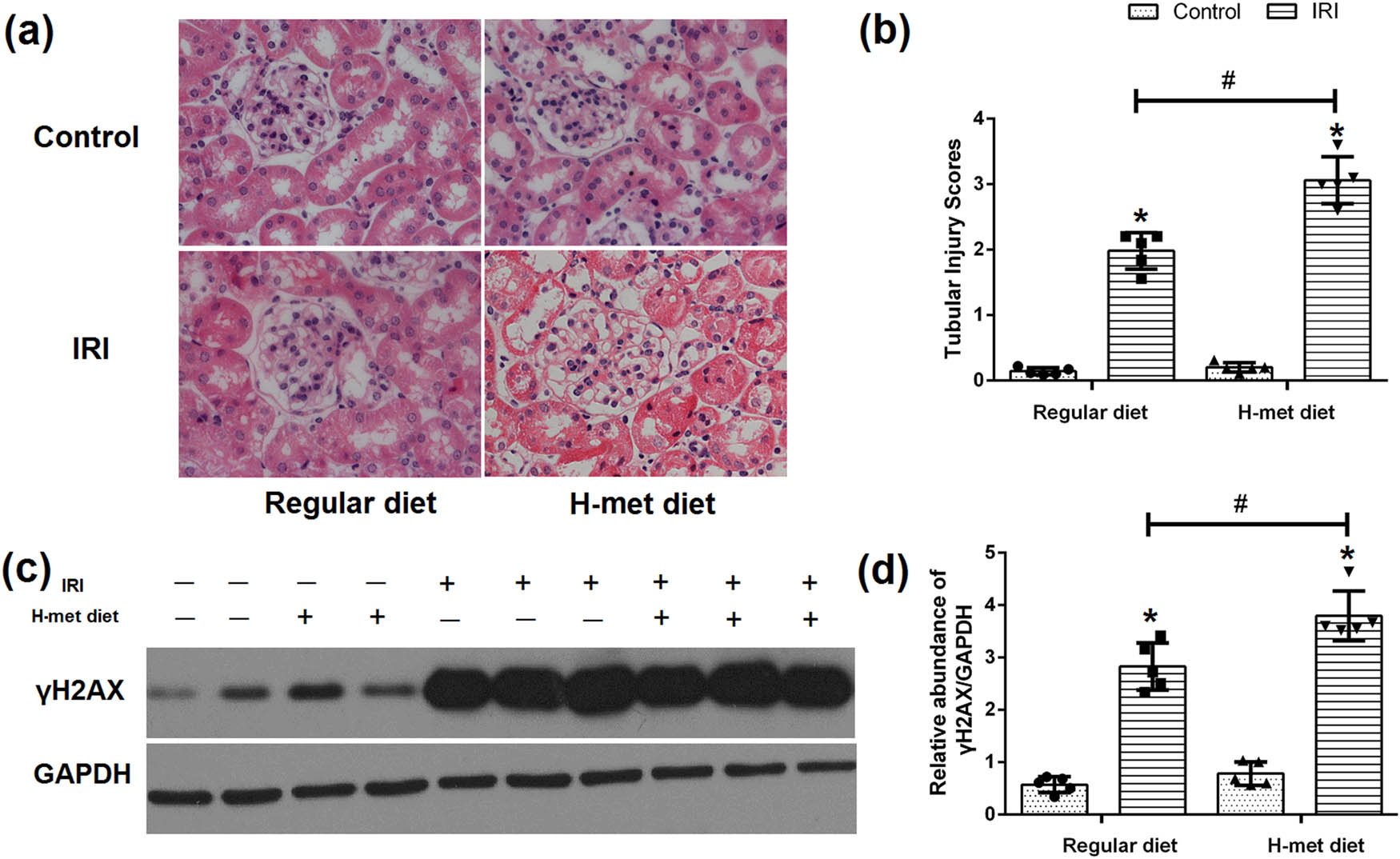
HHcy exacerbates IRI-induced tubular injury and DNA damage. (a) Representative figures of H&E staining show the tubular injury at 24 h after reperfusion. (b) Quantification assessment of tubular injury on the basis of H&E staining. (c) Representative western blot figures of γH2AX. (d) Graphic representation of relative expression of γH2AX normalized to GADPH. Data are expressed as mean ± SD, n = 5. *P < 0.05 vs the control group under same diet. # P < 0.05 vs the IRI group pretreated with regular diet. H-met diet: High methionine diet.
To determine the HHcy’s influence on the DNA damage, western blot analysis was performed to confirm the expression of γH2AX. As shown in Figure 3c and d, γH2AX was significantly increased in IRI mice, especially in H-Met diet pretreated IRI mice, indicating that preexisted HHcy condition can exacerbate IRI-induced DNA damage.
3.3 HHcy promotes the activation of JNK pathway in IRI-induced AKI mice
To determine the potential influence of HHcy on the JNK pathway, western blot analysis was performed to examine the expression of JNK and p-JNK. As shown in Figure 4a and b, JNK pathway activation manifested as increased expression of p-JNK was noticed in IRI mice, particularly in H-Met diet pretreated IRI mice. Our results suggest that preexisted HHcy condition can promote the activation of the JNK pathway caused by IRI.
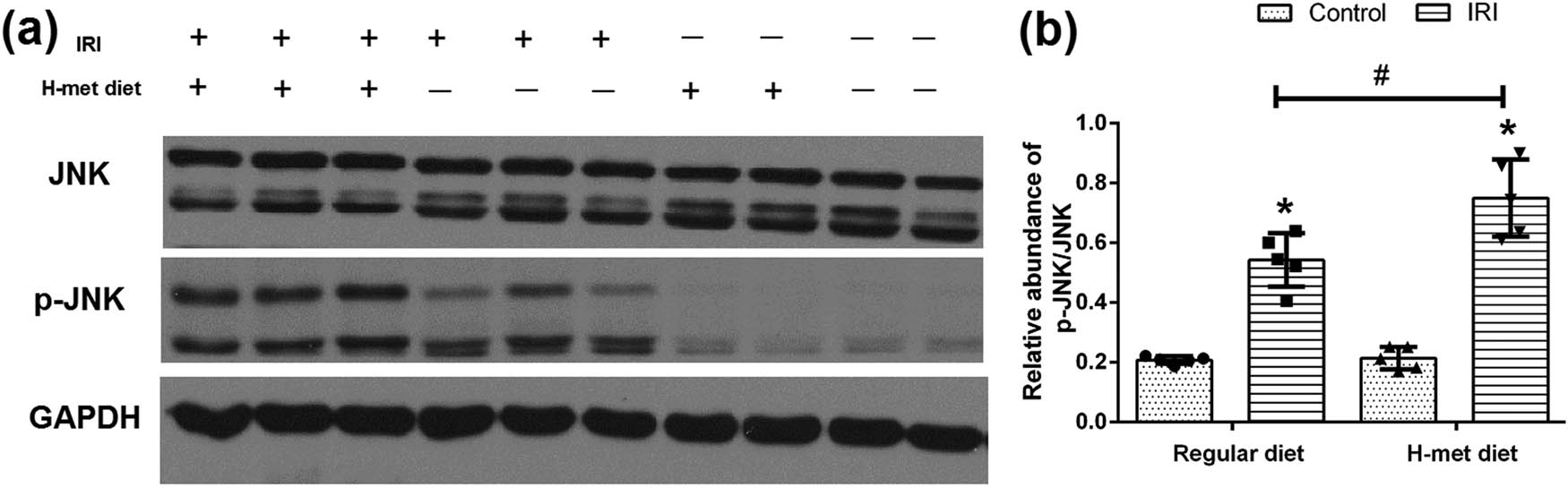
HHcy promotes IRI-induced JNK pathway activation. (a) Representative western blot figures of JNK and p-JNK. (b) Graphic representation of the ratio of p-JNK/JNK. Data are expressed as mean ± SD, n = 5. *P < 0.05 vs the control group under same diet. # P < 0.05 vs the IRI group pretreated with regular diet. H-met diet: High methionine diet.
3.4 HHcy exacerbates IRI-induced renal tubular epithelial cell apoptosis
To determine the potential effect of HHcy on the renal tubular epithelial cell apoptosis, TUNEL assay was performed. As shown in Figure 5a and b, only very few apoptotic cells were detected in control animals under both regular diet and H-Met diet pretreated conditions, while more tubular apoptotic cells were noticed in the H-Met diet pretreated IRI mice than regular diet pretreated IRI mice. Determination of cleaved caspase-3 by western blotting further confirmed that severer apoptosis was existed in the H-Met diet pretreated IRI mice (Figure 5c and d). Our results suggest that preexisted HHcy condition can exacerbate IRI-induced renal tubular epithelial cell apoptosis.
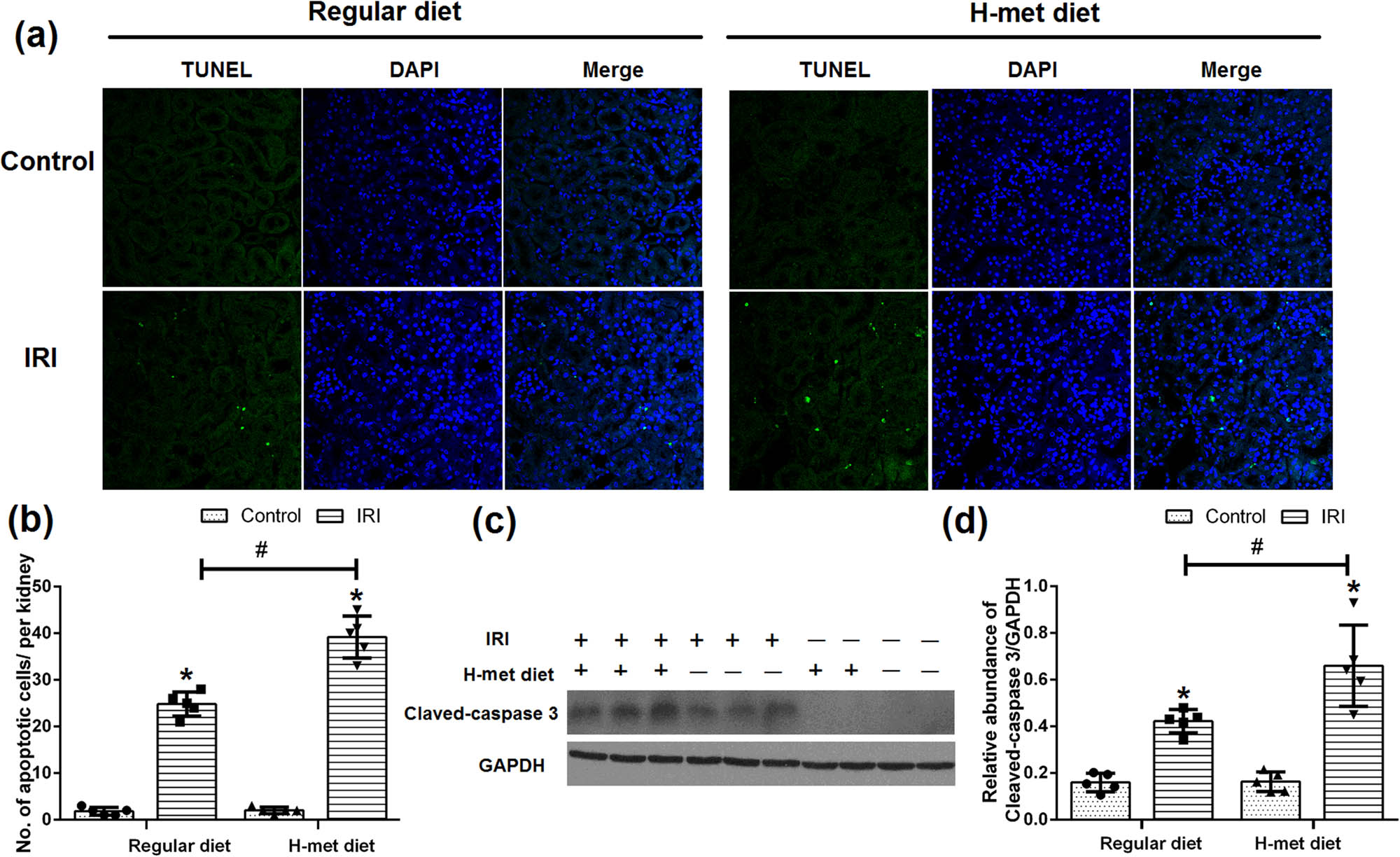
HHcy exacerbates IRI-induced renal tubular epithelial cell apoptosis. (a) Representative immunofluorescence figures show apoptotic cell death detected by TUNEL staining at 24 h after reperfusion. (b) Quantitative determination of apoptotic tubular cells. The number of apoptotic cells was counted in 10 fields per section and five sections per kidney. (c) Representative western blot figures of cleaved caspase-3. (d) Graphic representation of cleaved caspase-3 normalized to GADPH. Data are expressed as the mean ± SD, n = 5. *P < 0.05 vs the control group under same diet. # P < 0.05 vs the IRI group pretreated with regular diet. H-met diet: High methionine diet.
4 Discussion
In the present study, we used high methionine diet to generate HHcy mice; after that, the IRI-induced AKI model was employed to explore the effect and potential mechanisms of HHcy on IRI-induced AKI. Our results showed that the preexisted HHcy condition exerts very little influence on normal mice, but significantly exacerbates the renal damage, characterized by a decline in renal function and increase of tubular injury of IRI-induced AKI mice, which may be related to its potential in mediating oxidative stress, DNA damage, JNK pathway, and apoptosis.
In humans, two metabolism pathways, including remethylation and transsulfuration, are mainly involved in the metabolism of Hcy. It has been reported that up to 70% of plasma Hcy is removed from the kidney, mainly through transsulfuration [14]. Therefore, kidney injury can increase plasma Hcy concentration; in return, increased Hcy may be harmful to the kidney. In a study performed by Ye et al., the authors found that CKD patients with HHcy had higher incidence of renal damage than patients with normohomocysteinemia [15]. Liu and colleagues retrospectively analyzed 7,240 hypertensive patients and found that patients who developed HHcy had a higher long-term rate of renal function decline compared with patients who didn’t develop HHcy [16]. In an animal study, authors found renal dysfunction appeared in cystathionine β-synthase-deficient HHcy mice compared with wild-type mice [17]. In the present study, we noticed that HHcy almost doesn’t influence the renal function and tubular structure of control mice, which was consistent with the observations of Li et al. [18]. We speculate that the detrimental role of Hcy may not appear because of the moderate concentration and short action time of Hcy. While, on the other hand, our results showed that HHcy could exacerbate the renal damage of IRI-induced AKI mice.
Renal tubular epithelial cells account for about 70% of renal parenchymal cells in the kidney and are vulnerable to ischemia, hypoxia, nephrotoxin, and immune inflammation during acute renal injury because of their nature of high oxygen consumption. In the present study, significant renal damage, including tubular epithelial cell apoptosis and necrosis, was noticed in the IRI-induced AKI mice, consistent with previous studies [19,20]. Notably, the renal damage was severer in the H-Met diet pretreated IRI mice than regular diet pretreated IRI mice. To determine the potential mechanisms of this phenomenon, we further focused on the changes of oxidative stress, DNA damage, and the JNK pathway because they are closely related to tubular epithelial cell apoptosis.
As we know, oxidative stress is a well-known hallmark of IRI-induced AKI, which is believed to be one of the critical factors causing kidney injury during ischemia-reperfusion [21]. DNA damage, a deleterious event that occurs in the genome, may be induced under many conditions such as oxidative stress or free radical insult, irradiation, and UV exposure [22]. Increasing evidence indicated that oxidative stress and DNA damage occur in kidney tissues following ischemia-reperfusion [13,20]. In response to DNA damage, several pathways such as NF-κB and JNK pathways may be activated to maintain genome homeostasis [23]. Besides, endoplasmic reticulum stress, induced by oxidative stress, hypoxia, or energy deprivation, also existed in IRI-induced AKI, which can trigger the activation of the JNK pathway [5]. In the present study, our results showed that the expression of MDA, γH2AX, and p-JNK, as well as the number of apoptotic tubular epithelial cells, was significantly higher in the H-Met diet pretreated IRI mice than regular diet pretreated IRI mice. We speculate that HHcy might exhibit its detrimental role in IRI-induced AKI mice by promoting oxidative stress, which causes DNA damage and triggers apoptosis through activation of the JNK pathway. Considering the detrimental role of HHcy, close attention should be paid to it in patients vulnerable to IRI-induced AKI.
However, this study has limitations. The most noticeable one is that this in vivo study is not sufficient to make a definite conclusion. In the future, activation or inhibition experiments focused on oxidative stress, DNA damage, or JNK pathway are warranted to further explore the relationship between HHcy and IRI-induced AKI.
5 Conclusion
Our results demonstrated that preexisted HHcy condition could exacerbate IRI-induced AKI, which may be achieved through promoting oxidative stress, DNA damage, JNK pathway activation, and consequent apoptosis.
-
Funding information: This research was funded by grants from the National Natural Science Foundation of China (81760125), the Science & Technology Foundation of Guizhou Province (QKHJC[2016]1087), the Special Fund for Basic Scientific Research Operating of Central Public Welfare Research Institutes, the Chinese Academy of Medical Sciences (2019PT320003), and Guizhou high-level innovative talents program [QKHPTRC(2018)5636].
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Raup-Konsavage WM, Wang Y, Wang WW, Feliers D, Ruan H, Reeves WB. Neutrophil peptidyl arginine deiminase-4 has a pivotal role in ischemia/reperfusion-induced acute kidney injury. Kidney Int. 2018;93:365–74.10.1016/j.kint.2017.08.014Search in Google Scholar PubMed PubMed Central
[2] Li Y-M, Zhang J, Su L-J, Kellum JA, Peng Z-Y. Downregulation of TIMP2 attenuates sepsis-induced AKI through the NF-κb pathway. BBA-Mol Basis Dis. 2019;1865:558–69.10.1016/j.bbadis.2018.10.041Search in Google Scholar PubMed
[3] Perkins ZB, Captur G, Bird R, Gleeso L, Singer B, O’Brien B. Trauma induced acute kidney injury. PLoS One. 2019;14:e0211001.10.1371/journal.pone.0211001Search in Google Scholar PubMed PubMed Central
[4] McCullough PA, Choi JP, Feghali GA, Schussler JM, Stoler RM, Vallabahn RC, et al. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2016;68:1465–73.10.1016/j.jacc.2016.05.099Search in Google Scholar PubMed
[5] Xu Y, Guo M, Jiang W, Dong H, Han Y, An X-F, et al. Endoplasmic reticulum stress and its effects on renal tubular cells apoptosis in ischemic acute kidney injury. Ren Fail. 2016;38:831–7.10.3109/0886022X.2016.1160724Search in Google Scholar PubMed
[6] Xu X, Nie S, Liu Z, Chen C, Xu G, Zha Y, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephro. 2015;10:1510–8.10.2215/CJN.02140215Search in Google Scholar PubMed PubMed Central
[7] Kim J, Kim H, Roh H, Kwon Y. Causes of hyperhomocysteinemia and its pathological significance. Arch Pharm Res. 2018;41:372–83.10.1007/s12272-018-1016-4Search in Google Scholar PubMed
[8] Kong X, Ma X, Zhang C, Su H, Xu D. Hyperhomocysteinemia increases the risk of chronic kidney disease in a Chinese middle-aged and elderly population-based cohort. Int Urol Nephrol. 2017;49:661–7.10.1007/s11255-016-1452-3Search in Google Scholar PubMed
[9] Xu W, Tang S, Xiang M, Peng J. Serum Homocysteine, cystatin C as biomarkers for progression of diabetic nephropathy. Pteridines. 2019;30:183–8.10.1515/pteridines-2019-0024Search in Google Scholar
[10] Prathapasinghe GA, Siow YL, Karmin O. Detrimental role of homocysteine in renal ischemia-reperfusion injury. Am J Physiol-Renal Physiol. 2007;292:F1354–63.10.1152/ajprenal.00301.2006Search in Google Scholar PubMed
[11] Long Y, Zhen X, Zhu F, Hu Z, Lei W, Li S, et al. Hyperhomocysteinemia exacerbates cisplatin-induced acute kidney injury. Int J Bio Sci. 2017;13:219.10.7150/ijbs.16725Search in Google Scholar PubMed PubMed Central
[12] Li S, Qiu B, Lu H, Lai Y, Liu J, Luo J, et al. Hyperhomocysteinemia accelerates acute kidney injury to chronic kidney disease progression by downregulating heme oxygenase-1 expression. Antioxid Redox Sign. 2019;30:1635–50.10.1089/ars.2017.7397Search in Google Scholar PubMed
[13] Havakhah S, Sadeghnia HR, Mosa-Al-Reza Hajzadeh NM, Roshan SS, Hosseinzadeh H, Mohareri N, et al. Effect of Nigella sativa on ischemia-reperfusion induced rat kidney damage. Iran J Basic Med Sci. 2014;17:986.Search in Google Scholar
[14] Biasioli S, Schiavon R. Homocysteine as a cardiovascular risk factor. Blood Purificat. 2000;18:177–82.10.1159/000014416Search in Google Scholar PubMed
[15] Ye Z, Zhang Q, Li Y, Wang C, Zhang J, Ma X, et al. High prevalence of hyperhomocysteinemia and its association with target organ damage in chinese patients with chronic kidney disease. Nutrients. 2016;8:645.10.3390/nu8100645Search in Google Scholar PubMed PubMed Central
[16] Liu C, Lin L, Xu R. Elevated homocysteine and differential risks of the renal function decline in hypertensive patients. Clin Exp Hypertens. 2020;42:565–70.10.1080/10641963.2020.1739698Search in Google Scholar PubMed
[17] Pushpakumar S, Kundu S, Sen U. Hydrogen sulfide protects hyperhomocysteinemia-induced renal damage by modulation of caveolin and enos interaction. Sci Rep-UK. 2019;9:1–13.10.1038/s41598-018-38467-6Search in Google Scholar PubMed PubMed Central
[18] Li L, Hasegawa H, Inaba N, Yoshioka W, Chang D, Liu J, et al. Diet-induced hyperhomocysteinemia impairs vasodilation in 5/6-nephrectomized rats. Amino Acids. 2018;50:1485–94.10.1007/s00726-018-2626-3Search in Google Scholar PubMed
[19] Liang H, Liao M, Zhao W, Zheng X, Xu F, Wang H, et al. CXCL16/ROCK1 signaling pathway exacerbates acute kidney injury induced by ischemia-reperfusion. Biomed Pharmacother. 2018;98:347–56.10.1016/j.biopha.2017.12.063Search in Google Scholar PubMed
[20] Ko S-F, Chen Y-T, Wallace CG, Chen K-H, Sung P-H, Cheng B-C, et al. Inducible pluripotent stem cell-derived mesenchymal stem cell therapy effectively protected kidney from acute ischemia-reperfusion injury. Am J Transl Res. 2018;10:3053.Search in Google Scholar
[21] Melis N, Thuillier R, Steichen C, Giraud S, Sauvageon Y, Kaminski J, et al. Emerging therapeutic strategies for transplantation-induced acute kidney injury: protecting the organelles and the vascular bed. Expert Opin Ther Tar. 2019;23:495–509.10.1080/14728222.2019.1609451Search in Google Scholar PubMed
[22] Ma Z, Wei Q, Dong G, Huo Y, Dong Z. DNA damage response in renal ischemia–reperfusion and ATP-depletion injury of renal tubular cells. BBA-Mol Basis Dis. 2014;1842:1088–96.10.1016/j.bbadis.2014.04.002Search in Google Scholar PubMed PubMed Central
[23] Picco V, Pagès G. Linking JNK activity to the DNA damage response. Genes Cancer. 2013;4:360–8.10.1177/1947601913486347Search in Google Scholar PubMed PubMed Central
© 2021 Mei Zhang et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

