Abstract
Hop (Humulus lupulus) is a medicinal plant that has been used through centuries for its medicinal effects. A number of beneficial effects have been discovered. These may be useful not only in human nutrition but also in animal husbandry, specifically in swine husbandry. The objective of this study was to determine the effect of the inclusion of hops in diets on the growth performance of weaned piglets and finishing pigs. Dried hops were added in amounts of 0.5 and 0.8% of the total ration to feed mixes designed according to the nutritional requirements for weaned piglets and finishing pigs, respectively. Twelve piglets were fed the experimental mixture for 3 weeks after weaning. Their performance was compared with that of a control group fed the basic mixture without hops. At an average weight of 70 kg, the experimental group was then fed the mixture with the addition of 0.8% hops until slaughter (average weight 120 kg). Performance and meat quality were analysed. The addition of dried hops did not affect the intensity of piglet growth after weaning or on feed intake during this period. Hops in finishing pigs did not affect growth intensity but reduced feed consumption. This affected the ratio of feed consumption per unit weight gain. Chemical analysis of the produced meat revealed a change in the representation of several fatty acids (C14:0, C15:1n-5, C24:1n9). This is beneficial from the viewpoint of human nutrition.
1 Introduction
Hop (Humulus lupulus L.) is well known as an important ingredient in beer production. Hops are also used in the pharmaceutical and cosmetic industry. On the other hand, its positive effects on human health have been demonstrated due to its antimicrobial, anti-inflammatory, and anticancer properties, as well as some chronic disease effects [1,2,3,4]. Hops are a repository of bioactive substances useful in the treatment and prevention of a number of chronic diseases [5]. Most of these health benefits derive from three main components: polyphenols, bitter acids, and essential oils [6]. Hop flavonoids, especially xanthohumol, are substances with hypoglycaemic, anti-hyperlipidaemic, and anti-obesity effects [7,8] and exhibit antiviral activity against hepatitis C [9] and the porcine reproductive and respiratory syndrome virus [10]. The phenolic components of hops show anti-influenza activity [11], and their essential oil has demonstrated broad antimicrobial activity against Gram-positive bacteria, including Bacillus subtilis, Enterococcus faecalis, and Staphylococcus aureus, as well as Gram-negative bacteria, including Escherichia coli, Klebsiella oxytoca, and Salmonella typhimurium [12,13]. Chemoprotective effects of xanthohumol against the carcinogenic food contaminant aflatoxin B1 have also been demonstrated [14]. Hop components have the potential for use in treating diseases of the oral cavity [15], which can significantly affect the overall health of the organism. A mechanism of synergistic effect of antibacterial compounds from hops with some antibiotics has also been discovered [16]. Research on new compounds with antimicrobial properties represents a possible solution to the current global problem of bacterial resistance. Hops could thus find use as a source of potential antimicrobial substances usable in human and veterinary medicine [17].
With a view to the aforementioned properties, it can be anticipated that hops and their components might well find use in animal husbandry, not only in the form of medicines but also as part of regular diets, whereby their positive effects on animals’ health and condition can be beneficial. Although the effects of hops have often been studied in poultry, little is yet known about their use and effects in pigs. The antimicrobial effects of hops could be used in the context of searching for alternatives to zinc oxide at high therapeutic doses as a means of improving the condition and health of weaned piglets. In addition to antimicrobial effects, anti-inflammatory, chemoprotective, antioxidant, and immunomodulatory effects can be beneficial, too, and hops may also have a positive influence on meat’s quality and nutritional value.
The objective of this study was to determine the effect of hops inclusion in diets for weaned piglets and finishing pigs while examining effects on performance, health and condition of piglets, as well as on pig fattening performance and the composition of the meat.
2 Materials and methods
For the experiment, granulated hops (variety Žatecký červeňák G 90), which are made from pre-dried ground hops, were used. About 90 kg of granules are produced from 100 kg of hops [18].
The dried granulated hops were first ground to allow homogeneous mixing with the base diet in an amount of 0.5% for weaned piglets and 0.8% for animals in the final stage of fattening. The feed mixes were designed according to the nutritional requirements for weaning piglets and finishing pigs of less demanding breeds. The feed mixes consisted of components normally included in commercial feed mixes; they were based on barley, wheat, and soybean, as well as rapeseed meals with vitamin and mineral supplementation. Standard methods were used to determine feed composition. Nutrient compositions are shown in Table 1.
Average nutrient content (%) in feed mixes
| Item | Stage I | Stage II | ||
|---|---|---|---|---|
| Basic mixture | +0.5% hops | Basic mixture | +0.8% hops | |
| Dry matter | 90.06 | 89.61 | 88.10 | 87.78 |
| Crude protein | 15.79 | 16.23 | 12.33 | 12.52 |
| Ether extract | 4.21 | 4.54 | 3.67 | 3.86 |
| Crude fibre | 3.79 | 4.19 | 5.66 | 6.27 |
| Ash | 4.53 | 4.34 | 5.82 | 5.59 |
The addition of dried hops was evaluated in a feeding experiment. Twenty-four pigs (Prestice black-pied breed) were assigned into two groups of 12, each with an equal ratio of gilts and barrows. The weaning piglets were housed in pens with a partially slatted floor. Feed and water intake were ad libitum from feeders and nipple waterers. The fattening pigs were housed in pens with solid concrete floors. Water intake was ad libitum. Feeding into a trough was carried out twice daily in a semi-ad libitum manner to ensure that all of the feed mix provided was, in fact, consumed. Animals were weighed individually at weekly intervals and feed consumption was recorded at the same time. Average daily gain was calculated from the initial and final weights of individual animals. Feed intake and conversion were determined from group consumption of the mixed feed and recalculated to a per-pig value.
The average weights (kg) of animals in Experimental (E) and Control (C) groups at the beginning and end of stages I and II of the experiment are shown in Figure 1. The experiment started after weaning (Stage I) at 1 month of age when the average pig weight was 9.5 kg for the Experimental Group (E) and 9.15 kg for the Control Group (C). The Control Group was fed the basic mixture, and the Experimental Group was fed the mixture containing 0.5% dried hops for 21 days. Voluntary feed intake of the animals and their health status were both monitored. At the end of the trial period (after 3 weeks), faecal samples were collected randomly from six animals from each group, and dry matter, crude protein, ether extract, crude fibre, and ash were determined using standard methods.
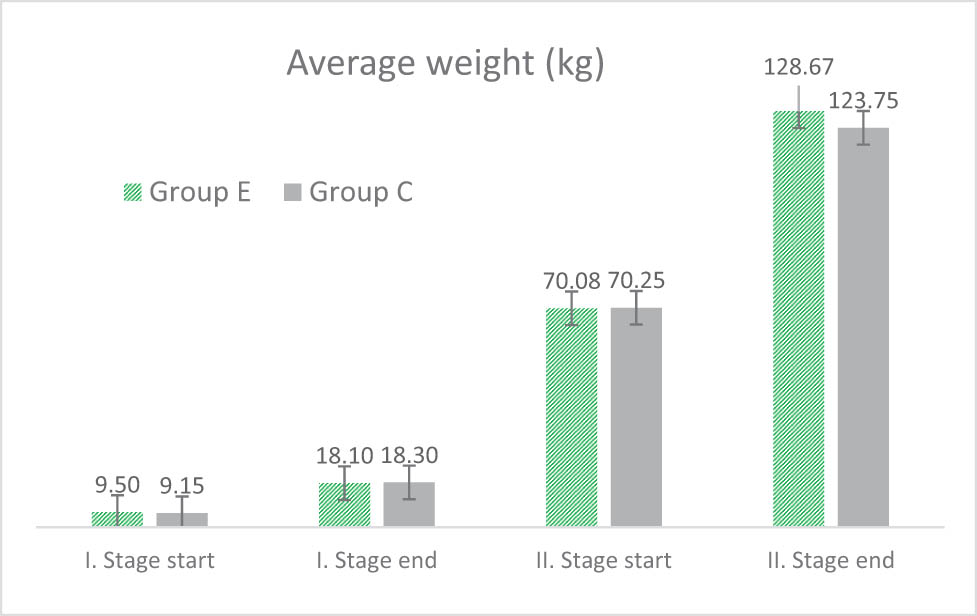
Average weight (kg) of animals in Experimental (E) and Control (C) groups at the beginning and end of stages I and II of the experiment.
The second phase of the experiment (Stage II) started at an average weight of approximately 70 kg (70.08 kg for Group E and 70.25 kg for Group C). Group C was fed with the basic feed mix, and Group E with a mix containing 0.8% dried hops. Fattening was terminated when an average weight exceeding 120 kg was reached in both groups simultaneously after the same experimental period (128.67 kg for Group E and 123.75 kg for Group C). The pigs were slaughtered at a slaughterhouse at the end of the trial, and meat samples were collected for chemical analysis. Samples of muscle (musculus longissimus lumborum et thoracis [MLLT]) were collected from the loin between the second and third to last rib 24 h after slaughter.
Standard methods were used to perform the basic chemical analysis of meat samples. Dry mass was determined gravimetrically after drying for 4 h at 105°C. Nitrogen was determined after converting nitrogen compounds to ammonium sulphate through mineralization with sulphuric acid and distilling ammonia through alkalimetric titration. Nitrogen substances were then determined by multiplying the amount of nitrogen by 6.25. The ether extract content was determined using dichloromethane extraction with the extracted fat determined gravimetrically. Ash was determined gravimetrically as the residue after the complete burning of organic matter at 550°C. Fatty acid composition in meat was determined after chloroform–methanol extraction of total lipids and alkaline trans-methylation of fatty acids. Gas chromatography of methyl esters was carried out using a Hewlett Packard 5890 Series II chromatograph and evaluated in accordance with the Supelco 37 Component FAME Mix standard. The amino acid content was determined using an AAA 400 analyser (INGOS s.r.o., Prague) after hydrolysis with 6 M HCl according to a standard method. To determine the oxidative stability of the meat, the samples were frozen at −20°C and then analysed upon thawing after 6 months of storage for thiobarbituric acid-reactive substance (TBARS) content [19]. The frozen meat samples were thawed, and the TBARS levels (measured as amount of malondialdehyde) were determined on days 1, 3, and 6 after thawing.
The atherogenic index (AI) and thrombogenicity index (TI) were calculated (Σg/100 g) according to Ulbricht and Southgate [20]:
where PUFA represents polyunsaturated fatty acids, and MUFA represents monounsaturated fatty acids.
The experimental data were statistically processed using a QC expert (TriloByte Statistical Software Ltd, Pardubice, Czech Republic). The data are presented as arithmetic means with standard deviation (SD). Student’s t-test was used for significance testing. Differences among means in the t-test were considered significant only if the F-test was significant. Differences between the assessed groups were considered significant at P < 0.05.
-
Ethical approval: The research has complied with all the relevant national regulations and institutional policies for the care and use of animals.
3 Results
The hops were first analysed and their nutrient content. The nutrient composition of dried hops is shown in Table 2.
Nutrient composition of dried hops
| Item | Unit | Value |
|---|---|---|
| Dry matter | % | 90.77 |
| Crude protein | % | 15.42 |
| Ash | % | 7.65 |
| Ether extract | % | 1.47 |
| Fibre | % | 39.42 |
| Copper | mg/kg | 30.20 |
Because of their bitter taste, granulated hops are rejected by pigs and must be well incorporated into the mix to ensure animal acceptance. Effort was made to ensure that the addition of hops did not cause a significant reduction in feed intake. The feed mix with the addition of 0.5% hops was well accepted by the piglets after weaning. The health status and condition of all animals in both groups were adequate and remained unchanged throughout the period.
In the first phase of the experiment, an average daily gain of 0.405 kg (SD 0.062) was achieved in Group E and 0.436 kg (SD 0.077) in Group C during 3 weeks after weaning. There was no statistically significant difference between the groups. The average daily intake of the feed mix was 0.789 kg in Group E and 0.827 kg in Group C. This represents a feed conversion ratio (FCR) of 1.95 kg of feed per 1 kg of weight gain (group E) and 1.90 kg of feed (group C). Analysis of faecal samples showed no statistically significant difference in the content of dry matter, crude protein, crude fibre, or ash. A statistically significant difference was found only in the ether extract content (P < 0.050), where that value was higher in Group E than in Group C. The results are shown in Table 3.
Average nutrient content (%) in faecal samples for Experimental (E) and Control (C) groups
| Item | Group C | Group E | Significance | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Dry matter | 25.49 | 1.530 | 23.61 | 1.579 | ns |
| Crude protein | 25.67 | 3.054 | 27.98 | 0.928 | ns |
| Ether extract | 8.25 | 0.633 | 9.63 | 0.776 | * |
| Crude fibre | 12.64 | 1.625 | 12.22 | 1.063 | ns |
| Ash | 9.06 | 0.731 | 8.22 | 0.566 | ns |
ns = not significant, *P < 0.05.
In the second phase of the experiment, the effect of the mixture with the addition of 0.8% hops on the growth intensity of pigs over 70 kg was monitored. No statistically significant difference was found between the groups. Group C achieved an average daily gain of 0.713 kg (SD 0.104), and Group E, with the addition of hops, achieved 0.781 kg (SD 0.073). The average feed intake was lower for the mixture with the addition of hops (2.73 kg vs 3.05 kg per animal per day). The FCR was 3.5 kg (group E) and 4.3 kg (group C) per 1 kg of weight gain. The determined average weights of the pigs during the experiment are shown in Figure 1, and performance parameters are shown in Table 4.
Performance parameters
| Item | Group C | Group E | Significance | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Average daily gain (kg/day) | |||||
| Stage I | 0.436 | 0.077 | 0.405 | 0.062 | ns |
| Stage II | 0.713 | 0.104 | 0.781 | 0.073 | ns |
| Average feed intake (kg/day) | |||||
| Stage I | 0.827 | 0.789 | |||
| Stage II | 3.050 | 2.730 | |||
| FCR (kg/kg) | |||||
| Stage I | 1,90 | 1,95 | |||
| Stage II | 4.30 | 3.50 | |||
ns = not significant.
A basic chemical analysis of the meat samples was carried out to determine the contents of dry matter, ether extract, crude protein, and ash (Table 5). A statistically significant difference was found between the groups only in dry matter content, which was 29.05% (SD 0.53) in Group E and 27.43% (SD 0.80) in Group C (P < 0.01). The contents of six saturated fatty acids (SFAs), five monounsaturated fatty acids (MUFAs), and six polyunsaturated fatty acids (PUFAs) detected in the samples are shown in Table 6. The analysis showed some significant differences between pigs fed with the control versus experimental diet. An increase in MUFA content was found in meat (47.46% vs 44.86% in Group C) and a decrease in SFAs (35.33% vs 36.36% in Group C), but these differences were not statistically significant. There was a statistically significant increase in two particular MUFAs (C15:1 and C 24:1) and a decrease in one SFA (C14:0).
Basic chemical composition (%) of meat samples for Experimental (E) and Control (C) groups
| Group C | Group E | ||||
|---|---|---|---|---|---|
| Item | Mean | SD | Mean | SD | Significance |
| Dry matter | 27.43 | 0.85 | 29.05 | 0.53 | * |
| Crude protein | 21.44 | 0.71 | 21.59 | 0.48 | ns |
| Ether extract | 4.43 | 1.36 | 5.03 | 0.33 | ns |
| Ash | 0.97 | 0.05 | 1.05 | 0.16 | ns |
ns = not significant, *P < 0.05.
Fatty acid profile (% of total) in muscle (musculus longissimus lumborum et thoracis) for Experimental (E) and Control (C) groups
| Fatty acid | Group C | Group E | Significance | ||
|---|---|---|---|---|---|
| (%) | Mean | SD | Mean | SD | |
| C14:0 | 1.24 | 0.10 | 1.06 | 0.04 | * |
| C15:1n-5 | 0.35 | 0.13 | 0.47 | 0.21 | ** |
| C16:0 | 21.45 | 1.01 | 21.04 | 0.18 | ns |
| C16:1n-7 | 2.28 | 0.19 | 2.49 | 0.31 | ns |
| C17:0 | 0.31 | 0.04 | 0.28 | 0.02 | ns |
| C18:0 | 12.36 | 0.92 | 12.17 | 0.96 | ns |
| C18:1n-9 | 40.85 | 2.54 | 42.79 | 0.25 | ns |
| C18:2n-6 | 9.48 | 0.80 | 10.06 | 0.36 | ns |
| C18:3n-3 | 1.02 | 0.60 | 0.97 | 0.05 | ns |
| C20:0 | 0.23 | 0.02 | 0.26 | 0.01 | ns |
| C20:1n-9 | 0.96 | 0.06 | 0.89 | 0.06 | ns |
| C20:2n-6 | 0.41 | 0.07 | 0.47 | 0.02 | ns |
| C20:3n-6 | 0.18 | 0.07 | 0.20 | 0.01 | ns |
| C20:4n-6 | 0.99 | 0.10 | 1.06 | 0.10 | ns |
| C20:3n-3 | 0.21 | 0.04 | 0.21 | 0.01 | ns |
| C24:0 | 0.31 | 0.05 | 0.30 | 0.02 | ns |
| C24:1n9 | 0.15 | 0.04 | 0.28 | 0.03 | * |
| ∑ SFAs | 36.36 | 1.28 | 35.33 | 2.33 | ns |
| ∑ MUFAs | 44.86 | 2.86 | 47.46 | 2.10 | ns |
| ∑ PUFAs | 12.76 | 1.03 | 13.50 | 0.55 | ns |
| ∑PUFA n-3 | 1.53 | 0.19 | 1.45 | 0.48 | ns |
| ∑PUFA n-6 | 11.22 | 0.96 | 11.84 | 0.36 | ns |
| PUFA n-6/PUFA n-3 ratio | 7.41 | 8.19 | |||
| AI | 0.46 | 0.42 | |||
| TI | 1.07 | 1.01 | |||
ns = not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
The meat samples were also analysed to determine the amino acid profile. Seventeen amino acids were determined, and differences between the groups as to their contents were evaluated. These values are listed in Table 7. No statistically significant difference was determined for 12 amino acids. Decreased content was found for three amino acids (Leu, Tyr, His) in Group E at a significance level of P < 0.05, and for one amino acid (Asp), this was at P < 0.01. Lysine is the only amino acid whose content increased in the muscle of Group E (P < 0.001).
Amino acid profile (g/100 g) of muscle (musculus longissimus lumborum et thoracis) for Experimental (E) and Control (C) groups
| Amino acid | Group C | Group E | |||
|---|---|---|---|---|---|
| g/100 g | Mean | SD | Mean | SD | Significance |
| ASP | 7.64 | 0.17 | 6.91 | 0.25 | ** |
| THR | 3.76 | 0.10 | 3.57 | 0.08 | ns |
| SER | 3.26 | 0.07 | 3.16 | 0.08 | ns |
| GLU | 12.75 | 0.16 | 11.95 | 0.55 | ns |
| PRO | 4.22 | 0.42 | 3.95 | 0.05 | ns |
| GLY | 3.40 | 0.14 | 3.27 | 0.10 | ns |
| ALA | 4.30 | 0.12 | 4.23 | 0.09 | ns |
| CYS | 0.44 | 0.06 | 0.47 | 0.03 | ns |
| VAL | 3.87 | 0.15 | 3.61 | 0.09 | ns |
| MET | 2.41 | 0.43 | 3.05 | 0.07 | ns |
| ILE | 3.56 | 0.12 | 3.35 | 0.07 | ns |
| LEU | 6.13 | 0.08 | 5.83 | 0.15 | * |
| TYR | 2.77 | 0.07 | 2.55 | 0.07 | * |
| PHE | 3.03 | 0.08 | 2.97 | 0.12 | ns |
| HIS | 3.52 | 0.20 | 2.90 | 0.19 | * |
| LYS | 5.41 | 0.22 | 6.45 | 0.11 | *** |
| ARG | 4.70 | 0.76 | 4.55 | 0.21 | ns |
ns = not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Malondialdehyde levels (mg/kg) were determined in thawed meat samples, and, in all cases, it was lower in Group E, but the differences were not statistically significant. The results are shown in Table 8.
TBARS content (measured as malondialdehyde) in meat samples (mg/kg) for Experimental (E) and Control (C) groups
| Day 1 | Day 3 | Day 6 | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Group C | 0.234 | 0.060 | 0.726 | 0.171 | 1.303 | 0.493 |
| Group E | 0.157 | 0.071 | 0.585 | 0.223 | 1.187 | 0.590 |
4 Discussion
Relatively high protein and crude fibre contents and a high level of copper (30.2 mg/kg) were found in hops. Commonly used cereal grains and their co-products in swine diets contain 4.4–38.4 mg/kg Cu on an as-fed basis. Espinosa and Stein [21] stated that the inclusion of doses above 75 mg/kg Cu in diets for pigs can improve feed intake and feed efficiency. They attribute improved growth performance upon Cu supplementation as likely resulting from an ability of dietary Cu to modulate intestinal microbial populations, indirectly improving immune response and increasing mRNA abundance of genes involved in the post-absorptive metabolism of lipids in pigs. Because only small amounts of hops were used in the experiment, this affected Cu content slightly in the resulting feed mixes. Fiesel et al. [22] stated that polyphenol-rich plant products (hops) had only a marginal effect on Fe, Zn, and Cu in piglets, which had received a diet supplemented with 1% spent hops for 4 weeks.
The available literature contains only sparse information on the feeding of hops in fattening pigs. The contents of polyphenols, bitter acids, and essential oils in hops, which have proven to have antimicrobial, anti-inflammatory, and chemoprotective effects, support the assumption of their positive effect on the health and condition of animals. A positive effect of hop extract was found in feeding another non-ruminant species (chickens). Bozkurt et al. [23] reported that the growth performance of birds fed with this extract was better than those receiving an antibiotic growth promoter. In an experiment by Williams et al. [24] in newly weaned piglets, no improvement in growth performance was found. Similarly, no positive effect of hops on the growth of piglets was observed either in the period after weaning or in the final stage of fattening. In another experiment carried out on growing pigs, Hanczakowska et al. [25] recorded no significant difference between the control and experimental groups.
A slight decrease in consumption of the feed mix with hops was recorded in the present experiment, especially in the initial stage of feeding. This could be due to the bitter substances from the hops affecting the taste of the feed mix. The slightly higher crude fibre content measured in the feed mixture with hops could have negatively affected nutrient digestibility and also feed intake [26,27]. Walker et al. [28] found that the extract of bitter hops can reduce food intake, suppress appetite, and stimulate the release of appetite-suppressing gut hormones in healthy men.
The decreased feed intake did not affect the growth intensity of Group E pigs in the experiment. This result slightly improved nutrient conversion compared with Group C in finishing pigs. According to Hanczakowska et al. [25], the hop extract showed a trend towards reducing feed conversion in comparison to control. In the experiment of Fiesel et al. [29] on piglets, supplementing spent hops significantly improved the gain/feed ratio, although protein and organic matter digestibility were decreased. According to Fiesel et al. [29], alterations in the microbial composition and anti-inflammatory properties might contribute to the beneficial effects.
The addition of hops possibly affected the digestibility of the diet, as indicated by the higher nutrient content in the faeces of Group E. These results suggest that it would be appropriate to determine the digestibility of the mixture with the addition of hops. In the faeces of Group E, an increased crude protein was observed, but without statistical significance. The highest difference was recorded in the faeces content of ether extract, which was increased in the group fed with hops. According to a study in rats [30], xanthohumol-rich hop extract promoted triacylglycerol excretion into faeces, probably caused by a decline in lipid absorption within the rats fed a high-fat diet.
An influence on the nutritional value of the produced meat was verified, especially with respect to the representation of fatty acids. A decrease in some SFAs (significantly only C14:0) is beneficial to human health in terms of the effect on plasma cholesterol concentration and coronary heart disease [31]. An increased level of MUFAs is also beneficial from a health perspective and contributes to better organoleptic sensations of pig fat [32]. The atherogenic and thrombogenicity indices were calculated according to Ulbricht and Southgate [20]. Both indices were lower in Group E supplemented with hops. This meat shows better potential effects in terms of the incidence of cardiovascular disease. The same effects of hops on pig meat were also noted by Hanczakowska et al. [25].
Unsaturated fatty acids are considered to be beneficial to human health, but they are also prone to oxidation, which can degrade the taste and nutritional quality of meat [33]. Oxidative changes that occur during storage can be prevented by antioxidant supplements in feed [34], and the bioactive components of hops are important antioxidants that delay or prevent oxidation [5]. The decrease in TBARS content (measured as malondialdehyde) was not statistically significant in the meat of Group E compared to that of Group C on days 1, 3, and 6 after thawing and even following 6 months of storage.
The analysis of amino acids in the meat showed a decrease in three amino acids and a significant increase in lysine within the E group. Lysine is important for protein synthesis and is the first limiting amino acid in pig nutrition and very important, too, in human nutrition [35]. The cause of the increase in meat of Group E is unclear, but gut microbiota may play a role. The addition of hops could affect the state of the intestinal microbiota and thus also the metabolism of lysine in which the microbiota participates [36]. This could also affect changes in the contents of other amino acids [37]. It would be appropriate to further investigate the effect of hops on the intestinal microbiome of pigs.
5 Conclusion
The pig fattening system with the addition of dried hops was verified, and the effect on fattening parameters and the quality of the final product was determined. Feeding the hop mixture had no statistically significant effect on the growth intensity of piglets after weaning, and feed intake during this period. Similarly, no statistically significant effect was found on the growth of fattening pigs, but nutrient conversion was slightly improved compared to that in the control group. Feeding with the tested mixture also enables the production of meat with a higher nutritional value. A change in the representation of some fatty acids was found, which is beneficial from the viewpoint of human nutrition. The addition of hops in fattening pigs can be beneficial for improving feed conversion and creating added value in the form of the quality of the meat produced.
-
Funding information: This work was supported by the Ministry of Agriculture of the Czech Republic institutional support RO 0723.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and consented to its submission to the journal, reviewed all the results and approved the final version of the manuscript. JB: data collection and evaluation, writing the article. MR: methodology, review, and editing.
-
Conflict of interest: Authors state no conflicts of interest.
-
Data availability statement: The datasets generated during the current study are available from the corresponding author on reasonable request.
References
[1] Van Cleemput M, Cattoor K, De Bosscher K, Haegeman G, De Keukeleire D, Heyerick A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J Nat Prod. 2009;72(6):1220–30. 10.1021/np800740m.Suche in Google Scholar PubMed
[2] Landete JM. Updated knowledge about polyphenols: functions, bioavailability, metabolism, and health. Crit Rev Food Sci Nutr. 2012;52:936–48.10.1080/10408398.2010.513779Suche in Google Scholar PubMed
[3] Cermak P, Olsovska J, Mikyska A, Dusek M, Kadleckova Z, Vanicek J, et al. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. J PatholMicrobiol Immunol. 2017;125(11):1033–8. 10.1111/apm.12747.Suche in Google Scholar PubMed
[4] Rutnik K, Knez Hrnčič M, Jože Košir I. Hop essential oil: chemical composition, extraction, analysis, and applications. Food Rev Int. 2021;38:529–51. 10.1080/87559129.2021.1874413.Suche in Google Scholar
[5] Zugravu CA, Bohiltea RE, Salmen T, Pogurschi E, Otelea MR. Antioxidants in hops: bioavailability, health effects and perspectives for new products. Antioxidants. 2022;11(2):241. 10.3390/antiox11020241, PMID: 35204124; PMCID: PMC8868281.Suche in Google Scholar PubMed PubMed Central
[6] Steenackers B, De Cooman L, De Vos D. Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: A review. Food Chem. 2015;172:742–56. ISSN 0308-8146, 10.1016/j.foodchem.2014.09.139.Suche in Google Scholar PubMed
[7] Liu M, Hansen PE, Wang G, Qiu L, Dong J, Yin H, et al. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules. 2015;20(1):754–79. 10.3390/molecules20010754, PMID: 25574819; PMCID: PMC6272297.Suche in Google Scholar PubMed PubMed Central
[8] Dostálek P, Karabín M, Jelínek L. Hop phytochemicals and their potential role in metabolic syndrome prevention and therapy. Molecules. 2017;22(10):1761. 10.3390/molecules22101761.Suche in Google Scholar PubMed PubMed Central
[9] Lou S, Zheng YM, Liu SL, Qiu J, Han Q, Li N, et al. ) Inhibition of hepatitis C virus replication in vitro by xanthohumol, a natural product present in hops. Planta Med. 2014;80(2–3):171–6. 10.1055/s-0033-1360172, Epub 2013 Dec 19 PMID: 24356905.Suche in Google Scholar PubMed
[10] Liu X, Bai J, Jiang Ch, Song Z, Zhao Y, Nauwynck H, et al. Therapeutic effect of Xanthohumol against highly pathogenic porcine reproductive and respiratory syndrome viruses. Vet Microbiol. 2019;238:108431. ISSN 0378-1135, 10.1016/j.vetmic.2019.108431.Suche in Google Scholar PubMed
[11] Di Sotto A, Checconi P, Celestino I, Locatelli M, Carissimi S, De Angelis M, et al. Antiviral and antioxidant activity of a hydroalcoholic extract from humulus lupulus L. Oxid Med Cell Longev. 2018;2018:5919237. 10.1155/2018/5919237, PMID: 30140367; PMCID: PMC6081516.Suche in Google Scholar PubMed PubMed Central
[12] Jirovetz L. Antimicrobial testings, gas chromatographic analysis and olfactory evaluation of an essential oil of hop cones (humulus lupulus L.) from Bavaria and some of its main compounds. Sci Pharm. 2006;74(4):189–201. 10.3797/scipharm.2006.74.189.Suche in Google Scholar
[13] Bocquet L, Sahpaz S, Rivi’ere C. An overview of the antimicrobial properties of hop. In: J. M. M´erillon, Riviere C, editors. Natural antimicrobial agents. Cham: Springer; 2018. 10.1007/978-3-319-67045-4_2.Suche in Google Scholar
[14] Štern A, Furlan V, Novak M, Štampar M, Kolenc Z, Kores K, et al. Chemoprotective effects of Xanthohumol against the carcinogenic mycotoxin aflatoxin B1. Foods. 2021;10(6):1331. 10.3390/foods10061331.Suche in Google Scholar PubMed PubMed Central
[15] Abiko Y, Paudel D, Uehara O. Hops components and oral health. J Funct Foods. 2022;92:105035. 10.1016/j.jff.2022.105035.Suche in Google Scholar
[16] Natarajan P, Katta S, Andrei VBR, Leonida M, Haas GJ. Positive antibacterial co-action between hop (Humulus lupulus) constituents and selected antibiotics. Phytomedicine. 2008;15(3):194–201. ISSN 0944-7113, 10.1016/j.phymed.2007.10.008.Suche in Google Scholar PubMed
[17] Bogdanova K, Kolar M, Langova K, Dušek M, Mikyška A, Bostikova V, et al. Inhibitory effect of hop fractions against Gram-positive multi-resistant bacteria. A pilot study. Biomed Pap. 2018;164:276–83. 10.5507/bp.2018.026.Suche in Google Scholar PubMed
[18] Kutňák M. Characteristics and use of hops and hops extract. Dissertation for the Bachelor’s Degree (Vlastnosti a využití chmele a chmelového extraktu, Bakalářská práce), Tomáš Bata University in Zlín. Accessible online at: Bc_Chmel25_5_final15 (utb.cz). 2011. 10.1080/10408398.2010.513779.Suche in Google Scholar PubMed
[19] Vít T, Červinková K. Institute of Animal Science Prague – Uhříněves. A Procedure for Determining the Oxidation Stability of Lipids in Muscle Tissues.(Způsob stanovení oxidační stability lipidů ve svalové tkáni). Patent registered in the Czech Republuic under CZ 305456 B6. 2015-08-19.Suche in Google Scholar
[20] Ulbricht TLV, Southgate DAT. Coronary disease: seven dietary factors. Lancet. 1991;338:985–92.10.1016/0140-6736(91)91846-MSuche in Google Scholar
[21] Espinosa CD, Stein HH. Digestibility and metabolism of copper in diets for pigs and influence of dietary copper on growth performance, intestinal health, and overall immune status: a review. J Anim Sci Biotechnol. 2021;12:13. 10.1186/s40104-020-00533-3.Suche in Google Scholar PubMed PubMed Central
[22] Fiesel A, Ehrmann M, Geßner DK, Most E, Eder K. Effects of polyphenol-rich plant products from grape or hop as feed supplements on iron, zinc and copper status in piglets. Arch Anim Nutr. 2015;69(4):276–84. 10.1080/1745039X.2015.1057065.Suche in Google Scholar PubMed
[23] Bozkurt M, Kucukyilmaz K, Catli AU, Cinar M. Effect of dietary mannan oligosaccharide with or without oregano essential oil and hop extract supplementation on the performance and slaughter characteristic of male broilers. South Afr J Anim Sci. 2009;39:223–32.10.4314/sajas.v39i3.49157Suche in Google Scholar
[24] Williams J, Stewart AH, Powles J, Rose SP, Mackenzie AM. The effect of different concentrations of hops on the performance, gut morphology, microflora and liver enzyme activity of newly weaned piglets. Book of Abstracts 56th Annual Meeting. Uppsala, Sweden: European Association for Animal Production (EAAP); 2005. p. 330.Suche in Google Scholar
[25] Hanczakowska E, Świątkiewicz M, Grela ER. Effect of dietary supplement of herbal extract from hop (Humulus lupulus) on pig performance and meat quality. Czech J Anim Sci. 2017;62:287–95.10.17221/49/2016-CJASSuche in Google Scholar
[26] Zhang W, Li D, Liu L, Zang J, Duan O, Yang W, et al. The effects of dietary fiber level on nutrient digestibility in growing pigs. J Anim Sci Biotechnol. 2013;4:17. 10.1186/2049-1891-4-17.Suche in Google Scholar PubMed PubMed Central
[27] Heyer CME, Wang LF, Beltranena E. Nutrient digestibility of extruded canola meal in ileal-cannulated growing pigs and effects of its feeding on diet nutrient digestibility and growth performance in weaned pigs. J Anim Sci. 2021;99:skab135. 10.1093/jas/skab135.Suche in Google Scholar PubMed PubMed Central
[28] Walker EG, Lo KR, Pahl MC, Shin HS, Lang C, Wohlers MW, et al. An extract of hops (Humulus lupulus L.) modulates gut peptide hormone secretion and reduces energy intake in healthy-weight men: a randomized, crossover clinical trial. Am J Clin Nutr. 2022;115:925–40. 10.1093/ajcn/nqab418.Suche in Google Scholar PubMed
[29] Fiesel A, Gessner DK, Most E, Eder K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet Res. 2014;10:196. 10.1186/s12917-014-0196-5.Suche in Google Scholar PubMed PubMed Central
[30] Yui K, Kiyofuji A, Osada K. Effects of xanthohumol-rich extract from the hop on fatty acid metabolism in rats fed a high-fat diet. J Oleo Sci. 2014;63(2):159–68. 10.5650/jos.ess13136, Epub 2014 Jan 14 PMID: 24420065.Suche in Google Scholar PubMed
[31] Webb EC, O’Neill HA. The animal fat paradox and meat quality. Meat Sci. 2008;80:28–36.10.1016/j.meatsci.2008.05.029Suche in Google Scholar PubMed
[32] Fallola A, Sanabria C, Sabio E, Vidal-Aragon MC, Esparrago F. The extensive pig quality. In: Flamant JC, Espejo Diaz M, editors. Basis of the quality of typical mediterranean animal products. Vol. 90, European Association for Animal Production Publication; 1998. p. 320–38.Suche in Google Scholar
[33] Sohaib M, Anjum FM, Sahar A, Arshad MS, Rahman UU, Imran A, et al. Antioxidant proteins and peptides to enhance the oxidative stability of meat and meat products: A comprehensive review. Int J Food Prop. 2017;20(11):2581–93. 10.1080/10942912.2016.1246456.Suche in Google Scholar
[34] Wood JD, Enser M. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Br J Nutr. 1997;78:49–60. 10.1079/BJN19970134.Suche in Google Scholar PubMed
[35] Tomé D, Bos C. Lysine requirement through the human life cycle. J Nutr. 2007;137(6):1642S–5S. ISSN 0022-3166, 10.1093/jn/137.6.1642S.Suche in Google Scholar PubMed
[36] Matthews DE. Review of lysine metabolism with a focus on humans. J Nutr. 2020;150(Supplement 1):2548S–55S. ISSN 0022-3166, 10.1093/jn/nxaa224.Suche in Google Scholar PubMed
[37] Hu J, Chen J, Ma L, Hou Q, Zhang Y, Kong X et al. Characterizing core microbiota and regulatory functions of the pig gut microbiome, ISME J, 2024;18:wrad037. 10.1093/ismejo/wrad037.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Optimization of sustainable corn–cattle integration in Gorontalo Province using goal programming
- Competitiveness of Indonesia’s nutmeg in global market
- Toward sustainable bioproducts from lignocellulosic biomass: Influence of chemical pretreatments on liquefied walnut shells
- Efficacy of Betaproteobacteria-based insecticides for managing whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), on cucumber plants
- Assessment of nutrition status of pineapple plants during ratoon season using diagnosis and recommendation integrated system
- Nutritional value and consumer assessment of 12 avocado crosses between cvs. Hass × Pionero
- The lacked access to beef in the low-income region: An evidence from the eastern part of Indonesia
- Comparison of milk consumption habits across two European countries: Pilot study in Portugal and France
- Antioxidant responses of black glutinous rice to drought and salinity stresses at different growth stages
- Differential efficacy of salicylic acid-induced resistance against bacterial blight caused by Xanthomonas oryzae pv. oryzae in rice genotypes
- Yield and vegetation index of different maize varieties and nitrogen doses under normal irrigation
- Urbanization and forecast possibilities of land use changes by 2050: New evidence in Ho Chi Minh city, Vietnam
- Organizational-economic efficiency of raspberry farming – case study of Kosovo
- Application of nitrogen-fixing purple non-sulfur bacteria in improving nitrogen uptake, growth, and yield of rice grown on extremely saline soil under greenhouse conditions
- Digital motivation, knowledge, and skills: Pathways to adaptive millennial farmers
- Investigation of biological characteristics of fruit development and physiological disorders of Musang King durian (Durio zibethinus Murr.)
- Enhancing rice yield and farmer welfare: Overcoming barriers to IPB 3S rice adoption in Indonesia
- Simulation model to realize soybean self-sufficiency and food security in Indonesia: A system dynamic approach
- Gender, empowerment, and rural sustainable development: A case study of crab business integration
- Metagenomic and metabolomic analyses of bacterial communities in short mackerel (Rastrelliger brachysoma) under storage conditions and inoculation of the histamine-producing bacterium
- Fostering women’s engagement in good agricultural practices within oil palm smallholdings: Evaluating the role of partnerships
- Increasing nitrogen use efficiency by reducing ammonia and nitrate losses from tomato production in Kabul, Afghanistan
- Physiological activities and yield of yacon potato are affected by soil water availability
- Vulnerability context due to COVID-19 and El Nino: Case study of poultry farming in South Sulawesi, Indonesia
- Wheat freshness recognition leveraging Gramian angular field and attention-augmented resnet
- Suggestions for promoting SOC storage within the carbon farming framework: Analyzing the INFOSOLO database
- Optimization of hot foam applications for thermal weed control in perennial crops and open-field vegetables
- Toxicity evaluation of metsulfuron-methyl, nicosulfuron, and methoxyfenozide as pesticides in Indonesia
- Fermentation parameters and nutritional value of silages from fodder mallow (Malva verticillata L.), white sweet clover (Melilotus albus Medik.), and their mixtures
- Five models and ten predictors for energy costs on farms in the European Union
- Effect of silvopastoral systems with integrated forest species from the Peruvian tropics on the soil chemical properties
- Transforming food systems in Semarang City, Indonesia: A short food supply chain model
- Understanding farmers’ behavior toward risk management practices and financial access: Evidence from chili farms in West Java, Indonesia
- Optimization of mixed botanical insecticides from Azadirachta indica and Calophyllum soulattri against Spodoptera frugiperda using response surface methodology
- Mapping socio-economic vulnerability and conflict in oil palm cultivation: A case study from West Papua, Indonesia
- Exploring rice consumption patterns and carbohydrate source diversification among the Indonesian community in Hungary
- Determinants of rice consumer lexicographic preferences in South Sulawesi Province, Indonesia
- Effect on growth and meat quality of weaned piglets and finishing pigs when hops (Humulus lupulus) are added to their rations
- Healthy motivations for food consumption in 16 countries
- The agriculture specialization through the lens of PESTLE analysis
- Combined application of chitosan-boron and chitosan-silicon nano-fertilizers with soybean protein hydrolysate to enhance rice growth and yield
- Stability and adaptability analyses to identify suitable high-yielding maize hybrids using PBSTAT-GE
- Phosphate-solubilizing bacteria-mediated rock phosphate utilization with poultry manure enhances soil nutrient dynamics and maize growth in semi-arid soil
- Factors impacting on purchasing decision of organic food in developing countries: A systematic review
- Influence of flowering plants in maize crop on the interaction network of Tetragonula laeviceps colonies
- Bacillus subtilis 34 and water-retaining polymer reduce Meloidogyne javanica damage in tomato plants under water stress
- Vachellia tortilis leaf meal improves antioxidant activity and colour stability of broiler meat
- Evaluating the competitiveness of leading coffee-producing nations: A comparative advantage analysis across coffee product categories
- Application of Lactiplantibacillus plantarum LP5 in vacuum-packaged cooked ham as a bioprotective culture
- Evaluation of tomato hybrid lines adapted to lowland
- South African commercial livestock farmers’ adaptation and coping strategies for agricultural drought
- Spatial analysis of desertification-sensitive areas in arid conditions based on modified MEDALUS approach and geospatial techniques
- Meta-analysis of the effect garlic (Allium sativum) on productive performance, egg quality, and lipid profiles in laying quails
- Review Articles
- Reference dietary patterns in Portugal: Mediterranean diet vs Atlantic diet
- Evaluating the nutritional, therapeutic, and economic potential of Tetragonia decumbens Mill.: A promising wild leafy vegetable for bio-saline agriculture in South Africa
- A review on apple cultivation in Morocco: Current situation and future prospects
- Quercus acorns as a component of human dietary patterns
- CRISPR/Cas-based detection systems – emerging tools for plant pathology
- Short Communications
- An analysis of consumer behavior regarding green product purchases in Semarang, Indonesia: The use of SEM-PLS and the AIDA model
- Effect of NaOH concentration on production of Na-CMC derived from pineapple waste collected from local society
Artikel in diesem Heft
- Research Articles
- Optimization of sustainable corn–cattle integration in Gorontalo Province using goal programming
- Competitiveness of Indonesia’s nutmeg in global market
- Toward sustainable bioproducts from lignocellulosic biomass: Influence of chemical pretreatments on liquefied walnut shells
- Efficacy of Betaproteobacteria-based insecticides for managing whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), on cucumber plants
- Assessment of nutrition status of pineapple plants during ratoon season using diagnosis and recommendation integrated system
- Nutritional value and consumer assessment of 12 avocado crosses between cvs. Hass × Pionero
- The lacked access to beef in the low-income region: An evidence from the eastern part of Indonesia
- Comparison of milk consumption habits across two European countries: Pilot study in Portugal and France
- Antioxidant responses of black glutinous rice to drought and salinity stresses at different growth stages
- Differential efficacy of salicylic acid-induced resistance against bacterial blight caused by Xanthomonas oryzae pv. oryzae in rice genotypes
- Yield and vegetation index of different maize varieties and nitrogen doses under normal irrigation
- Urbanization and forecast possibilities of land use changes by 2050: New evidence in Ho Chi Minh city, Vietnam
- Organizational-economic efficiency of raspberry farming – case study of Kosovo
- Application of nitrogen-fixing purple non-sulfur bacteria in improving nitrogen uptake, growth, and yield of rice grown on extremely saline soil under greenhouse conditions
- Digital motivation, knowledge, and skills: Pathways to adaptive millennial farmers
- Investigation of biological characteristics of fruit development and physiological disorders of Musang King durian (Durio zibethinus Murr.)
- Enhancing rice yield and farmer welfare: Overcoming barriers to IPB 3S rice adoption in Indonesia
- Simulation model to realize soybean self-sufficiency and food security in Indonesia: A system dynamic approach
- Gender, empowerment, and rural sustainable development: A case study of crab business integration
- Metagenomic and metabolomic analyses of bacterial communities in short mackerel (Rastrelliger brachysoma) under storage conditions and inoculation of the histamine-producing bacterium
- Fostering women’s engagement in good agricultural practices within oil palm smallholdings: Evaluating the role of partnerships
- Increasing nitrogen use efficiency by reducing ammonia and nitrate losses from tomato production in Kabul, Afghanistan
- Physiological activities and yield of yacon potato are affected by soil water availability
- Vulnerability context due to COVID-19 and El Nino: Case study of poultry farming in South Sulawesi, Indonesia
- Wheat freshness recognition leveraging Gramian angular field and attention-augmented resnet
- Suggestions for promoting SOC storage within the carbon farming framework: Analyzing the INFOSOLO database
- Optimization of hot foam applications for thermal weed control in perennial crops and open-field vegetables
- Toxicity evaluation of metsulfuron-methyl, nicosulfuron, and methoxyfenozide as pesticides in Indonesia
- Fermentation parameters and nutritional value of silages from fodder mallow (Malva verticillata L.), white sweet clover (Melilotus albus Medik.), and their mixtures
- Five models and ten predictors for energy costs on farms in the European Union
- Effect of silvopastoral systems with integrated forest species from the Peruvian tropics on the soil chemical properties
- Transforming food systems in Semarang City, Indonesia: A short food supply chain model
- Understanding farmers’ behavior toward risk management practices and financial access: Evidence from chili farms in West Java, Indonesia
- Optimization of mixed botanical insecticides from Azadirachta indica and Calophyllum soulattri against Spodoptera frugiperda using response surface methodology
- Mapping socio-economic vulnerability and conflict in oil palm cultivation: A case study from West Papua, Indonesia
- Exploring rice consumption patterns and carbohydrate source diversification among the Indonesian community in Hungary
- Determinants of rice consumer lexicographic preferences in South Sulawesi Province, Indonesia
- Effect on growth and meat quality of weaned piglets and finishing pigs when hops (Humulus lupulus) are added to their rations
- Healthy motivations for food consumption in 16 countries
- The agriculture specialization through the lens of PESTLE analysis
- Combined application of chitosan-boron and chitosan-silicon nano-fertilizers with soybean protein hydrolysate to enhance rice growth and yield
- Stability and adaptability analyses to identify suitable high-yielding maize hybrids using PBSTAT-GE
- Phosphate-solubilizing bacteria-mediated rock phosphate utilization with poultry manure enhances soil nutrient dynamics and maize growth in semi-arid soil
- Factors impacting on purchasing decision of organic food in developing countries: A systematic review
- Influence of flowering plants in maize crop on the interaction network of Tetragonula laeviceps colonies
- Bacillus subtilis 34 and water-retaining polymer reduce Meloidogyne javanica damage in tomato plants under water stress
- Vachellia tortilis leaf meal improves antioxidant activity and colour stability of broiler meat
- Evaluating the competitiveness of leading coffee-producing nations: A comparative advantage analysis across coffee product categories
- Application of Lactiplantibacillus plantarum LP5 in vacuum-packaged cooked ham as a bioprotective culture
- Evaluation of tomato hybrid lines adapted to lowland
- South African commercial livestock farmers’ adaptation and coping strategies for agricultural drought
- Spatial analysis of desertification-sensitive areas in arid conditions based on modified MEDALUS approach and geospatial techniques
- Meta-analysis of the effect garlic (Allium sativum) on productive performance, egg quality, and lipid profiles in laying quails
- Review Articles
- Reference dietary patterns in Portugal: Mediterranean diet vs Atlantic diet
- Evaluating the nutritional, therapeutic, and economic potential of Tetragonia decumbens Mill.: A promising wild leafy vegetable for bio-saline agriculture in South Africa
- A review on apple cultivation in Morocco: Current situation and future prospects
- Quercus acorns as a component of human dietary patterns
- CRISPR/Cas-based detection systems – emerging tools for plant pathology
- Short Communications
- An analysis of consumer behavior regarding green product purchases in Semarang, Indonesia: The use of SEM-PLS and the AIDA model
- Effect of NaOH concentration on production of Na-CMC derived from pineapple waste collected from local society

