Combined application of chitosan-boron and chitosan-silicon nano-fertilizers with soybean protein hydrolysate to enhance rice growth and yield
-
Apiradee Uthairatanakij
, Apichai Jenjob
Abstract
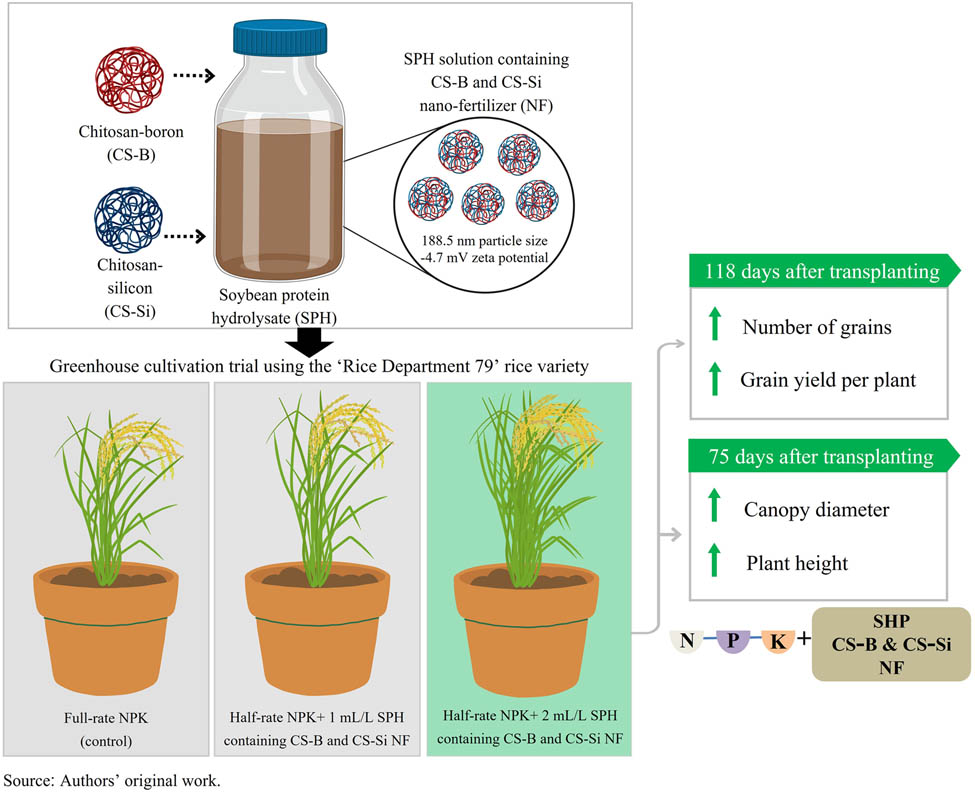
The effects of chitosan-boron (CS-B) and chitosan-silicon (CS-Si) combined with soybean protein hydrolysate (SPH) on the growth and yield of Rice Department 79 (RD 79) variety plants in a greenhouse were examined. Particle sizes were 331.9 nm (CS-B), 310.4 nm (CS-Si), and 188.5 nm (SPH solution containing CS-B and CS-Si), with zeta potentials of 30.8, 25.3, and −4.7 mV, respectively. Scanning electron microscopy and Fourier transform infrared spectroscopy confirmed the surface morphology and characteristic functional groups of these nano-fertilizers. A nitrogen–phosphorus–potassium (NPK) fertilizer was applied to rice plants at 1 g/pot (full rate) via the soil at 30 and 60 days after transplanting (DAT), and it served as the control group. The other treatments were applied with half-rate NPK fertilizer (0.5 g/pot) at the same time points, combined with a foliar spray (200 mL/plant) of a solution containing CS-B (0.5% w/v), CS-Si (0.1% w/v), and SPH (0.5:2:100 v/v/v ratio) diluted to final concentrations of 1 and 2 mL/L. At 75 DAT, applying half-rate NPK with the 2 mL/L SPH solution containing the CS-B and CS-Si nano-fertilizer significantly increased the canopy diameter (42 ± 2 cm) and plant height (129 ± 3 cm) compared to the full-rate NPK treatment (p < 0.05). At 118 DAT, this treatment also increased the number of rice grains (215 ± 14 grains) and grain yield per plant (127 ± 16 g) (p < 0.05). These results suggested that the CS-B and CS-Si nano-fertilizers conjugated with SPH could be a novel technique to enhance RD 79 rice growth and yield by reducing fertilizer usage.
Graphical abstract
Abbreviations
- B
-
boron
- CS
-
chitosan
- DAT
-
days after transplanting
- NPK
-
nitrogen–phosphorus–potassium
- RD 79
-
rice department 79
- Si
-
silicon
- SPH
-
soybean protein hydrolysate
1 Introduction
Rice (Oryza sativa L.) is an important food crop, especially in Asia [1]. In Thailand, rice production is important for both domestic and export markets. Rice Department 79 (RD 79) is a popular variety among farmers due to its high yield, disease resistance, and drought tolerance. Although macronutrients are important, micronutrients such as boron (B) and silicon (Si) are highly effective fertilizers to enhance rice growth and development. B is an essential micronutrient that plays a role in the cell wall structure, cell extension, photosynthesis, sugar translocation, carbohydrate metabolism, phenolics, polyamine accumulation, seed and pollen germination, and fruit development [2,3]. Deficiency of B has been shown to inhibit plant growth and development by affecting pollen germination, seed germination, fruit set, and yield [4]. B requirement for monocots is 5–10 mg kg−1, while that of dicots is 20–70 mg kg−1 [5]. Even though rice is less susceptible to B deficiency than dicotyledonous plants, B is still necessary for its growth, yield, and grain quality [6]. Similarly, Si has numerous beneficial effects on plant growth, especially under biotic or abiotic stress [7,8]. Si also enhances plant resistance to a variety of diseases [9]. Nonetheless, both B and Si are often applied as macro-sized particles that are sprayed on plant leaves or added to soil. This type of broad nutrient application may be inefficient and may lead to decreased absorption and translocation [10]. Moreover, the excess use of fertilizers can lead to several issues such as soil nutrient loss, soil acidification, and a reduction in the population of beneficial microbes [11].
The chelation of plant nutrients with amino acids has been promoted as a novel fertilizer formulation because it has the advantage of increasing the bioavailability of nutrients when applied to crops in trace amounts, as well as being eco-friendly, improving sustainability, and controlled release [12]. Protein hydrolysate is a biostimulant that contains peptides and free amino acids, which is produced by hydrolyzing proteins from plants or animals with heat, chemicals, or enzymes [13]. Soybean protein hydrolysate (SPH) from soybean meal is one of the plant protein sources that is rich in essential amino acids [14]. SPH has been shown to form complexes and chelates with essential plant nutrients due to its wide variety of amino acid residues with ionic and electron-rich side chains, which in turn improve the stability and solubility of nutrients [15]. However, SPH has some limitations associated with the efficiency of targeted distribution and slow release [16]. Therefore, the application of SPH combined with a nano-fertilizer provides an alternative approach to overcoming the limitations of chelated fertilizers, which would increase the fertilizer efficiency.
Nanoparticles exhibit unique morphological and structural characteristics [17]. The materials used for nanoparticle preparations can be made from inorganic, organic, synthetic, and natural polymers [18]. However, the use of natural polymers is an excellent choice because they are non-toxic, biodegradable, biocompatible, renewable, and environmentally friendly [19,20]. Chitosan (CS) is a linear polymer that contains N-acetyl-d-glucosamine and d-glucosamine linked by β-(1-4) bonds. Chitin is deacetylated to produce CS, which is a commercial product made from shrimp and crab shells. Modi et al. [21] reported that a CS-based nitrogen–phosphorus–potassium (NPK) nano-fertilizer increased the yield of cucumbers grown in a greenhouse. Wang and Nguyen [22] also found that a CS-based zinc (Zn)-B nano-fertilizer was more easily adsorbed by plants and enhanced the growth of coffee seedlings in a greenhouse. CS nanoparticles have demonstrated increased plant growth, seed germination, nutrient uptake, and antibacterial and antifungal activities [23].
The combined effects of CS-B and CS-Si nano-fertilizer with SPH on rice growth and yield have not been previously investigated. Therefore, the objective of this study was to evaluate this novel combination for enhancing the growth and yield of the RD 79 rice variety grown in a greenhouse, with the specific aim of reducing the required application of the conventional NPK fertilizer.
2 Materials and methods
2.1 Materials
CS was obtained from Sinudom Agriculture Products Limited Partnership, Thailand. The degree of deacetylation was 92%, and the molecular weight was 540 kDa. Food-grade sodium tripolyphosphate (TPP) and silicon dioxide were purchased from Krungthepchemi, Thailand. Analytical-grade boric acid and acetic acid were purchased from Merck, Germany. Soybean meal by-product was obtained from the soybean oil industry.
2.2 Preparation of CS-B and CS-Si nanoparticles
CS nanoparticles were prepared according to the modified ionic gelation method described by Nguyen et al. [24] and Saharan et al. [25]. In brief, a 0.1% CS solution was prepared by dissolving 0.1 g of CS powder in 100 mL of 1% acetic acid solution and kept overnight at room temperature. A 0.25% TPP solution was prepared by dissolving 0.25% TPP in deionized water and adding drop-wise to the CS solution at a 1:6 (w/w) ratio to prepare CS nanoparticles. The solution was then stirred at 900 rpm for 60 min at 25°C. The B nano-fertilizer was prepared by adding 0.5% boric acid, and the Si nano-fertilizer was prepared by adding 0.1% silicon dioxide to the CS nanoparticle solution. The samples were freeze-dried and kept in a desiccator until further use.
2.3 Preparation of SPH containing CS-B and CS-Si nano-fertilizers
Soybean meal powder (50 g) was mixed with 500 mL of 6N hydrochloric acid, following a modified procedure of Dzanic et al. [26]. The mixture was extracted at 95°C for 6 h in a water bath. Then, 6N calcium hydroxide was added to stop the reaction. Finally, the mixture was adjusted to a pH of 7 and then filtered using Whatman® filter paper No. 1. The CS-B and CS-Si nanoparticles were added into SPH at a ratio of 0.5:2:100 (by weight) based on the preliminary screening trials and ultrasonicated at 40 kHz for 20 min [27] (stock solution). The elemental composition of SPH containing CS-B and CS-Si nano-fertilizers was determined by atomic absorption spectroscopy (AAS) following the ASTM method (D5373-16), and the composition is shown in Table 1.
Elemental composition of SPH containing CS-B and CS-Si nano-fertilizers, as determined by AAS
| Elemental composition | Content | Unit |
|---|---|---|
| Carbon (C) | 3.21 | % |
| Nitrogen (N) | 0.22 | % |
| Calcium (Ca) | 95.63 | mg/L |
| Boron (B) | 7.54 | mg/L |
| Silicon (Si) | 35.81 | mg/L |
2.4 Characterization of CS-B, CS-Si, and SPH containing CS-B and CS-Si nano-fertilizers
2.4.1 Particle size, polydispersity index (PDI), and zeta potential analyses
The particle size, PDI, and zeta potential of CS-B, CS-Si, and SPH containing CS-B and CS-Si nano-fertilizers were determined by dynamic light scattering (DLS) at 25 and 90° scattering angles using a nanoparticle analyzer (HORIBA, SZ-100, Japan) with the manufacturer’s software.
2.4.2 Scanning electron microscopy (SEM)
CS, CS-B, CS-Si, SPH, and SPH containing CS-B and CS-Si nano-fertilizers were freeze-dried and mounted on stubs by coating them with a gold film on adhesive carbon tape [28]. The morphology was investigated using SEM (JEOL JSM-6610LV, USA) at 100× magnification and an accelerating voltage of 15 kV, with a working distance of 11.5 mm, a spot size of 4.0, and a pixel resolution of 1,200 dpi.
2.4.3 Fourier transform infrared (FTIR) spectroscopy
FTIR spectroscopy was performed to understand the functional groups of CS, CS-B, CS-Si, SPH, and SPH containing CS-B and CS-Si nano-fertilizers. Freeze-dried samples (3 mg) were mixed with potassium bromide powder (100 mg) [29]. The pellets were measured using an FTIR spectrometer (PerkinElmer, Spectrum One, USA), and data were collected from 550 to 4,000 cm−1 with a resolution of 4 cm−1.
2.5 Treatment applications
A greenhouse trial using the RD 79 rice variety was conducted from August 2023 to November 2023 (118 days of growing period) at King Mongkut’s University of Technology Thonburi, Thailand (latitude: 13.57672; longitude: 100.44240). The average air temperature was 32.0 ± 1.4°C, and the relative humidity was 75 ± 2%. Commercial RD 79 rice seeds were obtained from the Rice Department of Thailand and germinated in 104-cell seedling trays filled with a 1:1 (v/v) mixture of commercial peat moss and soil. After 30 days, the seedlings were transplanted into pots (39 cm × 54 cm × 20 cm) containing clay soil. Each pot contained one seedling, with 10 replicates per treatment. All pots were irrigated with tap water (6 L/pot) every 7 days. NPK soil fertilizer (16% NH4, 16% P2O5, and 16% K2O) was applied to the control group at a rate of 1 g/pot at 30 days (tillering stage) and 60 days (booting stage) after transplanting via soil application. The other treatment groups received a half-rate of NPK fertilizer (0.5 g/pot) via soil application in the same growth stages, supplemented with SPH containing CS-B and CS-Si nano-fertilizers applied via foliar spray at a rate of 200 mL/plant at 30 days (tillering stage) and 60 days (booting stage) after transplanting. The experimental treatments are summarized in Table 2.
Experimental treatments and application methods
| Treatment | NPK (soil) (g/pot) | SPH concentration (CS-B and CS-Si) (mL/L) | Nano-fertilizer (foliar) (mL/plant) | Timing (DAT) | Growth stage |
|---|---|---|---|---|---|
| Full-rate NPK (control) | 1 | 0 | 0 | 30 and 60 | Tillering and booting |
| Half-rate NPK + 1 mL/L SPH containing CS-B and CS-Si nano-fertilizers | 0.5 | 1 | 200 | 30 and 60 | Tillering and booting |
| Half-rate NPK + 2 mL/L SPH containing CS-B and CS-Si nano- fertilizers | 0.5 | 2 | 200 | 30 and 60 | Tillering and booting |
2.6 Measurement of rice plant growth and yield parameters
2.6.1 Canopy diameter
The width of each rice clump was measured at its widest point using a measuring tape and recorded in centimeters (cm).
2.6.2 Plant height
The height of the rice plant was measured from the soil surface in the pot to the top of the plant using a measuring tape and recorded in centimeters (cm).
2.6.3 Number and length of panicles per plant
The number of panicles per plant was counted. The length of each panicle on the rice plant was measured from the panicle base to the tip of the panicle using a measuring tape (cm).
2.6.4 Number of rice grains per panicle and yield
The number of rice grains per panicle was determined by counting the total number of seeds per panicle. Seeds of each plant were weighed and recorded as yield (g) per plant.
2.7 Statistical analysis
The experiments were conducted utilizing a completely randomized design with ten replications. All experimental data were analyzed by analysis of variance using version 9.0 of SAS software (SAS Institute, USA). The statistical significance of the mean ± standard deviation (SD) was compared with Duncan’s multiple range test at p < 0.05.
3 Results and discussion
3.1 Characterization of CS-B, CS-Si, and SPH containing CS-B and CS-Si nano-fertilizers
3.1.1 Particle size, PDI, and zeta potential analyses
The ionotropic gelation of CS with TPP was used to prepare CS-B and CS-Si nanoparticles because it was a non-toxic, cheap, and simple method. The synthesized CS-B and CS-Si nanoparticles, and their combined formulation within SPH, were characterized for their particle size and PDI using DLS. The results of this analysis are presented in Table 3. The particle sizes of CS-B and CS-Si nanoparticles were 331.9 and 310.4 nm, respectively. Ha et al. [30] reported that the particle size of the chitosan-based NPK nano-fertilizer produced by ionic gelation of the CS/TPP solution varied between 300 and 750 nm. Swati et al. [31] also reported that the size of the CS-based copper (Cu) nano-fertilizer ranged from 100 to 500 nm. While the CS-B and CS-Si nanoparticle sizes were within the range reported in other studies, the combination of these nanoparticles with SPH resulted in a smaller particle size. However, the SPH-based nano-fertilizer, prepared at a 0.5:2:100 volume ratio of CS-B/CS-Si/SPH following ultrasonication, showed a particle size of 188.5 nm (Table 2). This smaller particle size was explained by the electrostatic interaction between the positively charged chitosan matrices of the CS-B and CS-Si nanoparticles and the negatively charged amino acids and peptides in SPH [32] and the mechanical forces of acoustic cavitation generated during ultrasonication [33]. The decreased particle size can be expected to improve the bio-availability of the fertilizer. Such nanoparticles applied in agriculture, including nano-herbicides, nano-pesticides, and nano-fertilizers, fall within the accepted nanoparticle size range of up to 500 nm [34].
Particle size and PDI of CS-B, CS-Si, and SPH containing CS-B and CS-Si
| Nanoparticles | Particle size (nm) | PDI |
|---|---|---|
| CS-B | 331.9 | 0.4 |
| CS-Si | 310.4 | 0.5 |
| SPH containing CS-B and CS-Si | 188.5 | 0.5 |
The homogeneity of particle size within the dispersed system is indicated by PDI. The lower the PDI, the more homogeneous the nanoparticles are [35]. In this study, the PDI values of CS-B, CS-Si nanoparticles, and SPH containing CS-B and CS-Si nano-fertilizer were 0.4, 0.5, and 0.5, respectively (Table 3). Zeta potential is utilized to forecast the stability of nanoparticles because of van der Waals forces, hydrogen bonding, and electrostatic repulsive forces [36]. Nanoparticles with zeta potential greater than ±30 mV are regarded as exhibiting high stability and avoiding particle aggregation [37]. In this experiment, the zeta potentials of CS-B and CS-Si nanoparticles were 30.8 and 25.3 mV, respectively (Figure 1a and b), suggesting that the CS-B nanoparticles had good stability, while the CS-Si nanoparticles were more susceptible to aggregation. However, the zeta potential of the SPH containing CS-B and CS-Si nano-fertilizer was −4.7 mV (Figure 1c). This shift in zeta potential indicated that the CS-B and CS-Si nanoparticle adsorption onto the SPH was possibly the cause of the nanoparticles’ surface charge change [38].
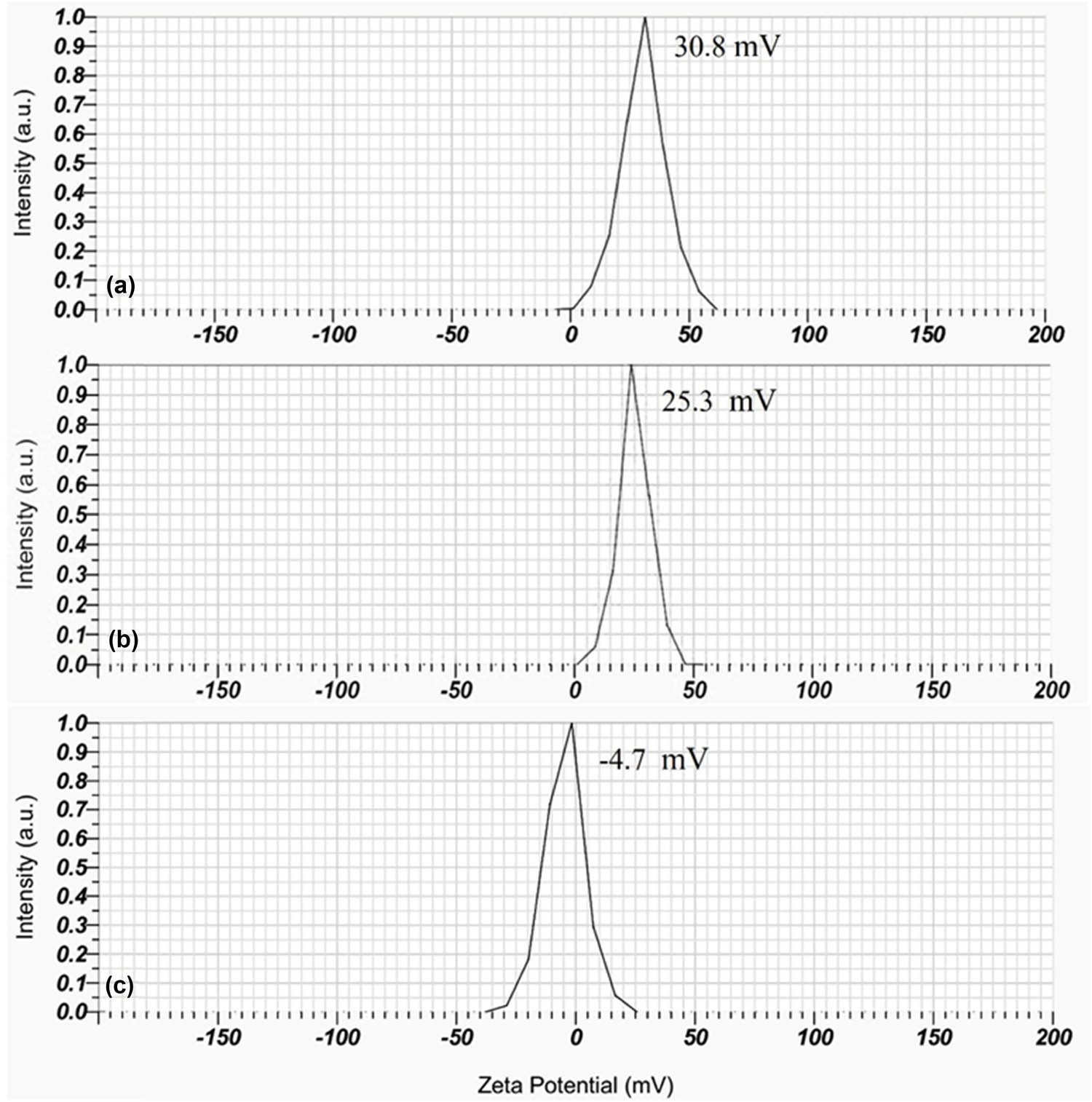
Zeta potentials of CS-B (a), CS-Si (b), and SPH containing CS-B and CS-Si (c). Source: Authors’ original work.
3.1.2 Surface morphology
The surface morphologies of CS, CS-B, and CS-Si nanoparticles were examined by SEM (Figure 2a–c). The results showed that the CS nanoparticles had a smooth surface, were aggregated like rocks, and were angular. A smooth surface of CS nanoparticles indicates a lower surface area compared to the porous surface of CS nanoparticles from other treatments. The strong attractive forces that cause their rock-like aggregation require either a large amount of energy input, such as sonication or surface modification for dispersion [39], which might potentially affect the entrapment of other molecules or nanoparticles. CS chains are held together by a network of hydrogen bonds between the amino and hydroxyl groups on adjacent chains, which is responsible for the ordered structure of the CS nanoparticles [40]. On the other hand, the CS-B nanoparticles exhibited a distinct crystal shape with sharp edges. The interaction of boron with chitosan involves a combination of chemical bonding and intermolecular forces. These interactions influenced the crystallinity of structures. These results are consistent with those of Özakar et al. [41]. The CS-Si nanoparticles showed a rough surface and irregular structures, possibly because the Si atoms disrupt the regular arrangement of CS chains by interfering with the hydrogen bonding network [42]. Moreover, the SPH showed a relatively smooth, film-like surface with visible wrinkles or folds (Figure 3a). The incorporation of CS-B and CS-Si in SPH significantly changed the appearance of SHP. SHP containing CS-B and CS-Si nano-fertilizers showed a rough surface, heterogeneous texture with visible particles and agglomerates, and had a more porous structure than SHP (Figure 3b). The change from the relatively smooth SPH surface to the rough, particulate-laden, and porous structure strongly evidences the successful formation of a nanocomposite. This conjugation disrupted the original SPH morphology, resulting in a new material. These modified structural properties are essential for the controlled release and targeted delivery of the nano-fertilizer system [43].
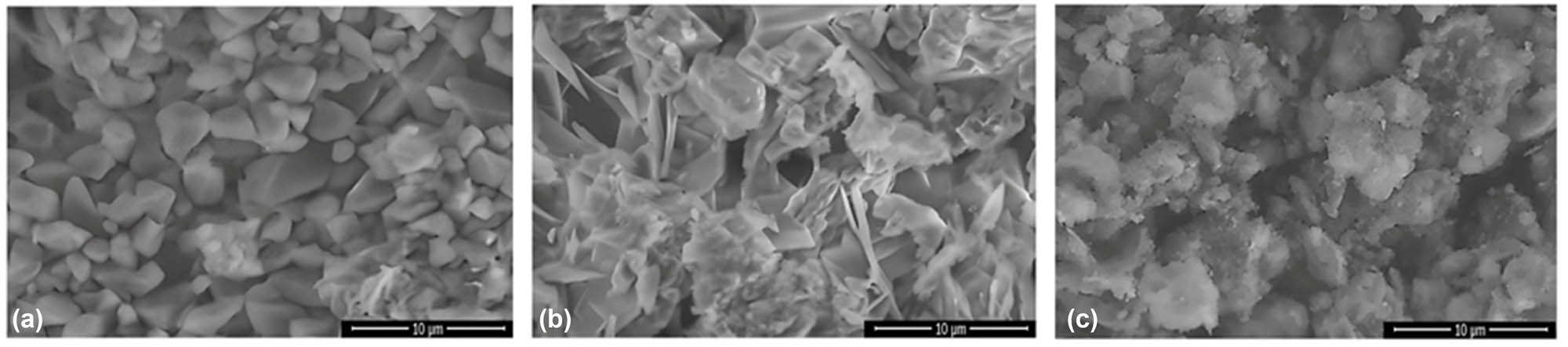
SEM images of CS (a), CS-B (b), and CS-Si (c) nanoparticles. Source: Authors’ original work.
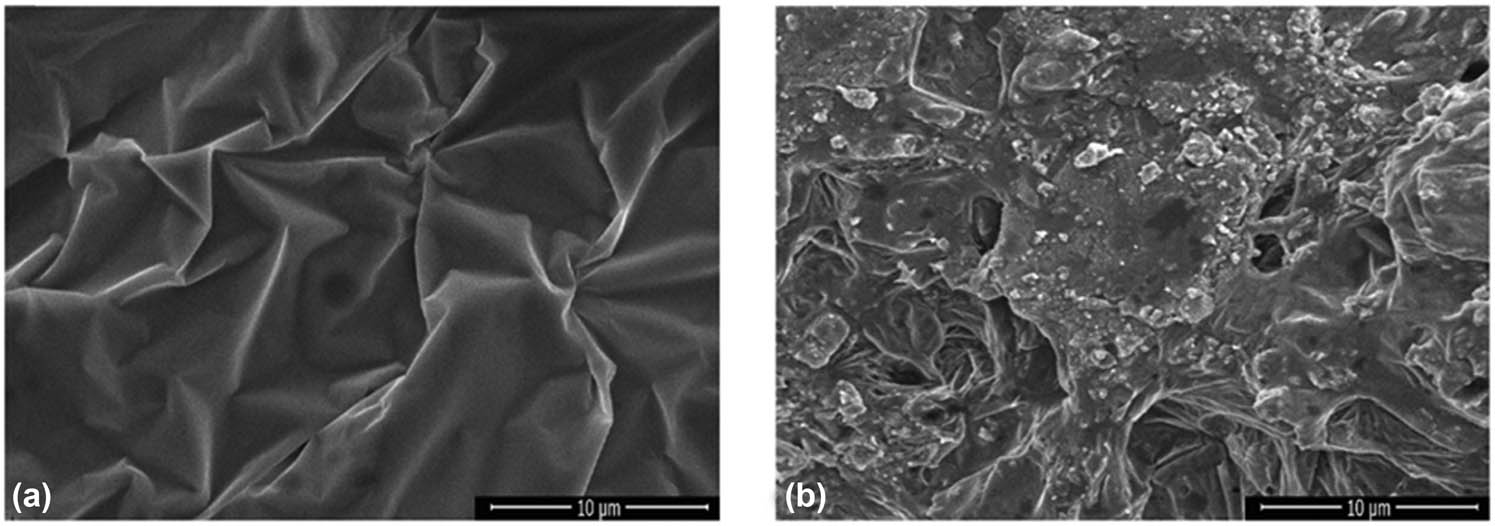
SEM images of SPH (a) and SPH containing CS-B and CS-Si, prepared at a volume ratio of 0.5:2:100 (CS-B/CS-Si/SPH) (b). Source: Authors’ original work.
3.1.3 Functional groups
The FTIR spectra of CS, CS-B, and CS-Si nanoparticles are illustrated in Figure 4. The CS nanoparticle exhibited a characteristic peak at 3,404 cm−1, which is attributed to –OH stretching vibrations. The additional peaks at 1,637 and 1,549 cm−1 are attributed to the CONH2 and NH2 groups, respectively. The other band for the CS nanoparticle was observed at 1,404 cm−1 and is due to –CH2 stretching. Interestingly, the peak at 903 cm−1 is attributed to the P−O−P stretching vibration due to cross-linking of TPP ions in the CS nanoparticle. The spectrum of the CS-B nanoparticle showed a strong peak at 3,196 cm−1, which corresponds to the OH stretching. The peak of the CS-B nanoparticle disappeared at 1,549 cm−1, but it displayed a broad band around 1,404 cm−1 and a sharp band at 1,190 cm−1, which is attributed to the B−O stretching and B−O−H bonding. These bands are associated with hydrogen bonds formed between molecules of boric acid and the amino groups found in the CS nanoparticle [44]. The FTIR spectrum of the CS-Si nanoparticle also showed a broad band at 3,404 cm−1, which is attributed to –OH stretching vibrations. The small peak at 1,637 cm−1 is attributed to the CONH2 group, while the peak at 1,592 cm−1 is attributed to vibrations of the NH2 group. The strong absorption peak at 1,098 cm−1 is attributed to the Si–O stretching vibration, which confirms that silicon dioxide is embedded with CS nanoparticles [45].
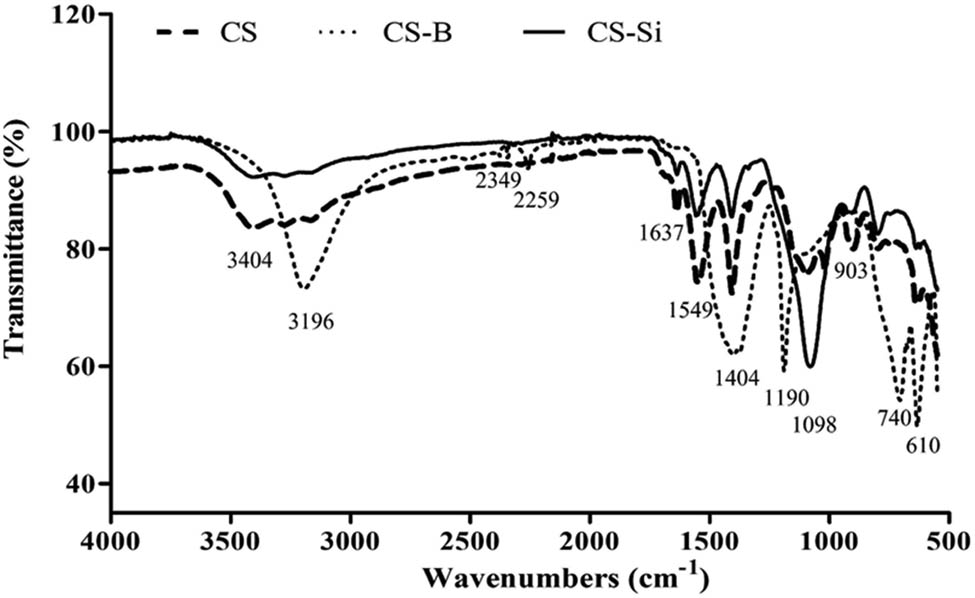
FTIR spectra of CS, CS-B and CS-Si nanoparticles. Source: Authors’ original work.
The FTIR spectra of SPH and SPH containing CS-B and CS-Si nano-fertilizer are illustrated in Figure 5. The SPH showed bands at the wavenumber 3,560 cm−1, which corresponds to –OH stretching vibrations, indicating the presence of hydroxyl groups [46]. The band located at 1,613 cm−1 is associated with the vibration of N–H bending (amide II). The bands at 650 cm−1 indicated the COH groups in the protein. The FTIR spectrum of SPH containing CS-B and CS-Si nano-fertilizer showed a broad peak at 3,560 cm−1 that was shifted and –OH stretching vibrations broader than SPH, indicating changes in hydrogen bonding. The small peak at 3,225 cm−1, possibly attributed to N–H stretching vibrations, indicates the presence of amino groups [47]. A strong peak at 1,613 cm−1 was likely due to N–H bending vibrations (amide II) or C═O stretching (amide I) from CS [48]. The new peak of SPH containing CS-B and CS-Si nano-fertilizer was observed at 1,404 cm−1. It was due to –CH2 stretching, potentially related to specific interactions between CS-B and CS-Si. The new peaks at 903 and 740 cm−1 are potentially related to Si–O–Si or B–O bonds, indicating the presence of CS-B and CS-Si in the SPH matrix.
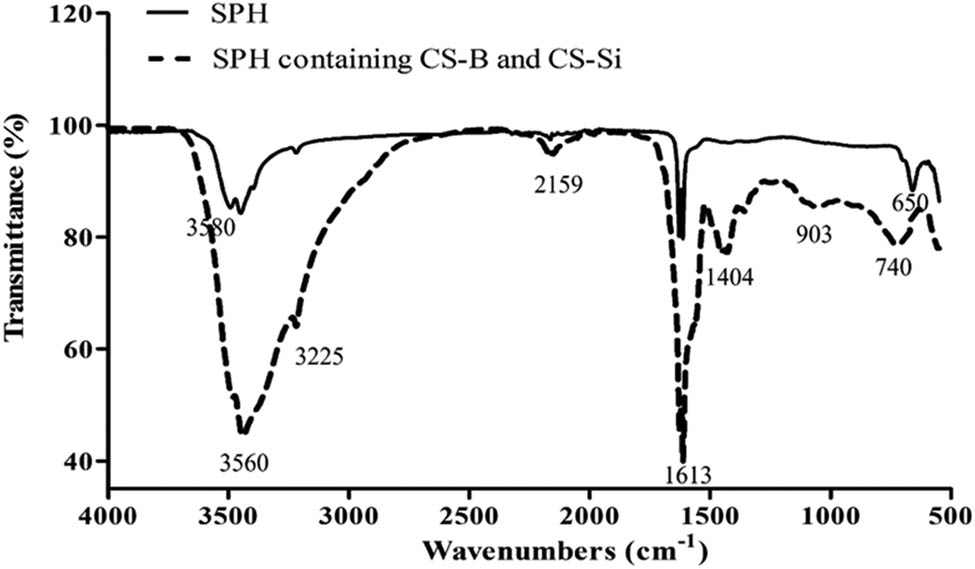
FTIR spectra of SPH and SPH containing CS-B and CS-Si. Source: Authors’ original work.
The FTIR spectra clearly showed that CS-B and CS-Si have been successfully incorporated into the SPH matrix. This is evidenced by the shifts in peak positions, changes in peak intensities, and the appearance of new peaks. These observations are consistent with the SEM findings, which revealed changes in surface morphology upon incorporation of CS-B and CS-Si. Additionally, the FTIR spectra indicated that the interactions between the components could be responsible for the decrease in particle size and changes in the zeta potential from positive values in CS-B and CS-Si nanoparticles to a negative value in the SPH containing CS-B and CS-Si nano-fertilizers. The formation of new bonds and changes in the surface morphologies of nano-fertilizers reinforce the hypothesis that the components are not merely physically mixed but actively interact to create a new composite material.
3.2 Effect of SPH containing CS-B and CS-Si nano-fertilizers on the growth and yield of rice plants grown in a greenhouse
Under greenhouse conditions, RD 79 rice plants reached the full tiller stage at 75 DAT (Figure 6a). The maturity stage was reached at 90 DAT, with complete grain filling and a predominantly golden yellow grain color, serving as key physiological markers [49] (Figure 6b). The plants were harvested 118 DAT (Figure 6c). The application of half-rate NPK with 2 mL/L SPH containing CS-B and CS-Si nano-fertilizers significantly increased the canopy diameter of rice plants, as shown in Table 4. The results showed that the canopy diameter of the rice plants treated with half-rate NPK with 2 mL/L SPH containing CS-B and CS-Si nano-fertilizers was 40 ± 3, 42 ± 2, 42 ± 2, and 42 ± 3 cm at 45, 60, 75, and 90 DAT, respectively (p < 0.05). Moreover, rice plants treated with half-rate NPK with 2 mL/L SPH containing CS-B and CS-Si nano-fertilizers also had significantly higher levels of plant height than other treatments (p < 0.05). The height of rice plants treated with half-rate NPK with 2 mL/L SPH containing CS-B and CS-Si nano-fertilizers was 129 ± 3 and 151 ± 4 cm at 75 and 90 DAT, respectively, as shown in Table 5. SPH was produced by breaking down the soy protein into smaller peptide fragments and amino acids through hydrolysis [50]. SPH promotes the assimilation, translocation, and utilization of other nutrients and stimulates the photosynthetic process in many plants, thereby supporting their growth and development [51]. The elemental analysis of the nano-fertilizer, detailed in Table 1, reveals the presence of key micronutrients that likely contributed to the enhanced growth observed. The SPH containing CS-B and CS-Si nano-fertilizers had the elemental composition of C (3.21%), N (0.22%), Ca (95.63 mg/L), B (7.54 mg/L), and Si (35.81 mg/L). The SEM images revealed a rough surface and a porous structure in the SPH containing CS-B and CS-Si nano-fertilizer, which could enhance adhesion and nutrient release. Furthermore, the FTIR spectra confirmed the presence of B and Si elements incorporated in the SPH matrix.
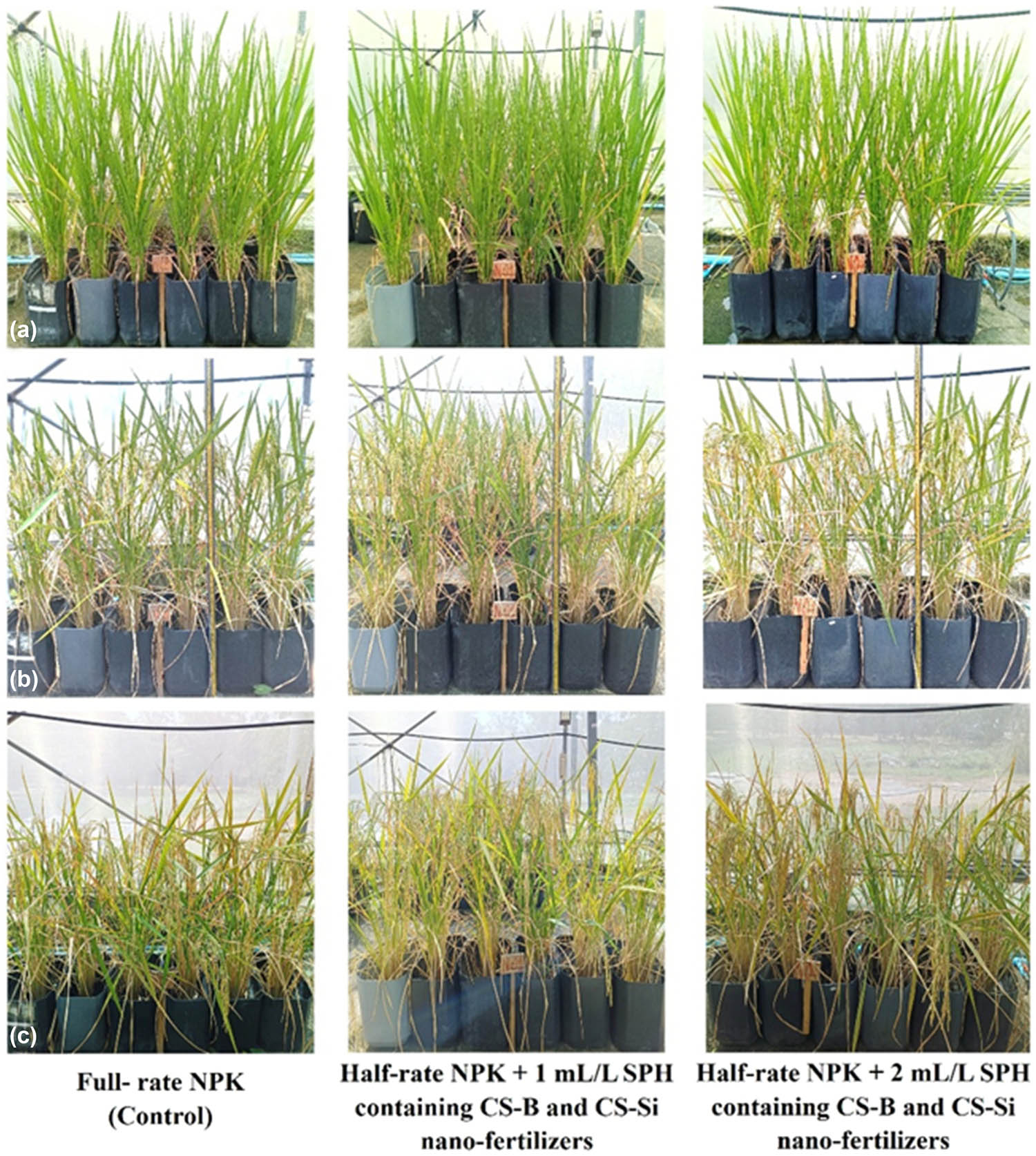
Effect of SPH containing CS-B and CS-Si nano-fertilizers on plant growth of the rice RD 79 variety at 75 (a), 90 (b), and 118 (c) days after transplanting in a greenhouse. Source: Authors’ original work.
Canopy diameter of RD 79 rice variety at different growth stages as affected by SPH-containing CS-B and CS-Si nano-fertilizer applications
| Treatment | Canopy diameter (cm) | ||||
|---|---|---|---|---|---|
| 30 DAT | 45 DAT | 60 DAT | 75 DAT | 90 DAT | |
| Full-rate NPK (Control) | 17 ± 1c | 34 ± 3c | 38 ± 3b | 38 ± 3b | 38 ± 3b |
| Half-rate NPK + 1 mL/L SPH containing CS-B and CS-Si nano-fertilizers | 18 ± 1bc | 36 ± 2bc | 38 ± 2b | 38 ± 3b | 42 ± 2a |
| Half-rate NPK + 2 mL/L SPH containing CS-B and CS-Si nano-fertilizers | 20 ± 2a | 40 ± 3a | 42 ± 2a | 42 ± 2a | 42 ± 3a |
Mean ± SD within each column followed by different letters are significantly different (p < 0.05).
Plant height of RD 79 rice variety at different growth stages as affected by SPH-containing CS-B and CS-Si nano-fertilizer applications
| Treatment | Plant height (cm) | ||||
|---|---|---|---|---|---|
| 30 DATns | 45 DATns | 60 DATns | 75 DAT | 90 DAT | |
| Full-rate NPK (control) | 76 ± 2 | 89 ± 2 | 106 ± 4 | 123 ± 3b | 141 ± 3b |
| Half-rate NPK + 1 mL/L SPH containing CS-B and CS-Si nano-fertilizers | 76 ± 1 | 90 ± 1 | 109 ± 2 | 127 ± 3ab | 147 ± 7ab |
| Half-rate NPK + 2 mL/L SPH CS-B and CS-Si nano-fertilizers | 75 ± 2 | 90 ± 2 | 110 ± 2 | 129 ± 3a | 151 ± 4a |
Mean ± SD within each column followed by different letters are significantly different (p < 0.05). ns = not significant.
The size of stomatal pores in rice typically ranges between 10 and 30 µm in diameter [52]. The small particle size of the SPH containing CS-B and CS-Si nano-fertilizers, as determined by DLS measurements, was 188.5 nm. The SPH containing CS-B and CS-Si nano-fertilizers could pass into the plant through foliar absorption as the nano-fertilizers have a small particle size and a high surface area, which allows them to more readily penetrate through stomata and deliver nutrients directly to the plant cell. B is crucial for cell wall formation, sugar transport, and reproductive development, whereas Si assists photosynthetic efficiency by strengthening the leaf structure and enhancing the chlorophyll content [53]. Therefore, SPH containing CS-B and CS-Si nano-fertilizers could improve the delivery and absorption of nutrients in plant cells.
Table 6 shows the effects of different fertilizer treatments on the yield components, including the number of panicles, length of the panicle, number of grains, and grain yield, of RD 79 at 118 DAT. The results showed that rice plants treated with half-rate NPK with 2 mL/L SPH containing CS-B and CS-Si nano-fertilizers had the highest number of grains (215 ± 14 grains) and grain yield per plant (127 ± 16 g) compared to other treatments (p < 0.05). However, the application of the half-rate NPK with 2 mL/L SPH containing CS-B and CS-Si nano-fertilizers not only promoted plant growth but also increased the yield of rice plants. This might be since SPH contained essential amino acids and peptides that supported grain development. Additionally, CS acted as a plant growth promoter, while the B and Si nano-fertilizers enhanced nutrient delivery and targeted the developing grains, resulting in higher yields. The nutrient delivery from SPH could be enhanced by B and Si nano-fertilizers, which promoted the grain and yield. These findings agree with Aljutheri et al. [54] who reported that nano-K, amino acids, and chelated superfertilizer enhanced the plant height, length of the spike, total chlorophyll content, grain yield, and grain weight of wheat. Munaro et al. [55] also observed nanoparticle formulations based on lecithin/CS as carriers of a protein hydrolysate from Arthrospira platensis, which enhanced the growth and yields of tomatoes. The application of half-rate NPK with a 2 mL/L solution of SPH containing CS-B and CS-Si nano-fertilizers enhanced the growth and yield of the RD 79 rice variety compared to the full rate of NPK fertilizer. This outcome demonstrates a potential reduction in conventional fertilizer usage. While the initial cost of nano-fertilizers may exceed that of conventional fertilizers, this economic consideration is mitigated by their reduced application volume and enhanced nutrient delivery efficiency [56]. The readily available sources of CS and SPH suggest an initial feasibility for large-scale production. However, the scalability of this nano-fertilizer is significantly contingent upon resolving challenges associated with achieving consistent nanoparticle quality during high-volume synthesis and ensuring the stability of the final formulation. Furthermore, the economic viability of this scale-up necessitates thorough evaluation through field trials conducted across diverse soil types, climates, and farming systems toward sustainable agricultural innovation [57]. The nano-fertilizer has the potential to slow the release of nutrients while ensuring more direct delivery to the plant in comparison to biofertilizers [58]. In this study, a developed nano-formulation effectively reduced conventional fertilizer (NPK) use by 50% and allowed for low-volume foliar application. This suggests that nano-fertilizers can reduce pollutant loads due to their improved nutrient absorption and the resulting need for smaller applications [59]. Moreover, the CS-based B and Si combined with SPH may present lower pollution risks due to its biopolymer composition and its potential for biodegradability. However, it is essential to undertake comprehensive long-term studies to rigorously evaluate and quantify potential pollution risks due to the recurrent use of these nano-formulations under a range of environmental conditions. Additionally, a limitation of this study is its confinement to greenhouse conditions. While these controlled environments facilitate precise monitoring of plant responses and nano-fertilizer behavior, they might not fully reflect the intricate and variable conditions encountered in the agriculture field. Variable temperatures, a variety of soil microbial communities, pests and diseases, and rainfall variations are all part of field conditions and could have an impact on the performance and effectiveness of the developed nano-fertilizer.
Number of panicles, length of the panicle, number of grains, and grain yield of RD 79 rice variety affected by SPH-containing CS-B and CS-Si nano-fertilizers at 118 days after transplanting (DAT)
| Treatment | Number of paniclesns (panicles/plant) | Length of the paniclens (cm) | Number of grains (grains) | Grain yield per plant (g) |
|---|---|---|---|---|
| Full-rate NPK (control) | 19 ± 1 | 33 ± 1 | 192 ± 15c | 98 ± 9c |
| Half-rate NPK + 1 mL/L SPH containing CS-B and CS-Si nano-fertilizers | 20 ± 1 | 33 ± 1 | 201 ± 16bc | 105 ± 8bc |
| Half-rate NPK + 2 mL/LSPH containing CS-B and CS-Si nano-fertilizers | 20 ± 2 | 33 ± 1 | 215 ± 14a | 127 ± 16a |
Mean ± SD within each column followed by different letters are significantly different (p < 0.05). ns = not significant.
4 Conclusions
This study successfully developed a novel nano-fertilizer in combination, CS-B and CS-Si with SPH. The components of the SPH containing CS-B and CS-Si nano-fertilizers formed a new composite material with a smaller particle size and modified surface charge. The SEM images clearly demonstrated that CS-B and CS-Si were incorporated into the SPH structure. FTIR spectra confirmed that B and Si were integrated within the SPH through specific functional groups. The application of half-rate NPK with 2 mL/L SPH containing CS-B and CS-Si nano-fertilizers resulted in significant improvements in the canopy diameter, plant height, and grain yield of the rice RD 79 variety compared to the conventional fertilizer under greenhouse conditions. These findings highlight the potential of SPH containing CS-B and CS-Si nano-fertilizer to enhance rice growth and yield under greenhouse conditions. However, future research should focus on evaluating the SPH containing CS-B and CS-Si nano-fertilizer under actual field conditions to validate its applicability and effectiveness in practical agriculture.
Acknowledgments
The authors acknowledge the support from King Mongkut’s University of Technology Thonburi, Thailand. We also sincerely thank The United Graduate School of Agricultural Science (UGSAS), Gifu University, Japan, for their support with research equipment.
-
Funding information: This research project was funded by King Mongkut’s University of Technology Thonburi, Thailand, Science Research and Innovation (TSRI) Basic Research Fund: Fiscal year 2023 under project number FRB660073/0164 (Program Sustainable Bioeconomy) and National Science, Research and Innovation Fund (NSRF) Fiscal year 2023.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal. All authors have read and agreed to the published version of the manuscript. AU: conceptualization, supervision, writing – review and editing; NL: conceptualization, writing – review and editing; AJ: software, methodology, data curation; RK: validation, methodology; PJ: supervision; JG: writing – review and editing; CC: methodology, writing – original draft, writing – review and editing.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during the current study are available from the corresponding author on reasonable request.
References
[1] Bandumula N. Rice production in Asia: Key to global food security. Proc Natl Acad Sci, India, Sect B. 2018;88:1323–8. 10.1007/s40011-017-0867-7.Suche in Google Scholar
[2] Williams JH, Reese JB. Evolution of development of pollen performance. Curr Top Dev Biol. 2019;131:299–336. 10.1016/bs.ctdb.2018.11.012.Suche in Google Scholar PubMed
[3] Jatav HS, Sharma LD, Sadhukhan R, Singh SK, Singh S, Rajput VD, et al. An overview of micronutrients: Prospects and implication in crop production. In: Aftab T and Hakeem KR, editors. Plant Micronutrients. Switzerland: Springer Nature; 2020. p. 1–30.10.1007/978-3-030-49856-6_1Suche in Google Scholar
[4] Hapuarachchi NS, Kämper W, Wallace HM, Hosseini Bai S, Ogbourne SM, Nichols J, et al. Boron effects on fruit set, yield, quality and paternity of Hass avocado. Agronomy. 2020;12(6):1479. 10.3390/agronomy12061479.Suche in Google Scholar
[5] Rashid A, Muhammad S, Rafique E. Genotypic variation in boron uptake and utilization in rice and wheat. In: Goldbach HE, Rerkasem B, Wimmer MA, Brown PH, Thellier M and Bell RW, editors. Boron in plant and animal nutrition. New York: Springer; 2002. p. 305–10.10.1007/978-1-4615-0607-2_29Suche in Google Scholar
[6] Rehman A, Farooq M, Rashid A, Nadeem F, Stuerz S, Asch F, et al. Boron nutrition of rice in different production systems: A review. Agron Sustain Dev. 2018;38(3):25. 10.1007/s13593-018-0504-8.Suche in Google Scholar
[7] Zargar SM, Mahajan R, Bhat JA, Nazir M, Deshmukh R. Role of silicon in plant stress tolerance: opportunities to achieve a sustainable cropping system. 3 Biotech. 2019;9:73. 10.1007/s13205-019-1613-z.Suche in Google Scholar PubMed PubMed Central
[8] Song D, Dehua G, Sun H, Qiao L, Zhao R. Chlorophyll content estimation based on cascade spectral optimizations of interval and wavelength characteristics. Comput Electr Agric. 2021;189(1):106413. 10.1016/j.compag.2021.106413.Suche in Google Scholar
[9] Islam MM, El-Sappah A, Ali H, Zandi P, Qiulan H, Soaud S, et al. Pathogenesis- related proteins (PRs) countering environmental stress in plants: A review. S Afr J Bot. 2023;160:414–27. 10.1016/j.sajb.2023.07.003.Suche in Google Scholar
[10] Schjoerring JK, Cakmak I, White PJ. Plant nutrition and soil fertility: synergies for acquiring global green growth and sustainable development. Plant Soil. 2019;434:1–6. 10.1007/s11104-018-03898-7.Suche in Google Scholar
[11] Hafez M, Popov A, Rashad M. Integrated use of bio-organic fertilizers for enhancing soil fertility-plant nutrition, germination status and initial growth of corn (Zea May L.). Env Technol Innov. 2021;21:101329. 10.1016/j.eti.2020.101329.Suche in Google Scholar
[12] Sales HBE, Carolino AS, Nunes RZA, Macalia CMA, Ruzo CM, Pinto CC, et al. Advances in agricultural technology: A review of slow-release nanofertilizers and innovative carriers. Comm Soil Sci Plant Anal. 2024;55(12):1849–82. 10.1080/00103624.2024.2326145.Suche in Google Scholar
[13] Malécange M, Sergheraert R, Teulat B, Mounier E, Lothier J, Sakr S. Biostimulant properties of protein hydrolysates: Recent advances and future challenges. Int J Mol Sci. 2023;24(11):9714. 10.3390/ijms24119714.Suche in Google Scholar PubMed PubMed Central
[14] Baslam M, Mitsui T, Hodges M, Priesack E, Herritt MT, Aranjuelo I, et al. Photosynthesis in a changing global climate: Scaling up and scaling down in crops. Front Plant Sci. 2020;11:882. 10.3389/fpls.2020.00882.Suche in Google Scholar PubMed PubMed Central
[15] Baakdah MM, Tsopmo A. Identification of peptides, metal binding and lipid peroxidation activities of HPLC fractions of hydrolyzed oat bran proteins. J Food Sci Technol. 2016;53(9):3593–601. 10.1007/s13197-016-2341-6.Suche in Google Scholar PubMed PubMed Central
[16] Singh U, Kaur D, Mishra V, Krishania M. Combinatorial approach to prepare antioxidative protein hydrolysate from corn gluten meal with dairy whey: Preparation, kinetics, nutritional study and cost analysis. LWT. 2022;153:112437. 10.1016/j.lwt.2021.112437.Suche in Google Scholar
[17] Ţălu Ş. Micro and nanoscale characterization of three-dimensional surfaces: basics and applications. Cluj-Napoca, Romania: Napoca Star Publishing House; 2015. https://books.google.co.th/books? id = oB1nAQAACAAJ.Suche in Google Scholar
[18] Delgadillo A, Carrillo González R, San Martín-Martínez E, Fonseca MR, Chacón A. Nanocapsules of urea in chitosan and polymethacrylic acid and their application to hydroponic culture of lettuce (Lactuca sativa L). Rev Mex Ing Quim. 2016;15:423–31. 10.24275/rmiq/Alim1137.Suche in Google Scholar
[19] Cholmaitri C, Uthairatanakij A, Laohakunjit N, Jitareerat P, Mingvanish W. Controlled release sachet of methyl salicylate from rice husk absorbents for delayed ripening in ‘Namwa’ bananas. Food Packag Shelf Life. 2022;32:100861. 10.1016/j.fpsl.2022.100861.Suche in Google Scholar
[20] Hameed AZ, Raj SA, Kandasamy J, Baghdadi MA, Shahzad MA. Chitosan: A sustainable material for multifarious applications. Polymers. 2022;14(12):335. 10.3390/polym14122335.Suche in Google Scholar PubMed PubMed Central
[21] Modi S, Kumar S, Dubey P. Dynamics of chitosan based NPK-nanofertilizers in greenhouse cucumber production system. J Env Biol. 2021;42:162–8. 10.22438/jeb/42/1/MRN-1251.Suche in Google Scholar
[22] Wang S, Nguyen AD. Effects of Zn/B nanofertilizer on biophysical characteristics and growth of coffee seedlings in a greenhouse. Res Chem Intermed. 2018;44:4889–901. 10.1007/s11164-018-3342-z.Suche in Google Scholar
[23] Liu R, Lal R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci Rep. 2014;4:5686. 10.1038/srep05686.Suche in Google Scholar PubMed PubMed Central
[24] Nguyen T, Nguyen T, Wang SL, Vo T, Dzung N. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle-amoxicillin complex. Res Chem Intermed. 2017;43:3527–37. 10.1007/s11164-016-2428-8.Suche in Google Scholar
[25] Saharan V, Kumaraswamy RV, Choudhary RC, Kumari S, Pal A, Raliya R, et al. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J Agric Food Chem. 2016;64(31):6148–55. 10.1021/acs.jafc.6b02239.Suche in Google Scholar PubMed
[26] Dzanic H, Mujic I, Sudarski-Hack V. Protein hydrolysate from soy grits and dehydrate alfalfa flour. J Agric Food Chem. 1985;33:683–5. 10.1021/jf00064a029.Suche in Google Scholar
[27] Fageria NK. Plant tissue test for determination of optimum concentration and uptake of nitrogen at different growth stages in lowland rice. Commun Soil Sci Plant Anal. 2003;34:259–70. 10.1081/CSS-120017430.Suche in Google Scholar
[28] Paopun Y. Freeze drying versus chemical fixation technique for scanning electron microscope of succulent and aquatic plant leaves. Microsc Microanal. 2017;30:28–32. 10.14456/microsc-microanal-res.2017.7.Suche in Google Scholar
[29] Krokida M, Pappa A, Agalioti M. Effect of drying on Aloe’s functional components. Procedia Food Sci. 2011;1:1523–7. 10.1016/j.profoo.2011.09.225.Suche in Google Scholar
[30] Ha N, Nguyen T, Wang SL, Dzung N. Preparation of NPK nano-fertilizer based on chitosan nanoparticles and its effect on biophysical characteristics and growth of coffee in green house. Res Chem Intermed. 2019;45:51–63. 10.1007/s11164-018-3630-7.Suche in Google Scholar
[31] Swati B, Choudhary MK, Joshi A, Saharan V. Assessment of Cu chitosan nanoparticles for its antibacterial activity against Pseudomonas syringae pv. Glycinea. Int J Curr Microbiol Appl Sci. 2017;6(11):1335–50. 10.20546/ijcmas.2017.611.160.Suche in Google Scholar
[32] Upadhyay SK, Chauhan PK. Optimization of eco-friendly amendments as sustainable asset for salt-tolerant plant growth-promoting bacteria mediated maize (Zea Mays L.) plant growth, Na uptake reduction and saline soil restoration. Env Res. 2022;211:113081. 10.1016/j.envres.2022.113081.Suche in Google Scholar PubMed
[33] Yang G, Lin Lai WH, Tong J, Lei J, Yuan M, Zhang Y, et al. Understanding the relationship between particle size and ultrasonic treatment during the synthesis of metal nanoparticles. Ultrason Sonochem. 2021;73:105497. 10.1016/j.ultsonch.2021.105497.Suche in Google Scholar PubMed PubMed Central
[34] Vert M, Doi Y, Hellwich KH, Hess M, Hodge P. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl Chem. 2012;84(2):377–410. 10.1351/PAC-REC-10-12-04.Suche in Google Scholar
[35] Salah R, Karmy M, Abdelraouf A, Kotb S. Evaluation of the bactericidal effect of silver nanoparticles against methicillin resistant Staphylococcus aureus (MRSA) and methicillin sensitive Staphylococcus aureus (MSSA) strains isolated from mastitic milk of small ruminants and their surrounding environment in Aswan. J Vet Med Res. 2021;27(2):143–51. 10.21608/jvmr.2021.55209.1027.Suche in Google Scholar
[36] Emelyanenko K. Analysis of Van der Waals interactions between nanoparticles with different geometries, with accounting for three-particle contributions to the total energy. Russ J Phys Chem. 2016;90(5):1057–62. 10.1134/S0036024416040087.Suche in Google Scholar
[37] Pochapski D, Santos C, Wosiak Leite G, Pulcinelli SH, Santilli C. Zeta potential and colloidal stability predictions for inorganic nanoparticle dispersions: Effects of experimental conditions and electrokinetic models on the interpretation of results. Langmuir. 2021;37(45):13379–89. 10.1021/acs.langmuir.1c02056.Suche in Google Scholar PubMed
[38] Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–51. 10.1038/nbt.3330.Suche in Google Scholar PubMed PubMed Central
[39] Chekh O, Bordunova O, Chivanov V, Yadgorova E, Bondarchuk L. Nanocomposite coatings for hatching eggs and table eggs. Open Agric. 2021;6(1):573–86. 10.1515/opag-2021-0046.Suche in Google Scholar
[40] Fusteș-Dămoc I, Măluțan T, Mija A. High content chitosan-based materials with high performance properties. Int J Biol Macromol. 2022;223(Part A):263–72. 10.1016/j.ijbiomac.2022.10.270.Suche in Google Scholar PubMed
[41] Özakar E, Bingöl MS, Özakar RS. Investigation of boron nanosized particles prepared with various surfactants and chitosan in terms of physical stability and cell viability. Turk J Chem. 2022;46(5):1429–49. 10.55730/1300-0527.3449.Suche in Google Scholar PubMed PubMed Central
[42] Zhong T, Xia M, Yao Z, Han C. Chitosan/silica nanocomposite preparation from shrimp shell and its adsorption performance for methylene blue. Sustainability. 2023;15(1):47. 10.3390/su15010047.Suche in Google Scholar
[43] Tasnim A, Roy A, Akash S, Ali H, Habib M, Barasarathi J, et al. Hibiscus sabdariffa L. petal biomass: A green source of nanoparticles of multifarious potential. Open Agric. 2024;9(1):20220332. 10.1515/opag-2022-0332.Suche in Google Scholar
[44] Nishiyama R, Watanabe Y, Fujita Y, Kojima M, Werner T, Vankova R, et al. Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell. 2011;23(6):2169–83. 10.1105/tpc.111.087395.Suche in Google Scholar PubMed PubMed Central
[45] Budnyak TM, Pylypchuk IV, Tertykh VA, Yanovska ES, Kolodynska D. Synthesis and adsorption properties of chitosan-silica nanocomposite prepared by sol-gel method. Nanoscale Res Lett. 2015;10:87. 10.1186/s11671-014-0722-1.Suche in Google Scholar PubMed PubMed Central
[46] Singha P, Singh SK, Muthukumarappan K. Textural and structural characterization of extrudates containing blends of apple pomace, defatted soy flour and corn grits. J Food Process Eng. 2019;42:e13046. 10.1111/jfpe.13046.Suche in Google Scholar
[47] Shu TP, Wen JL, Feng HM, Lei KW, Liang HZ. A novel 18-metallacrown-6 complex: Synthesis, structural characterization and magnetic properties. Solid State Sci. 2009;11(11):1919–22. 10.1016/j.solidstatesciences.2009.07.014.Suche in Google Scholar
[48] Hu Y, Du Y, Yang J, Tang Y, Li J, Wang X. Self-aggregation and antibacterial activity of N-acylated chitosan. Polymer. 2007;48(11):3098–106. 10.1016/j.polymer.2007.03.063.Suche in Google Scholar
[49] Mohapatra PK, Sarkar RK, Panda D, Kariali E. Staging of rice plant growth and development. In: Tillering behavior of rice plant. Singapore: Springer Nature Singapore; 2025. p. 105–39.10.1007/978-981-97-5235-5_4Suche in Google Scholar
[50] Barrada A, Delisle-Houde M, Nguyen TTA, Tweddell RJ, Dorais M. Drench application of soy protein hydrolysates increases tomato plant fitness, fruit yield, and resistance to a hemibiotrophic pathogen. Agronomy. 2022;12(8):1761. 10.3390/agronomy12081761.Suche in Google Scholar
[51] San D, Cai X, Hu C, Anwar A, Yang Y. Effects of shading on photosynthetic characteristics of wax apple leaves. Not Bot Horti Agrobot Cluj-Na. 2024;52(1):3367. 10.15835/nbha52113367.Suche in Google Scholar
[52] Raumjit N, Siwadon S, Sukanya S, Thirayut W. Comparison of root system and stomata in nine upland rice varieties. IJAT. 2019;15(5):769–78.Suche in Google Scholar
[53] Xu J, Guo L, Liu L. Exogenous silicon alleviates drought stress in maize by improving growth, photosynthetic and antioxidant metabolism. Env Exp Bot. 2022;201:104974. 10.1016/j.envexpbot.2022.104974.Suche in Google Scholar
[54] Aljutheri HW, Habeeb KH, Jawad F, Al-taee K, Al-Taey DK, Al-Tawaha ARM. Effect of foliar application of different sources of nano-fertilizers on growth and yield of wheat. Biosci Res. 2018;15(4):3988–97.Suche in Google Scholar
[55] Munaro D, Mazo C, Bauer C, Gomes L, Teodoro E, Mazzarino L, et al. A novel biostimulant from chitosan nanoparticles and microalgae-based protein hydrolysate: Improving crop performance in tomato. Sci Hortic. 2024;323(1):11249. 10.1016/j.scienta.2023.112491.Suche in Google Scholar
[56] Wadas W. Effect of foliar silicon application on nutrient content in early crop potato tubers. Agronomy. 2022;12:2706. 10.3390/agronomy12112706.Suche in Google Scholar
[57] González-Feijoo R, Martinez-Castillo C, Rodríguez-Seijo A, Pérez-Rodríguez P, Arenas-Lago D. Nanoagrochemicals versus conventional fertilizers: A field case study with tailor-made nanofertilizers for sustainable crop efficiency of Brassica oleracea L. convar. Capitata var. Sabauda Agron. 2024;14(9):1885. 10.3390/agronomy14091885.Suche in Google Scholar
[58] Srisha V, Prasad V. Effect of NPK and nano fertilizers on plant growth, yield and quality of Gladiolus (Gladiolus grandiflorus L.). Int J Plant Soil Sci. 2022;34:1529–34. 10.9734/IJPSS/2022/v34i2231528.Suche in Google Scholar
[59] Abioye OM, Okunola AA, Amodu MF, Olaseheinde DA, Yusuf KO. Nanobiofertilizer and its application in sustainable agriculture, crop specific nutrients delivery and environmental sustainability: A review. Appl Sci Eng Prog. 2024;17(3):7339. 10.14416/j.asep.2024.05.001.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Optimization of sustainable corn–cattle integration in Gorontalo Province using goal programming
- Competitiveness of Indonesia’s nutmeg in global market
- Toward sustainable bioproducts from lignocellulosic biomass: Influence of chemical pretreatments on liquefied walnut shells
- Efficacy of Betaproteobacteria-based insecticides for managing whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), on cucumber plants
- Assessment of nutrition status of pineapple plants during ratoon season using diagnosis and recommendation integrated system
- Nutritional value and consumer assessment of 12 avocado crosses between cvs. Hass × Pionero
- The lacked access to beef in the low-income region: An evidence from the eastern part of Indonesia
- Comparison of milk consumption habits across two European countries: Pilot study in Portugal and France
- Antioxidant responses of black glutinous rice to drought and salinity stresses at different growth stages
- Differential efficacy of salicylic acid-induced resistance against bacterial blight caused by Xanthomonas oryzae pv. oryzae in rice genotypes
- Yield and vegetation index of different maize varieties and nitrogen doses under normal irrigation
- Urbanization and forecast possibilities of land use changes by 2050: New evidence in Ho Chi Minh city, Vietnam
- Organizational-economic efficiency of raspberry farming – case study of Kosovo
- Application of nitrogen-fixing purple non-sulfur bacteria in improving nitrogen uptake, growth, and yield of rice grown on extremely saline soil under greenhouse conditions
- Digital motivation, knowledge, and skills: Pathways to adaptive millennial farmers
- Investigation of biological characteristics of fruit development and physiological disorders of Musang King durian (Durio zibethinus Murr.)
- Enhancing rice yield and farmer welfare: Overcoming barriers to IPB 3S rice adoption in Indonesia
- Simulation model to realize soybean self-sufficiency and food security in Indonesia: A system dynamic approach
- Gender, empowerment, and rural sustainable development: A case study of crab business integration
- Metagenomic and metabolomic analyses of bacterial communities in short mackerel (Rastrelliger brachysoma) under storage conditions and inoculation of the histamine-producing bacterium
- Fostering women’s engagement in good agricultural practices within oil palm smallholdings: Evaluating the role of partnerships
- Increasing nitrogen use efficiency by reducing ammonia and nitrate losses from tomato production in Kabul, Afghanistan
- Physiological activities and yield of yacon potato are affected by soil water availability
- Vulnerability context due to COVID-19 and El Nino: Case study of poultry farming in South Sulawesi, Indonesia
- Wheat freshness recognition leveraging Gramian angular field and attention-augmented resnet
- Suggestions for promoting SOC storage within the carbon farming framework: Analyzing the INFOSOLO database
- Optimization of hot foam applications for thermal weed control in perennial crops and open-field vegetables
- Toxicity evaluation of metsulfuron-methyl, nicosulfuron, and methoxyfenozide as pesticides in Indonesia
- Fermentation parameters and nutritional value of silages from fodder mallow (Malva verticillata L.), white sweet clover (Melilotus albus Medik.), and their mixtures
- Five models and ten predictors for energy costs on farms in the European Union
- Effect of silvopastoral systems with integrated forest species from the Peruvian tropics on the soil chemical properties
- Transforming food systems in Semarang City, Indonesia: A short food supply chain model
- Understanding farmers’ behavior toward risk management practices and financial access: Evidence from chili farms in West Java, Indonesia
- Optimization of mixed botanical insecticides from Azadirachta indica and Calophyllum soulattri against Spodoptera frugiperda using response surface methodology
- Mapping socio-economic vulnerability and conflict in oil palm cultivation: A case study from West Papua, Indonesia
- Exploring rice consumption patterns and carbohydrate source diversification among the Indonesian community in Hungary
- Determinants of rice consumer lexicographic preferences in South Sulawesi Province, Indonesia
- Effect on growth and meat quality of weaned piglets and finishing pigs when hops (Humulus lupulus) are added to their rations
- Healthy motivations for food consumption in 16 countries
- The agriculture specialization through the lens of PESTLE analysis
- Combined application of chitosan-boron and chitosan-silicon nano-fertilizers with soybean protein hydrolysate to enhance rice growth and yield
- Stability and adaptability analyses to identify suitable high-yielding maize hybrids using PBSTAT-GE
- Phosphate-solubilizing bacteria-mediated rock phosphate utilization with poultry manure enhances soil nutrient dynamics and maize growth in semi-arid soil
- Factors impacting on purchasing decision of organic food in developing countries: A systematic review
- Influence of flowering plants in maize crop on the interaction network of Tetragonula laeviceps colonies
- Bacillus subtilis 34 and water-retaining polymer reduce Meloidogyne javanica damage in tomato plants under water stress
- Vachellia tortilis leaf meal improves antioxidant activity and colour stability of broiler meat
- Evaluating the competitiveness of leading coffee-producing nations: A comparative advantage analysis across coffee product categories
- Application of Lactiplantibacillus plantarum LP5 in vacuum-packaged cooked ham as a bioprotective culture
- Evaluation of tomato hybrid lines adapted to lowland
- South African commercial livestock farmers’ adaptation and coping strategies for agricultural drought
- Spatial analysis of desertification-sensitive areas in arid conditions based on modified MEDALUS approach and geospatial techniques
- Meta-analysis of the effect garlic (Allium sativum) on productive performance, egg quality, and lipid profiles in laying quails
- Optimizing carrageenan–citric acid synergy in mango gummies using response surface methodology
- The strategic role of agricultural vocational training in sustainable local food systems
- Agricultural planning grounded in regional rainfall patterns in the Colombian Orinoquia: An essential step for advancing climate-adapted and sustainable agriculture
- Perspectives of master’s graduates on organic agriculture: A Portuguese case study
- Developing a behavioral model to predict eco-friendly packaging use among millennials
- Government support during COVID-19 for vulnerable households in Central Vietnam
- Citric acid–modified coconut shell biochar mitigates saline–alkaline stress in Solanum lycopersicum L. by modulating enzyme activity in the plant and soil
- Herbal extracts: For green control of citrus Huanglongbing
- Research on the impact of insurance policies on the welfare effects of pork producers and consumers: Evidence from China
- Investigating the susceptibility and resistance barley (Hordeum vulgare L.) cultivars against the Russian wheat aphid (Diuraphis noxia)
- Characterization of promising enterobacterial strains for silver nanoparticle synthesis and enhancement of product yields under optimal conditions
- Testing thawed rumen fluid to assess in vitro degradability and its link to phytochemical and fibre contents in selected herbs and spices
- Protein and iron enrichment on functional chicken sausage using plant-based natural resources
- Fruit and vegetable intake among Nigerian University students: patterns, preferences, and influencing factors
- Bioprospecting a plant growth-promoting and biocontrol bacterium isolated from wheat (Triticum turgidum subsp. durum) in the Yaqui Valley, Mexico: Paenibacillus sp. strain TSM33
- Quantifying urban expansion and agricultural land conversion using spatial indices: evidence from the Red River Delta, Vietnam
- Review Articles
- Reference dietary patterns in Portugal: Mediterranean diet vs Atlantic diet
- Evaluating the nutritional, therapeutic, and economic potential of Tetragonia decumbens Mill.: A promising wild leafy vegetable for bio-saline agriculture in South Africa
- A review on apple cultivation in Morocco: Current situation and future prospects
- Quercus acorns as a component of human dietary patterns
- CRISPR/Cas-based detection systems – emerging tools for plant pathology
- Short Communications
- An analysis of consumer behavior regarding green product purchases in Semarang, Indonesia: The use of SEM-PLS and the AIDA model
- Effect of NaOH concentration on production of Na-CMC derived from pineapple waste collected from local society
Artikel in diesem Heft
- Research Articles
- Optimization of sustainable corn–cattle integration in Gorontalo Province using goal programming
- Competitiveness of Indonesia’s nutmeg in global market
- Toward sustainable bioproducts from lignocellulosic biomass: Influence of chemical pretreatments on liquefied walnut shells
- Efficacy of Betaproteobacteria-based insecticides for managing whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), on cucumber plants
- Assessment of nutrition status of pineapple plants during ratoon season using diagnosis and recommendation integrated system
- Nutritional value and consumer assessment of 12 avocado crosses between cvs. Hass × Pionero
- The lacked access to beef in the low-income region: An evidence from the eastern part of Indonesia
- Comparison of milk consumption habits across two European countries: Pilot study in Portugal and France
- Antioxidant responses of black glutinous rice to drought and salinity stresses at different growth stages
- Differential efficacy of salicylic acid-induced resistance against bacterial blight caused by Xanthomonas oryzae pv. oryzae in rice genotypes
- Yield and vegetation index of different maize varieties and nitrogen doses under normal irrigation
- Urbanization and forecast possibilities of land use changes by 2050: New evidence in Ho Chi Minh city, Vietnam
- Organizational-economic efficiency of raspberry farming – case study of Kosovo
- Application of nitrogen-fixing purple non-sulfur bacteria in improving nitrogen uptake, growth, and yield of rice grown on extremely saline soil under greenhouse conditions
- Digital motivation, knowledge, and skills: Pathways to adaptive millennial farmers
- Investigation of biological characteristics of fruit development and physiological disorders of Musang King durian (Durio zibethinus Murr.)
- Enhancing rice yield and farmer welfare: Overcoming barriers to IPB 3S rice adoption in Indonesia
- Simulation model to realize soybean self-sufficiency and food security in Indonesia: A system dynamic approach
- Gender, empowerment, and rural sustainable development: A case study of crab business integration
- Metagenomic and metabolomic analyses of bacterial communities in short mackerel (Rastrelliger brachysoma) under storage conditions and inoculation of the histamine-producing bacterium
- Fostering women’s engagement in good agricultural practices within oil palm smallholdings: Evaluating the role of partnerships
- Increasing nitrogen use efficiency by reducing ammonia and nitrate losses from tomato production in Kabul, Afghanistan
- Physiological activities and yield of yacon potato are affected by soil water availability
- Vulnerability context due to COVID-19 and El Nino: Case study of poultry farming in South Sulawesi, Indonesia
- Wheat freshness recognition leveraging Gramian angular field and attention-augmented resnet
- Suggestions for promoting SOC storage within the carbon farming framework: Analyzing the INFOSOLO database
- Optimization of hot foam applications for thermal weed control in perennial crops and open-field vegetables
- Toxicity evaluation of metsulfuron-methyl, nicosulfuron, and methoxyfenozide as pesticides in Indonesia
- Fermentation parameters and nutritional value of silages from fodder mallow (Malva verticillata L.), white sweet clover (Melilotus albus Medik.), and their mixtures
- Five models and ten predictors for energy costs on farms in the European Union
- Effect of silvopastoral systems with integrated forest species from the Peruvian tropics on the soil chemical properties
- Transforming food systems in Semarang City, Indonesia: A short food supply chain model
- Understanding farmers’ behavior toward risk management practices and financial access: Evidence from chili farms in West Java, Indonesia
- Optimization of mixed botanical insecticides from Azadirachta indica and Calophyllum soulattri against Spodoptera frugiperda using response surface methodology
- Mapping socio-economic vulnerability and conflict in oil palm cultivation: A case study from West Papua, Indonesia
- Exploring rice consumption patterns and carbohydrate source diversification among the Indonesian community in Hungary
- Determinants of rice consumer lexicographic preferences in South Sulawesi Province, Indonesia
- Effect on growth and meat quality of weaned piglets and finishing pigs when hops (Humulus lupulus) are added to their rations
- Healthy motivations for food consumption in 16 countries
- The agriculture specialization through the lens of PESTLE analysis
- Combined application of chitosan-boron and chitosan-silicon nano-fertilizers with soybean protein hydrolysate to enhance rice growth and yield
- Stability and adaptability analyses to identify suitable high-yielding maize hybrids using PBSTAT-GE
- Phosphate-solubilizing bacteria-mediated rock phosphate utilization with poultry manure enhances soil nutrient dynamics and maize growth in semi-arid soil
- Factors impacting on purchasing decision of organic food in developing countries: A systematic review
- Influence of flowering plants in maize crop on the interaction network of Tetragonula laeviceps colonies
- Bacillus subtilis 34 and water-retaining polymer reduce Meloidogyne javanica damage in tomato plants under water stress
- Vachellia tortilis leaf meal improves antioxidant activity and colour stability of broiler meat
- Evaluating the competitiveness of leading coffee-producing nations: A comparative advantage analysis across coffee product categories
- Application of Lactiplantibacillus plantarum LP5 in vacuum-packaged cooked ham as a bioprotective culture
- Evaluation of tomato hybrid lines adapted to lowland
- South African commercial livestock farmers’ adaptation and coping strategies for agricultural drought
- Spatial analysis of desertification-sensitive areas in arid conditions based on modified MEDALUS approach and geospatial techniques
- Meta-analysis of the effect garlic (Allium sativum) on productive performance, egg quality, and lipid profiles in laying quails
- Optimizing carrageenan–citric acid synergy in mango gummies using response surface methodology
- The strategic role of agricultural vocational training in sustainable local food systems
- Agricultural planning grounded in regional rainfall patterns in the Colombian Orinoquia: An essential step for advancing climate-adapted and sustainable agriculture
- Perspectives of master’s graduates on organic agriculture: A Portuguese case study
- Developing a behavioral model to predict eco-friendly packaging use among millennials
- Government support during COVID-19 for vulnerable households in Central Vietnam
- Citric acid–modified coconut shell biochar mitigates saline–alkaline stress in Solanum lycopersicum L. by modulating enzyme activity in the plant and soil
- Herbal extracts: For green control of citrus Huanglongbing
- Research on the impact of insurance policies on the welfare effects of pork producers and consumers: Evidence from China
- Investigating the susceptibility and resistance barley (Hordeum vulgare L.) cultivars against the Russian wheat aphid (Diuraphis noxia)
- Characterization of promising enterobacterial strains for silver nanoparticle synthesis and enhancement of product yields under optimal conditions
- Testing thawed rumen fluid to assess in vitro degradability and its link to phytochemical and fibre contents in selected herbs and spices
- Protein and iron enrichment on functional chicken sausage using plant-based natural resources
- Fruit and vegetable intake among Nigerian University students: patterns, preferences, and influencing factors
- Bioprospecting a plant growth-promoting and biocontrol bacterium isolated from wheat (Triticum turgidum subsp. durum) in the Yaqui Valley, Mexico: Paenibacillus sp. strain TSM33
- Quantifying urban expansion and agricultural land conversion using spatial indices: evidence from the Red River Delta, Vietnam
- Review Articles
- Reference dietary patterns in Portugal: Mediterranean diet vs Atlantic diet
- Evaluating the nutritional, therapeutic, and economic potential of Tetragonia decumbens Mill.: A promising wild leafy vegetable for bio-saline agriculture in South Africa
- A review on apple cultivation in Morocco: Current situation and future prospects
- Quercus acorns as a component of human dietary patterns
- CRISPR/Cas-based detection systems – emerging tools for plant pathology
- Short Communications
- An analysis of consumer behavior regarding green product purchases in Semarang, Indonesia: The use of SEM-PLS and the AIDA model
- Effect of NaOH concentration on production of Na-CMC derived from pineapple waste collected from local society


