Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
Abstract
C40H19EuF8N4O9, triclinic,
Table 1 contains crystallographic data and Table 2 contains the list of the atoms including atomic coordinates and displacement parameters.
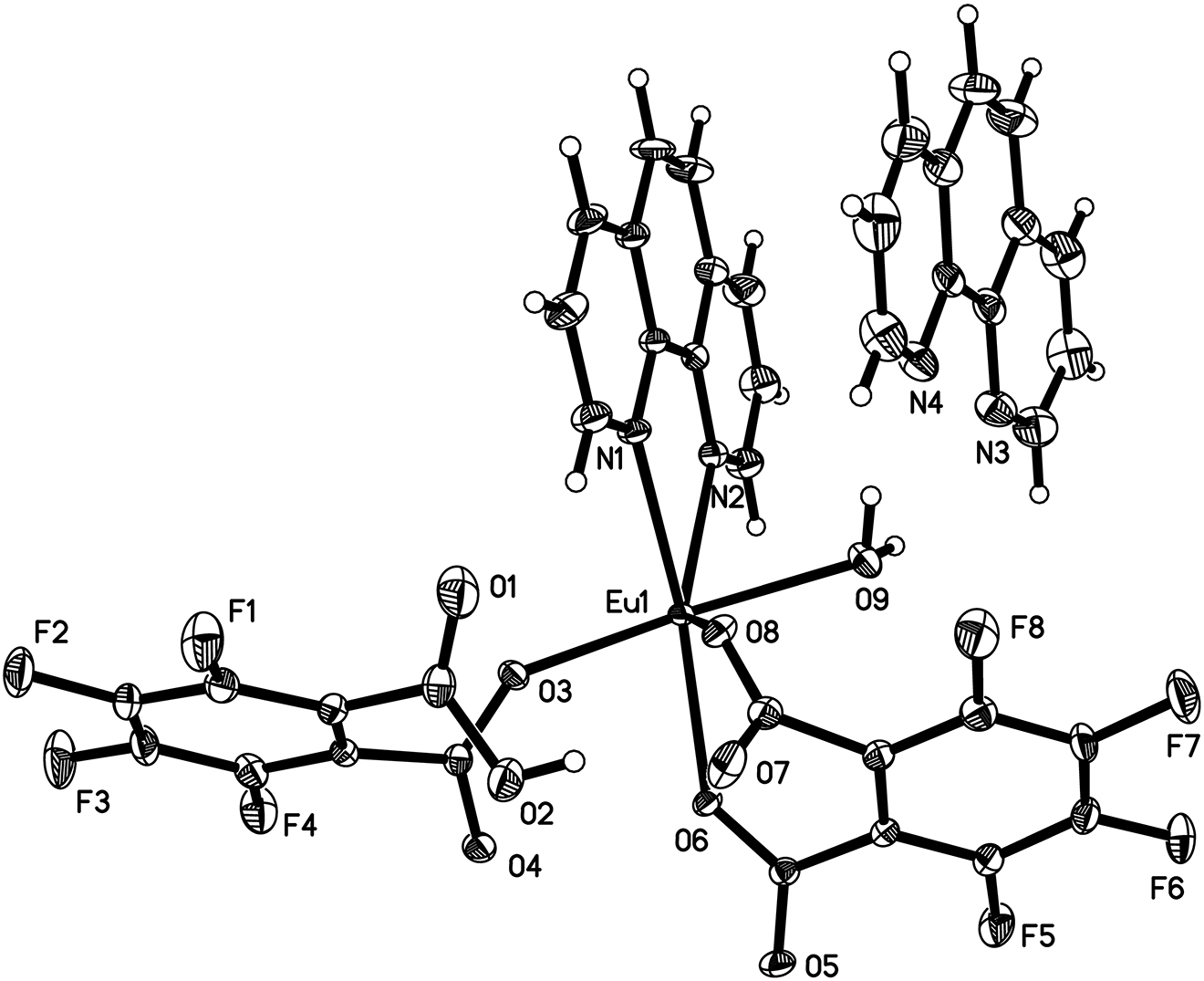
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.20 × 0.19 × 0.18 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.88 mm−1 |
| Diffractometer, scan mode: | SuperNova, ω |
| θmax, completeness: | 29.3°, >99% |
| N(hkl)measured, N(hkl)unique, Rint: | 15,476, 8124, 0.036 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 7356 |
| N(param)refined: | 561 |
| Programs: | CrysAlisPRO [1], Olex2 [2], SHELX [3, 4] |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2).
| Atom | x | y | z | Uiso*/Ueq |
|---|---|---|---|---|
| Eu1 | 0.41561 (2) | 0.83464 (2) | 0.44811 (2) | 0.01876 (5) |
| F1 | 0.46047 (18) | 0.79923 (18) | 0.99321 (15) | 0.0493 (5) |
| F2 | 0.70361 (19) | 0.87105 (17) | 1.08322 (14) | 0.0460 (5) |
| F3 | 0.86950 (19) | 1.01006 (19) | 1.00351 (17) | 0.0581 (6) |
| F4 | 0.79489 (17) | 1.07238 (17) | 0.83285 (15) | 0.0444 (5) |
| F5 | 0.17521 (18) | 1.12051 (16) | 0.31939 (15) | 0.0432 (5) |
| F6 | −0.04741 (18) | 0.99019 (18) | 0.20795 (14) | 0.0452 (5) |
| F7 | −0.17454 (18) | 0.80717 (18) | 0.26702 (16) | 0.0535 (6) |
| F8 | −0.06679 (18) | 0.74943 (17) | 0.43103 (17) | 0.0501 (5) |
| O1 | 0.3065 (2) | 0.7466 (2) | 0.7838 (2) | 0.0502 (7) |
| O2 | 0.3219 (2) | 0.9269 (2) | 0.78681 (18) | 0.0407 (6) |
| H2 | 0.2585 | 0.9008 | 0.7403 | 0.061* |
| O3 | 0.49453 (19) | 0.88944 (16) | 0.63749 (15) | 0.0252 (5) |
| O4 | 0.5758 (2) | 1.07722 (17) | 0.69839 (16) | 0.0300 (5) |
| O5 | 0.3492 (2) | 1.17246 (17) | 0.50666 (17) | 0.0322 (5) |
| O6 | 0.38206 (17) | 1.01242 (16) | 0.51217 (15) | 0.0221 (4) |
| O7 | 0.1417 (2) | 0.8916 (2) | 0.63660 (17) | 0.0390 (6) |
| O8 | 0.23770 (18) | 0.80148 (17) | 0.52914 (16) | 0.0268 (5) |
| O9 | 0.22775 (19) | 0.75510 (18) | 0.31263 (16) | 0.0321 (5) |
| H9A | 0.1898 | 0.6823 | 0.3082 | 0.048* |
| H9B | 0.2464 | 0.7512 | 0.2488 | 0.048* |
| N1 | 0.3694 (2) | 0.63497 (19) | 0.46945 (19) | 0.0238 (5) |
| N2 | 0.4643 (2) | 0.6908 (2) | 0.30306 (19) | 0.0250 (6) |
| C1 | 0.5858 (3) | 0.9623 (2) | 0.8163 (2) | 0.0229 (6) |
| C2 | 0.4994 (3) | 0.8915 (2) | 0.8583 (2) | 0.0245 (7) |
| C3 | 0.5413 (3) | 0.8650 (3) | 0.9493 (2) | 0.0285 (7) |
| C4 | 0.6646 (3) | 0.9028 (3) | 0.9981 (2) | 0.0317 (8) |
| C5 | 0.7484 (3) | 0.9727 (3) | 0.9584 (3) | 0.0342 (8) |
| C6 | 0.7078 (3) | 1.0036 (3) | 0.8688 (2) | 0.0286 (7) |
| C7 | 0.5471 (3) | 0.9781 (2) | 0.7070 (2) | 0.0218 (6) |
| C8 | 0.3637 (3) | 0.8447 (3) | 0.8053 (2) | 0.0302 (7) |
| C9 | 0.1773 (3) | 1.0028 (2) | 0.4353 (2) | 0.0219 (6) |
| C10 | 0.1139 (3) | 0.9047 (3) | 0.4619 (2) | 0.0235 (6) |
| C11 | −0.0040 (3) | 0.8430 (3) | 0.4064 (3) | 0.0299 (7) |
| C12 | −0.0600 (3) | 0.8717 (3) | 0.3214 (2) | 0.0321 (8) |
| C13 | 0.0022 (3) | 0.9648 (3) | 0.2933 (2) | 0.0302 (7) |
| C14 | 0.1176 (3) | 1.0302 (3) | 0.3509 (2) | 0.0270 (7) |
| C15 | 0.3093 (3) | 1.0691 (2) | 0.4884 (2) | 0.0215 (6) |
| C16 | 0.1702 (3) | 0.8630 (2) | 0.5501 (2) | 0.0253 (7) |
| C17 | 0.3238 (3) | 0.6063 (3) | 0.5500 (3) | 0.0335 (8) |
| H17 | 0.3120 | 0.6623 | 0.6015 | 0.040* |
| C18 | 0.2925 (3) | 0.4967 (3) | 0.5619 (3) | 0.0369 (8) |
| H18 | 0.2599 | 0.4808 | 0.6196 | 0.044* |
| C19 | 0.3101 (3) | 0.4143 (3) | 0.4888 (3) | 0.0363 (8) |
| H19 | 0.2908 | 0.3412 | 0.4963 | 0.044* |
| C20 | 0.3813 (3) | 0.3570 (3) | 0.3217 (3) | 0.0428 (9) |
| H20 | 0.3631 | 0.2828 | 0.3260 | 0.051* |
| C21 | 0.4289 (3) | 0.3851 (3) | 0.2418 (3) | 0.0448 (10) |
| H21 | 0.4432 | 0.3298 | 0.1911 | 0.054* |
| C22 | 0.5131 (3) | 0.5309 (3) | 0.1499 (3) | 0.0444 (9) |
| H22 | 0.5301 | 0.4783 | 0.0984 | 0.053* |
| C23 | 0.5408 (3) | 0.6399 (3) | 0.1465 (3) | 0.0439 (9) |
| H23 | 0.5768 | 0.6627 | 0.0926 | 0.053* |
| C24 | 0.5151 (3) | 0.7173 (3) | 0.2241 (3) | 0.0355 (8) |
| H24 | 0.5347 | 0.7918 | 0.2205 | 0.043* |
| C25 | 0.4374 (3) | 0.5815 (2) | 0.3077 (2) | 0.0248 (7) |
| C26 | 0.3863 (3) | 0.5513 (2) | 0.3944 (2) | 0.0237 (6) |
| C27 | 0.3575 (3) | 0.4393 (3) | 0.4019 (3) | 0.0302 (7) |
| C28 | 0.4588 (3) | 0.4978 (3) | 0.2310 (2) | 0.0326 (8) |
| N3 | 0.1721 (3) | 0.6067 (2) | 0.1003 (2) | 0.0401 (7) |
| N4 | 0.0919 (3) | 0.5328 (2) | 0.2676 (2) | 0.0404 (7) |
| C29 | 0.0470 (4) | 0.4953 (4) | 0.3439 (3) | 0.0522 (10) |
| H29 | 0.0420 | 0.5479 | 0.4023 | 0.063* |
| C30 | 0.0064 (4) | 0.3823 (4) | 0.3430 (3) | 0.0601 (12) |
| H30 | −0.0270 | 0.3600 | 0.3982 | 0.072* |
| C31 | 0.0168 (4) | 0.3055 (4) | 0.2600 (4) | 0.0560 (11) |
| H31 | −0.0068 | 0.2295 | 0.2586 | 0.067* |
| C32 | 0.0727 (4) | 0.2634 (3) | 0.0852 (4) | 0.0589 (12) |
| H32 | 0.0525 | 0.1872 | 0.0827 | 0.071* |
| C33 | 0.1101 (4) | 0.2983 (3) | 0.0034 (3) | 0.0600 (12) |
| H33 | 0.1142 | 0.2459 | −0.0555 | 0.072* |
| C34 | 0.1772 (4) | 0.4547 (4) | −0.0818 (3) | 0.0580 (12) |
| H34 | 0.1787 | 0.4042 | −0.1431 | 0.070* |
| C35 | 0.2069 (4) | 0.5661 (4) | −0.0758 (3) | 0.0600 (12) |
| H35 | 0.2289 | 0.5933 | −0.1327 | 0.072* |
| C36 | 0.2039 (4) | 0.6390 (3) | 0.0175 (3) | 0.0506 (10) |
| H36 | 0.2259 | 0.7156 | 0.0215 | 0.061* |
| C37 | 0.1404 (3) | 0.4954 (3) | 0.0947 (3) | 0.0340 (8) |
| C38 | 0.0991 (3) | 0.4565 (3) | 0.1818 (3) | 0.0327 (8) |
| C39 | 0.0630 (3) | 0.3407 (3) | 0.1762 (3) | 0.0419 (9) |
| C40 | 0.1438 (3) | 0.4150 (3) | 0.0047 (3) | 0.0436 (9) |
Source of material
A mixture of Eu(NO3)3·6H2O (0.0893 g, 0.2 mmol), 3,4,5,6-tetrafluorophthalic acid (0.054 g, 0.2 mmol) and phenanthroline (35.7 mg, 0.15 mmol) were dissolved in 8 mL of deionized water. The mixture was sealed in a 25 mL Teflon-lined steel autoclave after ultrasound treatment for 15 min and heated at 110 °C for 72 h. The mixture was cooled to room temperature at a rate of 2 °C/h. Colorless block crystals were isolated by filtration, washed with distilled water and dried in air (CCDC number 2153477).
Experimental details
An empirical absorption correction using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm was done [1].
Using Olex2 [2], the structure was solved with the ShelXT [3] structure solution program and refined with the ShelXL [4] refinement package.
The carbon bound hydrogen atoms were placed in calculated positions and refined using a riding model on attached atoms.
Comment
In recent decades, lanthanide complexes (Ln-CPs) have received particular attention because of their excellent luminescent properties such as high color purities, strong luminescence intensity, long lifetime and fast response time [5]. We are particularly interested in Eu-CPs because it can emit red fluorescence which is sensitive to naked eyes and can be used in multicolor displays and fluorescence probe [6, 7]. On the other hand, a perfluorinated multicarboxylate is an excellent ligand to construct Eu-CPs because it has a variety of coordination modes and can improve the luminescence properties of complexes [8, 9]. In addition, rigid N-containing ligands are versatile building blocks for luminescent materials and metal complexes [10], [11], [12], [13]. In this work, a binuclear Eu-CP was constructed with 2,3,4,5-tetrafluorophthalic acid (TFPA) and phenanthroline as main and auxiliary ligand, respectively. Both ligands can improve the luminescence properties of Eu-CP by antenna effect.
The asymmetric unit of the title compound consists of one Eu(III) ion, one phen ligand, one tetrafluorophthalato (TFP) ligand, one 2-carboxy-3,4,5,6-tetrafluorobenzoate ligand (TFPH) one coordinated water molecule and one cocrystallized phen molecule. Each Eu(III) ion is nine-coordinated [Eu1O7N2] by two N atoms of phen ligand, one O atoms from the coordinated water molecule and six O atoms from two TFP and two TFPH ligands. TFPH is mono-deprotonated and connected two adjacent Eu(III) cations with the carboxyl anion in mono coordination mode TFP is fully deprotonated and connected two adjacent Eu(III) cations in a chelating fashion (see the systematic name in the title). This coordination is different from similar lanthanide compound based on fluorine substituted ligands [8, 9]. The distances of Eu–O (carboxylate) and Eu–N range from 2.3900(19) to 2.683(2) and from 2.545(2) to 2.590(2) Å, respectively, and the distance of Eu–O (water) is 2.414(2) Å, which are consistent with the values for reported Eu(III) complexes [14, 15]. The carboxylate groups from TFP and TFPH form four bridges and link two adjacent Eu(III) cations, resulting in the dinuclear units with Eu···Eu separation of just 3.9813(2) Å. The cocrystallized phen molecules are linked to the {Eu2} dinuclear units via O–H···N hydrogen bonds. The dinuclear units are further connected into a three dimensional supramolecular structure via C–H···O hydrogen bonds and π–π interactions.
Funding source: Training Program for Young Cadre Teachers of Higher Education Institutions in Henan Province http://dx.doi.org/10.13039/501100009101, “Education Department of Henan Province”
Award Identifier / Grant number: 2018GGJS128
Funding source: Science and Technology Development Project in Henan Province http://dx.doi.org/10.13039/501100011447, “Science and Technology Department of Henan Province”
Award Identifier / Grant number: 172101410037
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This work was supported by the Training Program for Young Cadre Teachers of Higher Education Institutions in Henan Province (No. 2018GGJS128) and Science and Technology Development Project in Henan Province (No. 172101410037).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Oxford Diffraction Ltd. CrysAlisPro (version 1.171.39.6a); Rigaku Oxford Diffraction: England, 2018.Search in Google Scholar
2. Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K., Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. SHELXTL – integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Li, R. F., Li, R. H., Liu, X. F., Chang, X. H., Feng, X. Lanthanide complexes based on a conjugated pyridine carboxylate ligand: structures, luminescence and magnetic properties. RSC Adv. 2020, 10, 6192–6199; https://doi.org/10.1039/c9ra10975g.Search in Google Scholar PubMed PubMed Central
6. Zhang, X., Ma, Q., Liu, X., Niu, H., Luo, L., Li, R., Feng, X. A turn-off Eu-MOF@Fe2+ sensor for the selective and sensitive fluorescence detection of bromate in wheat flour. Food Chem. 2022, 382, 132379; https://doi.org/10.1016/j.foodchem.2022.132379.Search in Google Scholar PubMed
7. Liu, X., Du, L., Li, R., Ma, N., You, M., Feng, X. Different effects in the selective detection of aniline and Fe3+ by lanthanide-based coordination polymers containing multiple reactive sites. CrystEngComm 2020, 22, 2837–2844; https://doi.org/10.1039/d0ce00238k.Search in Google Scholar
8. Feng, X., Wang, R., Wang, X. Y., Zhang, Y. Y., Jia, X. X. Crystal structure of poly[diaqua-di-μ2-hydroxido-(μ4-3,4,5,6-tetrafluoro-1,2-phthalato-κ5O,O:O′:O″:O‴) disamarium(III)] – bipyridine (2/1), C21H11N F16O12Sm2. Z. Kristallogr. N. Cryst. Struct. 2019, 234, 55–57.10.1515/ncrs-2018-0138Search in Google Scholar
9. Li, J. J., Fan, T. T., Qu, X. L., Han, H. L., Li, X. Temperature-induced 1D lanthanide polymeric frameworks based on Lnn (n = 2, 2, 4, 6) cores: synthesis, crystal structures and luminescence properties. Dalton Trans. 2016, 45, 2924–2935; https://doi.org/10.1039/c5dt04262c.Search in Google Scholar PubMed
10. Chang, X. H., Qin, J. H., Ma, L. F., Wang, J. G., Wang, L. Y. Two-and three-dimensional divalent metal coordination polymers constructed from a new tricarboxylate linker and dipyridyl ligands. Cryst. Growth Des. 2012, 12, 4649–4657; https://doi.org/10.1021/cg3008602.Search in Google Scholar
11. Wang, Y. F., Tai, J. H., Yan, X. W., Zhao, M. Y., Wang, L. Y. Crystal structures and magnetic properties of two isomorphic frameworks based on 3-(1H-pyrazol-4-yl)-5-(pyridin-2-yl)-1, 2, 4-triazole and 1,2,4,5-benzenetetracarboxylic acid. Chin. J. Inorg. Chem. 2018, 34, 1121–1126.Search in Google Scholar
12. Xin, L. Y., Yang, H. B., Guo, H. Crystal structure of bis (phenanthroline)-zinc(II) 5-(4-carboxy-2-nitrophenoxy) isophthalic hydrate, Zn(C15H9NO9)(C12H8N2)2·(H2O). Z. Kristallogr. N. Cryst. Struct. 2012, 227, 407–409; https://doi.org/10.1524/ncrs.2012.0166.Search in Google Scholar
13. Li, J. X., Du, Z. X. A binuclear cadmium (II) cluster based on π···π stacking and halogen···halogen interactions: synthesis, crystal analysis and fluorescent properties. J. Cluster Sci. 2020, 31, 507–511; https://doi.org/10.1007/s10876-019-01666-w.Search in Google Scholar
14. Wang, H. R., Qin, J. H., Huang, C., Han, Y., Xu, W., Hou, H. Mono/bimetallic water-stable lanthanide coordination polymers as luminescent probes for detecting cations, anions and organic solvent molecules. Dalton Trans. 2016, 45, 12710–12716; https://doi.org/10.1039/c6dt02321e.Search in Google Scholar PubMed
15. Qin, J. H., Ma, B., Liu, X. F., Lu, H. L., Dong, X. Y., Zang, S. Q., Hou, H. Ionic liquid directed syntheses of water-stable Eu- and Tb-organic-frameworks for aqueous-phase detection of nitroaromatic explosives. Dalton Trans. 2015, 44, 14594–14603; https://doi.org/10.1039/c5dt02054a.Search in Google Scholar PubMed
© 2022 Xiao-Yu Zhang et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co
Articles in the same Issue
- Frontmatter
- New Crystal Structures
- Crystal structure of N-((3s,5s,7s)-adamantan-1-yl)-2-(3-benzoylphenyl)propanamide, C26H29NO2
- The crystal structure of bis(μ2-5-chloro-2-oxido-N-(1-oxidopropylidene)benzohydrazonato-κ5 N,O,O′:N′,O′′)-octakis(pyridine-κ1 N)trinickel(II) C60H56Cl2N12Ni3O6
- The crystal structure of 3-(4-chlorophenyl)-1,5-di-p-tolylpentane-1,5-dione, C25H23ClO2
- The crystal structure of 2,4,4-triphenyl-4H-benzo[b][1,4]oxaphosphinin-4-ium bromide – dichloromethane (1/1), C27H22BrCl2OP
- The crystal structure of 2-(3,6-di-tert-butyl-1,8-diiodo-9H-carbazol-9-yl)acetonitrile, C22H24I2N2
- Crystal structure of 3-phenylpropyl 2-(6-methoxynaphthalen-2-yl)propanoate, C23H24O3
- The crystal structure of (4-fluorophenyl)(5-(hydroxymethyl)furan-2-yl)methanol, C12H11FO3
- Crystal structure of the dihydrate of tetraethylammonium 1,3,5-thiadiazole-5-amido-2-carbamate, C11H27N5O4S
- Crystal structure of (Z)-4-[(p-tolylamino)(furan-2-yl)methylene]-3-phenyl-1-1-p-tolyl-1H-phenyl-1H-pyrazol-5(4H)-one, C28H23N3O2
- The crystal structure of (E)-3-(2-chlorophenyl)-1-ferrocenylprop-2-en-1-one, C19H15ClFeO
- The pseudosymmetric crystal structure of 3-((1R,2S)-1-methylpyrrolidin-1-ium-2-yl)pyridin-1-ium hexachloridostannate(IV), C10H16N2SnCl6
- Crystal structure of (2-(1-hydroxyheptyl)octahydro-8aH-chromene-5,8,8a-triol), C16H30O5
- The crystal structure of N-cyclohexyl-3-hydroxy-4-methoxybenzamide, C14H19NO3
- Crystal structure of 1-(4-hydroxybenzyl)-4-methoxy-9,10-dihydrophenanthrene-2,7-diol from Arundina graminifolia, C22H20O4
- The crystal structure of N-cyclopentyl-3-hydroxy-4-methoxybenzamide, C13H17NO3
- The crystal structure of 2,5,5-triphenyl-3,5-dihydro-4H-imidazol-4-one, C21H16N2O
- Crystal structure of 1H-1,2,3-Triazolo[4,5-b]-pyridin-4-ium nitrate, C5H5N5O3
- Crystal structure of (Z)-4-(((4-bromophenyl)amino)(furan-2-yl)methylene)-2,5-diphenyl-2,4-dihydro-3H-pyrazol-3-one, C26H18BrN3O2
- Crystal structure of 2-(4-methoxyphenyl)-3-methyl-1,8-naphthyridine, C16H14N2O
- The crystal structure of 3-([1,1′-biphenyl]-2-yl)-1,2-diphenylbenzo[b]phosphole-1-oxide, C32H23OP
- The crystal structure of ammonium (E)-4-((4-carboxyphenyl)diazenyl)benzoate, C14H13N3O4
- Crystal structure of bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane sulfate, C5H10N8O4S
- The crystal structure of phenantroline-κ2 N,N′-bis(6-phenylpyridine-2-carboxylato-κ2 N,O)copper(II), C36H24N4O4Cu
- The crystal structure of tris(6-methylpyridin-2-yl)phosphine oxide, C18H18N3OP
- The crystal structure of N-(2′-hydroxymethyl-5′-phenyl-3′,4′-dihydro-[1,1′:3′,1″-terphenyl]- 1′(2′H)-yl)-P,P-diphenylphosphinic amide, C37H34NO2P
- Crystal structure of (E)-4-(6-(4-(2-(pyridin-4-yl)vinyl)phenoxy)pyrimidin-4-yl)morpholine, C21H20N4O2
- Crystal structure of 5-(adamantan-1-yl)-3-[(4-trifluoromethylanilino)methyl]-2,3-dihydro-1,3,4-oxadiazole-2-thione, C20H22F3N3OS
- Crystal structure of 2,2-dichloro-1-(4-chloro-1H-indol-1-yl)ethan-1-one, C10H6Cl3NO
- The crystal structure of 4-(((3-bromo-5-(trifluoromethyl)pyridin-2-yl)oxy)methyl)benzonitrile, C28H16Br2F6N4O2
- The crystal structure of 1H-benzimidazole-2-carboxamide, C8H7N3O
- The crystal structure of Histidinium hydrogensquarate, C10H11N3O6
- The crystal structure of 3-amino-5-carboxypyridin-1-ium iodide, C6H7IN2O2
- Crystal structure of (E)-amino(2-(3-ethoxy-4-hydroxybenzylidene)hydrazineyl)methaniminium nitrate hemihydrate C10H16N5O5.5
- Crystal structure of 1,2-bis(4,5-dinitro-1H-imidazol-1-yl)ethane, C8H6N8O8
- The crystal structure of diaqua-bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)manganese(II), C14H12N6O6Mn
- The crystal structure of catena-poly[aqua-2,2′bipyridine-κ2N,N′-(μ2-5-ethoxyisophthalato-κ 4O,O′:Oʺ,O′ʺ)cadmium(II)] monohydrate, C20H20CdN2O7
- The crystal structure of (1S,3R)-1-(4-isopropylphenyl)-3-(methoxycarbonyl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-2-iumchloride monohydrate, C22H27ClN2O3
- Crystal structure of 1-isopropyl-3-(prop-1-en-2-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine, C11H15N5
- The crystal structure of (2,2′-bipyridine-κ2N,N′)- bis(6-phenylpyridine-2-carboxylate-κ2N,O)manganese(II)] monohydrate, C34H26N4O5Mn
- Crystal structure of the cocrystal 1,3,5,7-tetranitro-1,3,5,7-tetrazoctane ─ 2,3-dihydroindole (1/1), C12H17N9O8
- Crystal structure of 3-acetyl-6-hydroxy-2H-chromen-2-one monohydrate, C11H10O5
- Crystal structure of 6,9-diamino-2-ethoxyacridinium 3,5-dinitrobenozate — dimethylsulfoxide — water (1/1/1), C24H27N5O9S
- The crystal structure of 4,4′-bipyridinium bis-(2-hydroxy-3-methoxybenzoate), 2(C8H7.68O4)·C10H8.64N2
- Crystal structure of (Z)-4-(((4-fluorophenyl)amino)(furan-2-yl)methylene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one
- The crystal structure of bis(4-chloro-2-(((2-chloroethyl)imino)methyl)phenolato-κ2N,O)-oxidovanadium(IV), C18H16Cl4N2O3V
- The crystal structure of 17-(bromoethynyl)-17-hydroxy-10, 13-dimethyl- 1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3H-cyclopenta[a]phenanthren-3-one, C21H27BrO2
- The crystal structure of 4-((6-fluoropyridin-2-yloxy)methyl)benzonitrile, C13H9FN2O
- Crystal structure of (Z)-2-(1-bromo-2-phenylvinyl)-5-ethyl-2-methyl-1,3-dioxane-5-carboxylic acid, C15H17Br1O4
- Crystal structure of catena-poly[tribenzyl-κ1C-(μ2-6-oxidopyridin-1-ium-3-carboxylato-κ2O:O’)tin(IV)-dichloromethane-methanol (1/1/1), C29H31Cl2NO4Sn
- Crystal structure of bis{2-(tert-butyl)-6-((E)-((4-((E)-1-(methoxyimino)ethyl)phenyl)imino)methyl)phenolato-κ2N,O}zinc(II), C40H46N4O4Zn
- Crystal structure of diaqua-bis(μ2-2-carboxy-3,4,5,6-tetrafluorobenzoato-κ2O:O′)-bis(phenanthroline-κ2N,N′)-bis(μ2-3,4,5,6-tetrafluorophthalato-κ3O:O,O′)dieuropium(III) – phenanthroline (1/2), C40H19EuF8N4O9
- The crystal structure of diaqua-bis(6-phenylpyridine-2-carboxylato-κ2N,O) manganese(II) — water — dimethylformamide (1/2/1), C27H31N3O9Mn
- The crystal structure of bis(pyrazolo[1,5-a]pyrimidine-3-carboxylato-κ2N,O)-copper(ii), C14H8N6O4Cu
- Crystal structure of poly[(μ2-1-(1-imidazolyl)-4-(imidazol-1-ylmethyl)benzene-κ2N:N′)-(μ3-pyridazine-4,5-dicarboxylate-κ3O:O′:N)]copper(II) hydrate, C19H16CuN6O5
- Crystal structure of acrinidinium tetrafluorohydrogenphthalate, C21H11F4NO4
- Crystal structure of 2-(1H-pyrazol-3-yl-κN)pyridine-κN-bis(2-(2,4-difluorophenyl)pyridinato-κ2C,N)iridium(III) sesquihydrate, C30H18F4IrN5·1.5[H2O]
- Crystal structure of 2-(2-hydroxy-5-nitrophenyl)-5-methyl-1,3-dioxane-5-carboxylic acid, C12H13N1O7
- The crystal structure of 1,2-bis(pyridinium-4-yl)ethane diperchlorate, C12H14N2·2ClO4 – a second polymorph
- The crystal structure of [(1,10-phenantroline-κ2N,N′)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)manganese(II)] monohydrate, C36H26N4O5Mn
- Crystal structure of 1,2-bis(2,2,3,3,5,5,5-heptamethyl-1,1,4,4- tetrakis(trimethylsilyl)pentasilan-1-yl)ditellane, C38H114Si18Te2
- Crystal structure of 1,2-bis(2,4-dinitro-1H-imidazol-1-yl)ethane – dimethylformamide (1/1), C11H13N9O9
- Crystal structure of (Z)-3-((tert-butylamino) methylene)-2-(2-hydroxynaphthalen-1-yl) chroman-4-one, C24H23NO3
- Synthesis and crystal structure of (E)-1-(4-(((E)-3-(tert-butyl)-2-hydroxybenzylidene)amino)phenyl)ethan-1-one O-ethyl oxime, C21H26N2O2
- Crystal structure of the double salt bis(5-amino-1,2,4-triazol-4-ium-3-yl)methane hydrogen oxalate hemioxalate, C8H11N8O6
- Hydrothermal synthesis and crystal structure of catena-poly[diaqua-bis(μ2-4-[(4-pyridinylmethyl)amino]benzoato-κ2N:O)cobalt(II)]–1,2bi(4-pyridyl)ethene–water (1/1/1), C50H50N8O8Co
- Crystal structure of 3-(3-bromophenyl)-1′,3′-dimethyl-2′H,3H,4H-spiro[furo[3, 2-c]chromene-2,5′-pyrimidine]-2′,4,4′,6′(1′H,3′H) tetraone, C22H15BrN2O6
- The crystal structure of poly[aqua-(μ2-4,4′- bis(imidazolyl)biphenyl-κ2N:N′)-(μ2-3-nitrobenzene-1,2-dicarboxylato-κ2O:O′)]copper (II) hydrate, C26H21N5O8Cu
- The crystal structure of bis(4-(6-carboxy-8-ethyl-3-fluoro-5-oxo-5,8-dihydro-1,8-naphthyridin-2- yl)piperazin-1-ium) adipate tetrahydrate, C36H52F2N8O14
- Synthesis and crystal structure of poly[aqua(μ4-(1R,2S,4R)-4-hydroxy-1-((7-hydroxy-3-(4-hydroxy-3-sulfonatophenyl)-4-oxo-4H-chromen-8-yl)methyl)pyrrolidin-1-ium-2-carboxylate-κ4O:O′:O″:O‴)sodium(I)] monohydrate, C21H22NNaO12S
- Crystal structure of chlorido-(η6-toluene)(2,2′-bipyridine-κ2N,N′)ruthenium(II) hexafluorophosphate, C17H16ClN2RuPF6
- The crystal structure of (R)-6-hydroxy-8-methoxy-3-methylisochroman-1-one, C11H12O4
- Crystal structure of catena-poly[(5,5,7,12,12,14-hexamethyl -1,4,8,11-tetraazacyclotetradecane- κ4N,N′,Nʺ,N‴)nickel(II)-(μ2-perchlorato-κ2O:O′)] 3,5-dicarboxybenzoate – methanol (1/2), C27H49ClN4NiO12
- The crystal structure of 4-(chloromethyl)benzonitrile, C8H6ClN
- The crystal structure of dimethylammonium 8-[(7,9-dioxo-6,10-dioxaspiro[4.5]decan-8-ylidene)methyl]-9-oxo-6,10-dioxaspiro[4.5]dec-7-en-7-olate, C19H25NO8
- Crystal structure of (2R,3S,4S,5R,6S)-2-(acetoxymethyl)-6-((1-acetyl-5-bromo-4-chloro-1H-indol-3-yl)oxy)tetrahydro-2H-pyran-3,4,5-triyl triacetate hemihydrate C24H25BrClNO11
- The crystal structure of the co-crystal tetrakis[2-(tris(4-methoxyphenyl)stannyl)ethyl]silane – tetrahydrofuran – toluene – tetrahydrofurane (1/1/1), C103H116O13SiSn4
- Crystal structure of methyl 3-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)propanoate, C16H13NO4
- Crystal structure of ethyl (Z)-3-amino-2-cyano-3-(2-oxo-2H-chromen-3-yl)acrylate, C15H12N2O4
- Crystal structure of methyl 2-(1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)acetate, C15H11NO4
- Crystal structure of catena-poly[diaqua-bis(μ2-1,3-di(1H-imidazol-1-yl)propane-κ2N:N′)cobalt(II)] tetrafluoroterephthalate, C26H28N8O6F4Co

