Abstract
The aim of this study was to investigate the detailed role and molecular mechanism of long noncoding RNA (lncRNA) taurine upregulated gene 1 (TUG1) in cardiac hypertrophy. Cardiac hypertrophy was established by transverse abdominal aortic constriction (TAC) in vivo or angiotensin II (Ang II) treatment in vitro. Levels of lncRNA TUG1, miR-497 and myocyte enhancer factor 2C (MEF2C) mRNA were assessed by quantitative reverse transcriptase PCR (qRT-PCR). Western blot assay was performed to determine the expression of MEF2C protein. The endogenous interactions among TUG1, miR-497 and MEF2C were confirmed by dual-luciferase reporter and RNA immunoprecipitation assays. Our data indicated that TUG1 was upregulated and miR-497 was downregulated in the TAC rat model and Ang II-induced cardiomyocytes. TUG1 knockdown or miR-497 overexpression alleviated the hypertrophy induced by Ang II in cardiomyocytes. Moreover, TUG1 acted as a sponge of miR-497, and MEF2C was directly targeted and repressed by miR-497. miR-497 overexpression mediated the protective role of TUG1 knockdown in Ang II-induced cardiomyocyte hypertrophy. MEF2C was a functional target of miR-497 in regulating Ang II-induced cardiomyocyte hypertrophy. In addition, TUG1 regulated MEF2C expression through sponging miR-497. Knockdown of TUG1 rescued Ang II-induced hypertrophy in cardiomyocytes at least partly through targeting the miR-497/MEF2C axis, highlighting a novel promising therapeutic target for cardiac hypertrophy treatment.
1 Introduction
Cardiac hypertrophy is a common physiological compensatory response of the heart against a number of stressors to maintain normal cardiac function. However, enlargement of the heart in response to myocardial injury, hypertensive stress or excessive neurohumoral activation is associated with maladaptive remodeling and cardiac dysfunction and is classified as pathological hypertrophy [1]. Pathological cardiac hypertrophy is a major risk factor for diomyopathy, heart failure and sudden cardiac death [2,3]. Despite the improvements of the understanding of pathological regulators in cardiac hypertrophy [4,5], the molecular mechanisms of cardiac hypertrophy remain largely unclear.
Long noncoding RNAs (lncRNAs) are more than 200 nucleotide RNA molecules that perform various functions in a series of important biological processes [6]. They have been discovered to have relevance to human diseases, including cardiac hypertrophy [7]. For example, Wang et al. reported that cardiac hypertrophy-related factor (CHRF) regulated cardiac hypertrophy by acting as a sponge of microRNA (miRNA)-489 [8]. Wang et al. identified that cardiac hypertrophy-associated epigenetic regulator (Chaer) worked as an epigenetic checkpoint in cardiac hypertrophy [9]. A recent study demonstrated that taurine upregulated gene 1 (TUG1) was involved in the pathogenesis of cardiac hypertrophy by sponging miRNA-29b-3p [10]. Herein, we identified the functional role and the underlying mechanisms of TUG1 in cardiac hypertrophy.
miRNAs, a class of endogenous, small noncoding RNAs with 20–22 nucleotides, are known to be present in the RNA-induced silencing complexes (RISCs), where they silence gene expression [11]. Dysregulation of miRNAs is found to have relevance to human diseases, including cardiac hypertrophy [12]. miR-497, a member of the miR-15 family, was identified as a novel regulator of cardiac hypertrophy and fibrosis by the repression of the TGFβ-pathway [13]. A recent study demonstrated that the increased expression of miR-497 ameliorated cardiac hypertrophy in vitro and in vivo via targeting sirtuin 4 (Sirt4) [14]. Previous studies had reported that TUG1 exerted a regulatory function in human disease through the competing endogenous RNA (ceRNA) network via sponging miRNAs [15,16]. However, the effect of interplay between TUG1 and miR-497 in cardiac hypertrophy remains undefined. In the present study, our data supported that TUG1 was upregulated in the TAC rat model and angiotensin II (Ang II)-induced cardiomyocytes. Furthermore, TUG1 knockdown attenuated cardiomyocyte hypertrophy in vitro by targeting the miR-497/myocyte enhancer factor 2C (MEF2C) axis.
2 Materials and methods
2.1 Animal care and use
Female Sprague-Dawley (SD) rats (8 weeks old, 190–220 g) were purchased from Henan Research Center of Laboratory Animal (Zhengzhou, Henan, China) and housed in a specific pathogen-free environment in the animal facility of the Fourth Affiliated Hospital of Nanchang University. All rats were kept at a constant temperature (22 ± 2°C) with 60% humidity and a 12 h light–dark cycle and fed a standard chow diet for at least 1 week before experimentation. All animal experimental procedures were performed in accordance with the Agriculture Guidebook of the Care and Use of Laboratory Animals.
-
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals and was approved by the Animal Ethics Committee of the Fourth Affiliated Hospital of Nanchang University.
2.2 Animal model
Experimental rats were divided into two groups: (1) Sham group (n = 8), in which rats underwent exposure of abdominal aorta without ligation, and (2) transverse abdominal aortic constriction (TAC) group (n = 8), in which abdominal aorta of rats was exposed and ligated. The construct of the TAC rat model was performed as described previously [17]. Briefly, a 1.5–2.0 cm longitudinal incision was exposed to the left side of the rat abdomen. Then, a 4-0 suture was used to tie two circles around the abdominal aorta by a 12-gauge needle, after which the needle was eliminated. At 8 weeks after surgery, cardiac dimensions and function were analyzed using a Doppler echocardiography (Philios IE33 ultrasound system, Phillips Medical Systems, Andover, MA, USA) including the left ventricular end-diastolic dimension (LVEDD), the left ventricular end-systolic diameter (LVESD), the left ventricular end-systolic pressure (LVESP), the left ventricular end-diastolic pressure (LVEDP) and fractional shortening (FS). Subsequently, all rats were euthanized; the ratios of heart weight and body weight were determined; and heart tissues were collected for further evaluation.
2.3 Primary cardiomyocyte isolation, culture and treatment
Primary cardiomyocytes were isolated from the hearts of 1- to 3-day-old newborn SD rats as described previously with modification [18]. In brief, newborn rats were euthanized, and the hearts were immediately excised and minced. The heart tissues were digested with 0.1% collagenase type II (Gibco, Rockville, MD, USA) and 0.1% trypsin (Gibco) at 37°C. After centrifugation, cardiomyocytes were harvested and maintained in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and 1% penicillin/streptomycin (Gibco) in an incubator at 37°C with 5% CO2. To establish a cardiomyocyte hypertrophy model in vitro, the cardiomyocytes of ∼70% were treated with 1 mM of Ang II (Sigma-Aldrich, St. Louis, MO, USA) for 48 h.
2.4 Cell transfection
For TUG1 knockdown studies, cardiomyocytes were transiently introduced with siRNA targeting TUG1 (si-TUG1, GenePharma, Shanghai, China) or a nontarget siRNA (si-NC, GenePharma). For miR-497 overexpression, cardiomyocytes were transfected with the mature miR-497 mimic (GenePharma) or a scrambled oligonucleotide sequence (miR-NC mimic, GenePharma). miR-497 silencing was carried out using miR-497 inhibitor (in-miR-497, GenePharma), and in-miR-NC (GenePharma) was used as a negative control. For overexpression experiments, cardiomyocytes were introduced with pcDNA-based TUG1 or a MEF2C overexpression plasmid (pcDNA-TUG1 or pcDNA-MEF2C, GenePharma) or a negative control plasmid (pcDNA-NC, GenePharma). All transfections were performed using Lipofectamine 2000 transfection reagent (Invitrogen, Waltham, MA, USA) following the protocols of manufacturers.
2.5 Quantitative reverse transcriptase PCR
Total RNA was extracted from heart tissues and cardiomyocytes using RNA Purification kit (Invitrogen) in accordance with manufacturer’s instructions. The quality and quantity of RNA extracts were assessed by a NanoDrop ND-2000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The levels of atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), β-myosin heavy chain (β-MHC), TUG1 and MEF2C mRNA were detected by using quantitative reverse transcriptase PCR (qRT-PCR). Total RNA (1 µg) was reverse transcribed into cDNA using M-MLV reverse transcriptase (Invitrogen), and qRT-PCR was carried out using PowerUpTM SYBRTM Green PCR Master Mix (Applied Biosystems) on the ABI 7900HT sequence detector (Applied Biosystems) following the protocols of manufacturers. The indicated gene expression levels were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The level of mature miR-497 was determined by qRT-PCR using TaqMan reverse transcription kit (Applied Biosystems, Foster City, CA, USA) and TaqMan MicroRNA assay kit (Applied Biosystems) with snRNA RNU6B as an internal control. The amplification profile was denatured at 95°C for 10 min, followed by 40 cycles of 95°C for 20 s and 60°C for 1 min. Relative gene expression was calculated based on the 2−∆∆Ct method.
2.6 Western blot
Total protein was prepared in ice-cold RIPA buffer (150 mM Tris-HCl, pH = 7.6, 150 mM NaCl, 0.5% Triton X-100, 0.1% SDS, 1 mM phenylmethanesulfonyl fluoride, 1 mM Na3VO4) containing a cocktail of protease inhibitors (Thermo Fisher Scientific, Waltham, MA, USA) and then quantified using a BCA Protein assay kit (Thermo Fisher Scientific). Protein extracts were resolved on a 10% SDS-polyacrylamide gel and electrophoretically transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Bedford, MA, USA). The following primary antibodies were used: anti-ANP (Abcam, Cambridge, UK; dilution 1:1,000), anti-BNP (Abcam; dilution 1:500), anti-β-MHC (Abcam; dilution 1:1,000), anti-MEF2C (Abcam; dilution 1:1,000) and anti-β-actin (Abcam; dilution 1:3,000). The horseradish peroxidase-conjugated anti-rabbit (Abcam; dilution 1:5,000) or anti-mouse (Abcam; dilution 1:5,000) IgG was used as a secondary antibody. Protein bands were detected using the enhanced chemiluminescence (ECL) detection kit (Immobilon Western Chemiluminescent HRP Substrate, Millipore) and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
2.7 Bioinformatic analysis and dual-luciferase reporter assay
Online database LncBase Predicted v.2 (http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php?r=lncbasev2%2Findex-predicted) was used to help identify the miRNAs that potentially bind to TUG1. Bioinformatic analysis for the molecular targets of miR-497 was performed using microT-CDS software at http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/index. TUG1 and MEF2C 3′UTR luciferase reporter plasmids (TUG1-WT and MEF2C 3′UTR-WT) harboring the miR-497-binding sites and the site-directed mutations of the seed region (TUG1-MUT and MEF2C 3′UTR-MUT) were obtained from GenePharma. The cardiomyocytes were cotransfected with each reporter construct and miR-NC mimic or miR-497 mimic. After 48 h transfection, the luciferase activity was determined using a dual-luciferase reporter assay system (Promege, Madison, WI, USA) following manufacturer’s guidance.
2.8 RNA immunoprecipitation assay
RNA immunoprecipitation (RIP) assay was carried out using the Magna RIP RNA Immunoprecipitation kit (Millipore) with anti-Argonaute 2 (anti-Ago2, Abcam) antibody. Briefly, cell lysates were prepared using the ice-cold RIPA buffer and then incubated with anti-Ago2 or negative control IgG antibody at 4°C for 4 h, followed by the incubation with protein A/G agarose for 4 h. Beads were harvested by centrifugation and washed three times using ice-cold PBS. Next, total RNA was extracted, and the enrichment levels of TUG1 and MEF2C mRNA were detected by qRT-PCR.
2.9 Statistical analysis
All data were analyzed using GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA, USA) and expressed as mean ± standard deviation (SD). All experiments were carried out in triplicate. Differences between groups were compared by Student’s t-test or analysis of variance (ANOVA). A probability value of P < 0.05 was considered to be significant.
3 Results
3.1 TUG1 level was upregulated in the TAC rat model and Ang II-induced cardiomyocytes
To investigate the involvement of TUG1 in cardiac hypertrophy, we first established the cardiac hypertrophy model by TAC in vivo and Ang II treatment in vitro. By contrast, TAC led to a significant increase of LVEDD, LVESD and LVEDP and a striking reduction of LVESP and FS, as well as a strong elevation of the radio of heart weight and body weight (Figure A1a–f). Moreover, TAC caused a remarkable increase in the levels of hypertrophy-related genes ANP, BNP and β-MHC (Figure A1g and h), demonstrating the successful establishment for the TAC rat model. Furthermore, Ang II treatment remarkably increased the levels of ANP, BNP and β-MHC in cardiomyocytes (Figure A1i and j). Interestingly, as shown by qRT-PCR, the TUG1 level was significantly upregulated in the TAC model and Ang II-treated cardiomyocytes (Figure 1a and b).

TUG1 was upregulated in the TAC rat model and Ang II-treated cardiomyocytes. Relative TUG1 level by qRT-PCR in the untreated heart tissues (control), Sham model and TAC model (a), and untreated (Control), Mock-treated (Blank) and Ang II-treated cardiomyocytes (b). *P < 0.05.
3.2 Knockdown of TUG1 attenuated Ang II-induced cardiomyocyte hypertrophy
To further investigate the functional role of TUG1 in cardiac hypertrophy, we performed loss-of-function experiments with si-TUG1. Transient transfection of si-TUG1, but not a scrambled control sequence, resulted in a significant decrease in the level of TUG1 in Ang II-treated cardiomyocytes (Figure 2a). Subsequent qRT-PCR and western blot assays revealed that in comparison to the negative control, TUG1 knockdown triggered a remarkable reduction in the expression of ANP, BNP and β-MHC at both mRNA and protein levels in Ang II-treated cardiomyocytes (Figure 2b and c).
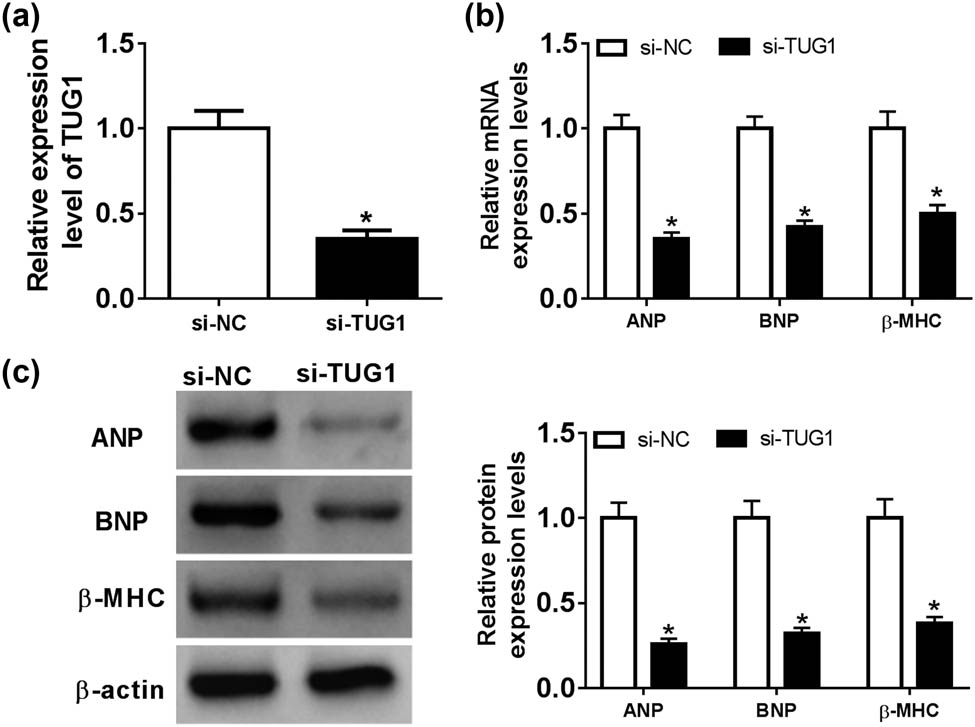
TUG1 knockdown attenuated Ang II-induced cardiomyocyte hypertrophy. Cardiomyocytes were transfected with si-NC or si-TUG1 for 24 h and then treated with 1 mM of Ang II for 48 h, followed by the determination of TUG1 expression by qRT-PCR (a), the mRNA levels of ANP, BNP and β-MHC by qRT-PCR (b) and their protein levels by western blot (c). *P < 0.05.
3.3 TUG1 acted as a molecular sponge of miR-497
To further understand the function of TUG1 in cardiac hypertrophy, we used online database LncBase Predicted v.2 to help identify the miRNAs that potentially bind to TUG1. Among these candidates, we selected several miRNAs (miR-93, miR-142-3p, miR-103, miR-631-5p, miR-16-5p and miR-497), which were related to cardiomyocyte hypertrophy and were downregulated in cardiomyocyte hypertrophy. Our results showed that miR-497 was the most significantly upregulated miRNA in TUG1-silencing cardiomyocytes (Figure A2), and thus, we selected miR-497 for further investigation. These data revealed a putative target sequence for miR-497 within TUG1 (Figure 3a). To validate this, we carried out dual-luciferase reporter assays. When we cloned the TUG1 segment harboring the miR-497-binding sequence into a luciferase reporter, the cotransfection of TUG1 wild-type reporter and miR-497 mimic into cardiomyocytes caused lower luciferase activity than cells cotransfected with miR-NC mimic (Figure 3b). However, when the target sites were mutated, no reduction was observed in luciferase in the presence of miR-497 mimic (Figure 3b). Ago2, a core component of the RISC, acts as a crucial regulator in the mature process of miRNA [11]. Thus, RIP experiments were done using anti-Ago2 antibody. The data showed that TUG1 enrichment was significantly elevated by miR-497 overexpression (Figure 3c), eliciting the potential endogenous interaction between TUG1 and miR-497. The data of qRT-PCR also revealed that miR-497 was prominently downregulated in the TAC model and Ang II-treated cardiomyocytes (Figure 3d and e). Furthermore, in comparison to their counterparts, miR-497 expression was significantly decreased by TUG1 overexpression plasmid, and it was remarkably increased in case of TUG1 depletion in cardiomyocytes (Figure 3f).

TUG1 acted as a molecular sponge of miR-497. (a) Schematic of the miR-497-binding sites within TUG1 and mutated miR-497-binding sites. (b) Relative luciferase activity in cardiomyocytes cotransfected with TUG1-WT or TUG1-MUT and miR-NC mimic or miR-497 mimic. (c) TUG1 enrichment in the RISC of cardiomyocytes transfected with miR-NC mimic or miR-497 mimic using anti-Ago2 antibody. TUG1 expression by qRT-PCR in the untreated heart tissues (control), Sham model and TAC model (d), and untreated (control), Mock-treated (Blank) and Ang II-induced cardiomyocytes (e) and cardiomyocytes transfected with pcDNA, pcDNA-TUG1, si-NC or si-TUG1 (f). *P < 0.05.
3.4 miR-497 overexpression mediated the protective role of TUG1 knockdown in Ang II-induced cardiomyocyte hypertrophy
To determine whether TUG1 modulated cardiomyocyte hypertrophy by miR-497, we reduced miR-497 expression in si-TUC1-transfected cardiomyocytes before Ang II treatment. The results of qRT-PCR showed that si-TUG1-mediated miR-497 upregulation was strikingly reversed by in-miR-497 transfection (Figure 4a). Moreover, the reduced effects of TUG1 knockdown on ANP, BNP and β-MHC expression were significantly abolished by miR-497 level restoration in Ang II-treated cardiomyocytes (Figure 4b and c).
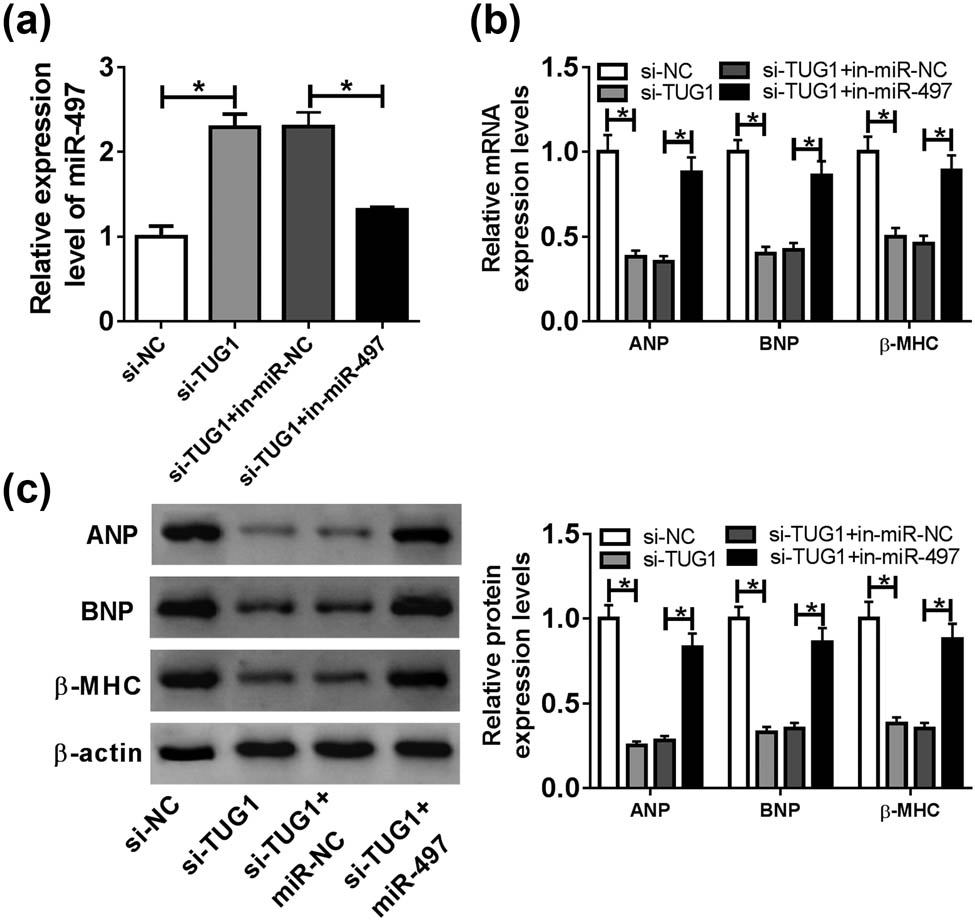
miR-497 overexpression mediated the protective role of TUG1 knockdown in Ang II-induced cardiomyocyte hypertrophy. Cardiomyocytes were transfected with si-NC, si-TUG1, si-TUG1 + in-miR-NC or si-TUG1 + in-miR-497 before Ang II exposure, followed by the measurement of miR-497 expression by qRT-PCR (a), the mRNA levels of ANP, BNP and β-MHC by qRT-PCR (b) and their protein levels by western blot (c). *P < 0.05.
3.5 TUG1-regulated MEF2C expression through sponging miR-497
miRNAs exert biological function by regulating their target genes. Herein, to further understand the underlying mechanism by which miR-497 regulated cardiac hypertrophy, we carried out a detailed analysis for its molecular targets. Using microT-CDS software, the predicted data showed a putative complementary sequence for miR-497 within the 3′UTR of MEF2C mRNA (Figure 5a). To confirm whether MEF2C was a direct target of miR-497, dual-luciferase reporter assays were performed using an MEF2C 3′UTR reporter (MEF2C 3′UTR-WT) harboring the miR-497-binding sequence and the site-directed mutation of the seed region (MEF2C 3′UTR-MUT). In contrast to the negative control, the transfection of miR-497 mimic significantly reduced the luciferase activity of MEF2C 3′UTR-WT (Figure 5b). However, the site-directed mutation of the miR-497-binding region strikingly abolished the suppression of miR-497 on reporter gene expression (Figure 5b). RIP assays revealed that compared with the negative control, the enrichment level of MEF2C mRNA was substantially elevated by miR-497 overexpression (Figure 5c). In addition, qRT-PCR assays demonstrated that the expression of MEF2C mRNA was remarkably upregulated in the TAC model and Ang II-treated cardiomyocytes (Figure 5d and e). Moreover, in contrast to their counterparts, MEF2C protein expression was significantly decreased by miR-497 overexpression, and it was highly increased with in-miR-497 transfection (Figure 5f). We further determined whether TUG1 modulated the expression of MEF2C in cardiomyocytes. As expected, the MEF2C level was prominently elevated by TUG1 overexpression, and this effect was strongly abolished by miR-497 mimic transfection (Figure 5g).

TUG1-regulated MEF2C expression through sponging miR-497. (a) Nucleotide resolution of the predicted miR-497-binding sites within the 3′UTR of MEF2C mRNA and the mutant of the seed region. (b) Relative luciferase activity in cardiomyocytes cotransfected with MEF2C 3′UTR-WT or MEF2C 3′UTR-MUT and miR-NC mimic or miR-497 mimic. (c) The enrichment of MEF2C mRNA in the RISC of cardiomyocytes transfected with miR-NC mimic or miR-497 mimic using anti-Ago2 antibody. MEF2C expression by qRT-PCR or western blot in TAC model (d), Ang II-treated cardiomyocytes (e) and cardiomyocytes transfected with miR-NC mimic, miR-497 mimic, in-miR-NC or in-miR-497 (f). (g) MEF2C expression by western blot in cardiomyocytes transfected with pcDNA, pcDNA-TUG1, pcDNA-TUG1 + miR-NC mimic or pcDNA-TUG1 + miR-497 mimic. *P < 0.05.
3.6 MEF2C was a functional target of miR-497 in regulating Ang II-induced cardiomyocyte hypertrophy
To provide further mechanistic insight into the link between miR-497 and MEF2C in cardiac hypertrophy, we cotransfected miR-497 mimic and pcDNA-MEF2C into cardiomyocytes before Ang II induction. In contrast to the negative control, the cotransfection of pcDNA-MEF2C significantly reversed the repressive effect of miR-497 overexpression on MEF2C expression (Figure 6a). Moreover, the restored level of MEF2C remarkably abolished the reduction of miR-497 overexpression on ANP, BNP and β-MHC levels in Ang II-treated cardiomyocytes (Figure 6b and c).

Overexpression of miR-497 exerted its antihypertrophic effect in Ang II-treated cardiomyocytes by MEF2C. Cardiomyocytes were transfected with miR-NC mimic, miR-497 mimic, miR-497 mimic + pcDNA-NC or miR-497 mimic + pcDNA-MEF2C before Ang II exposure, followed by the measurement of MEF2C protein expression by western blot (a), the mRNA levels of ANP, BNP and β-MHC by qRT-PCR (b) and their protein levels by western blot (c). *P < 0.05.
4 Discussion
Pathological cardiac hypertrophy has been widely known to cause the deposition of extracellular collagen, the loss of adrenergic responsivity and metabolic disorder, thereby leading to heart failure [19]. It was reported that cardiac hypertrophy could be established by Ang II induction in vitro and TAC surgery in vivo [17,20]. In the present study, we successfully established cardiac hypertrophy in vivo and in vitro, as evidenced by changes of hemodynamic parameters, the radio of heart weight and body weight and levels of ANP, BNP and β-MHC. Up to now, many lncRNAs have been identified as positive or negative regulators in cardiac hypertrophic pathways [21,22,23]. In this study, we first demonstrated that TUG1 alleviated cardiomyocyte hypertrophy in Ang II-induced cardiomyocytes through targeting the miR-497/MEF2C axis.
It was reported that TUG1 enhanced cardiac fibroblast transformation to myofibroblast by sponging miR-29c under hypoxia [24]. TUG1 had been found to play a crucial role in hypoxia-induced myocardial cell damage via regulating B-cell lymphoma 2 interacting protein 3 (Binp3) by sponging miR-145-5p [25]. In the current study, our data demonstrated that TUG1 was upregulated in the TAC rat model and Ang II-induced cardiomyocytes, and TUG1 knockdown attenuated Ang II-induced cardiomyocyte hypertrophy in agreement with the previous study [10].
Then, we used online database LncBase Predicted v.2 to help identify miRNAs that potentially bind to TUG1. Among these candidates, we selected miR-497 for further investigation, and we first verified that TUG1 acted as a miR-497 sponge. miR-497, a member of the miR-15 family, has been identified as a tumor-suppressive miRNA in a variety of human cancers, such as breast cancer [26], non-small cell lung cancer [27] and osteosarcoma [28]. Moreover, miR-497 was uncovered to be involved in the postnatal quiescence of skeletal muscle stem cells and osteoblast differentiation by targeting BMP signaling [29,30]. A previous document reported that miR-497 acted as a crucial regulator in cardiomyocyte mitotic arrest [31]. In the present study, our data revealed that miR-497 expression was downregulated in the TAC rat model and Ang II-induced cardiomyocytes, in accordance with a recent study [14]. Similar with the findings by Xiao et al. [14], we demonstrated that the enforced level of miR-497 rescued cardiomyocyte hypertrophy in vitro. Moreover, we first uncovered that miR-497 was a functional mediator of TUG1 in regulating Ang II-induced cardiomyocyte hypertrophy.
miRNAs are widely accepted to direct posttranscriptional repression of mRNA targets in cellular pathophysiology processes. Therefore, microT-CDS software was used to predict the potential targets of miR-497. Among these candidates, MEF2C was interesting in the present study owing to the crucial involvement of MEF2C in heart development, cardiomyocyte reprogramming and hypertrophic cardiomyopathy [32,33,34]. MEF2C has been emphasized as a signal-responsive mediator of the cardiac transcriptional program and plays a key role in the development of cardiac hypertrophy [35,36]. In addition, MEF2C deficiency alleviated the left ventricular hypertrophy induced by pressure overload through regulating the mTOR/S6K pathway in mice [37]. A previous study identified that MEF2C activation triggered by insulin-like growth factor-1 (IFG-1) mediated the pro-hypertrophic function of IGF-1 on the expression of cardiac gene [38]. In addition, the calcineurin-MEF2C pathway was demonstrated to participate in cardiac hypertrophy induced by endoplasmic reticulum stress in neonatal rat cardiomyocytes [39]. In the present study, we first verified that MEF2C was directly targeted and repressed by miR-497 in cardiomyocytes. Results of this study indicated that MEF2C was a functionally important target of miR-497 in modulating Ang II-induced cardiomyocyte hypertrophy. Similarly, Sato et al. reported that miR-214-3p played a repressive role in cardiac hypertrophy induced by Ang II via targeting MEF2C [29]. Xiao et al. demonstrated that the high level of miR-497 suppressed myocardial hypertrophy in vitro and in vivo through targeting Sirt4 [14]. More interestingly, we were first to validate that TUG1 modulated MEF2C expression through sponging miR-497. In addition, Zhao et al. highlighted that the silencing of TUG1 ameliorated diabetic cardiomyopathy-induced diastolic dysfunction via directly targeting miR-499-5p [40].
5 Conclusion
Our present study suggested that TUG1 knockdown alleviated Ang II-induced hypertrophy in cardiomyocytes at least in part through targeting the miR-497/MEF2C axis. The clinical significance of TUG1 and its potential value as a therapeutic target should be further explored.
Appendix

The establishment of cardiac hypertrophy model by TAC in vivo and Ang II treatment in vitro. Analysis for LVEDD (a), LVESD (b), LVESP (c), LVEDP (d) and FS (e), heart weight/body weight ratio (f) in the TAC rat model. mRNA levels of ANP, BNP and β-MHC by qRT-PCR (g), and their protein levels by western blot (h) in the TAC rat model. Primary cardiomyocytes were treated with 1 mM of Ang II for 48 h, followed by the detection of ANP, BNP and β-MHC mRNA levels by qRT-PCR (i) and their protein levels by western blot (j). *P < 0.05.

The effect of TUG1 on the expression of six miRNAs in cardiomyocytes. Relative expression levels of miR-93, miR-142-3p, miR-103, miR-631-5p, miR-16-5p and miR-497 by qRT-PCR in cardiomyocytes transfected with si-NC or si-TUG1. *P < 0.05.
-
Funding: The authors state no funding involved.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016;97:245–62.10.1016/j.yjmcc.2016.06.001Search in Google Scholar PubMed
[2] Anastasakis A, Theopistou A, Rigopoulos A, Kotsiopoulou C, Georgopoulos S, Fragakis K, et al. Sudden cardiac death: investigation of the classical risk factors in a community-based hypertrophic cardiomyopathy cohort. Hellenic J Cardiol. 2013;54(4):281–8.Search in Google Scholar
[3] Oka T, Akazawa H, Naito AT, Komuro I. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res. 2014;114(3):565–71. 10.1161/circresaha.114.300507.Search in Google Scholar PubMed
[4] Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128(1):191–227. 10.1016/j.pharmthera.2010.04.005.Search in Google Scholar PubMed
[5] Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010;61(4):269–80. 10.1016/j.phrs.2009.11.012.Search in Google Scholar PubMed
[6] Kirk JM, Kim SO, Inoue K, Smola MJ, Lee DM, Schertzer MD, et al. Functional classification of long non-coding RNAs by k-mer content. Nat Genet. 2018;50(10):1474–82. 10.1038/s41588-018-0207-8.Search in Google Scholar PubMed PubMed Central
[7] Kataoka M, Wang D-Z. Non-coding RNAs including miRNAs and lncRNAs in cardiovascular biology and disease. Cells. 2014;3(3):883–98. 10.3390/cells3030883.Search in Google Scholar PubMed PubMed Central
[8] Wang K, Liu F, Zhou L-Y, Long B, Yuan S-M, Wang Y, et al. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res. 2014;114(9):1377–88. 10.1161/CIRCRESAHA.114.302476.Search in Google Scholar PubMed
[9] Wang Z, Zhang X-J, Ji Y-X, Zhang P, Deng K-Q, Gong J, et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med. 2016;22(10):1131–9. 10.1038/nm.4179.Search in Google Scholar PubMed PubMed Central
[10] Zou X, Wang J, Tang L, Wen Q. lncRNA TUG1 contributes to cardiac hypertrophy via regulating miR-29b-3p. In Vitro Cell Dev Biol Anim. 2019;55(7):482–90. 10.1007/s11626-019-00368-x.Search in Google Scholar PubMed
[11] Iwakawa HO, Tomari Y. The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol. 2015;25(11):651–65. 10.1016/j.tcb.2015.07.011.Search in Google Scholar PubMed
[12] Wang J, Liew OW, Richards AM, Chen YT. Overview of microRNAs in cardiac hypertrophy, fibrosis, and apoptosis. Int J Mol Sci. 2016;17(5):749. 10.3390/ijms17050749.Search in Google Scholar PubMed PubMed Central
[13] Tijsen AJ, van der Made I, van den Hoogenhof MM, Wijnen WJ, van Deel ED, de Groot NE, et al. The microRNA-15 family inhibits the TGFbeta-pathway in the heart. Cardiovasc Res. 2014;104(1):61–71. 10.1093/cvr/cvu184.Search in Google Scholar PubMed
[14] Xiao Y, Zhang X, Fan S, Cui G, Shen Z. MicroRNA-497 inhibits cardiac hypertrophy by targeting Sirt4. PLoS One. 2016;11(12):e0168078. 10.1371/journal.pone.0168078.Search in Google Scholar PubMed PubMed Central
[15] Chen S, Wang M, Yang H, Mao L, He Q, Jin H, et al. lncRNA TUG1 sponges microRNA-9 to promote neurons apoptosis by up-regulated Bcl2l11 under ischemia. Biochem Biophys Res Commun. 2017;485(1):167–73. 10.1016/j.bbrc.2017.02.043.Search in Google Scholar PubMed
[16] Duan L-J, Ding M, Hou L-J, Cui Y-T, Li C-J, Yu D-M. Long noncoding RNA TUG1 alleviates extracellular matrix accumulation via mediating microRNA-377 targeting of PPARγ in diabetic nephropathy. Biochem Biophys Res Commun. 2017;484(3):598–604. 10.1016/j.bbrc.2017.01.145.Search in Google Scholar PubMed
[17] deAlmeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. J Vis Exp. 2010;38:e1729.10.3791/1729Search in Google Scholar PubMed PubMed Central
[18] Liu BL, Cheng M, Hu S, Wang S, Wang L, Tu X, et al. Overexpression of miR-142-3p improves mitochondrial function in cardiac hypertrophy. Biomed Pharmacother. 2018;108:1347–56. 10.1016/j.biopha.2018.09.146.Search in Google Scholar PubMed
[19] Tham YK, Bernardo BC, Ooi JY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol. 2015;89(9):1401–38. 10.1007/s00204-015-1477-x.Search in Google Scholar PubMed
[20] Kurdi M, Booz GW. New take on the role of angiotensin II in cardiac hypertrophy and fibrosis. Hypertension. 2011;57(6):1034–8. 10.1161/hypertensionaha.111.172700.Search in Google Scholar
[21] Xiao L, Gu Y, Sun Y, Chen J, Wang X, Zhang Y, et al. The long noncoding RNA XIST regulates cardiac hypertrophy by targeting miR-101. J Cell Physiol. 2019;234(8):13680–92. 10.1002/jcp.28047.Search in Google Scholar PubMed
[22] Liu L, An X, Li Z, Song Y, Li L, Zuo S, et al. The H19 long noncoding RNA is a novel negative regulator of cardiomyocyte hypertrophy. Cardiovasc Res. 2016;111(1):56–65. 10.1093/cvr/cvw078.Search in Google Scholar PubMed
[23] Chen Y, Liu X, Chen L, Chen W, Zhang Y, Chen J, et al. The long noncoding RNA XIST protects cardiomyocyte hypertrophy by targeting miR-330-3p. Biochem Biophys Res Commun. 2018;505(3):807–15. 10.1016/j.bbrc.2018.09.135.Search in Google Scholar PubMed
[24] Zhu Y, Feng Z, Jian Z, Xiao Y. Long noncoding RNA TUG1 promotes cardiac fibroblast transformation to myofibroblasts via miR‑29c in chronic hypoxia. Mol Med Rep. 2018;18(3):3451–60. 10.3892/mmr.2018.9327.Search in Google Scholar PubMed
[25] Wu Z, Zhao S, Li C, Liu C. lncRNA TUG1 serves an important role in hypoxia-induced myocardial cell injury by regulating the miR‑145‑5p‑Binp3 axis. Mol Med Rep. 2018;17(2):2422–30. 10.3892/mmr.2017.8116.Search in Google Scholar PubMed PubMed Central
[26] Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang Y, et al. Analysis of miR-195 and miR-497 expression, regulation and role in breast cancer. Clin Cancer Res. 2011;17(7):1722–30. 10.1158/1078-0432.ccr-10-1800.Search in Google Scholar
[27] Zhao WY, Wang Y, An ZJ, Shi CG, Zhu GA, Wang B, et al. Downregulation of miR-497 promotes tumor growth and angiogenesis by targeting HDGF in non-small cell lung cancer. Biochem Biophys Res Commun. 2013;435(3):466–71. 10.1016/j.bbrc.2013.05.010.Search in Google Scholar PubMed
[28] Song J, Wu X, Liu F, Li M, Sun Y, Wang Y, et al. Long non-coding RNA PVT1 promotes glycolysis and tumor progression by regulating miR-497/HK2 axis in osteosarcoma. Biochem Biophys Res Commun. 2017;490(2):217–24. 10.1016/j.bbrc.2017.06.024.Search in Google Scholar PubMed
[29] Sato T, Yamamoto T, Sehara-Fujisawa A. miR-195/497 induce postnatal quiescence of skeletal muscle stem cells. Nat Commun. 2014;5:4597. 10.1038/ncomms5597.Search in Google Scholar PubMed
[30] Grünhagen J, Bhushan R, Degenkolbe E, Jäger M, Knaus P, Mundlos S, et al. miR-497–195 cluster microRNAs regulate osteoblast differentiation by targeting BMP signaling. J Bone Miner Res. 2015;30(5):796–808.10.1002/jbmr.2412Search in Google Scholar PubMed
[31] Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, et al. miR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109(6):670–9. 10.1161/circresaha.111.248880.Search in Google Scholar
[32] Phan D, Rasmussen TL, Nakagawa O, McAnally J, Gottlieb PD, Tucker PW, et al. BOP, a regulator of right ventricular heart development, is a direct transcriptional target of MEF2C in the developing heart. Development. 2005;132(11):2669–78. 10.1242/dev.01849.Search in Google Scholar
[33] Wang L, Liu Z, Yin C, Asfour H, Chen O, Li Y, et al. Stoichiometry of Gata4, Mef2c, and Tbx5 influences the efficiency and quality of induced cardiac myocyte reprogramming. Circ Res. 2015;116(2):237–44. 10.1161/circresaha.116.305547.Search in Google Scholar
[34] Alonso-Montes C, Naves-Diaz M, Fernandez-Martin JL, Rodriguez-Reguero J, Moris C, Coto E, et al. New polymorphisms in human MEF2C gene as potential modifier of hypertrophic cardiomyopathy. Mol Biol Rep. 2012;39(9):8777–85.10.1007/s11033-012-1740-7Search in Google Scholar
[35] Molkentin JD, Markham BE. Myocyte-specific enhancer-binding factor (MEF-2) regulates alpha-cardiac myosin heavy chain gene expression in vitro and in vivo. J Biol Chem. 1993;268(26):19512–20.10.1016/S0021-9258(19)36545-7Search in Google Scholar
[36] Tang CM, Liu FZ, Zhu JN, Fu YH, Lin QX, Deng CY, et al. Myocyte-specific enhancer factor 2C: a novel target gene of miR-214-3p in suppressing angiotensin II-induced cardiomyocyte hypertrophy. Sci Rep. 2016;6:36146. 10.1038/srep36146.Search in Google Scholar PubMed PubMed Central
[37] Pereira AH, Clemente CF, Cardoso AC, Theizen TH, Rocco SA, Judice CC, et al. MEF2C silencing attenuates load-induced left ventricular hypertrophy by modulating mTOR/S6K pathway in mice. PLoS One. 2009;4(12):e8472. 10.1371/journal.pone.0008472.Search in Google Scholar PubMed PubMed Central
[38] Munoz JP, Collao A, Chiong M, Maldonado C, Adasme T, Carrasco L, et al. The transcription factor MEF2C mediates cardiomyocyte hypertrophy induced by IGF-1 signaling. Biochem Biophys Res Commun. 2009;388(1):155–60. 10.1016/j.bbrc.2009.07.147.Search in Google Scholar PubMed
[39] Zhang ZY, Liu XH, Hu WC, Rong F, Wu XD. The calcineurin-myocyte enhancer factor 2c pathway mediates cardiac hypertrophy induced by endoplasmic reticulum stress in neonatal rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2010;298(5):H1499–509. 10.1152/ajpheart.00980.2009.Search in Google Scholar PubMed
[40] Zhao L, Li WG, Zhao H. Inhibition of long non-coding RNA TUG1 protects against diabetic cardiomyopathy induced diastolic dysfunction by regulating miR-499-5p. Am J Transl Res. 2020;12(3):718–30.Search in Google Scholar
© 2021 Guorong Zhang and Xinghua Ni, published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells
Articles in the same Issue
- Biomedical Sciences
- Research progress on the mechanism of orexin in pain regulation in different brain regions
- Adriamycin-resistant cells are significantly less fit than adriamycin-sensitive cells in cervical cancer
- Exogenous spermidine affects polyamine metabolism in the mouse hypothalamus
- Iris metastasis of diffuse large B-cell lymphoma misdiagnosed as primary angle-closure glaucoma: A case report and review of the literature
- LncRNA PVT1 promotes cervical cancer progression by sponging miR-503 to upregulate ARL2 expression
- Two new inflammatory markers related to the CURB-65 score for disease severity in patients with community-acquired pneumonia: The hypersensitive C-reactive protein to albumin ratio and fibrinogen to albumin ratio
- Circ_0091579 enhances the malignancy of hepatocellular carcinoma via miR-1287/PDK2 axis
- Silencing XIST mitigated lipopolysaccharide (LPS)-induced inflammatory injury in human lung fibroblast WI-38 cells through modulating miR-30b-5p/CCL16 axis and TLR4/NF-κB signaling pathway
- Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats
- ABCB1 polymorphism in clopidogrel-treated Montenegrin patients
- Metabolic profiling of fatty acids in Tripterygium wilfordii multiglucoside- and triptolide-induced liver-injured rats
- miR-338-3p inhibits cell growth, invasion, and EMT process in neuroblastoma through targeting MMP-2
- Verification of neuroprotective effects of alpha-lipoic acid on chronic neuropathic pain in a chronic constriction injury rat model
- Circ_WWC3 overexpression decelerates the progression of osteosarcoma by regulating miR-421/PDE7B axis
- Knockdown of TUG1 rescues cardiomyocyte hypertrophy through targeting the miR-497/MEF2C axis
- MiR-146b-3p protects against AR42J cell injury in cerulein-induced acute pancreatitis model through targeting Anxa2
- miR-299-3p suppresses cell progression and induces apoptosis by downregulating PAX3 in gastric cancer
- Diabetes and COVID-19
- Discovery of novel potential KIT inhibitors for the treatment of gastrointestinal stromal tumor
- TEAD4 is a novel independent predictor of prognosis in LGG patients with IDH mutation
- circTLK1 facilitates the proliferation and metastasis of renal cell carcinoma by regulating miR-495-3p/CBL axis
- microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury
- Long noncoding RNA TUG1 regulates degradation of chondrocyte extracellular matrix via miR-320c/MMP-13 axis in osteoarthritis
- Duodenal adenocarcinoma with skin metastasis as initial manifestation: A case report
- Effects of Loofah cylindrica extract on learning and memory ability, brain tissue morphology, and immune function of aging mice
- Recombinant Bacteroides fragilis enterotoxin-1 (rBFT-1) promotes proliferation of colorectal cancer via CCL3-related molecular pathways
- Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis
- Gene therapy in PIDs, hemoglobin, ocular, neurodegenerative, and hemophilia B disorders
- Downregulation of circ_0037655 impedes glioma formation and metastasis via the regulation of miR-1229-3p/ITGB8 axis
- Vitamin D deficiency and cardiovascular risk in type 2 diabetes population
- Circ_0013359 facilitates the tumorigenicity of melanoma by regulating miR-136-5p/RAB9A axis
- Mechanisms of circular RNA circ_0066147 on pancreatic cancer progression
- lncRNA myocardial infarction-associated transcript (MIAT) knockdown alleviates LPS-induced chondrocytes inflammatory injury via regulating miR-488-3p/sex determining region Y-related HMG-box 11 (SOX11) axis
- Identification of circRNA circ-CSPP1 as a potent driver of colorectal cancer by directly targeting the miR-431/LASP1 axis
- Hyperhomocysteinemia exacerbates ischemia-reperfusion injury-induced acute kidney injury by mediating oxidative stress, DNA damage, JNK pathway, and apoptosis
- Potential prognostic markers and significant lncRNA–mRNA co-expression pairs in laryngeal squamous cell carcinoma
- Gamma irradiation-mediated inactivation of enveloped viruses with conservation of genome integrity: Potential application for SARS-CoV-2 inactivated vaccine development
- ADHFE1 is a correlative factor of patient survival in cancer
- The association of transcription factor Prox1 with the proliferation, migration, and invasion of lung cancer
- Is there a relationship between the prevalence of autoimmune thyroid disease and diabetic kidney disease?
- Immunoregulatory function of Dictyophora echinovolvata spore polysaccharides in immunocompromised mice induced by cyclophosphamide
- T cell epitopes of SARS-CoV-2 spike protein and conserved surface protein of Plasmodium malariae share sequence homology
- Anti-obesity effect and mechanism of mesenchymal stem cells influence on obese mice
- Long noncoding RNA HULC contributes to paclitaxel resistance in ovarian cancer via miR-137/ITGB8 axis
- Glucocorticoids protect HEI-OC1 cells from tunicamycin-induced cell damage via inhibiting endoplasmic reticulum stress
- Prognostic value of the neutrophil-to-lymphocyte ratio in acute organophosphorus pesticide poisoning
- Gastroprotective effects of diosgenin against HCl/ethanol-induced gastric mucosal injury through suppression of NF-κβ and myeloperoxidase activities
- Silencing of LINC00707 suppresses cell proliferation, migration, and invasion of osteosarcoma cells by modulating miR-338-3p/AHSA1 axis
- Successful extracorporeal membrane oxygenation resuscitation of patient with cardiogenic shock induced by phaeochromocytoma crisis mimicking hyperthyroidism: A case report
- Effects of miR-185-5p on replication of hepatitis C virus
- Lidocaine has antitumor effect on hepatocellular carcinoma via the circ_DYNC1H1/miR-520a-3p/USP14 axis
- Primary localized cutaneous nodular amyloidosis presenting as lymphatic malformation: A case report
- Multimodal magnetic resonance imaging analysis in the characteristics of Wilson’s disease: A case report and literature review
- Therapeutic potential of anticoagulant therapy in association with cytokine storm inhibition in severe cases of COVID-19: A case report
- Neoadjuvant immunotherapy combined with chemotherapy for locally advanced squamous cell lung carcinoma: A case report and literature review
- Rufinamide (RUF) suppresses inflammation and maintains the integrity of the blood–brain barrier during kainic acid-induced brain damage
- Inhibition of ADAM10 ameliorates doxorubicin-induced cardiac remodeling by suppressing N-cadherin cleavage
- Invasive ductal carcinoma and small lymphocytic lymphoma/chronic lymphocytic leukemia manifesting as a collision breast tumor: A case report and literature review
- Clonal diversity of the B cell receptor repertoire in patients with coronary in-stent restenosis and type 2 diabetes
- CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression
- WDR74 promotes proliferation and metastasis in colorectal cancer cells through regulating the Wnt/β-catenin signaling pathway
- Down-regulation of IGHG1 enhances Protoporphyrin IX accumulation and inhibits hemin biosynthesis in colorectal cancer by suppressing the MEK-FECH axis
- Curcumin suppresses the progression of gastric cancer by regulating circ_0056618/miR-194-5p axis
- Scutellarin-induced A549 cell apoptosis depends on activation of the transforming growth factor-β1/smad2/ROS/caspase-3 pathway
- lncRNA NEAT1 regulates CYP1A2 and influences steroid-induced necrosis
- A two-microRNA signature predicts the progression of male thyroid cancer
- Isolation of microglia from retinas of chronic ocular hypertensive rats
- Changes of immune cells in patients with hepatocellular carcinoma treated by radiofrequency ablation and hepatectomy, a pilot study
- Calcineurin Aβ gene knockdown inhibits transient outward potassium current ion channel remodeling in hypertrophic ventricular myocyte
- Aberrant expression of PI3K/AKT signaling is involved in apoptosis resistance of hepatocellular carcinoma
- Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer
- circ_CHFR regulates ox-LDL-mediated cell proliferation, apoptosis, and EndoMT by miR-15a-5p/EGFR axis in human brain microvessel endothelial cells
- Resveratrol pretreatment mitigates LPS-induced acute lung injury by regulating conventional dendritic cells’ maturation and function
- Ubiquitin-conjugating enzyme E2T promotes tumor stem cell characteristics and migration of cervical cancer cells by regulating the GRP78/FAK pathway
- Carriage of HLA-DRB1*11 and 1*12 alleles and risk factors in patients with breast cancer in Burkina Faso
- Protective effect of Lactobacillus-containing probiotics on intestinal mucosa of rats experiencing traumatic hemorrhagic shock
- Glucocorticoids induce osteonecrosis of the femoral head through the Hippo signaling pathway
- Endothelial cell-derived SSAO can increase MLC20 phosphorylation in VSMCs
- Downregulation of STOX1 is a novel prognostic biomarker for glioma patients
- miR-378a-3p regulates glioma cell chemosensitivity to cisplatin through IGF1R
- The molecular mechanisms underlying arecoline-induced cardiac fibrosis in rats
- TGF-β1-overexpressing mesenchymal stem cells reciprocally regulate Th17/Treg cells by regulating the expression of IFN-γ
- The influence of MTHFR genetic polymorphisms on methotrexate therapy in pediatric acute lymphoblastic leukemia
- Red blood cell distribution width-standard deviation but not red blood cell distribution width-coefficient of variation as a potential index for the diagnosis of iron-deficiency anemia in mid-pregnancy women
- Small cell neuroendocrine carcinoma expressing alpha fetoprotein in the endometrium
- Superoxide dismutase and the sigma1 receptor as key elements of the antioxidant system in human gastrointestinal tract cancers
- Molecular characterization and phylogenetic studies of Echinococcus granulosus and Taenia multiceps coenurus cysts in slaughtered sheep in Saudi Arabia
- ITGB5 mutation discovered in a Chinese family with blepharophimosis-ptosis-epicanthus inversus syndrome
- ACTB and GAPDH appear at multiple SDS-PAGE positions, thus not suitable as reference genes for determining protein loading in techniques like Western blotting
- Facilitation of mouse skin-derived precursor growth and yield by optimizing plating density
- 3,4-Dihydroxyphenylethanol ameliorates lipopolysaccharide-induced septic cardiac injury in a murine model
- Downregulation of PITX2 inhibits the proliferation and migration of liver cancer cells and induces cell apoptosis
- Expression of CDK9 in endometrial cancer tissues and its effect on the proliferation of HEC-1B
- Novel predictor of the occurrence of DKA in T1DM patients without infection: A combination of neutrophil/lymphocyte ratio and white blood cells
- Investigation of molecular regulation mechanism under the pathophysiology of subarachnoid hemorrhage
- miR-25-3p protects renal tubular epithelial cells from apoptosis induced by renal IRI by targeting DKK3
- Bioengineering and Biotechnology
- Green fabrication of Co and Co3O4 nanoparticles and their biomedical applications: A review
- Agriculture
- Effects of inorganic and organic selenium sources on the growth performance of broilers in China: A meta-analysis
- Crop-livestock integration practices, knowledge, and attitudes among smallholder farmers: Hedging against climate change-induced shocks in semi-arid Zimbabwe
- Food Science and Nutrition
- Effect of food processing on the antioxidant activity of flavones from Polygonatum odoratum (Mill.) Druce
- Vitamin D and iodine status was associated with the risk and complication of type 2 diabetes mellitus in China
- Diversity of microbiota in Slovak summer ewes’ cheese “Bryndza”
- Comparison between voltammetric detection methods for abalone-flavoring liquid
- Composition of low-molecular-weight glutenin subunits in common wheat (Triticum aestivum L.) and their effects on the rheological properties of dough
- Application of culture, PCR, and PacBio sequencing for determination of microbial composition of milk from subclinical mastitis dairy cows of smallholder farms
- Investigating microplastics and potentially toxic elements contamination in canned Tuna, Salmon, and Sardine fishes from Taif markets, KSA
- From bench to bar side: Evaluating the red wine storage lesion
- Establishment of an iodine model for prevention of iodine-excess-induced thyroid dysfunction in pregnant women
- Plant Sciences
- Characterization of GMPP from Dendrobium huoshanense yielding GDP-D-mannose
- Comparative analysis of the SPL gene family in five Rosaceae species: Fragaria vesca, Malus domestica, Prunus persica, Rubus occidentalis, and Pyrus pyrifolia
- Identification of leaf rust resistance genes Lr34 and Lr46 in common wheat (Triticum aestivum L. ssp. aestivum) lines of different origin using multiplex PCR
- Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry
- Morphological structures and histochemistry of roots and shoots in Myricaria laxiflora (Tamaricaceae)
- Transcriptome analysis of resistance mechanism to potato wart disease
- In silico analysis of glycosyltransferase 2 family genes in duckweed (Spirodela polyrhiza) and its role in salt stress tolerance
- Comparative study on growth traits and ions regulation of zoysiagrasses under varied salinity treatments
- Role of MS1 homolog Ntms1 gene of tobacco infertility
- Biological characteristics and fungicide sensitivity of Pyricularia variabilis
- In silico/computational analysis of mevalonate pyrophosphate decarboxylase gene families in Campanulids
- Identification of novel drought-responsive miRNA regulatory network of drought stress response in common vetch (Vicia sativa)
- How photoautotrophy, photomixotrophy, and ventilation affect the stomata and fluorescence emission of pistachios rootstock?
- Apoplastic histochemical features of plant root walls that may facilitate ion uptake and retention
- Ecology and Environmental Sciences
- The impact of sewage sludge on the fungal communities in the rhizosphere and roots of barley and on barley yield
- Domestication of wild animals may provide a springboard for rapid variation of coronavirus
- Response of benthic invertebrate assemblages to seasonal and habitat condition in the Wewe River, Ashanti region (Ghana)
- Molecular record for the first authentication of Isaria cicadae from Vietnam
- Twig biomass allocation of Betula platyphylla in different habitats in Wudalianchi Volcano, northeast China
- Animal Sciences
- Supplementation of probiotics in water beneficial growth performance, carcass traits, immune function, and antioxidant capacity in broiler chickens
- Predators of the giant pine scale, Marchalina hellenica (Gennadius 1883; Hemiptera: Marchalinidae), out of its natural range in Turkey
- Honey in wound healing: An updated review
- NONMMUT140591.1 may serve as a ceRNA to regulate Gata5 in UT-B knockout-induced cardiac conduction block
- Radiotherapy for the treatment of pulmonary hydatidosis in sheep
- Retraction
- Retraction of “Long non-coding RNA TUG1 knockdown hinders the tumorigenesis of multiple myeloma by regulating microRNA-34a-5p/NOTCH1 signaling pathway”
- Special Issue on Reuse of Agro-Industrial By-Products
- An effect of positional isomerism of benzoic acid derivatives on antibacterial activity against Escherichia coli
- Special Issue on Computing and Artificial Techniques for Life Science Applications - Part II
- Relationship of Gensini score with retinal vessel diameter and arteriovenous ratio in senile CHD
- Effects of different enantiomers of amlodipine on lipid profiles and vasomotor factors in atherosclerotic rabbits
- Establishment of the New Zealand white rabbit animal model of fatty keratopathy associated with corneal neovascularization
- lncRNA MALAT1/miR-143 axis is a potential biomarker for in-stent restenosis and is involved in the multiplication of vascular smooth muscle cells

