Abstract
C11H15N5, monoclinic, P21/c (no. 14), a = 9.5677(16) Å, b = 8.1755(15) Å, c = 14.846(3) Å, β = 93.783(4)°, V = 1158.7(4) Å3, Z = 4, R gt (F) = 0.0473, wR ref (F2) = 0.1425, T = 173 K.
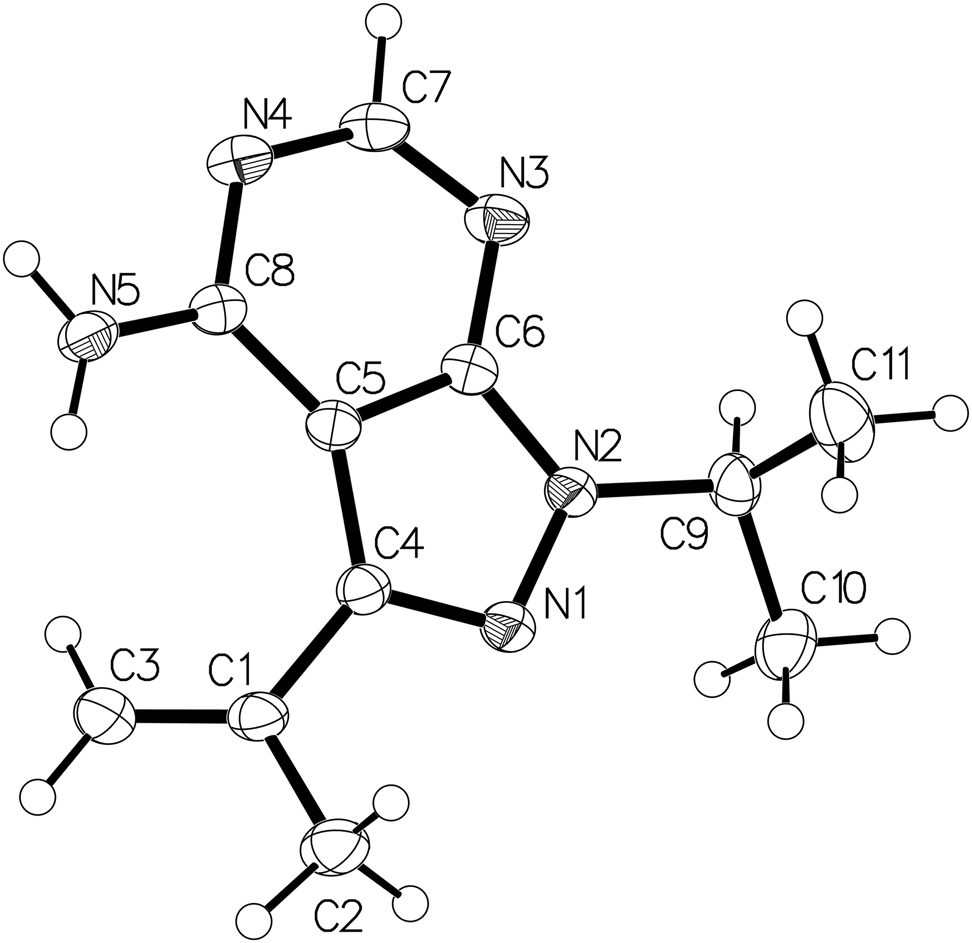
The molecular structure is shown in the figure. Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.28 × 0.15 × 0.12 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω |
| θmax, completeness: | 27.5°, 99% |
| N(hkl)measured, N(hkl)unique, Rint: | 7761, 2634, 0.033 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2078 |
| N(param)refined: | 148 |
| Programs: | Bruker [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| C1 | 0.29269 (16) | 0.38109 (19) | 0.81505 (10) | 0.0255 (4) |
| C2 | 0.39312 (17) | 0.4526 (2) | 0.88640 (11) | 0.0342 (4) |
| H2A | 0.4650 | 0.3714 | 0.9044 | 0.051* |
| H2B | 0.3422 | 0.4833 | 0.9390 | 0.051* |
| H2C | 0.4378 | 0.5497 | 0.8624 | 0.051* |
| C3 | 0.15865 (17) | 0.3502 (2) | 0.82834 (12) | 0.0349 (4) |
| H3A | 0.1007 | 0.2980 | 0.7825 | 0.042* |
| H3B | 0.1212 | 0.3804 | 0.8836 | 0.042* |
| C4 | 0.35130 (15) | 0.33392 (18) | 0.72866 (10) | 0.0212 (3) |
| C5 | 0.28863 (15) | 0.33403 (17) | 0.63892 (10) | 0.0205 (3) |
| C6 | 0.38771 (15) | 0.25893 (17) | 0.58742 (10) | 0.0212 (3) |
| C7 | 0.25267 (17) | 0.27983 (18) | 0.46146 (11) | 0.0269 (4) |
| H7 | 0.2349 | 0.2569 | 0.3990 | 0.032* |
| C8 | 0.16693 (14) | 0.39434 (17) | 0.58947 (10) | 0.0207 (3) |
| C9 | 0.63453 (15) | 0.15545 (19) | 0.61750 (11) | 0.0254 (4) |
| H9 | 0.6144 | 0.0745 | 0.5678 | 0.031* |
| C10 | 0.70884 (18) | 0.0665 (2) | 0.69639 (13) | 0.0388 (4) |
| H10A | 0.7315 | 0.1442 | 0.7455 | 0.058* |
| H10B | 0.7954 | 0.0174 | 0.6771 | 0.058* |
| H10C | 0.6477 | −0.0195 | 0.7176 | 0.058* |
| C11 | 0.72209 (19) | 0.2932 (2) | 0.58098 (14) | 0.0407 (5) |
| H11A | 0.6701 | 0.3448 | 0.5295 | 0.061* |
| H11B | 0.8103 | 0.2485 | 0.5615 | 0.061* |
| H11C | 0.7422 | 0.3747 | 0.6285 | 0.061* |
| N1 | 0.47968 (13) | 0.26930 (15) | 0.73023 (9) | 0.0233 (3) |
| N2 | 0.50004 (12) | 0.22216 (15) | 0.64367 (8) | 0.0215 (3) |
| N3 | 0.37534 (14) | 0.22748 (16) | 0.49757 (9) | 0.0257 (3) |
| N4 | 0.15002 (13) | 0.36040 (15) | 0.50025 (9) | 0.0247 (3) |
| N5 | 0.06744 (13) | 0.48352 (16) | 0.62477 (9) | 0.0266 (3) |
| H5A | −0.0051 | 0.5175 | 0.5903 | 0.032* |
| H5B | 0.0743 | 0.5084 | 0.6826 | 0.032* |
Source of material
A mixture of 3-iodo-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine (152 mg, 0.5 mmol), 4,4,5,5-tetramethyl-2-(prop-1-en-2-yl)-1,3,2-dioxaborolane (42 mg, 2.5 mmol), DMF (15 mL), Pd(dppf)Cl2 (20 mg, 0.03 mmol), Na2CO3 (159 mg, 1.5 mmol), and H2O (0.5 mL) were added and seald in a tube. The reaction system was heated to 100 °C for 2 h and monitored by thin-layer chromatography. After the reaction completed, saturated sodium chloride solution (20 mL) was added. The mixture was extracted with DCM (20 mL) three times. The organic phases were combined, then dried over anhydrous sodium sulfate, and evaporated under reduced pressure. The product was separated by silica gel column chromatography with methanol and dichloromethane (v/v = 5/95) as the eluent to obtain the title compound (yellow solid, 85.8 mg, yield 79%).
Experimental details
All hydrogen atoms were placed in theoretical positions and refined in riding model with the Uiso values set to 1.2 times those of the attached atoms.
Comment
Purine is a heterocyclic aromatic organic compound produced during metabolism in organisms with a crucial role in human health [5]. The purine derivatives have well antitumor and/or antiviral potential in drug field [6], and many researchers have developed new synthetic methods and effective drugs based on purine backbones [7], [8], [9]. For instance, ganciclovir [10] and abacavir [11] have been applied as antivirals, and 6-benzylaminopurine and isopentenyladenine have been developed for plant-growth-regulating pesticides [12]. The title compound contain an isomer of the aforementioned purine core, namely the 1H-pyrazolo[3,4-d]pyrimidine. Based on the above reasons, many 1H-pyrazolo[3,4-d]pyrimidine derivatives and their crystal structures have been reported [13], [14], [15], [16], [17], [18].
The figure shows the title compound. There are one isopropyl group, one prop-1-en-2-yl group and one amino group on the backbone, and the bond lengths of C4–C1, N2–C9, and C8–N5 bonds are 1.484(3) Å, 1.473(2) Å, and 1.333(2) Å, respectively. The pyrimidine group and pyrazole group in the structure are almost in the same plane. The amino substituent on the pyrimidine ring is in the same plane as the core system, and the dihedral angle between the plane formed by the amino group and the core system plane is 179.7. For the title molecule, all bond distances and angles are consistent with the expectation [13, 16, 17].
Funding source: Natural Science Foundation of Shaanxi Province http://dx.doi.org/10.13039/501100007128
Award Identifier / Grant number: 2021JM-540
Award Identifier / Grant number: 2021JQ-885
Funding source: Key Breeding Program by Collaborative Innovation Center of Green Manufacturing Technology for Traditional Chinese Medicine in Shaanxi Province http://dx.doi.org/10.13039/501100009103
Award Identifier / Grant number: 2019XT-1-05
Award Identifier / Grant number: 2019XT-2-05
Funding source: Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang City http://dx.doi.org/10.13039/501100011710
Award Identifier / Grant number: 2021QXNL-PT-0008
Funding source: Scientific research plan project of Shaanxi Provincial Department of Education http://dx.doi.org/10.13039/501100009103
Award Identifier / Grant number: 21JK0510
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This study was financially supported by the Natural Science Foundation of Shaanxi Province (2021JM-540, 2021JQ-885), Key Breeding Program by Collaborative Innovation Center of Green Manufacturing Technology for Traditional Chinese Medicine in Shaanxi Province (2019XT-1-05, 2019XT-2-05), Key Laboratory of Molecular Imaging and Drug Synthesis of Xianyang City (2021QXNL-PT-0008), Scientific research plan project of Shaanxi Provincial Department of Education (21JK0510).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruker. SMART APEX-II. Bruker AXS Inc.: Madison, WI, USA, 2006.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
5. Zhou, D., Xie, D., He, F., Song, B., Hu, D. Antiviral properties and interaction of novel chalcone derivatives containing a purine and benzenesulfonamide moiety. Bioorg. Med. Chem. Lett 2018, 28, 2091–2097; https://doi.org/10.1016/j.bmcl.2018.04.042.Search in Google Scholar PubMed
6. Wang, H. Y., Yu, K. K., Tan, C. Y., Li, K., Liu, Y. H., Shi, L., Lu, K., Yu, X.-Q. Three-in-one: information encryption, anti-counterfeiting and LD-tracking of multifunctional purine derivatives. J. Mater. Chem. C 2021, 9, 2864–2872; https://doi.org/10.1039/d0tc05221c.Search in Google Scholar
7. Avasthi, K., Kumar, A., Aswal, S., Kant, R., Raghunandan, R., Maulik, P. R., Khanna, R. S., Ravikumar, K. Role of arene interactions and substituent effects in conformational (syn/anti) control of 1,2-diarylethanes. CrystEngComm 2012, 14, 383–388; https://doi.org/10.1039/c1ce06001e.Search in Google Scholar
8. Liu, M., Li, J., Chai, H., Zhang, K., Yang, D., Zhang, Q., Shi, D. A convenient four-component one-pot strategy toward the synthesis of pyrazolo[3,4-d]pyrimidines. Beilstein J. Org. Chem. 2015, 11, 2125–2131; https://doi.org/10.3762/bjoc.11.229.Search in Google Scholar PubMed PubMed Central
9. Lu, S. H., Liu, P. L., Wong, F. F. Vilsmeier reagent-mediated synthesis of 6-[(formyloxy)methyl]-pyrazolopyrimidines via a one-pot multiple tandem reaction. RSC Adv. 2015, 5, 47098–47107; https://doi.org/10.1039/c5ra07707a.Search in Google Scholar
10. Sahu, P. K., Umme, T., Yu, J., Nayak, A., Kim, G., Noh, M., Lee, J. Y., Kim, D. D., Jeong, L. S. Selenoacyclovir and selenoganciclovir: discovery of a new template for antiviral agents. J. Med. Chem. 2015, 58, 8734–8738; https://doi.org/10.1021/acs.jmedchem.5b00804.Search in Google Scholar PubMed
11. Schlüter-Vorberg, L., Prasse, C., Ternes, T. A., Mückter, H., Coors, A. Toxification by transformation in conventional and advanced wastewater treatment: the antiviral drug acyclovir. Environ. Sci. Technol. Lett. 2015, 2, 342–346.10.1021/acs.estlett.5b00291Search in Google Scholar
12. Xu, F., Yang, Z., Chen, X., Jin, P., Wang, X., Zheng, Y. 6-Benzylaminopurine delays senescence and enhances health-promoting compounds of harvested broccoli. J. Agric. Food Chem. 2012, 60, 234–240; https://doi.org/10.1021/jf2040884.Search in Google Scholar PubMed
13. Zvoníček, V., Skořepová, E., Dušek, M., Žvátora, P., Šoóš, M. Ibrutinib polymorphs: crystallographic study. Cryst. Growth Des. 2018, 18, 1315–1326.10.1021/acs.cgd.7b00923Search in Google Scholar
14. Sprang, S., Scheller, R., Rohrer, D., Sundaralingam, M. Conformational analysis of 8-azanucleosides. Crystal and molecular structure of 8-azatubercidin monohydrate, a nucleoside analog exhibiting the “high anti” conformation. J. Am. Chem. Soc. 1978, 100, 2867–2872; https://doi.org/10.1021/ja00477a049.Search in Google Scholar
15. Al-Azmi, A. DFT study on two plausible mechanistic routes to pyrazolo[3,4-d]pyrimidine-4-amines from pyrazoloformimidate. Curr. Org. Chem. 2020, 24, 216–229.10.2174/1385272824666200203122450Search in Google Scholar
16. Wang, T., Xiong, J., Wang, W., Li, R., Tang, X., Xiong, F. Tunable regioselective synthesis of pyrazolo[3,4-d]pyrimidine derivatives via aza-Wittig cyclization and dimroth-type rearrangement. RSC Adv. 2015, 5, 19830–19837; https://doi.org/10.1039/c4ra15777j.Search in Google Scholar
17. Ogurtsov, V. A., Rakitin, O. A. 6-(Chloromethyl)-N,1-dimethyl-1H-pyrazolo[3,4-d] pyrimidin-4-amine. Molbank 2021, 2021, M1294; https://doi.org/10.3390/m1294.Search in Google Scholar
18. Oliveira-Campos, A. M. F., Sivasubramanian, A., Rodrigues, L. M., Seijas, J. A., Pilar Vázquez-Tato, M., Peixoto, F., Abreu, C. G., Cidade, H., Oliveira, A. E., Pinto, M. Substituted pyrazolo[3,4-d]pyrimidines: microwave-assisted, solvent-free synthesis and biological evaluation. Helv. Chim. Acta 2008, 91, 1336–1345.10.1002/hlca.200890145Search in Google Scholar
© 2022 Yanjiao Wang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

