Abstract
C103H116O13SiSn4, triclinic, P
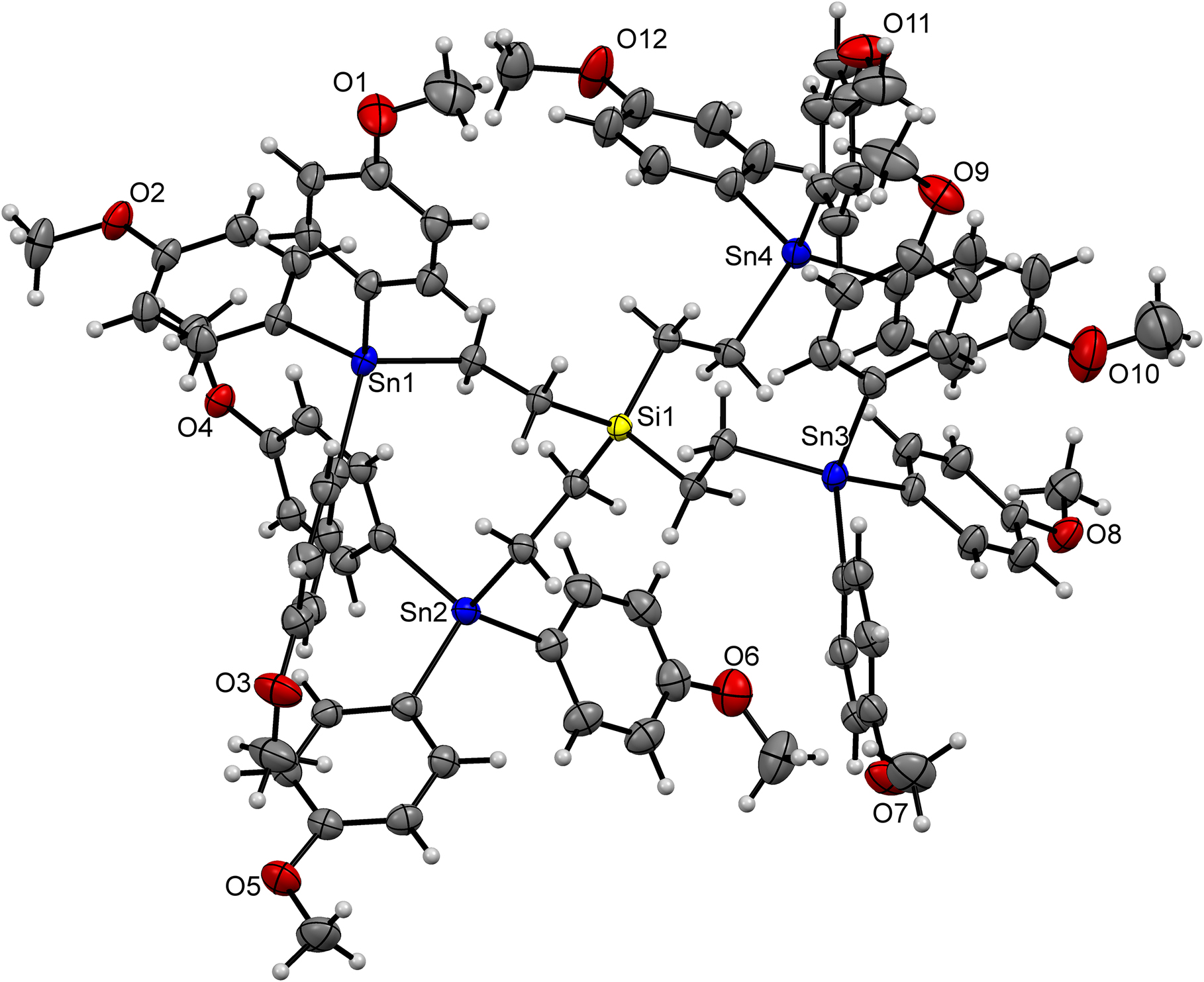
The molecular structure is shown in the figure (The DMF molecule as well as the toluene molecule are both not shown for clarity). Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.44 × 0.37 × 0.32 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.10 mm−1 |
| Diffractometer, scan mode: | Xcalibur, ω |
| θmax, completeness: | 25.0°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 37,597, 16,825, 0.021 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 14,534 |
| N(param)refined: | 1103 |
| Programs: | WinGX/ORTEP [1], SHELX [2], Mercury [3] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Sn1 | 0.463086 (17) | 0.665111 (14) | 0.439683 (12) | 0.02608 (6) |
| Sn2 | 0.875448 (17) | 0.919248 (14) | 0.574697 (13) | 0.02726 (6) |
| Sn3 | 0.787364 (18) | 0.629606 (14) | 0.839769 (12) | 0.02771 (7) |
| Sn4 | 0.614274 (18) | 0.943457 (15) | 0.889013 (13) | 0.02999 (7) |
| Si1 | 0.68738 (7) | 0.78356 (5) | 0.68810 (5) | 0.02201 (18) |
| O1 | 0.0969 (2) | 0.38438 (19) | 0.39913 (16) | 0.0578 (8) |
| O2 | 0.2628 (2) | 0.87890 (16) | 0.27855 (14) | 0.0417 (6) |
| O3 | 0.7583 (2) | 0.52011 (18) | 0.31405 (17) | 0.0559 (8) |
| O4 | 0.57430 (18) | 1.06794 (14) | 0.40286 (13) | 0.0332 (6) |
| O5 | 1.0416 (2) | 0.72702 (17) | 0.35449 (15) | 0.0479 (7) |
| O6 | 1.1731 (3) | 1.19859 (18) | 0.86854 (17) | 0.0578 (8) |
| O7 | 1.0929 (2) | 0.42722 (18) | 0.82442 (17) | 0.0522 (8) |
| O8 | 1.0558 (2) | 0.94760 (16) | 1.11227 (14) | 0.0440 (7) |
| O9 | 0.4448 (2) | 0.42605 (18) | 0.90062 (17) | 0.0558 (8) |
| O10 | 0.9324 (3) | 1.1834 (2) | 1.19529 (19) | 0.0817 (11) |
| O11 | 0.3144 (2) | 0.63865 (19) | 0.8888 (2) | 0.0620 (9) |
| O12 | 0.3944 (3) | 1.17991 (19) | 0.77599 (16) | 0.0638 (10) |
| C1 | 0.5953 (2) | 0.70304 (19) | 0.60042 (17) | 0.0255 (7) |
| H1A | 0.6308 | 0.6725 | 0.5741 | 0.031* |
| H1B | 0.5507 | 0.6629 | 0.6107 | 0.031* |
| C2 | 0.5332 (3) | 0.7413 (2) | 0.55211 (17) | 0.0271 (7) |
| H2A | 0.5765 | 0.7941 | 0.5567 | 0.032* |
| H2B | 0.4816 | 0.7543 | 0.5705 | 0.032* |
| C3 | 0.3451 (3) | 0.5630 (2) | 0.42338 (18) | 0.0278 (7) |
| C4 | 0.2539 (3) | 0.5422 (2) | 0.36838 (18) | 0.0314 (7) |
| H4 | 0.2474 | 0.5705 | 0.3354 | 0.038* |
| C5 | 0.1812 (3) | 0.4411 (2) | 0.4085 (2) | 0.0362 (8) |
| C6 | 0.1725 (3) | 0.4819 (2) | 0.36014 (19) | 0.0365 (8) |
| H6 | 0.1114 | 0.4686 | 0.3218 | 0.044* |
| C7 | 0.2711 (3) | 0.4584 (2) | 0.4627 (2) | 0.0377 (8) |
| H7 | 0.2775 | 0.4293 | 0.4950 | 0.045* |
| C8 | 0.3518 (3) | 0.5187 (2) | 0.4695 (2) | 0.0354 (8) |
| H8 | 0.4134 | 0.5301 | 0.5066 | 0.042* |
| C9 | 0.0951 (4) | 0.3620 (3) | 0.4587 (3) | 0.0704 (15) |
| H9A | 0.0278 | 0.3308 | 0.4493 | 0.106* |
| H9B | 0.1174 | 0.4120 | 0.5017 | 0.106* |
| H9C | 0.1390 | 0.3278 | 0.4664 | 0.106* |
| C10 | 0.4009 (3) | 0.7374 (2) | 0.38254 (18) | 0.0281 (7) |
| C11 | 0.3622 (3) | 0.7971 (2) | 0.41531 (19) | 0.0329 (7) |
| H11 | 0.3675 | 0.8068 | 0.4637 | 0.040* |
| C12 | 0.3163 (3) | 0.8424 (2) | 0.3791 (2) | 0.0356 (8) |
| H12 | 0.2903 | 0.8822 | 0.4025 | 0.043* |
| C13 | 0.3083 (3) | 0.8296 (2) | 0.30869 (19) | 0.0329 (7) |
| C14 | 0.3464 (3) | 0.7724 (2) | 0.2750 (2) | 0.0397 (8) |
| H14 | 0.3415 | 0.7637 | 0.2268 | 0.048* |
| C15 | 0.3927 (3) | 0.7272 (2) | 0.31214 (19) | 0.0380 (8) |
| H15 | 0.4193 | 0.6881 | 0.2885 | 0.046* |
| C16 | 0.2522 (4) | 0.8686 (3) | 0.2064 (2) | 0.0531 (12) |
| H16A | 0.2157 | 0.9049 | 0.1906 | 0.080* |
| H16B | 0.2160 | 0.8110 | 0.1752 | 0.080* |
| H16C | 0.3175 | 0.8828 | 0.2035 | 0.080* |
| C17 | 0.5665 (3) | 0.6196 (2) | 0.39863 (18) | 0.0288 (7) |
| C18 | 0.5512 (3) | 0.5339 (2) | 0.36903 (19) | 0.0335 (7) |
| H18 | 0.4955 | 0.4965 | 0.3683 | 0.040* |
| C19 | 0.6161 (3) | 0.5034 (2) | 0.3410 (2) | 0.0387 (8) |
| H19 | 0.6036 | 0.4454 | 0.3201 | 0.046* |
| C20 | 0.6988 (3) | 0.5566 (2) | 0.3431 (2) | 0.0379 (8) |
| C21 | 0.7168 (3) | 0.6417 (2) | 0.3732 (2) | 0.0370 (8) |
| H21 | 0.7738 | 0.6789 | 0.3753 | 0.044* |
| C22 | 0.6499 (3) | 0.6715 (2) | 0.40012 (19) | 0.0324 (7) |
| H22 | 0.6620 | 0.7296 | 0.4202 | 0.039* |
| C23 | 0.8477 (4) | 0.5737 (3) | 0.3189 (3) | 0.0654 (14) |
| H23A | 0.8824 | 0.5406 | 0.2949 | 0.098* |
| H23B | 0.8900 | 0.6040 | 0.3697 | 0.098* |
| H23C | 0.8318 | 0.6129 | 0.2955 | 0.098* |
| C24 | 0.7599 (2) | 0.8654 (2) | 0.66564 (17) | 0.0255 (7) |
| H24A | 0.7171 | 0.8980 | 0.6489 | 0.031* |
| H24B | 0.8160 | 0.9035 | 0.7099 | 0.031* |
| C25 | 0.7998 (3) | 0.8291 (2) | 0.60722 (18) | 0.0285 (7) |
| H25A | 0.7439 | 0.7882 | 0.5643 | 0.034* |
| H25B | 0.8456 | 0.7994 | 0.6254 | 0.034* |
| C26 | 0.7703 (3) | 0.9666 (2) | 0.51552 (18) | 0.0273 (7) |
| C27 | 0.8018 (3) | 1.0351 (2) | 0.49733 (18) | 0.0301 (7) |
| H27 | 0.8704 | 1.0602 | 0.5112 | 0.036* |
| C28 | 0.7349 (3) | 1.0668 (2) | 0.45970 (18) | 0.0305 (7) |
| H28 | 0.7579 | 1.1133 | 0.4482 | 0.037* |
| C29 | 0.6342 (3) | 1.0308 (2) | 0.43872 (18) | 0.0276 (7) |
| C30 | 0.6005 (3) | 0.9617 (2) | 0.45428 (19) | 0.0308 (7) |
| H30 | 0.5319 | 0.9357 | 0.4391 | 0.037* |
| C31 | 0.6695 (3) | 0.9313 (2) | 0.49261 (19) | 0.0308 (7) |
| H31 | 0.6464 | 0.8844 | 0.5035 | 0.037* |
| C32 | 0.4702 (3) | 1.0277 (3) | 0.3749 (2) | 0.0429 (10) |
| H32A | 0.4351 | 1.0589 | 0.3498 | 0.064* |
| H32B | 0.4489 | 1.0253 | 0.4147 | 0.064* |
| H32C | 0.4556 | 0.9715 | 0.3412 | 0.064* |
| C33 | 0.9409 (3) | 0.8601 (2) | 0.50396 (19) | 0.0303 (7) |
| C34 | 0.9134 (3) | 0.8576 (2) | 0.43314 (19) | 0.0311 (7) |
| H34 | 0.8697 | 0.8873 | 0.4179 | 0.037* |
| C35 | 0.9477 (3) | 0.8133 (2) | 0.3845 (2) | 0.0339 (7) |
| H35 | 0.9275 | 0.8129 | 0.3367 | 0.041* |
| C36 | 1.0120 (3) | 0.7693 (2) | 0.4059 (2) | 0.0344 (7) |
| C37 | 1.0414 (3) | 0.7712 (2) | 0.4759 (2) | 0.0354 (8) |
| H37 | 1.0854 | 0.7415 | 0.4910 | 0.043* |
| C38 | 1.0064 (3) | 0.8163 (2) | 0.5239 (2) | 0.0336 (7) |
| H38 | 1.0278 | 0.8176 | 0.5720 | 0.040* |
| C39 | 1.1080 (3) | 0.6815 (3) | 0.3741 (3) | 0.0592 (13) |
| H39A | 1.1262 | 0.6568 | 0.3332 | 0.089* |
| H39B | 1.0760 | 0.6377 | 0.3874 | 0.089* |
| H39C | 1.1674 | 0.7188 | 0.4152 | 0.089* |
| C40 | 0.9820 (3) | 1.0153 (2) | 0.6703 (2) | 0.0338 (7) |
| C41 | 0.9515 (3) | 1.0730 (2) | 0.7138 (2) | 0.0409 (8) |
| H41 | 0.8839 | 1.0709 | 0.6980 | 0.049* |
| C42 | 1.0158 (3) | 1.1326 (2) | 0.7787 (2) | 0.0442 (8) |
| H42 | 0.9926 | 1.1712 | 0.8064 | 0.053* |
| C43 | 1.1136 (3) | 1.1361 (2) | 0.8031 (2) | 0.0441 (8) |
| C44 | 1.1482 (3) | 1.0816 (2) | 0.7626 (2) | 0.0460 (9) |
| H44 | 1.2160 | 1.0845 | 0.7794 | 0.055* |
| C45 | 1.0821 (3) | 1.0213 (2) | 0.6957 (2) | 0.0436 (9) |
| H45 | 1.1062 | 0.9841 | 0.6674 | 0.052* |
| C46 | 1.2656 (4) | 1.1923 (3) | 0.9043 (3) | 0.0643 (14) |
| H46A | 1.2972 | 1.2370 | 0.9515 | 0.096* |
| H46B | 1.3068 | 1.1966 | 0.8757 | 0.096* |
| H46C | 1.2575 | 1.1388 | 0.9106 | 0.096* |
| C47 | 0.7715 (2) | 0.7348 (2) | 0.73876 (18) | 0.0261 (7) |
| H47A | 0.8125 | 0.7157 | 0.7116 | 0.031* |
| H47B | 0.8162 | 0.7768 | 0.7862 | 0.031* |
| C48 | 0.7130 (3) | 0.6613 (2) | 0.74994 (18) | 0.0294 (7) |
| H48A | 0.6915 | 0.6121 | 0.7055 | 0.035* |
| H48B | 0.6528 | 0.6731 | 0.7556 | 0.035* |
| C49 | 0.8871 (3) | 0.5588 (2) | 0.82693 (18) | 0.0297 (7) |
| C50 | 0.9819 (3) | 0.5933 (2) | 0.82992 (19) | 0.0345 (7) |
| H50 | 1.0013 | 0.6493 | 0.8336 | 0.041* |
| C51 | 1.0479 (3) | 0.5479 (2) | 0.82758 (19) | 0.0365 (8) |
| H51 | 1.1116 | 0.5725 | 0.8293 | 0.044* |
| C52 | 1.0205 (3) | 0.4662 (2) | 0.8227 (2) | 0.0369 (8) |
| C53 | 0.9267 (3) | 0.4298 (2) | 0.8177 (2) | 0.0379 (8) |
| H53 | 0.9071 | 0.3734 | 0.8127 | 0.045* |
| C54 | 0.8614 (3) | 0.4761 (2) | 0.82011 (19) | 0.0345 (7) |
| H54 | 0.7971 | 0.4507 | 0.8170 | 0.041* |
| C55 | 1.0708 (4) | 0.3458 (3) | 0.8267 (3) | 0.0616 (13) |
| H55A | 1.1279 | 0.3251 | 0.8285 | 0.092* |
| H55B | 1.0138 | 0.3090 | 0.7835 | 0.092* |
| H55C | 1.0556 | 0.3475 | 0.8697 | 0.092* |
| C56 | 0.8714 (3) | 0.7388 (2) | 0.93269 (18) | 0.0309 (7) |
| C57 | 0.9579 (3) | 0.7387 (2) | 0.9827 (2) | 0.0392 (8) |
| H57 | 0.9770 | 0.6895 | 0.9756 | 0.047* |
| C58 | 1.0162 (3) | 0.8085 (2) | 1.0423 (2) | 0.0432 (9) |
| H58 | 1.0740 | 0.8064 | 1.0760 | 0.052* |
| C59 | 0.9911 (3) | 0.8815 (2) | 1.05346 (19) | 0.0342 (8) |
| C60 | 0.9044 (3) | 0.8826 (2) | 1.0058 (2) | 0.0363 (8) |
| H60 | 0.8847 | 0.9315 | 1.0134 | 0.044* |
| C61 | 0.8463 (3) | 0.8113 (2) | 0.9465 (2) | 0.0363 (8) |
| H61 | 0.7867 | 0.8127 | 0.9142 | 0.044* |
| C62 | 1.0251 (3) | 1.0200 (2) | 1.1296 (2) | 0.0486 (11) |
| H62A | 1.0767 | 1.0631 | 1.1728 | 0.073* |
| H62B | 0.9641 | 1.0070 | 1.1388 | 0.073* |
| H62C | 1.0135 | 1.0397 | 1.0893 | 0.073* |
| C63 | 0.6733 (3) | 0.5582 (2) | 0.86088 (19) | 0.0311 (7) |
| C64 | 0.6688 (3) | 0.5769 (2) | 0.9295 (2) | 0.0380 (8) |
| H64 | 0.7189 | 0.6216 | 0.9690 | 0.046* |
| C65 | 0.5922 (3) | 0.5310 (2) | 0.9408 (2) | 0.0422 (8) |
| H65 | 0.5904 | 0.5441 | 0.9878 | 0.051* |
| C66 | 0.5182 (3) | 0.4661 (2) | 0.8835 (2) | 0.0401 (8) |
| C67 | 0.5210 (3) | 0.4466 (2) | 0.8153 (2) | 0.0414 (8) |
| H67 | 0.4707 | 0.4022 | 0.7758 | 0.050* |
| C68 | 0.5983 (3) | 0.4929 (2) | 0.8055 (2) | 0.0378 (8) |
| H68 | 0.5998 | 0.4792 | 0.7585 | 0.045* |
| C69 | 0.3626 (4) | 0.3628 (3) | 0.8427 (3) | 0.0692 (15) |
| H69A | 0.3131 | 0.3430 | 0.8613 | 0.104* |
| H69B | 0.3848 | 0.3168 | 0.8208 | 0.104* |
| H69C | 0.3334 | 0.3854 | 0.8064 | 0.104* |
| C70 | 0.6205 (2) | 0.8299 (2) | 0.74558 (18) | 0.0271 (7) |
| H70A | 0.5702 | 0.8490 | 0.7168 | 0.033* |
| H70B | 0.5854 | 0.7860 | 0.7580 | 0.033* |
| C71 | 0.6871 (3) | 0.9028 (2) | 0.81588 (18) | 0.0299 (7) |
| H71A | 0.7139 | 0.9500 | 0.8034 | 0.036* |
| H71B | 0.7435 | 0.8865 | 0.8410 | 0.036* |
| C72 | 0.7184 (3) | 1.0197 (2) | 0.9956 (2) | 0.0398 (8) |
| C73 | 0.7970 (3) | 1.0849 (3) | 1.0040 (2) | 0.0517 (10) |
| H73 | 0.8033 | 1.0929 | 0.9622 | 0.062* |
| C74 | 0.8647 (4) | 1.1375 (3) | 1.0702 (2) | 0.0607 (11) |
| H74 | 0.9163 | 1.1813 | 1.0738 | 0.073* |
| C75 | 0.8572 (4) | 1.1264 (3) | 1.1300 (3) | 0.0588 (10) |
| C76 | 0.7827 (4) | 1.0651 (3) | 1.1267 (2) | 0.0556 (10) |
| H76 | 0.7781 | 1.0590 | 1.1696 | 0.067* |
| C77 | 0.7106 (4) | 1.0093 (3) | 1.0567 (2) | 0.0499 (9) |
| H77 | 0.6585 | 0.9660 | 1.0533 | 0.060* |
| C78 | 0.9315 (6) | 1.1711 (5) | 1.2560 (3) | 0.101 (2) |
| H78A | 0.9729 | 1.2212 | 1.2978 | 0.151* |
| H78B | 0.9572 | 1.1247 | 1.2598 | 0.151* |
| H78C | 0.8637 | 1.1585 | 1.2547 | 0.151* |
| C79 | 0.5180 (3) | 0.8392 (2) | 0.8914 (2) | 0.0364 (8) |
| C80 | 0.4271 (3) | 0.8421 (3) | 0.8958 (2) | 0.0459 (9) |
| H80 | 0.4095 | 0.8916 | 0.8988 | 0.055* |
| C81 | 0.3613 (3) | 0.7751 (3) | 0.8958 (3) | 0.0513 (9) |
| H81 | 0.3003 | 0.7796 | 0.8999 | 0.062* |
| C82 | 0.3845 (3) | 0.7016 (3) | 0.8900 (2) | 0.0468 (9) |
| C83 | 0.4744 (3) | 0.6966 (3) | 0.8858 (2) | 0.0447 (9) |
| H83 | 0.4914 | 0.6467 | 0.8823 | 0.054* |
| C84 | 0.5398 (3) | 0.7651 (2) | 0.8868 (2) | 0.0418 (8) |
| H84 | 0.6015 | 0.7611 | 0.8842 | 0.050* |
| C85 | 0.3365 (4) | 0.5621 (3) | 0.8832 (3) | 0.0632 (13) |
| H85A | 0.2798 | 0.5212 | 0.8809 | 0.095* |
| H85B | 0.3941 | 0.5707 | 0.9255 | 0.095* |
| H85C | 0.3507 | 0.5420 | 0.8395 | 0.095* |
| C86 | 0.5305 (3) | 1.0189 (2) | 0.85138 (19) | 0.0344 (8) |
| C87 | 0.5418 (3) | 1.0986 (3) | 0.8957 (2) | 0.0519 (10) |
| H87 | 0.5822 | 1.1177 | 0.9453 | 0.062* |
| C88 | 0.4958 (4) | 1.1504 (3) | 0.8694 (2) | 0.0577 (11) |
| H88 | 0.5054 | 1.2046 | 0.9007 | 0.069* |
| C89 | 0.4356 (3) | 1.1235 (3) | 0.7976 (2) | 0.0452 (9) |
| C90 | 0.4200 (3) | 1.0447 (3) | 0.7531 (2) | 0.0462 (9) |
| H90 | 0.3772 | 1.0252 | 0.7040 | 0.055* |
| C91 | 0.4671 (3) | 0.9934 (2) | 0.7803 (2) | 0.0431 (9) |
| H91 | 0.4555 | 0.9387 | 0.7490 | 0.052* |
| C92 | 0.3467 (4) | 1.1602 (3) | 0.7009 (2) | 0.0645 (14) |
| H92A | 0.3214 | 1.2054 | 0.6927 | 0.097* |
| H92B | 0.2919 | 1.1091 | 0.6809 | 0.097* |
| H92C | 0.3940 | 1.1525 | 0.6771 | 0.097* |
| O13 | 0.1527 (4) | 0.6799 (3) | 0.0591 (2) | 0.1061 (14) |
| C100 | 0.1066 (5) | 0.5940 (4) | 0.0231 (3) | 0.0869 (15) |
| H10A | 0.0603 | 0.5826 | −0.0261 | 0.104* |
| H10B | 0.0689 | 0.5726 | 0.0496 | 0.104* |
| C101 | 0.2401 (6) | 0.6986 (5) | 0.0437 (4) | 0.1161 (19) |
| H10C | 0.2863 | 0.7531 | 0.0778 | 0.139* |
| H10D | 0.2244 | 0.6975 | −0.0062 | 0.139* |
| C102 | 0.2817 (6) | 0.6285 (5) | 0.0538 (4) | 0.123 (2) |
| H10E | 0.3168 | 0.6391 | 0.1054 | 0.147* |
| H10F | 0.3269 | 0.6194 | 0.0277 | 0.147* |
| C103 | 0.1847 (6) | 0.5531 (5) | 0.0197 (3) | 0.1052 (19) |
| H10G | 0.1734 | 0.5204 | −0.0307 | 0.126* |
| H10H | 0.1878 | 0.5165 | 0.0480 | 0.126* |
| C93 | 0.7983 (5) | 0.3304 (4) | 0.3307 (3) | 0.0979 (17) |
| C94 | 0.7236 (6) | 0.2598 (5) | 0.3123 (4) | 0.134 (2) |
| H94 | 0.6929 | 0.2229 | 0.2636 | 0.160* |
| C95 | 0.6931 (6) | 0.2422 (5) | 0.3635 (4) | 0.134 (2) |
| H95 | 0.6441 | 0.1918 | 0.3508 | 0.161* |
| C96 | 0.7327 (5) | 0.2965 (5) | 0.4323 (3) | 0.111 (2) |
| H96 | 0.7095 | 0.2856 | 0.4676 | 0.133* |
| C97 | 0.8056 (5) | 0.3665 (4) | 0.4506 (3) | 0.1010 (18) |
| H97 | 0.8334 | 0.4044 | 0.4990 | 0.121* |
| C98 | 0.8396 (5) | 0.3836 (4) | 0.4013 (3) | 0.1009 (17) |
| H98 | 0.8920 | 0.4323 | 0.4154 | 0.121* |
| C99 | 0.8357 (7) | 0.3493 (5) | 0.2748 (4) | 0.128 (3) |
| H99A | 0.9076 | 0.3732 | 0.2961 | 0.193* |
| H99B | 0.8057 | 0.3888 | 0.2586 | 0.193* |
| H99C | 0.8179 | 0.2982 | 0.2335 | 0.193* |
Source of material
Tri(4-methoxyphenyl)tin hydride (33.08 g; 75.0 mmol) was treated with tetravinylsilane (2.04 g; 15.0 mmol) and five drops of a 0.1 M solution of hexachloroplatinic acid in 2-propanol. After vigorously stirring the reaction mixture at room temperature for 12 h, pentane was added to the viscous product to give a solid, which was filtered and washed with pentane. Recrystallization from toluene-tetrahydrofuran yielded the product as colorless crystals.
Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms. Some disordered C atoms (C72–C77 and C93–C98) were refined using tools available from SHELXL [2].
Comment
The synthesis and characterization of well-defined heteroatomic macromolecules such as single-source organometallic precursors [4], [5], [6], [7], [8], [9], [10], [11] or metallodendrimers has developed very rapidly in recent years due to their diverse application possibilities in combination with very special properties [4]. Metallodendrimers are specified by the incorporation of metal atoms as the central unit, as branching centers, in the branches themselves or at the periphery of the dendritic skeleton [12]. We systematically study the synthesis and properties of dendritic organotin compounds [13], [14], [15], [16], [17]. The title compound tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane combines the properties of a dendritic molecule with the potential functionalizable organotin moities by replacing peripheral 4-methoxyphenyl groups with halogens and other modifications. The title compoud thereby is an appreciable key intermediate for new approaches to further derivatization and syntheses of higher-order organotin dendrimers with metal atoms as branch sites.
X-ray structure analysis reveals crystallographic data of a first-generation Si–Sn dendrimer. Four dendritic molecules are present in the unit cell. The central Si atom is tetrahedrally coordinated by four dendritic branches. The title compound shows a twisted internal geometry instead of an elongated dendritic backbone, indicating a conformational restriction that is likely due to the tendency to utilize the interior of the dendrimer when large substituents are present on the surface. The C–Si–C angles in the range from 107.37(14)° to 110.81(15)° evidence further an increased conformational restriction. All Sn–C bond lengths are comparable with that of closely related tetrakis[2-(triphenylstannyl)-ethylene]silane (2.144 Å(mean)) [18] and other similar compounds in literature [18, 19]. In addition, all bond lengths and bond angles are in the normal range. The structure of the title compound contains tetrahydrofuran and toluene as independent molecules.
Funding source: Scientific Research Projects Coordination Unit of Akdeniz University (BAP) http://dx.doi.org/10.13039/501100005703
Award Identifier / Grant number: FBA-2021-5531
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Scientific Research Projects Coordination Unit of Akdeniz University (BAP) (Grant No. FBA-2021–5531).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Farrugia, L. J. WinGX and ORTEP for Windows: an update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Suche in Google Scholar
2. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Suche in Google Scholar
3. Macrae, C. F., Bruno, I. J., Chisholm, J. A., Edgington, P. R., McCabe, P., Pidcock, E., Rodriguez-Monge, L., Taylor, R., van de Streek, J., Wood, P. A. Mercury CSD 2.0 – new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470; https://doi.org/10.1107/s0021889807067908.Suche in Google Scholar
4. Kirste, R., Aksu, Y., Wagner, M. R., Khachadorian, S., Jana, S., Driess, M., Thomsen, C., Hoffmann, A. A Raman and photoluminescence spectroscopic detection of surface-bound Li+O−2 defect sites in Li-doped ZnO nanocrystals derived from molecular precursors. ChemPhysChem 2011, 12, 1189–1195; https://doi.org/10.1002/cphc.201000852.Suche in Google Scholar PubMed
5. Samedov, K., Aksu, Y., Driess, M. Heterotermetallic Indium lithium halostannates: low-temperature single-source precursors for tin-rich indium tin oxides and their application for thin-film transistors. Chem. Eur J. 2012, 18, 7766–7779; https://doi.org/10.1002/chem.201103594.Suche in Google Scholar PubMed
6. Heitz, S., Aksu, Y., Merschjann, C., Driess, M. Unprecedented alkylzinc- magnesium alkoxide clusters as suitable organometallic precursors for magnesium- containing ZnO nanoparticles. Chem. Eur J. 2011, 17, 3904–3910; https://doi.org/10.1002/chem.201002743.Suche in Google Scholar PubMed
7. Krackl, S., Company, A., Aksu, Y., Avnir, D., Driess, M. Entrapment of heteropolyacids in metallic silver matrices: unique heterogenized acid catalysts. ChemCatChem 2011, 3, 227–232; https://doi.org/10.1002/cctc.201000239.Suche in Google Scholar
8. Arndt, S., Aksu, Y., Driess, M., Schomacker, R. The catalytic activity of zinc oxides from single source pecursors with additives for the C–H acitivation of lower alkanes. Catal. Lett. 2009, 131, 258–265; https://doi.org/10.1007/s10562-009-0055-3.Suche in Google Scholar
9. Samedov, K., Aksu, Y., Driess, M. Facile molecular approach to transparent thin-film field-effect transistors with high-performance using new homo- and heteroleptic indium(III)-tin(II) single-source precursors. Chem. Mater. 2012, 24, 2078–2090; https://doi.org/10.1021/cm300510y.Suche in Google Scholar
10. Tsaroucha, M., Aksu, Y., Epping, J. D., Driess, M. Facile low-temperature approach to tin-containing ZnO nanocrystals with tunable tin concentrations using heterobimetallic Sn–Zn single source precursors. ChemPlusChem 2013, 78, 62–69; https://doi.org/10.1002/cplu.201200259.Suche in Google Scholar
11. Krackl, S., Ma, J. G., Aksu, Y., Driess, M. Facile access to homo- and heteroleptic, triply bonded dimolybdenum hexaalkoxides with unsaturated alkoxide ligands. Eur. J. Inorg. Chem. 2011, 11, 1725–1732; https://doi.org/10.1002/ejic.201001236.Suche in Google Scholar
12. Bosmann, A. W., Janssen, H. M., Meijer, E. W. About dendrimers: structure, physical properties and applications. Chem. Rev. 1999, 99, 1665–1688; https://doi.org/10.1021/cr970069y.Suche in Google Scholar PubMed
13. Aksu, Y., Aksu, S., Schumann, H. Synthesis and characterization of alpha, omega-bis[tri-(w-triphenylstannyl)butylstannyl]alkanes as starting materials for organotin dendrimers. Appl. Organomet. Chem. 2007, 7, 521–530; https://doi.org/10.1002/aoc.1251.Suche in Google Scholar
14. Schumann, H., Wassermann, B. C., Schutte, S., Velder, J., Aksu, Y., Krause, W., Raduchel, B. Synthesis and characterization of water-soluble tin-based metallodendrimers. Organometallics 2003, 22, 2034–2041; https://doi.org/10.1021/om021011z.Suche in Google Scholar
15. Schumann, H., Aksu, Y., Schutte, S., Wassermann, B. C., Muehle, S. H. Synthesis and characterization of new silicon-centred tin-dendrimers Si[CH2CH2SnR3]4 Single-crystal X-ray structure of the tetrahydrofuran adduct of tetrakis [2-(tribromostannyl)ethyl]silane. J. Organomet. Chem. 2006, 691, 4717–4724; https://doi.org/10.1016/j.jorganchem.2006.07.017.Suche in Google Scholar
16. Schumann, H., Aksu, Y., Wassermann, B. C. Convergent synthesis and characterization of organotindendrimers Sn{(CH2)nSn[(CH2)4SnPh3]}4 (n = 3, 4). Organometallics 2006, 25, 3428–3434; https://doi.org/10.1021/om0602005.Suche in Google Scholar
17. Schumann, H., Wassermann, B. C., Frackowiak, M., Omotowa, B., Schutte, S., Velder, J., Muehle, S. H., Krause, W. Si(CH2CH2SnH3)4—a unique organotin hydride featuring 12 SnH units in a dendritic molecule. Single- crystal X-ray structures of tetrakis(2-stannylethylene)silane and tetrakis[2- (triphenylstannyl)ethylene]silane. J. Organomet. Chem. 2000, 609, 189–195; https://doi.org/10.1016/s0022-328x(00)00188-1.Suche in Google Scholar
18. Schager, F., Goddard, R., Seevogel, K., Poerschke, K.-R. Synthesis, structure and properties of {(Me3Si)2CH}2SnH(OH). Organometallics 1998, 17, 1546–1551; https://doi.org/10.1021/om970997n.Suche in Google Scholar
19. Blom, R., Haaland, A. A modification of the Schomaker—Stevenson rule for prediction of single bond distances. J. Mol. Struct. 1985, 128, 21–27; https://doi.org/10.1016/0022-2860(85)85036-5.Suche in Google Scholar
© 2022 Yilmaz Aksu et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Artikel in diesem Heft
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

